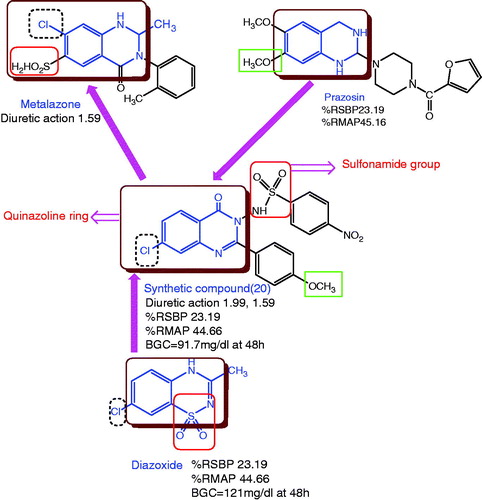
Abstract
To explore the pharmacological and structure–activity relationship of a series of N-substituted-(4-oxo-2-substituted-phenylquinazolin-3-(4H)-yl), substituted benzene sulfonamide derivatives (1–25) were synthesized from substituted anthranilic acids derived amino quinazolines and substituted benzene sulphonamides. All the synthesized compounds were evaluated for their diuretic (by Lipschitz et al. method), antihypertensive activity by non-invasive blood pressure (NIBP) using the tail-cuff method and anti-diabetic potential in rats. Six compounds showing significantly excellent activity were compared with metolazone, prazosin and diazoxide as standards. Compound N-[7-chloro-2-(4-methoxyphenyl)-4-oxoquinazolin-3(4H)-yl]-4 nitrobenzenesulfonamide (20) exhibited most potent of the series.
Introduction
According to Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 6) Guidelines and JNC 7 Guidelines recommended diuretics as first-line drugs in the treatment of uncomplicated hypertensionCitation1, but the major problems associated with the clinical use of diuretics are hypokalemia, hypomagnesaemia, glucose tolerance, hyperuricemia, azotemia, hypocalcaemia, and renal impairment. Metolazone is one of the clinically available diuretic associated with acute liver failure and pancreatitisCitation2, whereas a vasodilator, diazoxide (Potassium channel activator) interferes with insulin release and causes hyperglycaemia as a major side effectCitation3. Additionally, prazosin, a α1 adrenergic receptor antagonist, has received considerable attention in the treatment and management of severe hypertensive disorder. However, the “first dose phenomenon” is a most serious side effect of prazosin which can sometimes lead to syncope (a spontaneous loss of consciousness caused by insufficient blood to the brain)Citation4. Interestingly, all the above-mentionerd compounds possess similar or closely related to quinazoline moiety. Quinazoline derivatives, which belong to the N-containing heterocyclic compounds, have universal concerns due to their wide and distinct biological activities such as diureticCitation5–7, antihypertensiveCitation8, antihistaminicCitation9,Citation10 analgesic and anti-inflammatoryCitation11,Citation12 anticancerCitation13 and anti-HIVCitation14 activities. On the other hand, sulphonamide exhibits acidity in the molecule; these acidic protons enable the formation of the corresponding water-soluble sodium salt and also prevent the carbonic acid from acquiring deprotonated state due to the Zn(II) ion within the enzyme active site which causes diuresisCitation15,Citation16. Recent trends in medicinal chemistry show the popularity of molecular hybridization. It is a new concept in drug design and development based on the combination of pharmacophoric moieties of different bioactive substances to produce a new hybrid compound with improved affinity and efficacy, as compared to the parent drugs. In the present study, we have reported 25 hybridized molecules containing quinazoline and benzene sulphonamide moietiesCitation17.
Considering the above facts and in addition to our work on antihypertensiveCitation18 and diureticsCitation19, we have synthesized a hybrid molecule possessing both diuretic and anti-hypertensive activities with minimum side effects ().
Materials
All the chemicals used were of laboratory grade and procured from E. Merck (Darmstadt, Germany) and S.D. Fine Chemicals (Mumbai, India). Melting points were determined by open capillary tubes in a Hicon melting point apparatus (Hicon, New Delhi, India) and are uncorrected. Purity of the compounds was checked by thin-layer chromatography (TLC) plates (silica gel G), which were visualized by exposing to iodine vapours and UV light. The FTIR spectra were recorded on Bio-rad FTS-135 spectrophotometer (Bio-rad, Cambridge, CA) using KBr pellets; νmax values are given in cm−1. The 1H NMR spectra were taken on a Bruker 400 Ultra shield™ (400 MHz) NMR spectrometer (Bruker, Fremont, CA). Chemical shifts (δ) are expressed in ppm relative to tetramethylsilane (TMS) as an internal standard and coupling constants (J values) are expressed in Hz. Mass spectra recorded on UPLC-MS/MS (Water (Billerica, MA), Q-TOF-ESI and Mass Lyns v 4.1) mass serial no. JAA-272 (Synapt MS-G1) are presented as m/z. Elemental analysis was carried out on CHN Elemental (PerkinElmer 240 analyser, Waltham, MA) using sulphanilic acid as a standard and tungsten (VI) oxide as a combusting agent. Analyses for C, H, N were within ±0.4% of the theoretical values.
Methods
Chemistry
Substituted 2-benzaimidobenzoic acid (a1–a25)
A mixture of substituted anthranilic acid (2 mmol) and substituted benzoyl chloride (2.2 mmol) was stirred at room temperature in the presence of 10% sodium hydroxide (10 ml) for 1 h. The reaction mixture was poured in cold water. The obtained residue was washed with dilute HCl followed by water and recrystallized from ethanol. Yield: 90–95%.
2-(Substituted) phenyl-(substituted)-4H-benzo[d]oxazin-4-one (b1–b25)
Substituted 2-benzaimidobenzoic acid (2 mmol) was heated under reflux in acetic anhydride (10 ml) for 2 h. The reaction mixture was poured onto crushed ice. The solid so obtained was filtered, dried and recrystallized from ethanol. Yield = 85–90%.
3-Amino-(substituted)-2-(substituted) phenylquinazolin-4(3H)-one (c1–c25)
Substituted benzoxazine (2 mmol) was dissolved in methanol and heated under reflux in the presence of hydrazine hydrate (2 ml) for 3 h. The solid obtained was filtered while hot and dried and recrystallized from ethanol. Yield = 70–88%.
N-(substituted-4-oxo-2-substituted-phenylquinazolin-3-(4H)-yl)substituted benzenesulfonamide (1–25) (Hinsberg’s method)
Quinazolin-4(3H)-one derivatives (c1–c25) (1.5 mmol) were dissolved in 10% potassium hydroxide solution and substituted-benzenesulfonylchloride (2 mmol) was added drop-wise with heating until oily layers disappear. The reaction mixture was filtered, acidified with hydrochloric acid and the precipitate so obtained was filtered, dried and recrystallized from ethanol.
N-(4-oxo-2-phenylquinazolin-3(4H)-yl)benzenesulfonamide (1)
Yield: 80%; m.p. 140–142 °C; IR(KBr) νmax (cm−1): 3250 (N–H), 3032 (Ar–CH), 2190 (C–N), 1685 (CO), 1630 (C=N), 1610, 1425 (C=C), 1290, 1150 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 8.06–8.04 (m, 5H, Ar–H), 8.17–8.15 (m, 4H, Ar–H), 8.45–8.36 (m, 5H, Ar–H), 9.45 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 63.65 (63.67), H 4.01 (4.03), N 11.13 (11.12); Ms (m/z): 376.41(M − 1).
N-[2-(3-bromophenyl)-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (2)
Yield: 85%; m.p. 188–190 °C; IR(KBr) νmax (cm−1): 3245 (N–H), 3040 (Ar–CH), 2188 (C–N), 1684 (CO), 1632 (C=N), 1583, 1480 (C=C), 1295, 1140 (–SO2–), 550 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 7.92–7.81 (m, 5H, Ar–H), 7.57 (d, 1H, J = 4.4 Hz, Ar–H), 7.46-7.30 (m, 3H, Ar–H), 8.17–8.15(m, 4H, Ar–H), 9.32(bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 52.64 (52.60), H 3.09 (3.05), N 9.21(9.23); Ms (m/z): 457.31 (M + 1).
N-[2-(4-nitrophenyl)-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (3)
Yield: 70%; m.p. 180–182 °C; IR(KBr) νmax (cm−1): 3235 (N–H), 3070 (Ar–CH), 2178 (C–N), 1694 (CO), 1642 (C=N), 1540 (NO2), 1600, 1485 (C=C), 1293, 1130 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 7.47–7.45 (m, 5H, Ar-H), 8.10–8.06 (m, 4H, Ar–H), 8.35–8.32(m, 4H, Ar–H), 10.09 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 56.87 (56.85), H 3.34 (3.30), N 13.26 (13.25); Ms (m/z): 422.41 (M+).
N-[2-(2-chlorophenyl)-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (4)
Yield: 60%; m.p. 195–197 °C; IR(KBr) νmax (cm−1): 3250 (N–H), 3029 (Ar–CH), 2168 (C–N), 1684 (CO), 1652 (C=N), 1603, 1488 (C=C), 1297, 1142 (–SO2–), 650 (C–Cl); 1H NMR (DMSO-d6) δ (ppm): 7.12–7.10 (m, 5H, Ar–H), 7.58–7.55 (m, 4H, Ar–H), 8.04–7.99 (m, 4H, Ar–H), 9.19 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 58.32 (58.30), H 3.43 (3.46), N 10.20 (10.24); Ms (m/z): 412.86 (M + 1).
N-[2-(4-methylphenyl)-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (5)
Yield: 90%; m.p. 197–199 °C; IR(KBr) νmax (cm−1): 3250 (N–H), 3043 (Ar–CH), 2850 (C–H), 2168 (C–N), 1684 (CO), 1652 (C=N), 1593, 1470 (C=C), 1297, 1142(–SO2–); 1H NMR (DMSO-d6) δ (ppm): 2.23 (s, 3H, CH3), 7.22–7.20 (m, 5H, Ar–H), 7.47–7.44 (m, 3H, Ar–H), 7.48 (m, 1H, Ar–H), 8.12–8.11(m, 4H, Ar–H), 9.08 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 64.43 (64.40), H 4.38 (4.35), N 10.73 (10.75); Ms (m/z): 390.44 (M − 1).
N-(7-fluoro-4-oxo-2-phenylquinazolin-3(4H)-yl)benzenesulfonamide (6)
Yield: 60%; m.p. 130–132 °C; IR(KBr) νmax (cm−1): 3262 (N–H), 3012 (Ar–CH), 2158 (C–N), 1674 (CO), 1672 (C=N), 1598, 1472 (C=C), 1360 (C–F), 1295, 1152 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 7.08–7.02 (m, 5H, Ar–H), 7.12(d, 1H, J = 6, Ar–H), 7.15–7.13 (m, 4H, Ar-H), 8.12 (t, 1H, J = 7.2 Hz, J = 11.2 Hz, Ar–H), 8.82 (d, 1H, J = 9.2 Hz, Ar–H), 10.02 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 60.75 (60.72), H 3.57 (3.54), N 10.63 (10.62); Ms (m/z): 396.40 (M + 1).
N-[2-(3-bromophenyl)-7-fluoro-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (7)
Yield: 54%; m.p. 158–160 °C; IR(KBr) νmax (cm−1): 3310 (N–H), 3053 (Ar–CH), 2198 (C–N), 1714 (CO), 1622 (C=N), 1588, 1480 (C=C), 1260 (C–F), 1275, 1142 (–SO2–), 670 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 7.13–7.12 (m, 5H, Ar–H), 7.21 (d, 1H, J = 8.4, Ar–H), 7.45 (d, 1H, J = 4.8, Ar–H), 7.84–7.72 (m, 3H, Ar–H), 8.12 (t, 1H, J = 7.6 Hz, J = 6.4 Hz, Ar–H), 10.12 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 50.65 (50.67), H 2.76 (2.74), N 8.86 (8.83); Ms (m/z): 476.30 (M + 2).
N-[7-fluoro-2-(4-nitrophenyl)-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (8)
Yield: 65%; m.p. 146–148 °C; IR(KBr) νmax (cm−1): 3298 (N–H), 3065 (Ar–CH), 2195 (C–N), 1720 (CO), 1632 (C=N), 1590, 1486 (C=C), 1505 (NO2), 1270 (C–F), 1278, 1188 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 7.59–7.40 (m, 5H, Ar–H), 7.42 (d, 1H, J = 8 Hz, Ar–H), 8.08 (d, 1H, J = 6.8 Hz, Ar–H), 8.15 (t, 1H, J = 1.2 Hz, J = 4.8 Hz, Ar–H), 8.23–8.22 (m, 4H, Ar–H), 10.09 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 54.54 (54.52), H 2.98 (2.95), N 12.72 (12.70); Ms (m/z): 441.40 (M + 1).
N-[2-(2-chlorophenyl)-7-fluoro-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (9)
Yield: 70%; m.p. 118–120 °C; IR(KBr) νmax (cm−1): 3268 (N–H), 3103 (Ar–CH), 2210 (C–N), 1688 (CO), 1622 (C=N), 1598, 1482 (C=C), 1269 (C–F), 1288, 1198 (–SO2–), 660 (C–Cl); 1H NMR (DMSO-d6) δ (ppm): 7.40–7.34 (m, 5H, Ar–H), 7.42(d, 1H, J = 8.4 Hz, Ar–H), 8.38–8.32 (m, 4H, Ar–H), 8.54 (t, 1H, J = 6 Hz, J = 10 Hz, Ar–H), 8.58 (d, 1H, J = 7.6 Hz, Ar–H), 10.14 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 55.88 (55.86), H 3.05 (3.06), N 9.78 (9.76); Ms (m/z): 431.85 (M + 2).
N-[7-fluoro-2-(4-methylphenyl)-4-oxoquinazolin-3(4H)-yl]-4-methylbenzenesulfonamide (10)
Yield: 78%; m.p. 160–162 °C; IR(KBr) νmax (cm−1): 3218 (N–H), 3066 (Ar–CH), 2890 (C--H), 2224 (C--N), 1712 (CO), 1632 (C=N), 1596, 1474 (C=C), 1279 (C–F), 1298, 1168 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 2.00 (s, 3H, CH3), 2.03 (s, 3H, CH3), 7.94–7.92 (m, 4H, Ar–H), 8.22 (d, 1H, J = 7.6 Hz, Ar–H), 8.31–8.30 (m, 4H, Ar–H), 8.44 (t, 1H, J = 12 Hz, J = 5.2 Hz, Ar–H), 8.52 (d, 1H, J = 6 Hz, Ar–H), 11.04 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 62.40 (62.42), H 4.28 (4.26), N 9.92 (9.90); Ms (m/z): 424.46 (M + 1).
4-methyl-N-(7-nitro-4-oxo-2-phenylquinazolin-3(4H)-yl)benzenesulfonamide (11)
Yield: 64%; m.p. 215–217 °C; IR(KBr) νmax (cm−1): 3210 (N–H), 3023 (Ar–CH), 2882 (C–H), 2194 (C–N), 1722 (CO), 1642 (C=N), 1595, 1484 (C=C), 1360 (NO2), 1295, 1178 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 2.05 (s, 3H, CH3), 7.92–7.90 (m, 5H, Ar–H), 7.75(d, 1H, J = 6.8 Hz, Ar–H), 8.18–8.16 (m, 5H, Ar–H), 8.14 (t, 1H, J = 4.8 Hz, J = 11.6 Hz, Ar–H), 8.85 (d, 1H, J = 8.8 Hz, Ar–H), 9.32 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 57.79 (57.77), H 3.70 (3.71), N 12.84 (12.86); Ms (m/z): 435.44 (M_1).
N-[2-(3-bromophenyl)-7-nitro-4-oxoquinazolin-3(4H)-yl]-4-methylbenzenesulfonamide (12)
Yield: 55%; m.p. 165–167 °C; IR(KBr) νmax (cm−1): 3215 (N–H), 3033 (Ar–CH), 2888 (C–H), 2182 (C–N), 1690 (CO), 1620 (C=N), 1600, 1475 (C=C), 1363 (NO2), 1282, 1188 (–SO2–), 650 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 2.04 (s, 3H, CH3), 7.98–7.84 (m, 4H, Ar–H), 7.75 (d, 1H, J = 6.8 Hz, Ar–H), 7.59 (d, 1H, J = 4.4 Hz, Ar–H), 7.46–7.30 (m, 3H, Ar–H), 8.12 (t, 1H, J = 7.2 Hz, J = 11.2 Hz, Ar--H), 9.13 (d, 1H, J = 7.2 Hz, Ar–H), 11.84 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 48.94 (48.92), H 2.93 (2.91), N 10.87 (10.85); Ms (m/z): 516.33 (M + 1).
4-Methyl-N-[7-nitro-2-(4-nitrophenyl)-4-oxoquinazolin-3(4H)-yl]benzenesulfonamide (13)
Yield: 74%; m.p. 176–178 °C; IR(KBr) νmax (cm−1): 3202 (N–H), 3011 (Ar–CH), 2878 (C–H), 2198 (C–N), 1722 (CO), 1613 (C=N), 1592, 1482 (C=C), 1362, 1296 (NO2), 1286, 1192 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 2.05 (s, 3H, CH3), 7.44–7.02 (m, 4H, Ar–H), 7.81 (s, 1H, Ar–H), 8.09 (d, 1H, J = 9.6 Hz, Ar–H), 8.21 (t, 1H, J = 8.4 Hz, J = 8 Hz, Ar–H), 8.45 (m, 4H, Ar–H), 9.44 (bs, 1H, NH, D2O exchangeable); % Anal Cald (found) = C 52.39 (52.37), H 3.14 (3.12), N 14.55 (14.57); Ms (m/z): 480.43 (M − 1).
N-[2-(2-chlorophenyl)-7-nitro-4-oxoquinazolin-3(4H)-yl]-4-methylbenzenesulfonamide (14)
Yield: 56%; m.p. 162–164 °C; IR(KBr) νmax (cm−1): 3311 (N–H), 3018 (Ar–CH), 2880 (C–H), 2174 (C–N), 1733 (CO), 1634(C=N), 1592, 1465 (C=C), 1372 (NO2), 1287, 1195 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 2.03 (s, 3H, CH3), 7.40–7.34 (m, 4H, Ar–H), 7.42 (d, 1H, J = 8.4 Hz, Ar–H), 8.38–8.32 (m, 4H, Ar–H), 8.54 (t, 1H, J = 6 Hz, J = 10 Hz, Ar–H), 8.58 (d, 1H, J = 7.6 Hz, Ar–H), 9.83 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 53.56 (53.54), H 3.21 (3.23), N 11.90 (11.92); Ms (m/z): 471.88 (M + 1).
N-[2-(4-methoxyphenyl)-7-nitro-4-oxoquinazolin-3(4H)yl]-4-methylbenzenesulfonamide (15)
Yield: 60%; m.p. 180–182 °C; IR(KBr) νmax (cm−1): 3282 (N–H), 3056 (Ar–CH), 2877 (C–H), 2184 (C–N), 1723 (CO), 1631 (C=N), 1590, 1476 (C=C), 1378 (NO2), 1289, 1185 (–SO2–); 1H NMR (DMSO-d6) δ (ppm): 2.01(s, 3H, CH3), 3.75 (s, 3H, OCH3), 7.75–7.74 (m, 4H, Ar–H), 7.78 (s, 1H, Ar–H), 8.13 (d, 1H, J = 8.8 Hz, Ar–H), 8.17 (t, 1H, J = 6 Hz, J = 7.6 Hz, Ar–H), 8.35–8.33 (m, 4H, Ar–H), 10.03 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 56.65 (56.63), H 3.89 (3.87), N 12.01 (12.03); Ms (m/z): 465.46 (M − 1).
N-(7-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-methylbenzenesulfonamide (16)
Yield: 78%; m.p. 150–152 °C; IR(KBr) νmax (cm−1): 3302 (N–H), 3068 (Ar–CH), 2887 (C–H), 2197 (C–N), 1698 (CO), 1611 (C=N), 1598, 1480 (C=C), 1279, 1193 (–SO2–), 560 (C–Cl); 1H NMR (DMSO–d6) δ (ppm): 2.05 (s, 3H, CH3), 7.18–7.16 (m, 4H, Ar–H), 7.18 (d, 1H, J = 5.6, Ar–H), 7.21–7.18 (m, 5H, Ar–H), 8.10 (t, 1H, J = 8.8 Hz, J = 7.2 Hz, Ar–H), 8.32 (d, 1H, J = 8 Hz, Ar–H), 10.34 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 59.22 (59.20), H 3.79 (3.77), N 9.87 (9.85); Ms (m/z): 426.88 (M + 1).
N-[2-(3-bromophenyl)-7-chloro-4-oxoquinazolin-3(4H)-yl]-4-methylbenzenesulfonamide (17)
Yield: 60%; m.p. 185–187 °C; IR(KBr) νmax (cm−1): 3308 (N–H), 3070 (Ar–CH), 2880 (C–H), 2187 (C–N), 1720 (CO), 1618 (C=N), 1596,1455 (C=C), 1269, 1191 (–SO2–), 730 (C–Cl), 520 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 2.03 (s, 3H, CH3), 7.08–7.04 (m, 4H, Ar–H), 7.12 (d, 1H, J = 8 Hz, Ar–H), 7.17 (d, 1H, J = 5.2 Hz, Ar–H), 7.21–7.19 (m, 3H, Ar–H), 8.17 (t, 1H, J = 5.6 Hz, J = 4.4 Hz, Ar–H), 10.44 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 49.57 (49.55), H 3.00 (3.02), N 8.32 (8.34); Ms (m/z): 506.78 (M + 2).
N-[7-chloro-2-(4-methylphenyl)-4-oxoquinazolin-3(4H)-yl]-4-methylbenzenesulfonamide (18)
Yield: 88%; m.p. 176–178 °C; IR(KBr) νmax (cm−1): 3311 (N–H), 3056 (Ar–CH), 2876 (C–H), 2189 (C–N), 1690 (CO), 1612 (C=N), 1598, 1480 (C=C), 1279, 1194 (–SO2–), 670 (C–Cl); 1H NMR (DMSO-d6) δ (ppm): 2.05 (s, 3H, CH3), 2.34 (s, 3H, CH3), 7.04–7.02 (m, 4H, Ar–H), 8.10 (d, 1H, J = 8.8 Hz, Ar–H), 8.14–8.12 (m, 4H, Ar–H), 8.18 (t, 1H, J = 4.4 Hz, J = 5.6 Hz, Ar–H), 8.32 (d, 1H, J = 8.8 Hz, Ar–H), 10.40 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 60.07 (60.05), H 4.12 (4.10), N 9.55 (9.57); Ms (m/z): 440.91 (M + 1).
N-[7-chloro-2-(2-chlorophenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (19)
Yield: 90%; m.p. 122–124°C; IR(KBr) νmax (cm−1): 3304 (N–H), 3038 (Ar–CH), 2192 (C–N), 1699 (CO), 1614 (C=N), 1594, 1487 (C=C), 1375 (NO2), 1288, 1198 (–SO2–), 710 (C–Cl); 1H NMR (DMSO-d6) δ (ppm): 7.10–7.14 (m, 4H, Ar–H), 7.19 (d, 1H, J = 6.4 Hz, Ar–H), 8.28–8.30 (m, 4H, Ar–H), 8.42 (t, 1H, J = 9.6 Hz, J = 7.2 Hz, Ar–H), 8.48 (d, 1H, J = 9.2 Hz, Ar–H), 10.06 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 48.89 (48.87), H 2.46 (2.45), N 11.40 (11.38); Ms (m/z): 493.30 (M + 2).
N-[7-chloro-2-(4-methoxyphenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (20)
Yield: 72%; m.p. 190–193 °C; IR(KBr) νmax (cm−1): 3218 (N–H), 3012 (Ar–CH), 2886 (C–H), 2197 (C–N), 1688 (CO), 1610 (C=N), 1590, 1476 (C=C), 1378 (NO2), 1289, 1192 (–SO2–), 612 (C–Cl); 1H NMR (DMSO-d6) δ (ppm): 3.15 (s, 3H, OCH3), 7.54–7.51 (m, 4H, Ar–H), 7.62 (d, 1H, J = 9.6 Hz, Ar–H), 8.02 (d, 1H, J = 5.6 Hz, Ar–H), 8.10 (t, 1H, J = 7.2 Hz, J = 6 Hz, Ar–H),8.16–8.18(m, 4H, Ar–H), 9.34 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 51.80 (51.78), H 3.11 (3.08), N 11.51 (11.58); Ms (m/z): 487.88 (M + 1).
N-[7-bromo-2-(4-methylphenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (21)
Yield: 68%; m.p. 157–160 °C; IR(KBr) νmax (cm−1): 3310 (N–H), 3072 (Ar–CH), 2890 (C–H), 2178 (C–N), 1680 (CO), 1602 (C=N), 1588, 1465 (C=C), 1382 (NO2), 1267, 1190 (–SO2–), 520 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 2.04 (s, 3H, CH3), 7.12–7.13 (m, 4H, Ar–H), 8.02 (d, 1H, J = 7.2 Hz, Ar–H), 8.10–8.08 (m, 4H, Ar–H), 8.14(t, 1H, J = 9.2 Hz, J = 5.2 Hz, Ar–H), 8.18 (d, 1H, J = 8.8 Hz, Ar–H), 9.19 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 48.94 (48.96), H 2.93 (2.95), N 10.87 (10.85); Ms (m/z): 516.33 (M + 1).
N-[7-bromo-2-(2-chlorophenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (22)
Yield: 75%; m.p. 200–202 °C; IR(KBr) νmax (cm−1): 3270 (N–H), 3097 (Ar–CH), 2170 (C–N), 1688 (CO), 1603 (C=N), 1595, 1465 (C=C), 1380 (NO2), 1268, 1192 (–SO2–), 715 (C–Cl), 620 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 7.32–7.33 (m, 4H, Ar–H), 7.37 (d, 1H, J = 6.4 Hz, Ar–H), 8.18–8.22 (m, 4H, Ar–H), 8.64 (t, 1H, J = 6.4 Hz, J = 7.2 Hz, Ar–H), 8.84 (d, 1H, J = 7.2 Hz, Ar–H), 9.54 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 44.84 (44.82), H 2.26 (2.22), N 10.46 (10.42); Ms (m/z): 537.75 (M + 2).
N-[7-bromo-2-(3-bromophenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (23)
Yield: 62%; m.p. 290–292 °C; IR(KBr) νmax (cm−1): 3285 (N–H), 3085 (Ar–CH), 2174 (C–N), 1685 (CO), 1602 (C=N), 1595, 1460 (C=C), 1382 (NO2), 1275, 1190 (–SO2–), 615 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 7.22–7.21(m, 4H, Ar–H), 7.31(d, 1H, J = 6.4 Hz, Ar–H), 7.62 (d, 1H, J = 7.2 Hz, Ar–H), 7.70–7.68 (m, 3H, Ar–H), 8.76 (t, 1H, J = 8.8 Hz, J = 8.4 Hz, Ar–H), 9.80 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 41.40 (41.38), H 2.08 (2.06), N 9.66 (9.64); Ms (m/z): 582.20 (M + 2).
N-(7-bromo-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-nitrobenzenesulfonamide (24)
Yield: 70%; m.p. 185–187 °C; IR(KBr) νmax (cm−1): 3280 (N–H), 3093 (Ar–CH), 2172 (C–N), 1695 (CO), 1606 (C=N), 1597, 1478 (C=C), 1377 (NO2), 1285, 1195 (–SO2–), 612 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 7.21–7.20 (m, 4H, Ar–H), 7.28(d, 1H, J = 9.2, Ar–H), 8.01–8.08 (m, 5H, Ar–H), 8.12 (t, 1H, J = 5.2 Hz, J = 8.8 Hz, Ar–H), 8.38 (d, 1H, J = 8.4 Hz, Ar–H), 9.09 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 47.92 (47.90), H 2.61 (2.65), N 11.18 (11.15); Ms (m/z): 502.31 (M + 1).
N-[7-bromo-2-(4-nitrophenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (25)
Yield: 58%; m.p. 190–192 °C; IR(KBr) νmax (cm−1): 3290 (N–H), 3074 (Ar–CH), 2184 (C–N), 1698 (CO), 1600 (C=N), 1598, 1480 (C=C), 1510 (NO2), 1288, 1198 (–SO2–), 625 (C–Br); 1H NMR (DMSO-d6) δ (ppm): 7.52–7.50 (m, 4H, Ar–H), 7.52 (d, 1H, J = 7.6 Hz, Ar–H), 8.14 (d, 1H, J = 9.2 Hz, Ar–H), 8.18 (t, 1H, J = 9.2 Hz, J = 8 Hz, Ar–H), 8.33–8.30 (m, 4H, Ar–H), 9.92 (bs, 1H, NH, D2O exchangeable); % Anal Calcd (found) = C 43.97 (43.95), H 2.21 (2.18), N 12.82 (12.80); Ms (m/z): 547.30 (M + 1).
Pharmacology
All the experimental protocols were carried out with the permission from Institutional Animal Ethics committee (IAEC), project proposal no. 781 and the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments in Animal (CPCSEA). Animals were obtained from Central Animal House Facility, Hamdard University, New Delhi – 62. Registration no. and date of registration is 173/CPCSEA, 28 January 2000. All rats were housed in a temperature- and humidity-controlled room at an ambient temperature of 25 ± 2 °C with 12 h light/dark cycle. Animals were provided with pellets diet (Lipton, Calcutta, India) and water ad libitum.
Diuretic activity
Sixty healthy adult albino rats weighing 200–250 g were used. Each group comprised six animals (n = 6). Fifteen hours prior to the experiment food and water were withdrawn. Diuretic activity was measured by collecting total excreted urine (0–5 h) of rat kept in metabolic cage. The cages together with the funnel and measuring cylinder used in the studies were coated with liquid paraffin before each experiment to facilitate the collection of urine with a minimum loss. Each animal was placed in metabolic cage provided with a wire mesh at the bottom and a funnel to collect the urine. Stainless steel sieves were placed in the funnel to retain faeces and to allow the urine to pass. Rats were placed in metabolic cages individually as soon as the treatments started. The urine sample was collected for a total period of 5 h (urine collected for initial 20 min was discarded). The test compounds were administered orally at two dose levels of 2 mg and 1 mg/kg body weights in 5 mL of 0.9% NaCl solution. Control group received 5 mL of 0.9% NaCl solution per kg body weight. The test compounds were compared with two standard diuretics: urea (1 g/kg body weight in 5 mL of 0.9% NaCl solution) and metolazone (1 mg/kg body weight in 5 mL of 0.9% NaCl solution). Animal was reused after 2-week washout period for remaining test compounds.
Antihypertensive activity
Conditioning/training of animals
For conducting the BP measurement studies, the animals were kept in restrainers for 10 min every day for one week. This exercise was done to avoid the fluctuation in blood pressure due to aggressive behaviour of animal while keeping into the restrainers for measuring the activity.
Induction of hypertension in albino rats
After recording the initial BP of rats, the animals were divided into groups of six animals each. One group was taken as control. Hypertension was induced in the remaining groups by subcutaneous injection of methyl prednisolone acetate (20 mg/kg body wt./wk) for 2 weeks as per the method reported by Krakoff et alCitation20.
Measurement of systolic and mean arterial blood pressure of rats
Systolic and mean arterial blood pressure was measured in conscious rats using CODA Non-Invasive Blood Pressure Recorder by the Tail–Cuff method (Kent Scientific Corporation, Torrington, CT). The restrainer carrying the rat was placed in the BP instrument with tail protruding out. The tail was gently placed in contact with a transducer membrane, which was connected to the digital BP display panel. The instrument was then turned on and allowed to stabilize until steady pulse rate was observed. Once the ‘‘pulse level ready’’ signal appeared, the BP recording button was pressed and the mean arterial BP was recorded. Albino rats (body weight 200–250 g) were used in study. Rats were assigned to groups of six animals in each group. Each compound (5 mg/kg body weight) was injected intraperitoneally after suspending in 1% carboxymethyl cellulose (CMC) solution. The blood pressure was recorded at various time intervals.
Evaluation of anti-diabetic potential
Test animals
Animals were obtained from Central Animal House Facility, Hamdard University, New Delhi – 62. Prior to experimental treatments, animals were fasted overnight but were allowed free access to water. Six animals were used for each group of study.
Collection of blood samples and determination of the blood glucose levels
Collection of blood samples and determination of the blood glucose levels Blood samples were collected from the tip of tail. Blood glucose concentration (mg/dl) was determined using a Glucometer-elite commercial test (Bayer), based on the Glucose oxidize method.
Experimental procedure
Effect in normoglycaemic animals fasting blood sugar level of each animal was determined at zero-time, after overnight fasting with free access to water. Control group animals received 0.5% CMC. Test group animals were treated with the test samples suspended in the same vehicle. Blood samples were collected at 0, 1, 12, 24, 48 h after the oral administration of test samplesCitation21.
Statistical analysis of data
The statistical analysis was performed using GRAPHPAD INSTAT 3 software (Graph Pad Software Inc, San Diego, CA). Data obtained from animal experiments were expressed as arithmetic mean ± SEM. The comparison between various groups was performed by one-way analysis of variance (ANOVA), and the effects in treatment groups were compared with toxic control or control group by the Dunnet multiple comparison test. p < 0.05 was considered to be significant [*p < 0.05; **p < 0.01].
Result and discussion
Chemistry
The synthesis of some N-(substituted-4-oxo-2-substituted-phenylquinazolin-3-(4H)-yl) substituted benzenesulfonamide derivatives (1–25) were carried out according to . The benzoylation of substituted anthranilic acids leads to the formation of substituted amides, these substituted amides after refluxing with acetic anhydride gave substituted benzoxazinesCitation22,Citation23. The resulting substituted benzoxazines on hydrazinolysis gave the quinazolinone derivatives. The aminoquinazolinone derivatives were subjected to Hinsberg’s reaction with substituted benzene sulfonylchlorideCitation24–26 to yield the final compounds. The physicochemical data of all the synthesized compounds are recorded.
Scheme 1. Synthesis of N-(substituted-4-oxo-2-substituted-phenylquinazolin-3-(4H)-yl)substituted benzenesulfonamide derivatives.
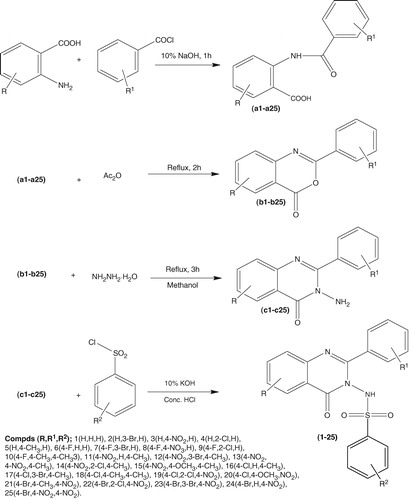
All the synthesized compounds were obtained in good yield, which were purified and characterized by spectral data and elemental analyses, explained with the example of compound 12. The IR spectra of the mentioned molecule showed characteristic absorption bands at 3215 cm−1 (N–H), 3033 cm−1 (Ar–CH), 2888 cm−1 (C–H), 2182 cm−1 (C–N), 1690 cm−1 (CO), 1620 cm−1 (C=N), 1600, 1475 cm−1 (C=C), 1363 cm−1 (NO2), 1282, 1188 cm−1 (–SO2–). The 1H NMR spectra showed two doublet at δ7.75, J = 6.8 and 9.13, J = 7.2 confirming the positions 5 and 8 in quinazolinone ring, one doublet at δ7.59, J = 4.4 confirmed the position of 2′ in phenyl ring B. One triplet at δ 8.12, J = 7.2, 11.2 confirmed the position of 6 in quinazolinone ring. The multiplets at δ7.46–7.30 and δ7.98–7.84 indicated 3 protons of phenyl ring-B and 4 protons of phenyl ring-C, respectively. The one broad singlet corresponding to NH was obtained at δ11.84 that was D2O exchangeable. The mass spectrum showed the presence of molecular ion peak at m/z 516 (M + 1) in accordance to the molecular formula C21H15BrN4O5S. The structure was also supported by elemental analysis data, which was ± 0.4%. The other compounds were also characterized in the similar manner.
Diuretic activity
The final compounds (1--25) were evaluated for diuretic activity by the Lipschitz et al. methodCitation27. The results at a dose of 2 mg after oral administration of test drug and 1 mg of standard drug metolazone are also observed. Compounds 7, 9, 14, 15, 19 and 20 showed good cumulative urine output, among which N-[7-chloro-2-(4-methoxyphenyl)-4-oxoquinazolin-3(4H)-yl]-4-nitrobenzenesulfonamide (20) was highly substantial (11.46 ± 0.257) (p < 0.01), i.e. increased by >200% with respect to control. Diuretic action of compound 20 was found to be 1.99 (1.25 times more potent than metolazone at a double dose). The Lipschitz value (the ratio T/U, in which T is the response of the test compound and U is that of urea treatment, indices of 1.0 and higher are regarded as a positive effect in terms of diuretic activity) shows that compound 20 is 1.41 times potent than urea. Further, compound 20 showed a substantial increase in the Na+ excretion (p < 0.01), i.e. 4.10 ± 0.012, which was greater than standards, i.e. urea 2.49 ± 0.023 and metolazone (3.58 ± 0.018). The diuretic action of compounds showing significant activity were further analysed at dose equivalent to metolazone (1mg/kg body weight). Among the tested compounds, compound 20 was found to be almost equally potent to metolazone. Interestingly, the change/fall in diuretic action were not equal to half of the effect of double dose of test compound 20 – it may be due to unchanged excretion of drug at double strength. However, Na+ and K+ excretion potential was higher than metolazone shown in and , respectively. Compound 20 was also found to possess significant kaliuretic property (p < 0.01), i.e. 1.84 ± 0.008 with regards to the Na+/K+ ratio.
Table 1. Diuretic activity data shown by compounds 1–25.
Table 2. Diuretic activity data shown by some active compound.
The presence of sulphonamide group like –SO2NH– at the 3rd position in quinazolinone seems to be essential for diuretic activity similar to metolazone. SAR showed that compounds bearing electron-withdrawing groups (like NO2) were more active than the compounds having electron-releasing groups (CH3).
Antihypertensive activity
The final compounds 1--25 were evaluated for antihypertensive activity by the non-invasive Tail Cuff method. The results are compared with standard drug, prazosinCitation28 and diazoxideCitation29 shown in . All compounds exhibited good decrease in the systolic blood pressure up to 6 h after the administration of test compounds and a moderate rise afterwards, which may be due to the excretion of compounds from body after 6 h. The titled compounds 7, 9, 14, 15 and 19 were found to show good reduction in systolic blood pressure and mean arterial blood pressure, whereas compound 20 showed significant antihypertensive activity as compared to standard drugs prazosin and diazoxide. Rest of the compounds showed moderate decrease in hypertension ().
Figure 2. Graph showing decrease in the systolic blood pressure (SBP) exhibited by compounds (1–25) and standard as compared to control.
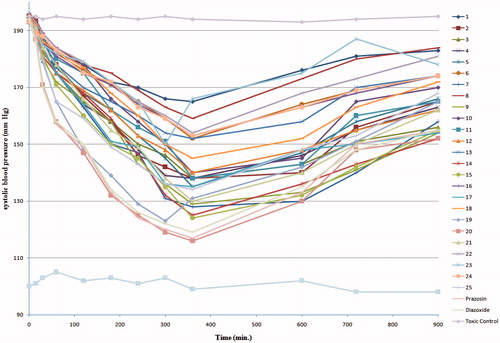
Table 3. Antihypertensive activity data shown by compounds 1–25.
The structure–activity relationship of quinazolinone derivatives showed that, substitution of peripheral phenyl rings had a great influence on the antihypertensive activity of the compounds. These variations might be through the stereolectronic properties of the substituents. In general, substitution of electron-withdrawing groups like NO2 and Cl in the phenyl ring attached with quinazolinone moiety increases the antihypertensive activity irrespective of their positions, for example compounds 20 and 19 showed excellent antihypertensive activity. On the other hand, the electron-releasing group like CH3 in the phenyl ring causes decrease in the activity, as shown in compound 18. However, unsubstituted phenyl ring leads to loss in activity, as shown in compound 1, thus indicating the significance of electron-withdrawing groups in the quinazolinone series.
Evaluation to assess the effect on insulin secretion (as diazoxide causes hyperglycaemia)
The compounds which showed good antihypertensive activity (7, 9, 14, 15, 19 and 20) were further evaluated for their anti-diabetic potential using a Glucometer-elite commercial test (Bayer), based on the Glucose oxidize method and the activity was compared with diazoxide (a potassium channel activator with antihypertensive activity). The results are shown in . All the tested compounds did not show any significant fall/increase in the normal glucose level; however, diazoxide showed significant increase in the normal glucose level, as shown in the graph of .
Figure 3. Graph showing changing in blood glucose concentration exhibited by some potent compounds and compared with diazoxide.
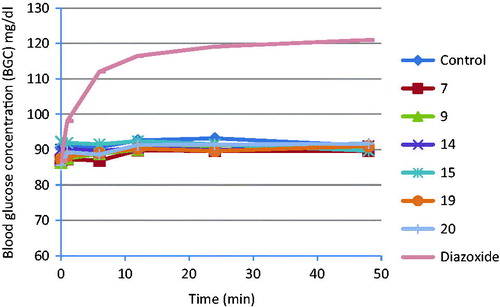
Table 4. Evaluation of anti-diabetic potential.
Conclusion
We have presented an easy method for the formation of N-(substituted-4-oxo-2-substituted-phenylquinazolin-3-(4H)-yl) substituted benzenesulfonamide derivatives. The hybrid molecules did show good to moderate biological activities. The synthetic methodology and the compounds developed may open a new door towards the synthesis of interesting biologically active compounds.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. One of the authors (Mujeeb Ur Rahman) is also thankful to UGC (MANF) for providing financial assistance.
Supplementary materials online only Physicochemical parameters of synthesized compounds are available in Table 5 and electrolyte concentration data range of synthesized compounds are available in Tables 6 and 7 at 2 mg and 1 mg/kg/body weight.
Acknowledgements
We are thankful to Jamia Hamdard, New Delhi (India), for providing research facilities.
References
- Doshi A, Miller GE. Medical Expenditure Panel Survey 2006. Available from: http://hyper.ahajournals.org/content/42/6/1206 [last accessed Feb 2013]
- Moutinho-Ribeiro P, Figueira P, Lopes SS, et al. Metolazone-associated acute liver failure and pancreatitis: first case report. Am J Gastroenterol 2001;96:S258
- Sturgess NC, Kozlowski RZ, Carrington CA, et al. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol 1988;95:83–94
- Gavero I, Roach AG. A new antihypertensive agent with a peripheral site of action: in vivo pharmacological studies. Life Sci 1980;27:1525–40
- Shetty BV, Campenella LA, Thomas TL, et al. Synthesis and activity of some 3-Aryl-3-Aralkyl-1,2,3,4-tetrahydro-4-oxo-6-quinazolinesulfonamides. J Med Chem 1970;13:886–95
- Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91
- Ekinci D, Kurbanoglu NI, Salamci E, et al. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzyme Inhib Chem 2012;27:845–8
- El-Sabbagh OI, Shabaan MA, Kadry HH, Al-Din ES. New octahydroquinazoline derivatives: synthesis and hypotensive activity. Eur J Med Chem 2010;45:5390–6
- Alagarsamy V, Venkatesaperumal R, Vijayakumar S, et al. Synthesis and pharmacological investigation of some novel 2-phenyl-3-(substituted methyl amino) quinazolin-4(3H)-ones as H1-receptor blockers. Pharmazie 2002;57:306–7
- Alagarsamy V. Synthesis and pharmacological investigation of some novel 2-methyl-3-(substituted methylamino)-(3H)-quinazolin-4-ones as histamine H1-receptor blockers. Pharmazie 2004;59:753–5
- Alagarsamy V, Solomon VR, Vanikavitha G, et al. Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-phenyl-3-substituted quinazolin-4(3H) ones. Bio Pharm Bull 2002;25:1432–5
- Alagarsamy V, Shankar D, Solomon VR, et al. Synthesis and pharmacological evaluation of 3-cyclohexyl-2-substituted hydrazino-3H-quinazolin-4-ones as analgesic and anti-inflammatory agents. Acta Pharm 2009;59:75–88
- Hour MJ, Huang LJ, Kuo SC, et al. 6-Alkylamino- and 2,3-dihydro-3′-methoxy-2-phenyl-4-quinazolinones and related compounds: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem 2000;43:4479–87
- Alagarsamy V, Giridhar R, Yadav MR, et al. AntiHIV, antibacterial and antifungal activities of some novel 1,4-disubstituted-1,2,4-triazolo[4,3-a] quinazolin-5(4 h)-ones. Ind J Pharm Sci 2006;68:532–5
- Cragoe EJ. (Ed.). Chemistry and pharmacology of drugs, Vol. 2: diuretics: chemistry, pharmacology and medicine. New York: Wiley; 1983
- Krungkrai J, Scozzafava A, Reungprapavut S, et al. Carbonic anhydrase inhibitors. Inhibition of Plasmodium falciparum carbonic anhydrase with aromatic sulfonamides: towards antimalarials with a novel mechanism of action. Bioorg Med Chem 2005;13:483–9
- Siddiqui N, Ahsan W, Alam MS, et al. Design, synthesis and evaluation of anticonvulsant activity of pyridinyl-pyrrolidones: a pharmacophore hybrid approach. Archiv Pharmazie 2012;345:185–94
- Siddiqui AA, Mishra R, Shaharyar M. Synthesis, characterization and antihypertensive activity of pyridazinone derivatives. Eur J Med Chem 2010;45:2283–90
- Shahar yar M, Ansari ZH. Synthesis and in vivo diuretic activity of biphenyl benzothiazole-2-carboxamide derivatives. Acta Polo Pharm – Drug Res 2009;66:387–92
- Krakoff LR, Selvadurai R, Sytter E. Effect of methylprednisolone upon arterial pressure and the renin angiotensin system in the rat. Am J Physiol 1975;228:613–17
- Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci 2005;76:1223–38
- Mosaad SM, Mohammed KI, Ahmed MA, Abdel-Hamide SG. Synthesis of certain new 6-iodoquinazolines as potential antitubercular agents. J Appl Sci 2004;4:302–7
- El-Azab AS, Al-Omar MA, Abdel-Aziz AA, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur J Med Chem 2010;45:4188–98
- Aslan HG, Karacan N. Aromatic sulfonyl hydrazides and sulfonyl hydrazones: antimicrobial activity and physical properties. Med Chem Res 2013;22:1330–8
- Alterio V, Di Fiore A, D'Ambrosio K, Supuran CT, De Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms. Chem Rev 2012;112:4421–68
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Chem 2012;27:759–72
- Lipschitz WL, Haddian Z, Kerpscar A. Bioassay of diuretics. J Pharmacol Exp Ther 1943;79:97–110
- El-Sabbagh OI, Shabaan MA, Kadry HH, Al-Din ES. New octahydroquinazoline derivatives: synthesis and hypotensive activity. Eur J Med Chem 2010;45:5390–6
- Pohl JEF, Thurston H. Use of diazoxide in hypertension with renal failure. Bri Med J 1971;4:142–5