Abstract
In this study, a new affinity gel for the purification of bovine testicular hyaluronidase (BTH) was synthesized. l-Tyrosine was added as the extension arm to the Sepharose-4B activated with cyanogen bromide. m-Anisidine is a specific inhibitor of BTH enzyme. m-Anisidine was clamped to the newly formed Sepharose-4B-l-tyrosine as a ligand. As a result, an affinity gel having the chemical structure of Sepharose-4B-l-tyrosine-m-anisidine was obtained. BTH purified by ammonium sulfate precipitation and affinity chromatography was obtained with a 16.95% yield and 881.78 degree of purity. The kinetic constants KM and VMax for BTH were determined by using hyaluronic acid as a substrate. KM and VMax values obtained from the Lineweaver–Burk graph were found to be 2.23 mM and 19.85 U/mL, respectively. In vitro effects of some chemicals were determined on purified BTH enzyme. Some chemically active ingredients were 1,1-dimethyl piperidinium chloride, β-naphthoxyacetic acid and gibberellic acid. Gibberellic acid showed the best inhibition effect on BTH.
Introduction
Hyaluronidase, which acts on hyaluronan (HA), chondroitin (Ch), chondroitin 4-sulfate (Ch4S), chondroitin 6-sulfate (Ch6S) and partially on dermatan sulfate, is distributed widely in animal tissues, especially human testis and liverCitation1,Citation2. The enzyme is an endo-β-N-acetylhexosaminidase. The final reaction products obtained by digestion with the enzyme are primarily tetra- and hexasaccharides having an N-acetylhexosamine residue at the reducing terminus and a glucuronic acid (GlcUA) residue at the non-reducing terminusCitation2.
Hyaluronidase (hyaluronoglucosaminidase; EC 3.2.1.35) is an endo-β-N-acetyl-d-hexosaminidase that hydrolyzes HA at the β1,4-N-acetylglucosaminide bonds [GlcNAc-β-1 → 4)-(GlcUA)]Citation3–6. Besides that, the enzyme also hydrolyzes Ch/Ch4S/Ch6S/DS at the β-1,4-N-acetylgalactosaminide bonds [GalNAc-β1 → 4-GlcUA], but at a lower yield, which is dependent on the structure of the Ch sulfateCitation3,Citation7. The wide substrate specificity of bovine testicular hyaluronidase (BTH) is very useful for glycotechnological applications, such as the preparation of glycosaminoglycan oligosaccharides of variable chain lengths. Exhaustive digestion with this enzyme yields primarily a mixture of tetrasaccharides and hexasaccharides with GlcUA at the nonreducing endCitation8. Simultaneously, BTH displays both hydrolytic and transglycosylation activitiesCitation9–12. Furthermore, preparations of BTH have been applied therapeutically in the fields of orthopaedia, ophthalmology and internal medicine for many yearsCitation13,Citation14. A common field of application of BTH is its addition to local anesthetic agents for ophthalmic anesthesia, as it is known to improve the rapidity of onset, dispersion, depth and duration of the local anesthesiaCitation15.
In this study, for the inhibition studies, three compounds named as of 3-methoxyaniline; (m-anisidine), mepiquat chloride (1,1-dimethylpiperidinium chloride (PIX); 2-naphthoxyacetic acid (β-NOA); and gibberellic acid were used to determine the efficacy of purified BTH.
Mepiquat chloride (PIX), well known as PIX, is a potential systemic plant growth regulator. The effects of PIX on plant height, stem elongation, leaf area, net photosynthetic rates, chlorophyll content, sucrose and starch levels and ribulose bisphosphate carboxylase (RuBP) carboxylase activity in cotton (Gossypium hirsutum L. cv. DES 119) plants have been demonstratedCitation16. β-NOA is a kind of plant growth regulator, particularly for grapes, apples and tomatoes. It is included in auxino-similar phytodrugs because its structure resembles that of auxine, a plant hormone, which controls the growth of stems, roots, flowers and fruits, and can also improve the color of fruits and vegetables. Furthermore, auxino-similar phytodrugs guarantee a relatively low environmental impactCitation17. Gibberellic acid, a tetracyclic dihydroxylactonic acid, C19H22O6, produces marked shoot elongation in many plants. Unlike other auxins, its stimulation of growth of intact plants often results in substantial increases in height and in fresh and dry weightsCitation18.
In this study, a new affinity gel for the purification of BTH was synthesized. And using the structure of sepharose 4B-tyrosine-m-anisidine new affinity gel, in vitro effects of the below chemicals were studied on purified BTH enzyme.
Materials and methods
Sepharose-4B, l-tyrosine, m-anisidine (3-methoxyaniline), HA (sodium salt; from Streptococcus equi), protein assay reagents and chemicals for electrophoresis were obtained from Sigma Chem. Co (Milan, Italy). The other chemicals were obtained from Merck & Co (Darmstadt, Germany).
Bovine testis tissue was cut into small pieces and homogenized in 100 mM sodium acetate buffer (pH 5.4) containing 150 mM NaCl and 0.25 mM phenylmethanesulfonyl fluoride. The homogenate was centrifuged at 26 916 g for 75 min at 4 °C and the supernatant was then recovered. First, crude BTH was isolated by ammonium sulfate precipitation (40–60%) of the supernatant. The precipitate was collected by centrifugation at 26 916 g for 45 min and then redissolved in 50 mM sodium phosphate buffer (pH 7.0).
The redissolved precipitate obtained by ammonium sulfate precipitation was then subjected to affinity chromatography. It was loaded on to an affinity column comprised of sepharose-4B-l-tyrosine-m-anisidine. The affinity column was prepared as follows. Cyanogen bromide (CNBr) was added at 10% (w/v) to a 1:1 dilution of Sepharose-4B in water. The mixture was titrated to pH 11 with NaOH in an ice bath and maintained at that pH for 8–10 min. The reaction was then stopped by filtering out the gel on a Buchner funnel and washing with cold 0.1 M NaHCO3 buffer, pH 10. The linker l-tyrosine was then coupled to the CNBr-activated Sepharose-4B using saturated l-tyrosine solution in the same buffer. The reaction was completed by stirring with a magnet for 90 min. In order to remove the excess of l-tyrosine from the Sepharose-4B-l-tyrosine gel, it was washed with distilled water. The final affinity gel was obtained by diazotization of m-anisidine and coupling of this compound to the Sepharose-4B-l-tyrosineCitation19. The pH was adjusted to 9.5 with 1 M NaOH and, after gentle stirring for 3 h at room temperature, the coupled red Sepharose 4B derivative was washed with 1 L of water and then 200 ml of 0.05 M Tris–sulfate, pH 7.5. The final affinity column was then equilibrated with 50 mM sodium phosphate buffer (pH 7.0), and the crude BTH enzyme preparation was added in the same buffer. After washing extensively with buffer, the BTH was eluted with 25 mM sodium phosphate buffer, pH 4.0, containing 250 mM Na2SO4 and 50 mM m-anisidine. The purified BTH enzyme was stored at +4 °C.
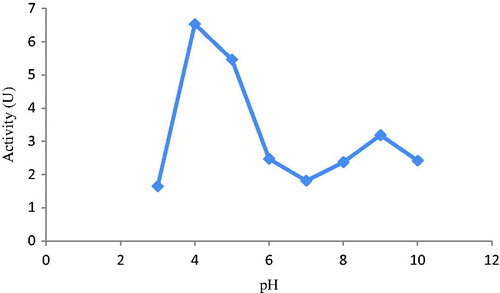
In addition, the maximum inhibition values for BTH with m-anisidine were determined in pH 3.0 and pH 7.0 buffers (). These pH values were so important for purification of BTH by affinity chromatography that after precipitation of BTH with ammonium sulfate, the precipitant was dissolved with this pH value buffer, because the protein was bound at this pH. That is why sepharose-4B-l-tyrosine-m-anisidine affinity column was equilibrated with the same pH. Meanwhile, the column was washed with this buffer. For elution of BTH, we made use of m-anisidine possible inhibitor of BTH and ionic strength with Na2SO4. pH 7.0 was preferred for loading and dissolving precipitate buffer of the mentioned protein because it is believed that some was denatured at pH 3.0. So much so that the enzyme activities obtained by purification, loading and dissolving precipitate buffer at pH 3.0 are lower than those obtained at pH 7.0.
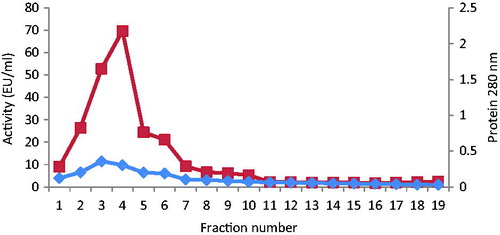
The absorbance at 280 nm was used to monitor the protein in the ammonium sulfate precipitation and column effluents (). Quantitative protein determination was achieved by absorbance measurements at 595 nm according to BradfordCitation20, with bovine serum albumin as standard. SDS polyacrylamide gel electrophoresis, under reducing conditions, was performed in order to verify the degree of purification of the BTH. It was carried out using 10% (w/v) and 3% (w/v) acrylamide concentrations, containing 0.1% SDS, for the running and stacking gels, respectively, according to the procedure of LaemmliCitation21.
Hyaluronidase activity can be quantified according to the definition of the International Union of Biochemistry, i.e. 1 unit (U) as the amount of enzyme that catalyses the liberation of 1 μmol of reducing terminal N-acetylhexosamine per minute under specified conditions. The hyaluronidase activity toward HA was quantified spectrophotometrically using the method described by GreilingCitation22. The reaction was followed for 1 min at 37 °C by monitoring the appearance of HA at 232 nm in Biotek automated recording spectrophotometer (Bad Friedrichshall, Germany). Final substrate concentration (12.3 mM) was used during enzyme assay. The velocity of the enzymatic reaction was calculated by using the following equation:
For the inhibition studies, different concentrations of m-anisidine, PIX, β-NOA and gibberellic acid were added to the enzyme. Activity % values of BTH in the presence of five different inhibitor concentrations were determined by regression analysis. BTH activity without any inhibitor was accepted as 100% activity. The inhibitor concentrations causing 50% inhibition (IC50 value) were determined from the graphs.
Results and discussion
In this study, BTH was purified from a crude ammonium sulfate-precipitated fraction of an extract of bovine testis using a Sepharose 4B-l-tyrosine-m-anisidine affinity column. shows the typical elution pattern of the enzyme activity from the affinity column. The enzyme activity showed a single peak and the peak fractions were pooled as purified BTH.
On SDS-polyacrylamide gel electrophoresis, after affinity chromatography, the major protein band showed a Mw of ∼55 kDa (), which corresponds to the previous studiesCitation23,Citation24. As listed in , at the end of the affinity chromatography, an 881.78-fold purification was achieved, which is a higher value than the previous study that has been carried out by Barsukov, A. et al. (40–42 factor of purity and 42–59 U mg−1 specific activity by affinity chromatography on Sepharose Blue; )Citation25.
Figure 3. SDS-PAGE of BTH. The pooled fractions from ammonium sulfate precipitation and affinity chromatography (sepharose-4B, l-tyrosine and m-anisidine) were analyzed by SDS-PAGE (10% and 3%) and revealed by Coomassie blue staining. Experimental conditions were as described in the method.
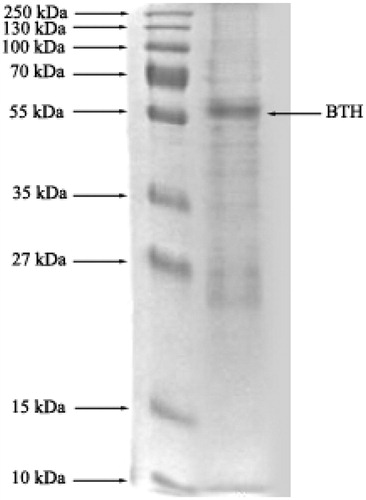
Table 1. Summary of the purification of BTH.
KM and VMax values for BTH were calculated from a Lineweaver–Burk plot using HA (sodium salt) as substrate and found to be 2.23 mM and 19.85 U/mL, respectively. These values, using HA (sodium salt), are close to other values reported for BTH from bovine testisCitation24,Citation26.
The literature shows that PIX, β-NOA and gibberellic acid have various effects, in different ways and at different levels on many enzymes and physiological systems. Reddy et al.Citation16 showed that plant height was clearly reduced by PIX treatment. Net photosynthetic rates were 25% less in PIX-treated leaves, though PIX-treated leaves had higher chlorophyll content. The activity of RuBP was decreased in PIX-treated plants. Starch accumulation was noticed in PIX-treated leaves, though sucrose content was unchanged.
In addition, in the study of Niakan et al.Citation27, the effect of different concentrations of PIX, as a plant growth regulator, were evaluated on the contents of soluble sugars, proline and phenolic compounds, and also antioxidant enzyme activities, such as catalase, peroxidase and polyphenol oxidase, in both leaf and root of cotton plant in vegetative phase mid under pots condition. Their results showed that PIX spray increased the amount of soluble sugars in the leaf and reduced proline content in the root, while the amount of phenolic compounds in the cotton plant was unaffected. Their data also showed that PIX application, at different levels, had no significant effects on catalase and peroxidase activities in the leaf, but decreased catalase activity and increased peroxidase activity in the root. No changes in polyphenol oxidase activity were seen in either the root or leaf.
Mapelli et al. identified the production of hydrolytic enzymes by embryo-less barley seeds in response to various gibberellins and abscisic acidCitation28. In contrast, there are no studies reported in the literature on the effects of PIX, β-NOA and gibberellic acid on BTH. This is important, considering that the cow is a grass-fed animal, and potential traces of commercial plant regulators in grass could be ingested with subsequent physiological effects. The data mentioned earlier suggests that these compounds could potentially have effects upon BTH and physiological systems. For this reason, the in vitro effects of PIX, β-NOA and gibberellic acid on BTH activity were investigated ().
Figure 4. Activity (%) graphs of mepiquat chloride (1,1-dimethylpiperidinium chloride (PIX), 2-naphthoxyacetic acid (β-NOA); and gibberellic acid on BTH.
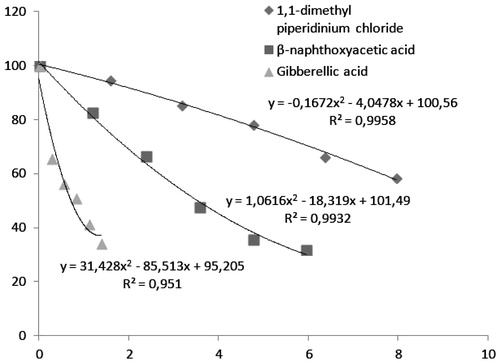
Our affinity column, used for the first time for the purification of BTH, has the chemical structure of Sepharose-4B-l-tyrosine-m-anisidine.Within the past two decades, many studies about the effects of anisidine derivatives on enzymes have been carried out. Thompson et al. found that anisidine derivatives are mutagenic and N-acetyltransferase has an important role in the metabolism of mutagenic species in Salmonella typhimurium test strains that have high levels of N-acetyltransferaseCitation29. Furthermore, the in vitro study conducted by Bacherikov et al. showed the cytotoxic effects of m-anisidine derivatives on the growth of various human tumor cellsCitation30. In a study carried out by Suciu, they determined that 4′-(9-acridinylamino) methansulfon-m-anisidine, a derivative of m-anisidine, is an inhibitor of DNA gyrase and topoisomerase IICitation31. However, there is no information available on m-anisidine as a possible inhibitor of BTH. Therefore, this study is the first in terms of the effect of m-anisidine compound on BTH. m-Anisidine is shown to be an inhibitor of BTH, with an IC50 value of 1.73 mg/mL at 37 °C, using a 12.3 mM stock substrate concentration in 0.2 M HCOONa/0.1 M NaCl buffer (pH 3.7) as listed in .
Table 2. IC50 values of 3-methoxyaniline; (m-anisidine) mepiquat chloride (1,1-dimethylpiperidinium chloride (PIX); 2-naphthoxyacetic acid (β-NOA); and gibberellic acid.
Acknowledgements
The authors thank Dr. Malcolm Lyon for his invaluable contribution on this paper.
Declaration of interest
The authors do not report any declaration of interest for this study.
This work was supported by a Balikesir University Research Project (2012/38) and carried out at the Balikesir University Research Center of Applied Sciences (BURCAS).
References
- Kreil G. Hyaluronidases: a group of neglected enzymes. Protein Sci 1995;9:1666–9
- Csoka TB, Frost GI, Stern R. Hyaluronidases in tissue invasion. Invasion Metastasis 1997;17:297–311
- Meyer K. Hyaluronidases in the enzymes. New York: Academic Press; 1971:307–20
- Meyer K, Hoffman P, Linker A. Hyaluronidases in the enzymes. New York: Academic Press; 1971:447–60
- Meyer K, Rapport MM. Hyaluronidases. Adv Enzymol Relat Subj Biochem 1952;13:199–236
- Takagaki K, Kakizaki I. Degradation of glycosaminoglycans. In: Kamerling JP, Boons GJ, Lee YC, et al., eds. Comprehensive glycoscience: from chemistry to systems biology. Amsterdam: Elsevier Science BV; 2007:171–92
- Meyer K, Rapport MM. The hydrolysis of chondroitin sulfate by testicular hyaluronidase. Arch Biochem 1950;27:287–93
- Takagaki K, Nakamura T, Izumi J, et al. Characterization of hydrolysis and transglycosylation by testicular hyaluronidase using ion-spray mass spectrometry. Biochemistry 1994;33:6503–7
- Highsmith S Jr, Garvin JH, Chipman DM. Mechanism of action of bovine testicular hyaluronidase. Mapping of the active site. J Biol Chem 1975;250:7473–80
- Hoffman P, Meyer K, Linker A. Transglycosylation during the mixed digestion of hyaluronic acid and chondroitin sulfate by testicular hyaluronidase. J Biol Chem 1956;219:653–63
- Saitoh H, Takagaki K, Majima M, et al. Enzymic reconstruction of glycosaminoglycan oligosaccharide chains using the transglycosylation reaction of bovine testicular hyaluronidase. J Biol Chem 1995;270:3741–7
- Weissmann B. The transglycosylative action of testicular hyaluronidase. J Biol Chem 1955;216:783–94
- Baumgartner G, Moritz A. Hyaluronidase: Anwendung in der Onkologie. Wien Berlin, Heidelberg, New York, Vienna: Springer-Verlag; 1988
- Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett 1998;131:3–11
- Kallio H, Paloheimo M, Maunuksela EL. Hyaluronidase as an adjuvant in bupivacaine–lidocaine mixture for retrobulbar/peribulbar block. Anesth Analg 2000;91:934–7
- Reddy AR, Reddy KR, Hodges HF. Mepiquat chloride (PIX)-induced changes in photosynthesis and growth of cotton. Plant Growth Regul 1996;20:179–83
- Casado-Terronesa S, Fernandez-Sanchez JF, Segura-Carreteroa A, Fernandez-Gutierrez A. The development and comparison of a fluorescence and a phosphorescence optosensors for determining the plant growth regulator 2-naphthoxyacetic acid. Sens Actuat B 2005;107:929–35
- Weller LE, Wittwer SH, Bukovac MJ, Sell HM. The effect of gibberellic acid on enzyme activity and oxygen uptake in bean plants. Plant Physiol 1957;32:371–2
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–74
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680
- Greiling H. Spectrophotometric method for the determination of bacterial hyaluronidase. Hoppe Seylers Z Physiol Chem 1957;309:239–42
- Meyer MF, Kreil G, Aschauer H. The soluble hyaluronidase from bull testes is a fragment of the membrane-bound PH-20 enzyme. FEBS Lett 1997;413:385–8
- Ashok M, Krishnapillai KD, Anthony T, et al. Quantick. Characterisation of Norway lobster (Nephrops norvegicus) hyaluronidase and comparison with sheep and bovine testicular hyaluronidase. Food Chem 1999;65:515–21
- Barsukov AK, Kozhevnikova OV, Khokhryakova AV. Isolation and purification of bovine testicular hyaluronidase. Appl Biochem Microbiol 2003;39:549–52
- Zhang LS, Mummert ME. Development of a fluorescent substrate to measure hyaluronidase activity. Anal Biochem 2008;379:80–5
- Niakan M, Habibi A, Ghorbanli M. Study of Pix regulator effect on physiological responses in cotton plant. Ann Biol Res 2012;3:5229–35
- Mapelli S, Lombardi L, Rocchi P. Gibberellin and abscisic acid effects on the activity of hydrolytic enzymes in de-embryonated barley seeds. Plant Growth Regul 1984;2:31–40
- Thompsona DC, Josephyb PD, Chub JWK, Eling TE. Enhanced mutagenicity of anisidine isomers in bacterial strains containing elevated N-acetyltransferase activity. Mutat Res Genet Toxicol 1992;279:83–9
- Bacherikova VA, Changb JYYWL, Chena CH, et al. Synthesis and antitumor activity of 5-(9-acridinylamino)anisidine derivatives. Bioorg Med Chem 2005;13:6513–20
- Suciu D. Inhibition of DNA synthesis and cytotoxic effects of some DNA topoisomerase II and gyrase inhibitors in Chinese hamster V79 cells. Mutat Res Lett 1990;243:213–18