Abstract
A new series of chiral thiourea derivatives (5a–5c) and thiourea containing benzimidazole moieties (9b–9e) were synthesized from different amino acids (l-valine, l-isoleucine, l-methionine, l-phenylalanine, and d-phenylglycine). The compounds were characterized and tested against the two most studied members of the pH regulatory enzyme family, carbonic anhydrase (CA, EC 4.2.1.1). KI values of the novel compounds were measured in the range of 3.4–73.6 μM for hCA I isozyme and 8.7–1.44.2 μM for hCA II isozyme, respectively. Phenol was also tested as standard in order to understand the structure activity relationship and the clinically used sulfonamide acetazolamide was tested for comparison reasons. All of the compounds exhibited competitive inhibition with 4-nitrophenylacetate as substrate.
Introduction
Benzimidazole derivatives incorporating thiourea moieties play important roles in medical field with various pharmacological activities such as antimicrobial, antiviral, antidiabetic, and anticancer activityCitation1. The potency of these clinically useful drugs in the treatment of microbial infections and other activities encouraged the development of some more potent and significant compoundsCitation2,Citation3. Benzimidazoles are remarkably effective compounds, and extensive biochemical and pharmacological studies have confirmed that these molecules are effective against various strains of microorganisms. The development of new antimicrobial and anticancer therapeutic agents is one of the fundamental goals in medicinal chemistryCitation4,Citation5.
Carbonic anhydrase (CA; carbonate hydrolase, EC 4.2.1.1) enzymes play important roles in several physiological and pathological processesCitation6. Sixteen CA isoforms have been identified in mammals that differ in subcellular localization and catalytic activityCitation7. CA isoforms take part in several vital biological processes such as acid–base balance, electrolyte secretion, carbon dioxide and ion transport, bone resorption, respiration, ureagenesis, gluconeogenesis, and lipogenesisCitation6–10. Inhibitors or activators of these enzymes have several medical applications, such as diuretics, in the treatment of glaucoma, in the management of several neurological disorders, including epilepsy, possibly in the treatment of Alzheimer’s disease. However, it is relatively difficult to design agents (inhibitors or activators) with specificity or selectivity for any of these isoforms, and many pharmacological agents belonging to the class of the CA inhibitors (CAIs) act as promiscuous inhibitors of most isozymes with physiological/pathological relevance resulting in undesired side effectsCitation10–14. So far inhibitory effects of different sulfonamide derivatives, phenols, anions, and drugs have been investigated against many CAsCitation15–19.
However, it is still important to discover further classes of potential CAIs in order to develop novel compounds with distinct inhibition profiles as compared to the known molecules. Therefore, in this study, we focused on the synthesis, characterization, and CA inhibitory properties of benzimidazole derivatives of thiourea compounds.
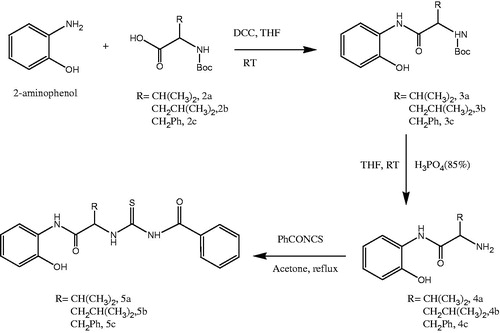
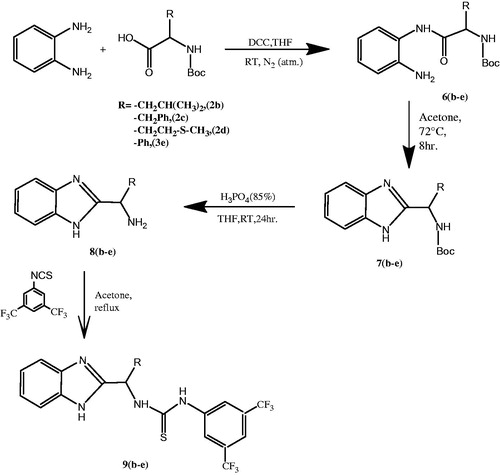
In this study, a new series of chiral thiourea derivatives (5a–c) () and chiral thiourea containing benzimidazol derivatives (9b–e) () were synthesized in good yields, and the structures of the compounds were confirmed by IR, 1H-NMR, 13C-NMR, and elemental analysis. In addition, CA inhibitory activity of the compounds was evaluated.
General procedure for the synthesis of N-Boc-amide derivatives (3a–c)
To a solution of N-Boc-amino acid (2a–c) (1 mmol) in 15 ml THF, 2-aminophenol (1 mmol) and a slight excess of N,N′-dicyclohexylcarbodiimide (1.2 mmol) were added. The mixture was stirred under inert atmosphere at room temperature overnight. The insoluble dicyclohexylurea was removed by filtration and the solvent was evaporated. Evaporation of the solvent provided a residue which was purified by column chromatography using dichloromethane and methanol (25:1) to give 3a–c.
CA inhibitory activity
Compounds 5a–c and 9b–d, as well as the standard, clinically used CAI acetazolamide (AZA), have been tested for the inhibition of two cytosolic ubiquitous isozymes of human origin, that is, hCA I and hCA II ().
Table 1. hCAs I and II inhibition data with studied compounds, phenol and acetazolamide, by an esterase assay with 4-nitrophenylacetate as substrate.
The following should be noted regarding the CA inhibitory data of :
The thiourea derivatives investigated here showed moderate to strong inhibitory properties against the slow cytosolic isoform hCA I. Compound 9b exhibited the lowest inhibition of this isoform, with KI value of 73.6 μM. Compounds 5a–c were much more effective inhibitors against hCA I, with KI-s in the range of 3.4–7.6 μM. These results demonstrate the contribution of the hydroxyl groups to the inhibition efficacy. Interestingly, benzimidazole containing compounds 9b–d were less effective than compound 5c. This trend also shows the attenuator potency of the benzimidazole moiety. As these compounds do not possess any of the zincanchoring groups present in known CAIs, presumably such compounds may bind in the coumarin/phenol binding site.
Similar to hCA I inhibition data, compound 9b acted as the weakest inhibitor against the ubiquitous and dominant rapid cytosolic isozyme hCA II. However, 5a–c derivatives acted as more effective hCA II inhibitors with a comparable potency as the reference compounds phenol (KI: 5.5 μM). The most potent inhibitor was compound 5a (KI: 8.7 μM). This trend again demonstrates the attenuator potency of the benzimidazole moiety. Also, hydroxyl group containing compounds 5a–c were much more effective compared to hydrophobic group containing compounds 9b–d.
Experimental
All chemicals were obtained commercially from Sigma-Aldrich (Bornem, Belgium). IR spectra were recorded on a Perkin-Elmer 100 FTIR spectrometer and all 1H-NMR and 13C-NMR spectra were recorded using an Oxford NMR 400 MHz spectrometer using TMS as the internal standard d-values in ppm at ambient temperature. Melting points were measured on variable heater.
Tert-butyl 1-(2-hydroxyphenylamino)-3-methyl-1-oxobutan-2-ylcarbamate (3a): Yellow oily product, 73% yield, IR (KBr): 3291(–OH), 3070(ArC–H), 2970(C–H), 2934(C–H), 1680(C=O), 1612(C=O), 1532(ArC=C), 1505(ArC=C), 1499(ArC=C), 1286(C–O), 1169(N–C), 749(mono subs. Ar). 1H-NMR (CDCl3, δ ppm) 8.67 (s, 1H), 8.59 (bs, 1H), 7.08 (d, J = 8 Hz, 2H), 6.98 (dd, J = 8.4 Hz and 1.2 Hz, 1H), 6.82 (td, J = 8 Hz and 1.2 Hz, 1H), 5.19 (d, J = 8 Hz, 1H), 4.42 (t, J = 7.2 Hz, 1H), 2.24 (m, 1H), 1.46 (s, 9H), 1.05 (d, J = 6.4 Hz, 3H) and 1.01 (d, J = 6.8 Hz).
Tert-butyl 1-(2-hydroxyphenylamino)-4-methyl-1-oxopentan-2-ylcarbamate (3b): Yellow oily product, 53% yield, IR (KBr): 3291(–OH), 3049(ArC–H), 2965(C–H), 2933(C–H), 1694(C=O), 1660(C=O), 1524(ArC=C), 1450(ArC=C), 1256(C–O), 1168(C–N) and 738(mono subst. Ar). 1H-NMR (CDCl3, δ ppm) 8.74 (bs, 1H), 7.08 (m, 2H), 6.98 (d, J = 7.6 Hz, 1H), 6.79 (t, J = 7.2 Hz, 1H), 5.73 (d, J = 8 Hz, 1H), 4.18 (m, 1H), 1.96 (m, 1H), 1.6 (m, 1H), 1.44 (s, 9H), 1.01 (d, J = 6.8 Hz, 3H), 0.93 (d, J = 7.6 Hz, 3H).
Tert-butyl 1-(2-hydroxyphenylamino)-1-oxo-3-phenylpropan-2-ylcarbamate (3c): White solid, 51% yield, mp = 126–128 °C, IR (KBr): 3360(N–H), 3311(N–H), 3064(ArC–H), 2978(C–H), 1668(C=O), 1616(C=O), 1599(C=O), 1543(ArC=C), 1455(ArC=C), 1279(C–O), 1165(C–N), 750(mono subs Ar). 1H-NMR (CDCl3, δ ppm), 8.47 (bs, 1H), 8.22 (bs, 1H), 7.33–7.22 (m, 5H), 7.11–7.07 (m, 1H), 6.97 (dd, J = 7.6, 1.2 Hz, 1H), 6.84–6.77 (m, 2H).
Deprotection reaction of 2-N-Boc-amide derivatives (4a–c)
About 1 mmol N-Boc-amide (3a–b) was dissolved in 2 ml THF and 2 ml 85% H3PO4 was added dropwise to the solution. The reaction mixture was stirred overnight at room temperature. To terminate the reaction, 5 ml distilled water was added and the reaction mixture was neutralized with saturated NaOH. The solution was extracted with ethyl acetate. The organic phase was dried using Na2SO4 and evaporated under vacuum and pure products (4a–c) were obtained.
2-Amino-N-(2-hydroxyphenyl)-3-methylbutanamide (4a): Yellow solid, 77% yield, mp = 140–142 °C, IR (KBr): 3411(–OH), 3329(NH2), 3274(NH2), 2966(C–H), 2918(C–H), 1676(C=O), 1596(C=O), 1525(ArC=C), 1456(ArC=C), 1284(C–O), 1004(C–N), 751(mono subs. Ar) cm−1. 1H-NMR (CDCl3, δ ppm) 9.81 (s, 1H), 7.11 (td, J = 8.4 Hz and 1.2 Hz, 1H), 7.02 (dd, J = 8 Hz and 1.2 Hz, 1H) 6.95 (dd, J = 8 Hz and 1.6 Hz, 1H), 6.84 (td, J = 8.8 Hz and 1.6 Hz, 1H), 3.47 (d, J = 3.6 Hz, 1H), 2.46 (m, 1H), 1.07 (d, J = 7.2 Hz, 3H) and 0.9 (d, J = 6.8 Hz, 3H).
2-Amino-N-(2-hydroxyphenyl)-4-methylpentanamide (4b): Yellow oily product, 85% yield, IR (KBr): 3287(–OH), 3064(ArC–H), 2963(C–H), 1695(C=O), 1596(C=O), 1499(ArC=C), 1429(ArC=C), 1282(C–O), 1105(C–N), 750(mono subs Ar). 1H-NMR (CDCl3, δ ppm), 9.81 (bs, 1H), 7.12 (td, J = 8 Hz and 1.6 Hz, 1H), 7.02 (d, J = 8 Hz, 1H), 6.95 (d, J = 6.8 Hz, 1H), 6.84 (td, J = 7.2 Hz and 0.8 Hz,1H), 3.52 (d, J = 3.6 Hz, 1H), 2.16 (m, 2H), 2.09 (s, 1H), 1.37 (m, 1H), 1.15 (m, 1H), 1.05 (d, J = 6.8 Hz, 3H) and 0.93 (t, 7.6 Hz, 3H).
2-Amino-N-(2-hydroxyphenyl)-3-phenylpropanamide (4c): White solid, 86% yield, mp = 170–173 °C, IR (KBr): 3399(−OH), 3296(NH), 3083(ArC–H), 2978(C–H), 1647(C=O), 1614(C=O), 1588(ArC=C), 1542(ArC=C), 1454(ArC=C), 1282(C–O), 1102(C–N), 748.6(mono subs. Ar), 700(mono subs. Ar). 1H-NMR (CDCl3, δ ppm), 9.69 (bs, 1H), 7.36–7.23 (m, 5H), 7.11 (t, J = 7.6 Hz, 1H), 7.18 (d, J = 8 Hz, 1H), 6.93 (dd, J = 8, 1.6 Hz, 1H), 6.83 (td, J = 8, 1.6 Hz, 1H) 3.81 (s, 1H), 3.35 (dd, J = 14, 3.6 Hz, 1H), 2.85 (J = 13.6, 8.8 Hz, 1H).
Synthesis of thiourea derivatives (5a–c)
About 1 mmol of potassiumthiocyanide (KSCN) was stirred in 15 ml dried acetone and 1 mmol benzoyl chloride was added dropwise under an argon atmosphere. Precipitation was observed immediately. The reaction mixture was refluxed until the color of the solution turned yellow (approximately 2 h). Then, the reaction mixture was cooled to room temperature and an amide (4a–b) solution in 5 ml dried acetone was added dropwise to the reaction flask which was then refluxed for 2 h. Reaction was monitored by TLC. The solution was filtered and evaporation of the solvent from the filtrate provided a residue which was crystallized from CH2Cl2: hexane to give bright yellow crystals.
N-(1-(2-hydroxyphenylamino)-3-methyl-1-oxobutan-2-ylcarbamothioyl)benzamide (5a): Bright yellow crystal, 79% yield, mp = 164.5–165.8 °C, IR (KBr): 3308(–OH), 3248(N–H), 3071(ArC–H), 2968(C–H), 2981(C–H), 1670(C=O amide), 1645(C=O), 1531(ArC=C), 1520(ArC=C), 1453(ArC=C), 1263(C–O), 1162(N–C), 1093(C=S), 748(mono subs. Ar), 714(mono subs. Ar). 1H-NMR (CDCl3, δ ppm) 11.23 (d, J = 7.2 Hz, 1H), 9.11 (s, 1H), 8.44 (s, 1H), 7.84 (d, J = 7.6 Hz, 2H), 7.64 (t, J = 1.2 Hz, 1H), 7.52 (t, J = 8 Hz, 2H), 7.17 (d, J = 1.6 Hz, 1H), 7.14 (t, J = 6.8 Hz, 1H), 7.09 (d, J = 1.6 Hz, 1H), 6.87 (t, J = 6.8 Hz, 1H), 4.93 (t, J = 7.2 Hz, 1H), 2.52 (m, 1H) and 1.15 (d, J = 6.8 Hz, 6H). 13C-NMR (CDCl3, δ ppm) 169.7, 167.3, 148.7, 134.1, 131.6, 129.4, 127.8, 127.4, 125.4, 122.9, 120.8, 119.4, 65.7, 30.6, 19.6, and 18.6. Anal. Calc. for C19H21N3O3S: C, 61.44; H, 5.70; N, 11.31; S, 8.63. Found: C, 61.57; H, 5.88; N, 10.60; S, 7.73%.
N-(1-(2-hydroxyphenylamino)-4-methyl-1-oxopentan-2-ylcarbamothioyl)benzamide (5b): Yellow solid, 79% yield, mp = 161–163 °C, IR (KBr): 3269(N–H), 3063(ArC–H), 2961(C–H), 2927(C–H), 1666(C=O), 1599(C=O) 1520(ArC=C), 1498(–ArC=C), 1458(–ArC=C), 1166(C–O)¸ 1157(N–C), 1084(C=S), 745(mono subs. Ar), 723(mono subs. Ar). 1H-NMR (CDCl3, δ ppm) 11.22 (d, J = 7.6 Hz, 1H), 9.09 (s, 1H), 8.4 (s, 1H), 8.29 (bs, 1H), 7.86 (d, J = 8 Hz, 2H), 7.64 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 8 Hz, 2H), 7.12 (t, J = 7.6 Hz, 2H), 7.08 (d, J = 7.2 Hz, 1H), 6.86 (t, J = 7.6 Hz, 1H), 4.99 (t, J = 7.6 Hz, 1H), 2.32 (m, 1H), 1.72 (m, 1H), 1.67 (s, 1H), 1.40 (m, 1H), 1.11 (d, J = 6.8 Hz, 3H), and 1.01 (t, J = 7.2 Hz, 3H). 13C-NMR (CDCl3, δ ppm), 181.3, 169.7, 167.3, 148.8, 134.1, 131.6, 129.4, 127.8, 127.5, 125.4, 122.9, 120.8, 119.6, 64.7, 36.5, 25.4, 15.9, and 11.4. Anal. Calc. for C20H23N3O3S: C, 63.32; H, 6.01; N, 10.90; S, 8.32. Found: C, 61.93; H, 6.03; N, 10.54; S, 7.72%.
N-(1-(2-hydroxyphenylamino)-1-oxo-3-phenylpropan-2-ylcarbamothioyl)benzamide (5c): White solid, 65% yield, mp = 164–167 °C IR (KBr): 3262(N–H), 3063(ArC=C), 2955(C–H), 2922(C–H), 1667(C=O), 1600(C=O), 1580(ArC=C), 1532(ArC=C), 1515(ArC=C), 1498(ArC=C), 1220(C–O), 1162(C–N), 743(mono subs. Ar), 714(mono subs. Ar). 1H-NMR (CDCl3, δ ppm) 11.31 (d, J = 6.8 Hz, 1H), 9.04 (s, 1H), 8.1 (s, 1H), 7.93–6.79 (m, 14H), 5.36 (dd, J = 14.8, 8 Hz, 1H), 3.37 (dd, J = 13.6, 6.4 Hz, 1H), and 3.29 (dd, J = 14, 8.4 Hz, 1H). 13C-NMR (CDCl3, δ ppm), 180.8, 169.11, 168.85, 149.07, 137.13, 133.72, 132.76, 130.09, 129.27, 129.09, 128.91, 127.38, 126.23, 125.78, 123.56, 119.59, 116.28, 60.39, and 38.12. Anal. Calc. for C23H21N3O3S: C, 65.85; H, 5.05; N, 10.02; S, 7.64. Found: C, 64.66; H, 5.40; N, 9.14; S, 6.89%.
Synthesis of N-Boc-amide derivatives (6b–e)
The general procedure described for compounds (3a–c) was carried out by using phenylenediamine (1 mmol). The resulting residue was purified by column chromatography using (hexane/ethyl acetate (EtOAc), 3:2) to give product (6b–e).
Tert-butyl 1-(2-aminophenylamino)-3-methyl-1-oxopentan-2-ylcarbamate (6b): Yellow oily product, 72% yield, IR (KBr): 3488.1, 3419.6, 3354.2, 3304.8, 3033.2, 2966.8, 2632.7, 2310.9, 1673.6, 1660.5, 1625.9, 1592.9, 1525.5, 1460.3, 1392.5, 1314.4, 1165.8, 1020.7, 987.8, 811.9, and 746.2. 1H-NMR (CDCl3, 400 MHz, δ ppm) 7.85 (bs, 1H), 7.20 (d, 1H, J = 7.2 Hz), 7.03 (d, 1H, J = 16 and 7.6 Hz), 6.75 (dd, 2H, J = 12 and 7.6 Hz), 5.21 (d, 1H, J = 7.6 Hz), 4.79 (t, 1H, J = 9.6 and 7.6), 4.06 (t, 1H, J = 15.2 and 7.2), 3.85 (s, 2H), 1.99 (m, 1H, J = 6.4 and 3.6 Hz), 1.44 (s, 9H), 1.36 (t, 1H, J = 7.6 and 4 Hz), and 1.03 (d, 3H, J = 6.8 Hz). 13C-NMR (CDCl3, 100 MHz, δ ppm) 171.57, 156.63, 141.45, 127.32, 126.19, 118.76, 117.30, 80.16, 60.08, 28.57, 25.20, 37.27, 15.79, and 11.38.
Tert-butyl 1-(2-aminophenylamino)-1-oxo-3-phenylpropan-2-ylcarbamate (6c): Yellow oily product, 66% yield, IR (KBr): 3283.2, 2963.2, 2935.3, 2852.4, 1663.9, 1661.5, 1527.3, 1366.1, 1342.3, 1332.5, 1256.9, 1167.3, 1019.3, 872.4, and 748.8. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 9.19 (bs, 1H), 7.29 (dd, 3H, J = 15.2 and 7.6 Hz), 7.20 (t, 1H, J = 13.2 and 6 Hz), 7.03 (dd, 2H, J = 22 and 8 Hz), 6.89 (dd, 2H, J = 15.2 and 7.6 Hz), 6.52 (dd, 2H, J = 14.8 and 7.2 Hz), 4.77 (s, 2H), 4.34 (d, 1H, J = 5.6 Hz), 3.31 (s, 1H), 3.03 (dd, 1H, J = 13.6 and 5.2 Hz), 2.88 (t, 1H, J = 22.4 and 9.2 Hz), 1.34 (s, 9H). 13C-NMR (DMSO-d6, 100 MHz, δ ppm) 171.35, 156.21, 143.34, 138.65, 130.01, 128.75, 126.97, 126.63, 123.35, 116.65, 116.22, 78.87, 56.94, 38.14, and 28.86.
Tert-butyl1-(2-aminophenylamino)-4-(methylthio)-1-oxobutan-2-ylcarbamate(6d): Yellow oily product, 71% yield, IR (KBr): 3285.3, 3072.7, 2976.8, 2934.3, 2928.8, 1664.3, 1634.9, 1533.2, 1500.1, 1457.6, 1366.4, 1250.7, 1166.3, 1049.9, 1042.1, 1018.8, 860.5, and 748.1. 1H-NMR (CDCl3, 400 MHz, δ ppm) 8.19 (bs, 1H), 7.17 (d, J = 8 Hz, 1H), 7.01 (t, 1H, J = 8 and 3.6 Hz), 6.72 (t, 2H, J = 8 and 6.8 Hz), 5.53 (bs, 1H), 4.41 (d, 1H, J = 6.8 Hz), 3.86 (s, 2H), 2.59 (t, 2H, J = 14.4 and 7.2 Hz), 2.16 (t.d, 1H, J = 14, 6.8 and 7.2 Hz), 2.00 (t.d, 1H, J = 13.6, 6.8 and 3.6 Hz), and 1.44 (s, 9H). 13C NMR (CDCl3, 100 MHz, δ ppm) 170.79, 156.29, 141.10, 127.50, 125.82, 123.52, 119.18, 117.58, 80.76 54.41, 31.59, 30.54, 28.57, and 15.59.
Tert-butyl 2-(2-aminophenylamino)-2-oxo-1-phenylethylcarbamate (6e): Yellow oily product, 69% yield, IR (KBr): 3324.7, 3274.3, 2976.9, 2964.3, 2930.3, 1685.1, 1498.1, 1458.6, 1366.2, 1246.9, 1167.5, 1052.6, 886.2, and 698.7. 1H-NMR (CDCl3, 400 MHz, δ ppm) 8.04 (s, 1H), 7.42 (d, 2H, J = 7.6 Hz), 7.32 (d, 3H, J = 5.6 Hz), 7.09 (d, 1H, J = 7.6 Hz), 6.98 (t, 1H, J = 15.6 and 7.6 Hz), 6.68 (dd, 2H, J = 14 and 7.6 Hz), 5.94 (d, 1H, J = 6.4 Hz), 5.37 (bs, 1H), 3.66 (s, 2H), and 1.41 (s, 2H). 13C-NMR (CDCl3, 100 MHz, δ ppm) 169.61, 155.70, 141.24, 138.14, 137.35, 128.71, 129.30, 128.71, 127.65, 127.48, 126.09, 123.40, 117.65, 80.64, 59.28, and 28.56.
Synthesis of 2-N-Boc-amide derivative of benzimidazoles (7b–e)
N-Boc-amide derivative (6b–e) was dissolved in 20 ml of acetic acid and the solution was stirred at 72 °C for 8 h. The acetic acid was removed under reduced pressure and the crude compound was purified by column chromatography (hexane/EtOAc, 3:2 by volume) to afford a white solid.
Tert-butyl 1-(1H-benzo[d]imidazol-2-yl)-2-methylbutylcarbamate (7b): White solid product, 75% yield, m.p = 226–228 °C, IR (KBr): 3202.9, 3060.8, 2966.8, 2985.8, 2877.5, 1674.8, 1623.3, 1584.7, 1487.5, 1456.1, 1318.2, 1173.4, 1011.6, 930.9, 877.8, and 740.5. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 12.10 (bs, 1H), 7.55 (d, 1H, J = 6.8), 7.45 (d, 1H, J = 7.2 Hz), 7.13 (dd, 2H, J = 12 and 5.6 Hz), 4.63 (t, 1H, J = 16.4 and 8 Hz), 1.95 (s, 1H), 1.48 (m, 1H, J = 13.6, 6.8 and 4 Hz), 1.37 (s, 9H), 0.85 (t, 3H, J = 14.8 and 7.6 Hz), and 0.74 (d, 3H, J = 6.8 Hz). 13C-NMR (CDCl3, 100 MHz, δ ppm) 156.70, 155.32, 122.51, 137.52, 117.25, 80.22, 54.88, 38.83, 28.54, 25.82, 15.86, and 11.19.
Tert-butyl 1-(1H-benzo[d]imidazol-2-yl)-2-phenylethylcarbamate (7c): White solid product, 68% yield, IR (KBr): 3315.7, 2930.2 2916.8, 1677.9, 1634.9, 1530.1, 1522.4, 1455.3, 1367.2, 1363.8, 1273.5, 1169.4, 1017.2, 730.1, and 697.9. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 8.19 (bs, 1H), 7.49 (dd, 2H, J = 6 and 3.6 Hz), 7.32 (d, 1H, J = 4 Hz), 7.23 (d, 4H, J = 4 Hz), 7.12 (dd, 2H, J = 3.6 and 1.2 Hz), 4.99 (d, 1H, J = 5.6 Hz), 3.34 (dd, 1H, J = 14 and 5.2 Hz), 3.08 (dd, 1H, J = 9.6 Hz), 1.71 (s, 1H), and 1.28 (s, 9H). 13C-NMR (CDCl3, 100 MHz, δ ppm) 155.93, 155.83, 138.81, 129.89, 128.72, 126.86, 122.13, 78.76, 51.53, 34.04, and 28.84.
Tert-butyl 1-(1H-benzo[d]imidazol-2-yl)-3-(methylthio)propylcarbamate (7d): White solid product, 82% yield, IR (KBr): 3298.3, 3072.7, 2916.8, 1634.9, 1527.4, 1457.6, 1439.5, 1367.1, 1362.4, 1307.8, 1270.5, 1250.7, 1165.2, 1043.4, 1171.4, 1024.7, 867.5, and 760.1. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 12.15 (bs, 1H), 7.33 (d, 2H, J = 8 Hz), 7.12 (d, 2H, J = 3.6 Hz), 4.90 (d, 1H, J = 6.4 Hz), 3.32 (s, 1H), 2.50 (t, 2H, J = 14.4 and 6.8 Hz), 2.19 (td, 1H, J = 14, 6.4 and 6.8 Hz), 2.07 (t, 1H, J = 15.2 and 7.6), 1.37 (s, 3H) and 1.21 (s, 9H). 13C-NMR (CDCl3, 100 MHz, δ ppm) 156.05, 143.69, 134.87, 122.43, 121.67, 112.00, 78.89, 49.17, 34.13, 30.50, 28.88, and 15.38.
Tert-butyl 1-(1H-benzo[d]imidazol-2-yl)-3-(methylthio)propylcarbamate (7e): White solid product, 76% yield, mp = 195–198 °C, IR (KBr): 3408.7, 2975.8, 2964.8, 2874.5, 1671.2, 1622.4, 1585.6, 1439.6, 1247.8, 1163.9, 1052.6, 750.1 and 740.5. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 12.22 (bs, 1H), 7.76 (s, 1H), 7.54 (d, 1H, J = 6.8 Hz), 7.41 (d, 1H, J = 6.4 Hz), 7.25 (td, 1H, J = 8.4, 7.2 and 6.4 Hz), 7.23 (dd, 2H, J = 12.8 and 6 Hz), 7.12 (d, 2H, J = 0.8 Hz), 5.99 (d, 1H, J = 3.6 Hz) and 1.37 (s, 9H). 13C-NMR (DMSO-d6, 100 MHz, δ ppm) 157.34, 154.97, 140.86, 129.04, 128.16, 127.96, 122.36, 79.27, 28.84, and 53.95.
Deprotection of 2-N-Boc-amide derivative benzimidazoles (8b–e)
The general procedure described for compound (4a–c) was carried out with benzimidazole derivative (8b–e).
1-(1H-Benzo[d]imidazol-2-yl)-2-methylbutan-1-amine (8b): White solid product, 70% yield, mp = 162–164 °C, IR (KBr): 2965.4, 2928.7, 1625.9, 1592.8, 1519.9, 1447.9, 1392.3, 1342.4, 1311.3, 1272.9, 1149.7, 1016.5, 839.6, 768.1, 742.1 and 609.8. 1H-NMR (CDCl3, 400 MHz, δ ppm) 7.54 (d, 2H, J = 5.2 and 2.8 Hz), 7.16 (dd, 2H, J = 7.2 and 4 Hz), 5.75 (bs, 2H), 4.21 (d, 1H, J = 6 Hz), 2.01 (s, 1H), 1.54 (m, 1H) and 1.24 (s, 1H). 13C-NMR (CDCl3, 100 MHz, δ ppm) 157.35, 138.49, 122.42, 115.19, 55.67, 40.82, 24.82, 15.76, and 11.69.
1-(1H-Benzo[d]imidazol-2-yl)-2-phenylethanamine (8c): White solid product, 88% yield, mp = 169–172 °C, IR (KBr): 3167.2, 2930.4, 2776.3, 1452.8, 1274.8, 1121.5, 764.3, 751.2, 749.8 and 700.09. 1H-NMR (CDCl3, 400 MHz, δ ppm) 8.20 (bs, 1H), 7.48 (s, 2H), 7.22 (d, 2H, J = 7.2 Hz), 7.12 (dd, 5H, J = 18.4 and 6.8 Hz), 7.10 (dd, 2H, J = 3.2 and 1.2 Hz), 4.27 (t, 1H, J = 7.6 and 5,6 Hz), 3.25 (dd, 1H, J = 13.2 and 6 Hz), 2.96 (dd, 1H, J = 13.2 and 7.6 Hz). 13C-NMR (DMSO-d6, 100 MHz, δ ppm) 159.32, 139.45, 129.94, 128.80, 126.74, 121.83, 43.81, and 52.67.
1-(1H-Benzo[d]imidazol-2-yl)-3-(methylthio)propan-1-amine (8d): Yellow oily, 66% yield, IR (KBr): 3321.3, 2922.1, 2966.4, 2918.8, 1675.3, 1575.5, 1437.2, 1271.8, 1252.4, 1022.1, 752.4, and 743.4. 1H-NMR (CDCl3, 400 MHz, δ ppm) 7.55 (dd, 2H, J = 6 and 3.2 Hz), 7.21 (dd, 2H, J = 7.2 and 2.8 Hz), 4.99 (s, 2H), 4.42 (t, 1H, J = 13.6 and 6.8), 2.80 (td, 1H, J = 14.8 and 2.8 Hz), 2.56 (t, 2H, J = 7.2 and 2.8 Hz), 2.30 (td, 1H, J = 13.2 and 6.8), 2.16 (s, 1H), and 2.03 (s, 3H). 13C-NMR (CDCl3, 100 MHz, δ ppm) 157.27, 138.47, 122.70, 115.27, and 49.92.
(1H-Benzo[d]imidazol-2-yl)(phenyl)methanamine (8e): White solid product, 65% yield, mp = 201–203 °C, IR (KBr): 3380.7, 2935.3, 2925.1, 1564.2, 1413.6, 1275.3, 1312.4, 1019.7, 1012.2, 750.1, 742.1 and 653.1. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 7.84 (dd, 4H, J = 6.8, 3.2 and 3.6 Hz), 7.30 (dd, 2H, J = 7.6, 2 and 1.2 Hz), 7.20 (m, 1H, J = 6.8, 2 and 1.2 Hz), 7.11 (dd, 2H, J = 6.8, 4 and 3.2 Hz), 5.74 (s, 1H), 5.29 (s, 2H), 2.48 (t, 1H, J = 4.4 and 2 Hz). 13C-NMR (DMSO-d6, 100 MHz, δ ppm) 158.99, 144.59, 128.86, 127.60, 121.91, and 55.36.
Synthesis of thiourea derivatives (9b–e)
2-N-Boc-amide derivative benzimidazole (1 mmol) (8b–e) was dissolved in 5 ml of dry THF. Then 1-isothiocyanato-3,5-bis(trifluoromethyl)benzene (1 mmol) was added at 0 °C to the solution. The mixture was stirred for 10 min at 0 °C then allowed to reach room temperature, and stirred for a further 24 h. The solvent was removed in vacuum and the resulting material was purified by column chromatography (hexane/EtOAc, 3:2 by volume) to get a bright viscous product.
1-(1-(1H-Benzo[d]imidazol-2-yl)-2-methylbutyl)-3-(3,5-bis(trifluoromethyl)phenyl) thiourea (9b): Bright yellow oily product, 65% yield, mp = 195–197 °C, IR (KBr): 3027.8, 2874.2, 1738.1, 1541.5, 1457.3, 1381.5, 1357.8, 1276.2, 1180.1, 1131.3, 886.3, 764.3 and 680.9. 1H-NMR (DMSO-d6, 400 MHz, δ ppm) 12.42 (bs, 1H), 10.32 (s, 1H), 8.76 (d, 1H, J = 7.6 Hz), 8.29 (s, 2H), 7.75 (s, 1H), 7.59 (d, 1H, J = 6.8 Hz), 7.47 (d, 1H, J = 6.8 Hz), 7.21 (dd, 5H, J = 8 and 7.6 Hz), 7.19 (dd, 2H, J = 8 and 6.8 Hz), 4.04 (t, 1H, J = 7.6 and 6.8 Hz), 3.45 (dd, 1H, J = 14 and 6.8 Hz) and 3.34 (dd, 1H, J = 13.6 and 6.8 Hz). 13C-NMR (DMSO-d6, 100 MHz, δ ppm) 180.88, 154.38, 142.34, 137.80, 131.10, 130.78, 129.84, 128.88, 125.26, 122.71, 122.55, 112.12, 54.52, and 39.86.
1-(1-(1H-benzo[d]imidazol-2-yl)-2-phenylethyl)-3-(3,5-bis(trifluoromethyl)phenyl) thiourea (9c): Bright yellow oily product, 65% yield, mp = 175–177 °C, IR (KBr): 3267.4, 2968.9, 1663.7, 1542.1, 1458, 1382.7, 1277.1, 1179.6, 1133.1, 1000.6, 955.4, 886.7, 848.1, 745.1, and 681.3. 1H-NMR (CDCl3, 400 MHz, δ ppm) 8.92 (bs, 1H), 7.43 (s, 3H), 7.32 (d, 2H, J = 9.6 Hz), 7.16 (s, 2H), 5.80 (s, 1H), 2.31 (t, 1H, J = 6.8 Hz), 2.08 (s, 1H), 1.84 (s, 1H), 1.54 (t, 1H, J = 13.6 and 5.6 Hz), 1.26 (m, 1H, J = 10.8, 6.8 and 3.2 Hz) and 1.12 (d, 3H, J = 6 Hz). 13C-NMR (CDCl3, 100 MHz, δ ppm) 182.22, 156.33, 139.49, 131.52, 131.19, 124.32, 124.10, 123.83, 121.60, 118.52, 59.32, 40.13, 26.66, 14.38, and 11.26.
1-(1-(1H-Benzo[d]imidazol-2-yl)-3-(methylthio)propyl)-3-(3,5-bis(trifluoromethyl) phenyl)thiourea (9d): Bright yellow oily product, 69% yield, mp = 105–108 °C, IR (KBr): 3247.2, 2968.9, 2981.5, 2892.1, 1738.5, 1642.4, 1542.1, 1457.9, 1381.1, 1280.7, 1180.5, 1372.5, 1265.2, 1130.6, 886.9, and 681.1. 1H-NMR (CDCl3, 400 MHz, δ ppm) 9.10 (bs, 1H), 7.99 (s, 1H), 7.63 (s, 1H), 7.47 (s, 1H), 7.34 (s, 1H), 7.20 (dd, 2H, J = 5.6 and 3.6 Hz), 6.14 (s, 1H), 2.76 (td, 1H, J = 13.2 and 5.6 Hz), 2.65 (td, 1H, J = 12.8 and 6.4 Hz), 2.47 (d, 2H, J = 6.8 Hz) and 1.95 (s, 1H). 13C-NMR (CDCl3, 100 MHz, δ ppm) 182.23, 156.46, 139.46, 131.64, 131.30, 123.99, 123.60, 121.61, 118.54, 52.79, 34.43, 30.93, and 15.50.
1-((1H-Benzo[d]imidazol-2-yl)(phenyl)methyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea (9e): Bright yellow oily product, 66% yield, mp = 104–106 °C, IR (KBr): 3277.3, 3031.4, 1704.9, 1662.6, 1541.6, 1457.7, 1382.5, 1277.9, 1179.5, 1132.1, 957.8, 885.8 and 739.9. 1H-NMR (CDCl3, 400 MHz, δ ppm) 10.18 (bs, 1H), 9.69 (s, 2H), 7.75 (s, 2H), 7.40 (s, 1H), 7.32 (dd, 3H, J = 8.8 and 5.6 Hz), 7.22 (dd, 2H, J = 6.8 and 4.2 Hz), 7.19 (dd, 4H, J = 8.4 and 2.8 Hz), and 4.10 (d, 1H, J = 6.4 Hz). 13C-NMR (CDCl3, 100 MHz, δ ppm) 181.89, 155.07, 140.03, 136.94, 131.98, 131.65, 131.31, 130.97, 129.47, 129.19, 124.48, 123.94, 123.65, 121.78, 118.32, and 57.05.
CA inhibition
Enzyme activity was determined spectrophotometrically by following the change in absorbance at 348 nm of 4-nitrophenylacetate to 4-nitrophenylate over a period of 3 min at 25 °CCitation20–23. The enzymatic reaction contained 1.4 ml 0.05 M Tris-SO4 buffer (pH 7.4), 1 ml 3 mM 4-nitrophenylacetate, 0.5 ml H2O and 0.1 ml enzyme solution, in a total volume of 3.0 mlCitation24. Inhibitory effects of compounds 5a–c and 9b–d were compared with phenol and AZA. Different inhibitor concentrations were used and all compounds were tested in triplicate at each concentration used. Control cuvette activity was acknowledged as 100% in the absence of inhibitor. An Activity% – [Inhibitor] graph was drawn for each inhibitorCitation21–23. The curve-fitting algorithm allowed to obtain the IC50 values, working at the lowest concentration of substrate of 0.15 mM, from which KI values were calculatedCitation22–23. The catalytic activity of these enzymes was calculated from Lineweaver-Burk plots, as reported earlierCitation25, and represents the mean from at least three different determinations. The CAI and II isoenzymes used here were purified from human blood as described earlierCitation8–13,Citation26–28.
Conclusion
In summary, synthesis of chiral thiourea containing benzimidazol derivatives 5a–c and the first synthesis of 9b–d have been achieved. In addition, several benzimidazol compounds including novel derivatives have been assayed for the inhibition of the physiologically relevant human CA isozymes hCA I and II. These compounds showed inhibition constants in the range of 3.4–73.6 μM for hCA I and 8.7–44.2 μM for hCA II. In general, the compounds had comparable inhibitory activity with the clinically used sulfonamide AZA. Interaction of most CA isozymes with several types of phenols, such as simple phenol and its substituted derivatives, clioquinol, salicyclates, and some of their derivatives has been recently investigated. Here, we extend these earlier investigations to a novel series of chiral thiourea containing benzimidazol derivatives. The novel benzimidazols represent a promising class of CAIs and the results discussed in this study may help medicinal chemists to design new analogs with enhanced activity and other tailored properties.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Zhang M, Hao P, Zuo W, et al. 2-(Benzimidazol-2-yl)-1,10-phenanthrolyl metal (Fe and Co) complexes and their catalytic behaviors toward ethylene oligomerization. J Organomet Chem 2008;693:483–91
- Takasu K, Azuma T. Synthesis of trifunctional thioureas bearing 1,5-disubstituted triazole tether by Ru-catalyzed Huisgen cycloaddition. Tetrahedron Lett 2010;51:2737–40
- Demir SA, Eymur, S. Self-assembly of organocatalysts for the enantioselective Michael addition of aldehydes to nitroalkenes. Tetrahedron-Asymmetr 2010;21:112–15
- Tumerdem R, Topal G, Turgut Y. Asymmetric reduction of acetophenone using lithium aluminium hydride modified with some novel amino alcohol Schiff bases. Tetrahedron-Asymmetr 2005;16:865–8
- Saeed S, Rashid N, Jones P, et al. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur J Med Chem 2010;45:1323–31
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Ekinci D, Al-Rashida M, Abbas G, et al. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzym Inhib Med Chem 2012;27:744–7
- Cavdar H, Ekinci D, Talaz O, et al. alpha-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzym Inhib Med Chem 2012;27:148–54
- Senturk M, Ekinci D, Goksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzym Inhib Med Chem 2012;27:365–9
- Ekinci D, Karagoz L, Ekinci D, et al. Carbonic anhydrase inhibitors: in vitro inhibition of α isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J Enzym Inhib Med Chem 2013;28:283–8
- Özdemir ZO, Senturk M, Ekinci D. Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules. J Enzym Inhib Med Chem 2013;28:316–19
- Demirdag R, Comakli V, Senturk M, et al. Purification and characterization of carbonic anhydrase from sheep kidney and effects of sulfonamides on enzyme activity. Bioorg Med Chem 2013;21:1522–5
- Ekinci D, Ceyhun SB, Sentürk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–8
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Reis O, Eymur S, Reis B, Demir AS. Direct enantioselective aldol reactions catalyzed by a proline-thiourea host-guest complex. Chem Commun 2009;9:1088–90
- Demirdag R, Yerlikaya E, Senturk M, et al. Heavy metal ion inhibition studies of human, sheep and fish α-carbonic anhydrases. J Enzym Inhib Med Chem 2013;28:278–82
- Balaydin HT, Soyut H, Ekinci D, et al. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzym Inhib Med Chem 2012;27:43–50
- Ekinci D, Ceyhun SB, Senturk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–8
- Ekinci D, Cavdar H, Talaz O, et al. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–63
- Ekinci D, Cavdar H, Durdagi S, et al. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73
- Alp C, Ekinci D, Gultekin MS, et al. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis for carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–74
- Ekinci D, Senturk M, Kufrevioglu OI. Salicylic acid derivatives: synthesis, features and usage as therapeutic tools. Expert Opin Ther Pat 2011;21:1831–41
- Durdagi S, Şentürk M, Ekinci D, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–9
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–66
- Balaydin HT, Durdagi S, Ekinci D, et al. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, methoxy and bromophenol compounds. J Enzym Inhib Med Chem 2012;27:467–75
- Ceyhun SB, Senturk M, Yerlikaya E, et al. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74
- Ekinci D, Kurbanoglu NI, Salamci E, et al. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzym Inhib Med . 2012;27:845–8