Abstract
Plectranthus madagascariensis is used as a traditional medicine in Southern Africa. In search of compounds and activities supporting the medicinal use, the chemical profile of the methanolic extract was studied by high-performance liquid chromatography with diode array detection (HPLC-DAD). Four major constituents were isolated and identified as rosmarinic acid (1), 7β,6β-dihydroxyroyleanone (2), 7β-acetoxy-6β-hydroxyroyleanone (3) and coleon U quinone (4). The two abietane diterpenoids (2 and 3) were isolated for the first time from this species. Antimicrobial, cholinesterase and α-glucosidase inhibitory activities of these compounds were studied. The compounds exhibited inhibitory activity on α-glucosidase with IC50 values from 33 to 275 μM. Abietanes showed potent antibacterial activity against Staphylococcus aureus and Enterococcus faecalis.
Introduction
Plectranthus (Lamiaceae) is a widespread and large genus distributed from Africa to Asia and Australia. Plectranthus madagascariensis (Pers.) Benth is a perennial aromatic herb native to Southern Africa and cultivated in the Czech Republic as an ornamental plant. It has been used as a traditional medicine in the treatment of scabies and small wounds. Decoction of the whole plant is used for the treatment of cold, cough and asthmaCitation1.
The main compound of the plant methanolic extract of Plectranthus spp. is rosmarinic acid, a common compound in the family Lamiaceae. This compound is in relation to the antioxidant, anti-acetylcholinesterase and anti-inflammatory activityCitation2. Flavonoids and caffeic acid derivatives nepetoidin A and B have also been reported in a chemotaxonomic studyCitation3. Abietane diterpenoids of Plectranthus spp. have especially antimicrobial activityCitation4,Citation5. Extracts of P. madagascariensis showed antibacterial and insect-antifeedant effectCitation6. The main component of the oil was diterpenic 6,7-dehydroroyleanoneCitation7.
Alzheimer’s disease (AD), a very common form of dementia among the elderly, is in relation to brain deficits in acetylcholine. Acetylcholinesterase (AChE) predominates in healthy brain, with butyrylcholinesterase (BuChE) considered to play role in regulating brain acetylcholine levels. However, BuChE activity progressively increases in patients with AD while AChE activity remains unchanged or declines. Both enzymes therefore represent legitimate therapeutic targets for symptomatic treatment of ADCitation8.
α-Glucosidase inhibitors are currently promising drugs in the treatment of type 2 diabetes mellitus. α-Glucosidase is an intestinal enzyme that releases glucose from larger carbohydrates. The enzyme inhibiton leads to a decrease of the α-linkage cleavage at the anomeric center in the sugar molecule and as a result less glucose is absorbedCitation9.
This study is focused on the isolation, enzyme inhibitory activities and antimicrobial activity of the isolated compounds, and is a part of our search for bioactive constituents from less known but traditionally used Plectranthus species.
Methods
Chemicals
AChE from electric eel, BuChE from equine serum, dithio-bis-(2-nitrobenzoic acid) (DTNB), acetyl thiocholine iodide (ATCI), butyryl thiocholine iodide (BTCI), α-glucosidase type I from baker‘s yeast, 4-nitrophenyl α-D-glucopyranoside (α-PNPG), doxycycline, acarbose, galanthamine and rosmarinic acid were purchased from Sigma-Aldrich (Prague, Czech Republic). Organic solvents (of analytical grade) were purchased from Merck (Prague, Czech Republic).
Apparatus
Extract and compounds were analyzed using high-performance liquid chromatography with diode array detection (HPLC-DAD) (HP 1100, Agilent Technologies, Prague, Czech Republic, column ABZ + Plus 150 × 4.6 mm, 3 μm particle size, gradient elution: 10% acetonitrile (ACN) and 90% formic acid 0.2% in 0 min to 100% ACN at 36 min, flow rate 1 mL/min, temperature 40 °C) and HPLC–mass spectrometry (HP 1100, Agilent Technologies, Prague, Czech Republic, LC–MSD Trap VL combined with electrospray ionization, negative mode, nebulizer pressure 50 psi, dry gas 10 L/min, dry temparature 350 °C). Compounds were identified by NMR (Bruker Avance 200, Bruker, Coventry, UK) and FT-IR (Nicolet Impact 410, ThermoScientific, Vienna, Austria). The results of bioassays were measured using a microplate-reader (Bio-Tek, Bad Friedrichshall, Germany).
Plant collection and extract preparation
Aerial sections of P. madagascariensis were collected in October 2010 in the greenhouse of the Faculty of Pharmacy, Brno, Czech Republic. A voucher specimen (PM 2010) is deposited in the herbarium of the faculty. The fresh aerial sections (700 g) were extracted with methanol (3000 ml) at room temperature for 30 min in an ultrasonic cleaning bath. The methanolic extract was concentrated in vacuo to a dark gum and lyophilized.
Isolation of compounds from extract
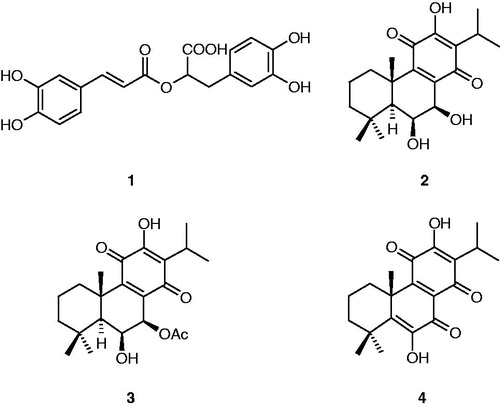
The lyophilized extract (8 g) was subjected to column chromatography on silica gel by eluting with mobile phase petrolether–ethylacetate (9:1–1:1). FrB yielded 10 mg of 7β-acetoxy-6β-hydroxyroyleanone (3), FrD provided 15 mg of 7β,6β-dihydroxyroyleanone (2) and FrI yielded 20 mg of coleon U quinone (4). These compounds were identified by HPLC-ESI–MS, NMR and FT-IR and by comparison with literature data. Rosmarinic acid (1.7 mg) was obtained by eluting of the column with MeOH and was identified by HPLC-ESI–MS, FT-IR and co-HPLC with standard.
Antibacterial activity
The compounds were dissolved in dimethyl sulfoxide (DMSO) and subjected to an in vitro antimicrobial activity assay using the broth microdilution methodCitation10,Citation11. Eleven successive 2-fold dilutions of each compound were mixed with appropriate Mueller–Hinton broth to provide a concentration range from 128 down to 0.063 µg/mL. Each of these aliquots was then inoculated with 3 µL of suspension of a standard bacteria strains: Gram-positive S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212 and Gram-negative Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 [deposited at the Department of Infectious Diseases and Microbiology, University of Veterinary and Pharmaceutical Sciences (VFU), Brno, Czech Republic] at a density of 0.5 McF. After the inoculation, the plates were incubated at 37 °C for 24 h. The growth of the microorganisms was observed using a UV-Vis spectrophotometer monitoring absorbance at 600 nm. The minimum inhibitory concentrations (MICs) were established based on the ratio of the absorbance of the growth of the negative control to the absorbance of the sample, and were calculated as the lowest concentration of the compound that resulted in an 80% reduction in the growth of the microorganism. The DMSO solvent solution was tested simultaneously as a negative control. The antibiotic doxycycline was used as the positive control.
AChE and BuChE inhibitory activity
AChE and BuChE inhibitory activity was measured by method developed by Ellman with slight modificationCitation12. Assays were performed in a 96-well microplate reader. One hundred and seventy microliter of 0.1 M phosphate buffer (pH 7.0), 20 μL of enzyme solution (2.3 U/mL), 20 μL of tested compound and 20 μL of 10 mM DTNB were mixed and incubated at 37 °C for 15 min. Then, 20 μL of 7.5 mM ATCI/BTCI were added. The absorbance was measured at 405 nm. One set of mixtures prepared with an equivalent volume of methanol instead of tested samples was used as a control. Another set of mixtures prepared with an equivalent volume of phosphate buffer instead of enzyme was used as a blank. The inhibitory rates (%) were calculated according to the formula:
Assay for α-glucosidase inhibitory activity
The inhibition of α-glucosidase was performed on a microplate reader according to the standard methodCitation13 with slight modification: 170 μL of 0.1 M phosphate buffer (pH 7.0), 20 μL of enzyme solution (0.2 U/mL) and 20 μL of tested compound were mixed. After the incubation time (15 min, 37 °C) the reaction was initiated by adding 20 μL of 2.5 mM α-PNPG. After an additional 15 min at 37 °C, the reaction was stopped by adding 80 μL of 0.2 M Na2CO3 and absorbance was measured at 405 nm. One set of mixtures prepared with an equivalent volume of methanol instead of tested samples was used as a control. Another set of mixtures prepared with an equivalent volume of phosphate buffer instead of enzyme was used as a blank. The inhibitory rates (%) were calculated according to the formula:
Results
Supplementary Figure 1 shows the HPLC profile of the crude methanolic extract recorded at 254 nm. Four compounds were obtained by the column chromatography of the extract, three of them [7β,6β-dihydroxyroyleanone (2), 7β-acetoxy-6β-hydroxyroyleanone (3) and coleon U quinone (4)] were identified using obtained NMR spectral data by comparison with literature. Rosmarinic acid was identified by co-HPLC with the standard. Structures of the isolated compounds are presented in .
Isolated compounds were investigated for their antibacterial activities. The MIC of each compound was determined and the results are given in . Abietane diterpenoids were active against the Gram-positive bacteria S. aureus and E. faecalis. Coleon U quinone has high activity against S. aureus and moderate activity against E. faecalis, but showed no activity against the Gram-negative bacteria.
Table 1. Effect of the isolated compounds on bacterial growth.
The four isolated compounds were tested for inhibition of AChE, BuChE (with galanthamine as the reference compound) and α-glucosidase (with acarbose as the reference compound). Among the contituents of P. madagascariensis, rosmarinic acid (1) exhibited the strongest inhibition of AChE with IC50 527.8 μM and 7β-acetoxy-6β-hydroxyroyleanone was effective inhibitor of BuChE with IC50 256.4 μM ().
Table 2. Enzyme inhibition activities of the isolated compounds.
In our study of the α-glucosidase inhibition, the inhibitory concentration IC50 was estimated to be 131.2 μM for acarbose. 7β-acetoxy-6β-hydroxyroyleanone (3) demonstrated stronger activity than acarbose with IC50 108.2 μM, the best α-glucosidase inhibition was caused by rosmarinic acid (1) with IC50 33.0 μM.
Discussion
The isolated compounds 7β,6β-dihydroxyroyleanone (2) and 7β-acetoxy-6β-hydroxyroyleanone (3) are highly oxidized abietanes with high antibacterial activity and were previously found in extracts of Coleus zeylanicusCitation14. Two known stereoisomers of compunds (2) and (3), reported as 7α,6β-dihydroxyroyleanone and 7α-acetoxy-6β-hydroxyroyleanone, showed strong antimicrobial activity and cytotoxic activity on different human cancer cell linesCitation5,Citation15.
Abietane diterpenoids coleon U quinone (4) together with coleon U were previously identified in the aerial parts of Plectranthus forsteri “Marginatus”. Previous study by Wellsow et al.Citation6 suggested that coleon U quinone is an oxidation product of coleon U based on the fact that it was not detected by HPLC in the crude extract. According to our results, coleon U quinone is present also in the extract of the fresh plant (Supplementary Figure 1). The quantity of coleon U quinone in the extract is increased by drying or lyophilizing. Coleon U quinone is an antibacterial compound with a comparable effect with that of the positive control chloramphenicol.
According to earlier study by Gaspar-Marques et al.,Citation5 the antibacterial activity is dependent on the presence of 12-hydroxy-p-benzoquinone moiety in the ring C. Based on our results, the stereochemistry of the hydroxyl group (2) and the acetoxy group (3) at C-7 in the ring B had no effect on the antibacterial activity (MIC values 4–60 µg/mL).
Rosmarinic acid is the main compound in the extracts of Plectranthus species and the AChE inhibitory activity exhibited by the plant extracts is in correlation to its content (2). Abietanes, especially 7β-acetoxy-6β-hydroxyroyleanone (3), acted as inhibitors of BuChE. The present ester bond may contribute to this activity. However, the inhibitory activity of these compounds is approximately two times lower than that of the standard galanthamine and ten times lower than the activity of some flavonoidsCitation16.
In the Plectranthus species, antidiabetic activity of the ethanolic extract of P. amboinicus mediated through regulation of carbohydrate metabolic enzyme activities was confirmedCitation17. The most potent α-glucosidase inhibitor, rosmarinic acid, has the same activity as the flavonoid quercetin earlier used as positive controlCitation18. Based on our results, high antioxidant activity and significant anti-glycation inhibitory activity of rosmarinic acidCitation19, this compound is a promising drug for the treatment of diabetic complications.
Conclusions
In this study, we focused on the biological activities of the constituents from P. madagascariensis (antibacterial, AChE, BuChE and α-glucosidase inhibitory activity). Our results confirm high potential of this plant in the treatment of antibacterial diseases. Plectranthus madagascariensis can be also used for isolation of compounds for the treatment of diabetes mellitus and AD in view of the fact that it has components with BuChE and α-glucosidase inhibitory activities (7β-acetoxy-6β-hydroxyroyleanone and rosmarinic acid).
Declaration of interest
This work was supported by the Internal Grant Agency, University of Veterinary and Pharmaceutical Sciences (IGA VFU) (Grant Nos. 6/2010/FaF and 7/2010/FaF).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplementary material available online
Supplementary Figure 1
Supplementary Material
Download PDF (54.8 KB)References
- Lukhoba CW, Simmonds MSJ, Paton AJ. Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol 2006;103:1–24
- Falé PLV, Madeira PJA, Florôencio MH, et al. Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food Funct 2011;2:130–6
- Grayer RJ, Eckert MR, Veitch NC, et al. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry 2003;64:519–28
- Teixeira AP, Batista O, Simoes MF, et al. Abietanes diterpenoids from Plectranthus grandidentatus. Phytochemistry 1997;44:325–7
- Gaspar-Marques C, Rijo P, Simoes MF, et al. Abietanes from Plectranthus grandidentatus and P. hereroensis against methicillin- and vancomycin-resistant bacteria. Phytomedicine 2006;13:267–71
- Wellsow J, Grayer RJ, Veitch NC, et al. Insect-antifeedant and antibacterial activity of diterpenoids from species of Plectranhus. Phytochemistry 2006;67:1818–25
- Ascensão L, Figueiredo AC, Barroso JG, et al. Plectranthus madagascariensis: morphology of the glandular trichomes, essential oil composition, and its biological aktivity. Int J Plant Sci 1998;159:31–8
- Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int Psychogeriatr 2002;14:77–91
- Kumar S, Narwal S, Kumar V, Prakash O. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev 2011;5:19–29
- Jorgensen JH, Turnidge JD, Washington, JA. Antibacterial susceptibility tests: dilution and disc-diffusion methods. In: Murray PR, Baron EJ, Pfaller MA, et al, eds. Manual of clinical microbiology. 7th ed. Washington, DC: ASM Press; 1999:1526–43
- Schwalbe R, Steele-Moore L, Goodwin AC. Antimicrobial susceptibility testing protocols. Boca Raton (FL): CRC Press; 2007
- Fan P, Terrier L, Hay AE, et al. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010;81:124–31
- Valentová M, Marek R, Švajdlenka E, et al. A new isoflavanone from Iresine herbstii. Fitoterapia 2011;82:272–5
- Mehrotra R, Vishwakarma RA, Thakur RS. Abietane diterpenoids from Coleus zeylanicus. Phytochemistry 1989;28:3135–7
- Gaspar-Marques C, Pedro M, Simoes MF, et al. Effect of abietane diterpenes from Plectranthus grandidentatus on the growth of human cancer cell lines. Planta Medica 2002;68:839–40
- Katalinić M, Rusak G, Domaćinović BJ, et al. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem 2010;45:186–92
- Vidwanathaswamy AHM, Koti BC, Ore A, et al. Antihyperglycemic and antihyperlipidemic activity of Plectranthus amboinicus on normal and alloxan induced diabetic rats. Indian J Pharm Sci 2011;73:139–45
- Ha TJ, Lee JH, Lee MH, et al. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem 2012;135:1397–403
- Al-Musayeib N, Perveen S, Fatima I, et al. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules 2011;16:10214–26