Abstract
Context: Asthma is multifaceted disease where many targets contribute towards its development and progression. Among these, adenosine receptor subtypes play a major role.
Objective: MCD-KV-10, a novel thiazolo-thiophene was designed and evaluated pre-clinically for its implication in management of asthma.
Materials and methods: This compound showed good affinity and selectivity towards A2A/A3 adenosine receptor (AR) subtypes. Furthermore, MCD-KV-10 was evaluated for in vitro lipoxygenase inhibition activity; in vivo mast cell stabilization potential and in vivo anti-asthmatic activity was done in ovalbumin-induced airway inflammation model in guinea pigs.
Results: The compound showed good (>57%) inhibition of lipoxygenase enzyme and also effectively protected mast cell degranulation (>63%). The compound showed good anti-asthmatic activity as inferred from the in vivo studies.
Discussion: These results indicate that MCD-KV-10 has an inhibitory effect on airway inflammation.
Conclusion: Though, we have identified a potential candidate for management of asthma, further mechanistic studies are needed.
Introduction
Adenosine receptors are G-protein-coupled receptors which mediate its biological action via its four subtypes, A1, A2A, A2B and A3-adenosine receptors (AR)Citation1. The biochemical interaction of adenosine with its receptors initiates signals transduction pathways, including the adenylyl cyclase effector system, utilizing cAMP as a second messenger. A decrease in intracellular levels of cAMP occurs due to coupling of A1 and A3 adenosine receptors (A1-AR, A3-AR) to Gi proteins followed by inhibition of adenylyl cyclase. However, an upsurge of cAMP levels occurs upon coupling of A2A/A2B adenosine receptor to Gs proteins leading to stimulation of adenylyl cyclaseCitation2. Studies have demonstrated the synergy between adenosine receptor activation and allergens in inducing mast cell degranulationCitation3,Citation4. The involvement of A1-ARs have been identified on a number of different human cell types that are important in the pathophysiology of asthma, including human airway epithelial and bronchial smooth muscle cells, lymphocytes, mast cells, neutrophils, monocytes, macrophages, fibroblasts and endothelial cellsCitation5–19. Activation of A1-ARs subsequently induces the release of mediators and cytokines that lead to airway hyper-reactivity, inflammation and airway remodeling. It has been found that activation of A1-ARs on human asthmatic bronchial tissue produces bronchoconstrictionCitation20. A2A also play a major role in development and progression of asthma. The specific contribution of A2A-AR in relevance to asthma is: selective activation of the A2A receptor inhibits histamine and tryptase release from mast cellsCitation21–23, adherence of neutrophil to the endotheliumCitation6,Citation24, integrin upregulationCitation25, respiratory burstCitation26,Citation27 and degranulation of mast cellsCitation28. Furthermore, the A2A receptor suppresses the activation and expansion of T-lymphocytesCitation29. It has been predicted that a selective A2A receptor agonist may have broad-spectrum anti-inflammatory effects similar to glucocorticosteroids, by exerting inhibitory effects on multiple inflammatory cell types.
A3-ARs also play a crucial role in the progression and development of asthma. Activation of A3-ARs induces the release of basophils and produces bronchoconstriction, eosinophil migration into airways and mucus hypersecretion in animals. Thus, A3-AR antagonists have been suggested for development as anti-asthma drugsCitation30,Citation31.
Our efforts in developing novel ligands for AR subtypes resulted in the identification of 2-aminoimidazole as a privileged structure to achieve selectivity towards A2B AR subtype (unpublished data). Along with this, we have also identified 2-amino-5-benzoyl-4-phenylthiazole as a potential AR1-AR receptor antagonistCitation32. Based on our previous findings, a series of novel thiazole derivatives were synthesized with a view of exploring their potential as ligands for AR subtypes. Our efforts resulted in the development of MCD-KV-10, a novel thiazolo-thiophene derivative which showed good selectivity towards A2A and A3 receptor subtypes. This compound was then screened for its in vivo mast cell stabilizing activity as it is well documented that degranulation of mast cells in diseased condition results in the release of number of constituents and de novo synthesized mediators which include histamine, leukotrienes and prostaglandinsCitation33. Therefore, mast cell stabilization is considered as a key factor in controlling the occurrence of asthma. Along with this, the compound was screened for its lipoxygenase (LOX) inhibition activity as LOX catalyses the conversion of arachidonic acid into leukotrienes which is also considered as an important inflammatory mediator leading to the progression and development of asthmaCitation34. Subsequently, it was evaluated for its in vivo efficacy against ovalbumin-induced chronic airway inflammation model in guinea pigs. The activity of the compound in the guinea pig model was quantified in terms of its effect on respiratory parameters, cytokine levels in broncheo-alveolar lavage (BAL) fluid and serum, cellular counts in BAL fluid and blood, histamine level in lungs and histological evaluation of lung tissue.
Materials and methods
Chemicals
The reactions leading to the synthesis of MCD-KV-10 were carried on magnetic stirrer (Remi 2MLH, Mumbai, India) and heating stirrer (Radleys Discovery technology, RR98074, Essex, UK), as per the required experimental conditions. Buchi Rotavapor (R-210, Buchi India Private Ltd., Mumbai, India) was used for concentrating fractions under reduced pressure. Melting points were determined on a Veego VMP-DS apparatus (Veego, Mumbai, India). The FT-IR spectrum was recorded using IR Affinity 1 by Shimadzu, Kyoto, Japan. Mass spectra were obtained using LC-ESI-MS (Perkin Elmer mass spectrometer, Waltham, MA). The NMR spectra were recorded using Brucker biospin 500 MHz NMR spectrophotometer with chemical shift value represented in ppm relative to the internal standard tetramethyl silane. All reactions were monitored by TLC plates, pre-coated with silica gel 60 F254 TLC plates (E. Merck, Darmstadt, Germany; 0.2 mm thickness) with aluminum sheet support and the compounds were detected by examination under UV lamp.
All the solvents and chemicals were obtained from Qualigens Fine Chemicals Ltd. (Mumbai, India) and were of analytical grade. Aluminium hydroxide gel was obtained from Merck Specialties Pvt. Ltd., Mumbai, India. O-Phthalaldehyde (OPT), acetylcholine (Ach), linoleic acid, lipoxygenase enzyme and histamine were purchased from M. P. Biomedicals, Solon, OH. Ovalbumin (OVA) was obtained from Himedia laboratories, Mumbai, India. Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-1β and IL-6 were purchased from Krishgen Biosystems, Mumbai, India for cytokine assay. Isoflurane (Raman and Weil Pvt. Ltd., Daman, India) was used for anesthetizing animals. Triple antigen was obtained from Biological E. Ltd., Hyderabad, India. Toluidine blue was obtained from Merck, Darmstadt, Germany. Quercetin was purchased from Sigma Aldrich, St. Louis, MO. Ketotifen fumarate was obtained as a gift sample from Torrent Pharmaceuticals, Ahmedabad, India. Dexamethasone was obtained from Cadila Pharmaceuticals, Ahmedabad, India. Deionized water used for the experiment was prepared in-house using a water purification system (Millipore Elix, Darmstadt, Germany).
Animals
Male Dunkin–Hartley guinea pigs weighing 300–400 g were obtained from the animal house of B. V. Patel PERD Centre, Ahmedabad, India. Animal housing and handling were performed in accordance with Good Laboratory Practice (GLP) mentioned in CPCSEA guidelines. The animal house is registered with the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India, vide registration no. 1661/PO/a/12/CPCSEA, dated 21/11/2012. All experimental protocols were reviewed and accepted by the Institutional Animal Ethics Committee prior to initiation of the experiment. The animals were housed in stainless steel cages (three animals per cage) which were then placed in the experimental room where they were allowed to acclimatize for a week before experiment. A 10% air exhaust conditioning unit was maintained along with a relative humidity of 60 ± 5% and a temperature of 25 ± 3 °C in the animal house facility. A 10:14 h light:dark cycle was also regulated for the experimental animals. Amrut certified rodent diet (Maharashtra Chakan Oil Mill Ltd) and 0.01% vitamin C solution were provided ad libitum to the experimental animals.
Synthesis and characterization of MCD-KV-10
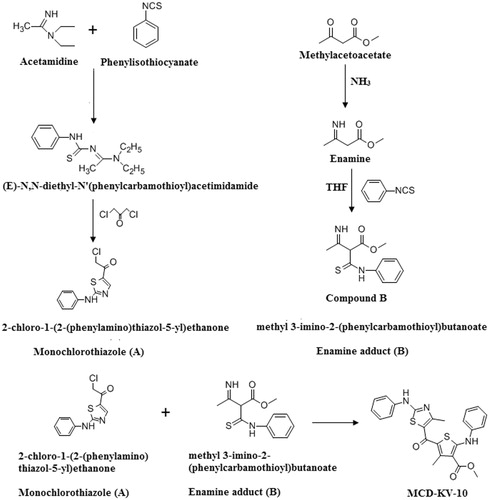
MCD-KV-10 was synthesized by dissolving monochlorothiazole (A) (1 equivalent) in dimethylformamide, to which an enamine adduct (B), i.e. methyl 3-imino-2-(phenylcarbomathioyl) butanoate (1 equivalent) was added drop wise. The reaction was carried on a magnetic stirrer with constant stirring for 4 h. Progress of the reaction was monitored by TLC visualization under UV lamp, where the plate was developed in a mobile phase with a composition of ethyl acetate:hexane (3:7). The reaction mixture was added slowly along with continuous stirring into a beaker containing water. A yellow solid precipitate was obtained which was then filtered. The solid precipitate was given solvent retreatment to remove non-polar impurities and then recrystallized in dichloromethane to obtain MCD-KV-10. The detailed synthesis scheme for MCD-KV-10 is shown in . The compound was then characterized by FT-IR, mass spectrum and NMR spectrum analysis.
Figure 1. Synthesis scheme of MCD-KV-10. Synthesis of compound A: phenylisothiocyanate reacts with acetamidine to form of an adduct which further reacts with dichloroacetone to form compound A. Synthesis of compound B: methylacetoacetate reacts with ammonia to form enamine which on subsequent reaction with phenylisothiocyanate forms compound B. Synthesis of MCD-KV-10: Compound A reacts with compound B in the presence of dimethylformamide at room temperature for 4 h to produce MCD-KV-10.
Adenosine receptor binding assay
The ligand affinity at A1, A2A, A2B and A3 AR subtypes was determined by following the previously reported protocolsCitation35,Citation36. Briefly, the membranes for radioligand binding were prepared from Chinese Hamster Ovary (CHO) cells stably transfected with human adenosine receptor subtypes. This sample was then centrifuged at 1000 × g for 10 min at 4 °C, to remove cell fragments and nuclei. This was followed by centrifugation at 100 000 × g for 1 h at 4 °C to obtain the crude membrane fraction. The resulting membrane pellets were then suspended in 50 mM Tris-HCl, pH 7.4 and assayed for radioligand binding. The radioligands for binding experiments were 1 nM [3H] CCPA for A1 AR; 10 nM [3H] NECA for A2A receptor subtype; 1 nM [3H] HEMADO for A3 receptor subtype. Non-specific binding of [3H] CCPA was determined in the presence of 1 mM of theophylline whereas R-phenylisopropyl adenosine was used for [3H] NECA and [3H] HEMADO. Inhibition of NECA stimulated adenylyl cyclase activity at A2B AR was determined as a measurement of affinity of the new compound. The Ki values from the radioligand competition experiments were calculated using the program SCTFITCitation37. IC50 values from adenylyl cyclase experiments were converted to Ki values using the equation derived by Cheng and PrusoffCitation38.
Lipoxygenase assay
The lipoxygenase assay was carried out as reported previously with some modificationsCitation39–41. MCD-KV-10 was screened at three different concentrations (25, 50 and 100 µg/mL). Absorbance readings were collected at 254 nm. Quercetin (Concentration: 15 µg/mL) was used as a positive control. The percentage inhibition of lipoxygenase was calculated using formula:
Mast cell stabilization assay
The mast cell stabilization activity was carried out as reported previouslyCitation42. Briefly, guinea pigs were sensitized by injecting subcutaneously 0.5 mL sheep serum along with 0.5 mL of triple antigen. Animals were divided into three groups (six animals per group). Group I served as a control (non-sensitized) and received vehicle (0.2% agar solution), Group II (sensitized disease control group received 0.2% agar solution), Group III (Positive control received ketotifen fumarate), Dose: 1 mg/kg bodyweight, orally), Group IV (Treatment with MCD-KV-10, Dose: 20 mg/kg bodyweight, orally). All the drugs were administered orally as a suspension in 0.2% of agar. Treatment was continued for 14 days. On the 14th day, 2 h after the assigned treatment, animals were sacrificed; intestinal mesenteries were taken out and kept in Ringer Locke solution for 30 min. These mesenteric fans were challenged with 5% sheep serum for 10 min and then fixed, dried, stained with toluidine blue and examined microscopically for the number of intact and degranulated mast cells in at least 20 randomly selected high power fields. The percentage inhibition of the mast cell degranulation was calculated by using formula:
Evaluation of anti-asthmatic activity in ova-albumin induced airway inflammation in guinea pigs
Sensitization and treatment of animals
Guinea pigs used in this study were divided into four different groups of six animals each: group I, non-sensitized controls received 0.2% agar solution (control group); group II (disease control group), OVA sensitized and then received 0.2% agar solution orally (vehicle for dexamethasone [DXM] and MCD-KV-10 treatments); group III, OVA sensitized and then received DXM (Dose: 2.5 mg/kg body weight orally) as reference standard; group IV, OVA sensitized and then received MCD-KV-10 (Dose: 20 mg/kg body weight orally). All animals (except group I) were sensitized following a previously reported methodCitation43, using a subcutaneous injection of 100 mg of ovalbumin (which had been adsorbed onto 100 mg of aluminum hydroxide in saline) on day 0 as the first sensitization. Second sensitization was then carried out using the same dose of antigen 2 weeks later (i.e. on day 14). The daily doses of drug or vehicle were initiated on day 18 and continued until day 21 by oral route.
Ovalbumin exposure
The animals were challenged with 0.5% of aerosolized OVA for 10 min, 2.5 h after receiving their drug or vehicle treatment, from day 18 to day 21. For the challenge, conscious animals were placed into a plastic circular chamber (diameter ¼ 70 cm, height ¼ 40 cm) connected to a nebulizer (CX4-Omron Healthcare, Kyoto, Japan). Animals in the non-sensitized group (group I) were exposed to aerosolized saline following the same protocol.
Lung function and bronchoconstriction test
On day 21, tidal volume and respiration rate of animals were measured after 2 h of 10 min of OVA exposure using a data acquisition system (MP-35, Biopac Systems, Santa Barbara, CA). The means to measure airway resistance directly was unavailable hence; the changes in respiratory rate and tidal volume were used as an indirect surrogate. All animals were subjected to a bronchoconstriction test immediately, after measurement of lung function parameters, where all OVA-sensitized animals were exposed to 0.25% Acetylcholine (Ach) solution for 30 s using a nebulizer (CX4-Omron Healthcare). Guinea pigs in the non-sensitized control group were exposed to normal saline in place of Ach. Tidal volume and respiration rate of each animal were recorded before and after Ach exposure.
Cellular count and serum preparation
On day 22, blood samples (3 mL) were collected from all the animals under isoflurane anesthesia. Each sample was then divided into two portions. The first aliquot (2.5 mL) was placed in a non-heparinized tube for serum; the separated serum was stored at −80 °C for later quantitative determination of cytokines. The second portion (0.5 mL) was placed in a heparinized tube and used for total and differential leukocyte counts. Detailed procedures for total and differential leukocyte counts were the same as those described for the BAL fluid (see below).
Bronchoalveolar lavage
At the end of experiment (i.e. day 22), all the animals were anaesthetized with isoflurane for the collection of BAL fluid. A polypropylene cannula was then inserted into the trachea, and normal saline solution (10 mL), maintained at 37 °C was introduced into lungs via a 10-mL syringe and then collected 5 min later. The recovered lavage fluid (5 mL) was centrifuged at 500 × g for 10 min at 4 °C; the resultant supernatant was collected and stored at −80 °C until used in cytokine determinations. The cells in the pellet were washed in 0.5 mL of normal saline and total cell counts were then performed in an automated hematology analyzer (Abaxis Inc., Union City, CA).
Cytokines in serum and BAL fluid
The levels of TNF-α, IL-1β and IL-6 in collected serum and BAL samples were measured using ELISA kits. The standard and samples were processed following the manufacturer’s protocol and all plates were analyzed on an automated plate reader (BIOTEK ELx800-MS ELISA Plate Reader, India). Linearity for the calibration curve was found in the range of 62.5–2000 pg/mL as mentioned in the manufacturer’s protocol.
Histamine assay in lavaged lung tissue
Lung tissue lobe from each animal was dissected out separately immediately following BAL fluid collection. Lung tissue (500 ± 20 mg) was placed in 2.5 mL of normal saline for the preparation of homogenate. The presence of histamine was determined fluorometrically (SL-174, Elico, India) by the modified method of Shore et al.Citation44.
Histological evaluation of lung tissue
Histological studies were done on excised lung tissue of guinea pigs from all animal groups. Five micrometer sectioning of the lung was done using a rotary microtome (Model 0126, Yorco, India) followed by staining with haematoxylin and eosin. Tissue sections were observed for all possible changes in the anatomy under Olympus IX51 optical microscope (Olympus Optical Co. Ltd., Tokyo, Japan). The photographs were taken with an Olympus TL4 camera. The observations were compared to detect significant histological changes, if any.
Statistical analysis
Statistical analysis was performed using One-way ANOVA followed by Bonferroni’s multiple comparison test where probability values, p ≤ 0.05 were considered to be significant. The activity of normal control, positive control and test compounds were compared with disease control group.
Results
Characterization of the designed molecules
The characterization of the synthesized compound was done using; FT-IR, mass spectra analysis and proton NMR data (data represented in supplementary material) and yields were calculated. The characterization data of MCD-KV-10 is discussed below.
MCD-KV-10
Chemical formula: Methyl-4-methyl-5-(4-methyl-2-(phenyl amino)thiazole-5-carbonyl)-2-(phenyl amino)thiophene-3-carboxylate.
Yield: 58.32%; molecular formula: C24H21N3O3S2; yellow color solid; molecular weight: 463 g/mol; LC-ESI-MS (m/z): 464 (M + 1)+; melting point: 155–156 °C; Rf: 0.35 (hexane:ethyl acetate – 7:3); IR (KBr, cm−1): 3213.41 (NH stretch), 1587.42 (NH bend), 1660.50 (O=C=O), 1658.78 (C=O) 1537.27 (C=C), 3176.76 (aromatic CH stretch), 1238.30 (C–O stretch); 1H-NMR: (500 MHz, DMSO)δ: 10.7 (s, 1H), 10.06 (s, 1H), 7.67–7.61 (m, 3H), 7.41–7.31 (m, 4H), 7.19–7.11 (m, 3H), 4.01 (s, 3H), 2.48 (s, 3H), 2.42 (s, 3H).
Adenosine receptor binding assay
MCD-KV-10 showed the highest affinity for A3-ARs (Ki = 797 nM) followed by A2A binding with a Ki of 4820 nM. For the A1 subtype, Ki value of 9680 nM was determined, whereas limited solubility prevented an exact determination of A2B affinity (Ki > 10 000 nM). The detailed radioligand binding data is shown in .
Table 1. Ki values from radioligand binding experiment for MCD-KV-10 at A1, A2A and A3 receptor subtypes.
Lipoxygenase assay
It has been reported that compounds inhibiting lipoxygenases can be employed in the treatment of allergy/asthmaCitation39. Based on these evidences, MCD-KV-10 was also evaluated for its ability to inhibit lipoxygenase enzyme at three different concentrations 25, 50 and 100 µg/mL. The compound showed inhibitory activity at 25, 50 and 100 µg/mL with percentage inhibition value of 38.1, 46.8 and 57.1%, respectively. The percentage inhibition for the positive control quercetin at 15 µg/mL was 61.9%.
Mast cell stabilization assay
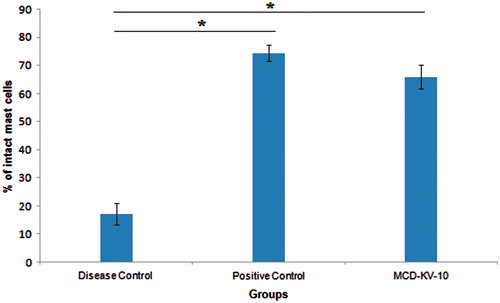
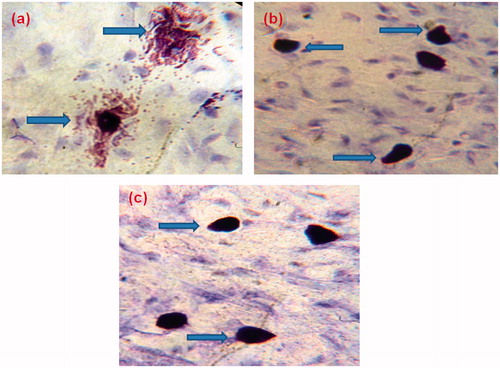
During degranulation, mast cells release a number of mediators which include histamine, leukotrienes and prostaglandins. Therefore, mast cell stabilization is a key factor in controlling the occurrence of asthmaCitation33. Mast cell degranulation data as shown in implicates that MCD-KV-10, at dose 20 mg/kg showed significant protection (p ≤ 0.05) to mast cells degranulation (66.0 ± 2.8%) when compared to the untreated disease group animals. shows the difference between the intact and degranulated mast cell in different animal groups.
Evaluation of anti-asthmatic activity in ova-albumin induced airway inflammation in guinea pigs
Effect of MCD-KV-10 on body weight
No animals in the disease control group (group II) and drug regimen groups (groups III and IV) exhibited any significant differences in body weight compared with those of the non-sensitized controls during the experimental period (data not shown). Furthermore, there were no apparent effects on appetite/water consumption or outward appearance (i.e. fur coat, eyes) of these animals in each treatment group.
Effect of MCD-KV-10 on lung function and bronchoconstriction test
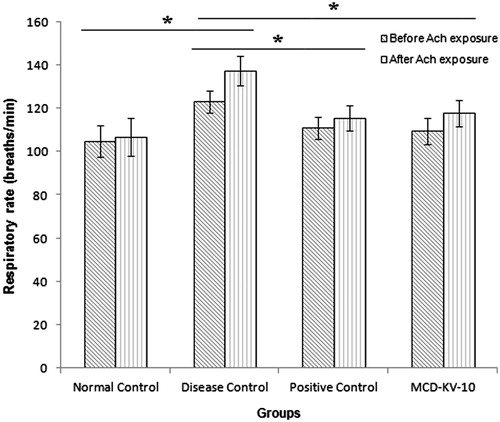
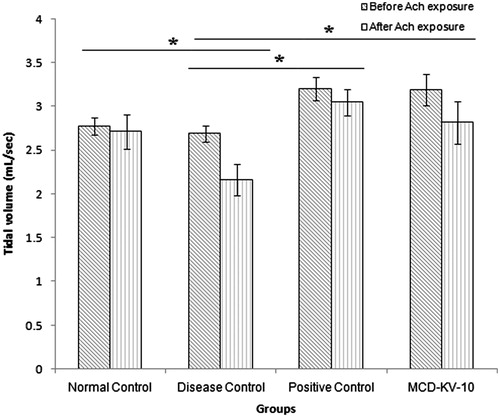
It was observed that MCD-KV-10 prevents the changes in respiratory parameters due to acetylcholine inhalation observed in the disease control group. MCD-KV-10 inhibited the increase in respiratory rate significantly after acetylcholine challenge as shown in . A similar response was observed in case of tidal volume measurement when the compound prevented the significant decrease in tidal volume as seen in the disease control group ().
Effect of MCD-KV-10 on cellular count in blood and BAL fluid
Infiltration of proinflammatory cells into the airways is an important characteristic of asthmaCitation45. The infiltration of proinflammatory cells into the airways was evaluated by collection of BAL fluid after 24 h of last provocation. shows the total WBC count in BAL fluid of different animal groups. The results of differential count of BAL fluid samples showed a significant difference (p ≤ 0.05) in lymphocyte and neutrophil counts among the animals of different groups as shown in . Total WBC count in blood was done on day 17 as a baseline and on day 22 as shown in which also showed significantly high WBC count (p ≤ 0.05) in disease control group as compared to other groups.
Table 2. Total WBC count in blood and BAL fluid of guinea pigs.
Table 3. Differential count in BAL fluid of guinea pigs.
Effect of MCD-KV-10 on cytokine production in serum and BAL fluid
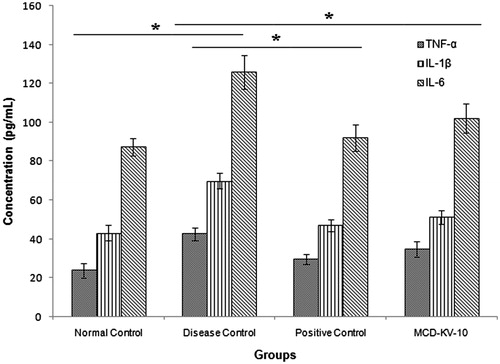
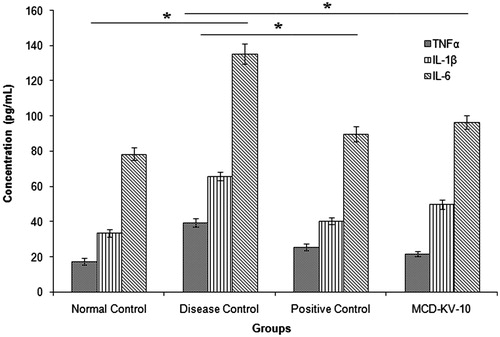
The airway inflammation underlying asthma is regulated by a network of mutually interacting cytokines. However, the exact functional role of each individual cytokine in the pathogenesis of the disease remains to be completely recognizedCitation46. Levels of IL-1β in BAL fluids of patients with asthma were found to be increased compared with those of non-asthmatic individual. TNF-α may have an important amplifying effect on asthmatic inflammation and it is proof that its expression is increased in asthmatic airways. Role of IL-6 in asthma pathophysiology is still controversial. It has been accepted that IL-6 is released from alveolar macrophages from asthmatic patients after allergen challenge and increased basal release, compared with non-asthmatic subjectsCitation47,Citation48. The levels of TNF-α, IL-1β and IL-6 in serum () and BAL fluid () of guinea pigs of disease control group were significantly (p ≤ 0.05) higher than those in the non-sensitized controls, positive control and test group (animals treated with MCD-KV-10). DXM and MCD-KV-10 treatment significantly reduced TNF-α, IL-1β and IL-6 levels as compared with the disease controls.
Effect of MCD-KV-10 on histamine levels
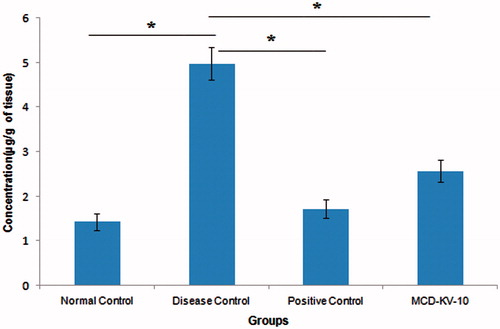
Histamine levels in lung homogenates of the previously lavaged lungs were assayed (). The levels of histamine in disease control animals were significantly (p ≤ 0.05) higher than those in the non-sensitized controls. Both positive control and treatment with MCD-KV-10 showed a significant (p ≤ 0.05) decrease in the degree of histamine release that was significantly increased in disease control animals compared to the normal control.
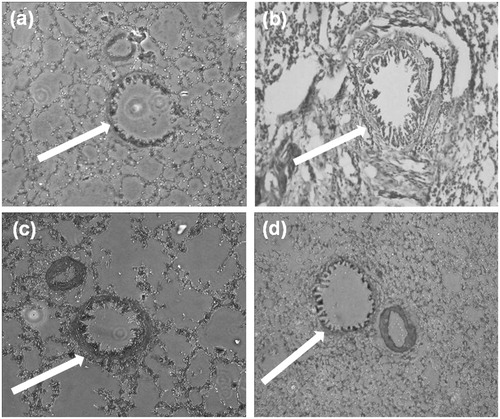
Histological evaluation of lung tissue
Histological evaluation of lungs of guinea pigs as shown in showed no significant difference amongst different animal groups.
Discussion
Allergic mediators are very well known to exert individual effects which combine to generate asthma symptoms and may also interact to produce the inflammatory characteristics of the disease. These interactions subsequently lead to bronchial hyperactivity and increased release of mediators from inflammatory cells, as well as for the generation of bronchospasmCitation49–52. The receptor-based studies pertaining to the development and progression of asthma show involvement of many targets of which adenosine receptor subtypes have a significant contribution. Thus realizing the significant involvement of AR in asthma, we tried to develop a potential lead which may be used for management of asthma by targeting the adenosine receptor subtypes. We synthesized the novel thiazolo-thiophene derivative, MCD-KV-10. Prior to its in vivo pharmacological evaluation, we conducted in vitro studies to determine the ligand binding affinity at AR subtypes using membranes from Chinese Hamster Ovary (CHO) cells stably transfected with the human adenosine receptor subtypes. The results showed that this compound has highest affinity and selectivity towards A2A and A3 AR receptor subtypes. Along with this, the compound was evaluated for its in vitro lipoxygenase inhibition activity because lipoxygenase-derived metabolic products interact with other mediators for the production of asthmatic symptomsCitation39. The compound showed good lipoxygenase inhibition activity with an inhibition of 57.1% at a concentration of 100 µg/mL.
Based on these promising results, the compound was evaluated for its in vivo pharmacological activity. We initially started with evaluation of its potential for inhibition of mast cell degranulation in intestinal mesenteries of guinea pigs. MCD-KV-10 (Dose: 20 mg/kg) showed significant protection to mast cells degranulation (66.0 ± 2.8%), which was considered as an important finding for this compound to have potential anti-asthmatic activity. Finally, the compound was screened for its anti-asthmatic activity using ovalbumin-induced airway inflammation in a guinea pig model of asthma. The animals in the test group were dosed with MCD-KV-10 (20 mg/kg bodyweight). Dexamethasone (5 mg/kg bodyweight) was used at positive control in this experiment. Initially, we determined the effect of treatment on hyper-responsiveness by evaluating the lung function in acetylcholine induced bronchoconstriction test. This was done as it is well documented that hyper-responsiveness is another important characteristic of asthma and widely used as efficacious tool for pharmacological screening of various anti-asthmatic agents in animal model of asthmaCitation53. The results showed that MCD-KV-10 prevented the increase in respiratory rate and decrease in tidal volume, which were significantly different as compared to disease control animals.
An upsurge of pro-inflammatory cells is associated with asthma. These generally infiltrate into the airways and are present in the circulation which can be monitored by determining WBC count and differential counts in the BAL fluid and blood samplesCitation54. We found that the animals treated with MCD-KV-10 showed a significantly lower WBC count in both BAL fluid and blood samples as compared to the disease control group at the end of the study period indicating its potential to control the rise and infiltration of pro-inflammatory cells in the airways.
It is well-documented that allergen-specific Th2 responses with the subsequent release of cytokines are responsible for the cascade of events resulting in asthmaCitation55. Thus, it may be presumed that a potential anti-asthmatic compound should be able to reduce cytokine levels. In our study, we determined the levels of three primary cytokines, namely, TNF-α, IL-1β and IL-6 in BAL fluid and serum samples as previous reports suggest their primary involvement in the pathophysiology of asthma. The results obtained were quite promising as the animals treated with MCD-KV-10 showed significantly lower cytokines levels as compared to the disease control animals in both BAL fluid and serum samples. However, no significant differences in cytokine levels were observed among the test and positive control groups.
Along with the cytokines, histamine is also considered as an important marker in asthma. Previous reports emphasize the importance of histamine in the development of the asthmatic response, and show that newer antihistamines might be helpful to reduce asthma symptomsCitation48. Based on these findings, we evaluated the anti-histaminic potential of MCD-KV-10 in the same guinea pig model as discussed above. The results inferred that animals treated with MCD-KV-10 showed significantly lower histamine concentration in lung tissues as compared to the disease control animals, thus confirming its anti-histaminic potential. However, the histamine levels in positive control animals were comparable to that of test group. This study further emphasized the effect of MCD-KV-10 to inhibit histamine release. The result of histological evaluation of the lungs of guinea pigs showed no significant difference among different animal groups.
Conclusion
The findings of this study suggest regarding the probable implication of MCD-KV-10 in management of asthma, by primarily modulating A2A and A3-AR receptor subtypes. In this study, we highlighted lipoxygenase inhibition and mast cell stabilization potential of MCD-KV-10 where it showed good activity. MCD-KV-10 also showed good anti-asthmatic potential in guinea pig model of asthma, which was confirmed from its efficacy against T-cell-derived cytokine production, subsequent cytokine-mediated cellular infiltration, its bronchodilatory potential and capacity to block mediator release into the local tissues like lung. This study gave an implication for exploration of thiazolo-thiophene class of compounds with other substitutions for management of asthma. However, further mechanistic studies in future will give us a better insight about the role of this class of compounds on other targets and inflammatory mediators underlying asthma.
Declaration of interest
The authors declare that there are no conflicts of interest.
Supplementary material available online
SUPPLEMENTAL
Download PDF (251.8 KB)Acknowledgements
The authors wish to acknowledge NIPER – Ahmedabad for providing all the facilities to carry out this work and Dr. Sonja Kachler and Dr. Karl-Norbert Klotz from Institut für Pharmakologie und Toxikologie, Julius-Maximilians-Universität, Germany for their assistance in in vitro studies. The authors are also grateful to Torrent Pharmaceuticals (Ahmedabad, India) for providing ketotifen fumarate as a gift sample.
Notes
* NIPER-Ahmedabad Submission No – NIPERA250213.
References
- Fredholm BB, IJzerman AP, Jacobson KA, et al. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–52
- Fredholm BB, Abbracchio MP, Burnstock G, et al. Nomenclature and classification of purinoceptors. Pharmacol Rev 1994;46:143–6
- Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol 2001;107:595–601
- Meade CJ, Worrall L, Hayes D, Protin U. Induction of interleukin 8 release from the HMC-1 mast cell line: synergy between stem cell factor and activators of the adenosine A(2b) receptor. Biochem Pharmacol 2002;64:317–25
- Cronstein BN, Daguma L, Nichols D, et al. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest 1990;85:1150–7
- Cronstein BN, Levin RI, Philips M, et al. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol 1992;148:2201–6
- Salmon JE, Cronstein BN. Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol 1990;145:2235–40
- Marone G, Petracca R, Vigorita S, et al. Adenosine receptors on human leukocytes IV. Characterization of an A1/Ri receptor. Int J Clin Lab Res 1992;22:235–42
- Salmon JE, Brogle N, Brownlie C, et al. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J Immunol 1993;151:2775–85
- Ahmed AH, Jacobson KA, Kim J, Heppel LA. Presence of both A1 and A2A adenosine receptors in human cells and their interactions. Biochem Biophys Res Commun 1995;208:871–8
- Peachell PT, Columbo M, Kagey-Sobotka A, et al. Adenosine potentiates mediator release from human lung mast cells. Am Rev Respir Dis 1988;138:1143–51
- Forsythe P, McGarvey LPA, Heaney LG, et al. Adenosine induces histamine release from human bronchoalveolar lavage mast cells. Clin Sci 1999;96:349–55
- Murakami S, Hashikawa T, Saho T, et al. Adenosine regulates the IL-1β induced cellular functions of human gingival fibroblasts. Int Immunol 2001;13:1533–40
- Panther E, Idzko M, Herouy Y, et al. Expression and function of adenosine receptors in human dendritic cells. FASEB J 2001;15:1963–70
- Wilson CN, Batra VK. Lipopolysaccharide binds to and activates A1 adenosine receptors on human pulmonary artery endothelial cells. J Endotoxin Res 2002;8:263–71
- McNamara N, Gallup M, Khong A, et al. Adenosine up-regulation of the mucin gene, MUC2, in asthma. FASEB J 2004;18:1770–2
- Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 2006;35:496–502
- Clark AN, Youkey R, Liu X, et al. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ Res 2007;101:1130–8
- Brown RA, Clarke GW, Ledbetter CL, et al. Elevated expression of adenosine A1 receptor in bronchial biopsies from asthmatic subjects. Eur Respir J 2008;31:1–9
- Bjorck T, Gustafsson LE, Dahlen SE. Isolated bronchi from asthmatics are hyper responsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis 1992;145:1087–91
- Hughes PJ, Holgate ST, Church MK. Adenosine inhibits and potentiates IgE-dependent histamine release from human lung mast cells by an A2-purinoceptor mediated mechanism. Biochem Pharmacol 1984;33:3847–52
- Peachell PT, Lichtenstein LM, Schleimer RP. Differential regulation of human basophil and lung mast cell function by adenosine. J Pharmacol Exp Ther 1991;256:717–26
- Suzuki H, Takei M, Nakahata T, Fukamachi H. Inhibitory effect of adenosine on degranulation of human cultured mast cells upon cross-linking of Fc epsilon RI. Biochem Biophys Res Commun 1998;242:697–702
- Felsch A, Stocker K, Borchard U. Phorbol ester-stimulated adherence of neutrophils to endothelial cells is reduced by adenosine A2 receptor agonists. J Immunol 1995;155:333–8
- Wollner A, Wollner S, Smith JB. Acting via A2 receptors, adenosine inhibits the upregulation of Mac-1 (Cd11b/CD18) expression on FMLP-stimulated neutrophils. Am J Respir Cell Mol Biol 1993;9:179–85
- Fredholm BB, Zhang Y, Van der Ploeg I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn Schmiedebergs Arch Pharmacol 1996;354:262–7
- Hannon JP, Bray-French KM, Phillips RM, Fozard JR. Further pharmacological characterization of the adenosine receptor subtype mediating inhibition of oxidative burst in human isolated neutrophils. Drug Dev Res 1998;43:214–24
- Bouma MG, Jeunhomme TM, Boyle DL, et al. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J Immunol 1997;158:5400–8
- Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2A extracellular adenosine receptor-mediated signaling in adenosine mediated inhibition of T-cell activation and expansion. Blood 1997;90:1600–6
- Fishman P, Bar-Yehuda S. Pharmacology and therapeutic applications of A3 receptor subtypes. Curr Topics Med Chem 2003;3:463–9
- Nadeem A, Mustafa SJ. Adenosine receptor anatagonists and asthma. Drug Discov Today Ther Strat 2006;3:269–75
- Scheiff AB, Yerande SG, Tayeb AE, et al. 2-Amino-5-benzoyl-4-phenylthiazoles: development of potent and selective adenosine A1 receptor antagonists. Bioorg Med Chem 2010;18:2195–203
- Slater JW, Zechnich AD, Haxby DG. Second generation antihistamines: a comparative review. Drugs 1999;57:31–47
- Gilman A, Goodman L, Rall T, Murad F. Goodman and Gilman’s – the pharmacological basis of therapeutics. USA: Macmillan; 2010:659–60
- Klotz KN, Hessling J, Hegler J, et al. Comparative pharmacology of human adenosine receptor subtypes – characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg's Arch Pharmacol 1998;357:1–9
- Klotz KN, Falgner N, Kachler S, et al. [3H]HEMADO – a novel tritiated agonist selective for the human adenosine A3 receptor. Eur J Pharmacol 2007;556:14–18
- De Lean A, Hancock AA, Lefkowitz RJ. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol 1982;21:5–16
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099–108
- Sircar JC, Schwender CF, Johnson EA. Soyabean lipoxygenase inhibition by nonsteroidal anti-inflammatory drugs. Prostaglandins 1983;25:393–6
- Gaffney BJ. Lipoxygenases: structural principles and spectroscopy. Annu Rev Biophys Biomol Struct 1996;25:431–59
- Mahesha HG, Singh SA, Rao AGA. Inhibition of lipoxygenase by soy isoflavones: evidence of isoflavones as redox inhibitors. Arch Biochem Biophys 2007;461:176–85
- Harish MS, Nagur M, Badami S. Antihistaminic and mast cell stabilizing activity of Striga orobanchioides. J Ethnopharmacol 2001;76:197–200
- Duan W, Kua IC, Selvarajan S, et al. Anti-inflammatory effects of genistein, a tyrosine kinase inhibitor, on guinea pig model of asthma. Am J Respir Crit Care Med 2003;167:185–92
- Shore PA, Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther 1959;127:182–6
- Busse WW, Lemanske JR. Advances in immunology: asthma. N Engl J Med 2001;344:350–62
- Kips JC. Cytokines in asthma. Eur Respir J 2001;18:S24–33
- Chung KF, Barnes PJ. Cytokines in asthma. Thorax 1999;54:825–57
- Mahajan SG, Mehta AA. Role of cytokines in pathophysiology of asthma. Ir J Pharmacol Ther 2006;5:1–14
- Schellenberg RR, Foster A, Duff MJ. Anti-IgE induced contraction of human bronchus in vitro. J Allergy Clin Immun 1985;75:126–30
- Phillips GD, Holgate ST. Interaction of inhaled LTC4 with histamine and PGD2 on airway caliber in asthma. J Appl Physiol 1989;66:304–12
- Engineer DM, Morris HR, Piper PJ, Sirois P. The release of prostaglandins and thromboxanes from guinea-pig lung by slow reacting substance, of anaphylaxis, and its inhibition. Br J Pharmacol 1978;64:211–18
- Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 2008;7:759–70
- Eum SY, Karim M, Hamid Q, et al. Involvement of the cysteinyl-leucotrienes in allergen-induced airways eosinophilia and hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol 2003;8:25–32
- Mahajan SG, Banerjee A, Chauhan BF, et al. Inhibitory effect of n-butanol fraction of Moringa oleifera Lam. seeds on ovalbumin-induced airway inflammation in a guinea pig model of asthma. Int J Toxicol 2009;28:519–27
- Ngoc LP, Gold DR, Tzianabos AO, et al. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol 2005;5:161–6