Abstract
Pseudoalteromonas sp. strain 1020R produces prodigiosin and its closely related congeners, which differ in the length of their alkyl side chains. These red-pigmented compounds were found to exhibit cytotoxicity against human leukemia cell lines. The compounds also showed dose-dependent inhibitory effects on protein phosphatase 2A and protein tyrosine phosphatase 1B (PTP1B), while remaining relatively inactive against protein kinases, including protein tyrosine kinase, Ca2+/calmodulin-dependent protein kinase and protein kinases A and C. Comparative studies of the individual pigmented compounds on PTP1B inhibition showed that as the chain length of the alkyl group at the C-3 position of the compound increased, the inhibitory effect on PTP1B decreased. These results suggest that protein phosphatases but not protein kinases might be involved in the cytotoxicity of the prodigiosin family of compounds against malignant cells.
Introduction
Pseudoalteromonas sp. strain 1020R isolated from the Pacific coast of Japan produces seven red-pigmented compounds from the prodigiosin family. Structural analyses of four of these compounds have revealed that they represent the prodigiosin, 2-methyl-3-pentylprodiginine and its closely related congeners, namely 2-methyl-3-butylprodiginine, 2-methyl-3-hexylprodiginine and 2-methyl-3-heptylprodiginine, differing from each other only by their alkyl side chain length ()Citation1. It is well known that compounds from the prodigiosin family have a wide variety of pharmacological activities including antibacterial, immunosuppressive and cytotoxic against malignant tumor cellsCitation2,Citation3. Although some molecular mechanisms of apoptotic cell death induced by prodigiosin have already been proposedCitation4, the detailed mechanisms of action are still elusive and require further investigation.
Protein phosphatases and protein kinases, which are known to play significant roles in intracellular signal transduction pathways, are the focus of our research. Fürstner and coworkers were the first to show that roseophilin and some synthetic prodigiosin analogs inhibit protein tyrosine phosphatase activityCitation5. They also suggested that this compound-induced inhibitory activity may be employed to cure malignant diseases. However, the properties of phosphatase inhibition by the prodigiosin family of compounds from natural sources have not been fully investigated. In a previous study, we purified and identified four prodigiosin family compounds from strain 1020RCitation1. These compounds have been shown to differ in the length of the alkyl side chain attached to the C ring and be cytotoxic toward cancer cells through the induction of apoptosis.
Herein, we describe the effects of these prodigiosins on the activities of protein kinases and protein phosphatases as possible targets in the process of apoptosis programmed cell death of tumor cells. Selected members of kinase and phosphatase enzymes were tested in vitro to assess the inhibitory effects of the compounds and to evaluate the structure-activity relationship (SAR) responsible for the inhibitory activity.
Materials and methods
Enzymes and chemicals
Protein tyrosine phosphatase 1B (PTP1B) (human, recombinant) and protein phosphatase 2A dimer (PP2A) (human, recombinant) were from CycLex (Nagano, Japan) and Wako Pure Chemical Industries (Osaka, Japan), respectively. Src kinase (human, recombinant) and Ca2+/calmodulin-dependent protein kinase II (CaM kinase) (from rat brain) were purchased from Enzo Life Sciences (Farmingdale, NY). Catalytic subunits of protein kinase A (PKA) (from bovine heart) and protein kinase C (PKC) (from rat brain) were from Sigma-Aldrich (St. Louis, MO). [γ-32P]ATP used for the protein kinase assay was purchased from Perkin Elmer (Waltham, MA).
Production and purification of the pigments
The culture of Pseudoalteromonas sp. 1020R and the following extraction and purification of prodigiosin compounds were carried out according to Wang et al.Citation1 with some modifications. The culture was incubated at pH 7.0 instead of pH 7.8, which reduced the concentration of pigments in the supernatant and thus increased the amount of prodigiosin compounds in the pellet after the centrifugation of cultured medium. The pigments in the pellet were extracted with ethanol and purified by silica gel followed by ODS open column separation. The fraction containing a mixture of prodigiosin family compounds from the silica gel column and the separated individual compounds from the ODS column were dissolved in dimethyl sulfoxide (DMSO) and used in the cytotoxicity assay and the enzyme inhibition assay.
The purity of the pigments was examined using a Nanospace SI-2 HPLC system equipped with an ODS Capcell Pak C-18 MGII (1.5 mm × 150 mm, 5 μm) column (Shiseido, Tokyo, Japan) using an isocratic mobile phase of 55:45 methanol:water (v/v) acidified with 0.2% acetic acid at 100 µL/min.
Cytotoxicity assay
U937, K562 and HL60 human leukemia cell lines (registry numbers JCRB9021, JCRB0019 and JCRB0085, respectively) were purchased from Health Science Research Resources Bank (Osaka, Japan) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum. Cells at a concentration of approximately 1 × 106 cells/mL were incubated with different concentrations (0.1–10 μM) of prodigiosin compounds for 24, 48 and 72 h at 37 °C under humidified conditions with 5% CO2 and cytotoxicity was determined using a CellTiter 96® Aqueous One Solution Cell Proliferation Assay Kit (MTS assay) (Promega, Fitchburg, WI). Non-treated cells and cells treated with DMSO instead of prodigiosins were used as controls.
Protein phosphatase assay
PTP1B enzyme activity was assayed with the CycLex® Protein Tyrosine Phosphatase (PTP1B) Fluorometric Assay Kit (CycLex, Nagano, Japan). The experiments were performed in 96-well plates according to the manufacturer’s protocol. The reaction medium contained recombinant PTP1B enzyme and the compounds dissolved in DMSO at different concentrations. After the reaction, the fluorescence intensity was measured using a microplate reader at an excitation of 490 nm and emission of 530 nm. Samples containing DMSO but not prodigiosin pigments were used as positive controls, and samples without enzyme and inhibitor (included in the kit) were negative controls.
PP2A enzyme was assayed using the SensoLyte® pNPP Colorimetric Protein Phosphatase Assay Kit (AnaSpec, Fremont, CA) according to the manufacturer’s protocol. Reactions were performed in a 96-well plate containing PP2A and the prodigiosin pigment dissolved in DMSO at different concentrations. p-Nitrophenol formed as a result of dephosphorylation was measured at 405 nm. Samples containing DMSO but not the prodigiosin compounds were used as positive controls.
Protein kinase assay
Protein tyrosine kinase (Src kinase) and CaM kinase were assayed according to the manufacturer’s instructions. The substrates of p60c-src for Src kinase and autocamtide for CaM kinase, and [γ-32P]ATP were used. PKA and PKC were assayed according to the method of Toomik and EkCitation6 using [γ-32P]ATP as a substrate. Kemptide and PKC [Ser-25] (19–31) peptide were also used as enzyme substrates for PKA and PKC, respectively.
Results
The pigments extracted from the bacterial pellet were first purified by silica gel column chromatography. The red-coloured fraction obtained after silica gel column chromatography contained seven prodigiosin family compounds (P1–P7) according to HPLC analysis where P4 was the major component which occupied 27–35% of the whole red pigments (). This fraction was further purified by ODS column chromatography to obtain four individual prodigiosin-like compounds, P2, P4, P5 and P6. By mass spectrometry performed according to the previously described methodCitation1, these compounds were confirmed as 2-methyl-3-butylprodiginine (P-2, m/z 310.1 [M+H]+), 2-methyl-3-pentylprodiginine (prodigiosin) (P-4, m/z 324.1), 2-methyl-3-hexylprodiginine (P-5, m/z 338.1) and 2-methyl-3-heptylprodiginine (P-6, m/z 352.1), which differ from each other only by the length of the alkyl chain at the C-3 position in the pyrrolyldipyrromethene core ()Citation1. Other red pigments, P-1, P-3 and P-7, were not purified and characterized due to their instability or small quantity.
Figure 1. HPLC analysis of the red pigment fraction after silica gel column chromatography. Pigments were subjected to HPLC using an isocratic mobile phase of 55% aqueous methanol solution containing 0.2% acetic acid. Seven pigments are indicated as P-1 to P-7 according to the order of elution.
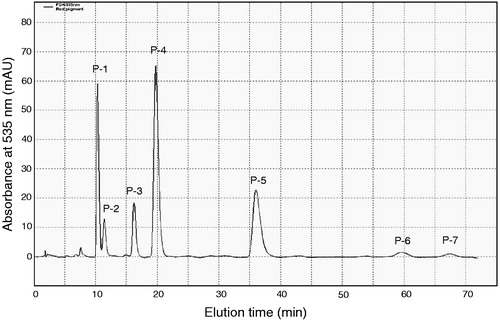
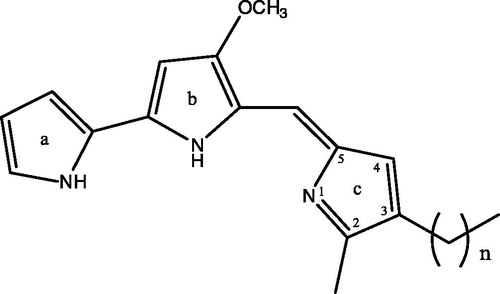
Figure 2. Chemical structures of prodigiosin congeners: n = 3, 2-methyl-3-butylprodiginine (P-2); n = 4, 2-methyl-3-pentylprodiginine (prodigiosin) (P-4); n = 5, 2-methyl-3-hexylprodiginine (P-5); n = 6, 2-methyl-3-heptylprodiginine (P-6).
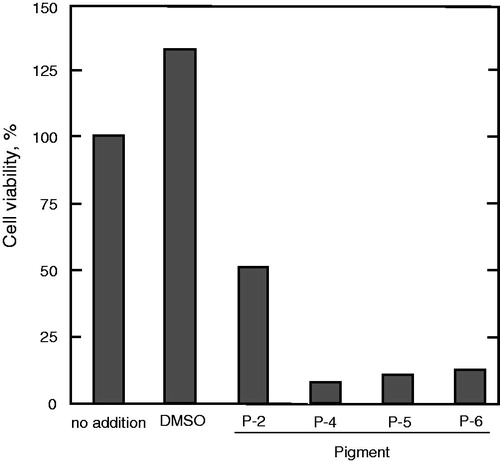
Cytotoxicity of prodigiosin compounds against human leukemia cell lines
The cytotoxicities of the prodigiosin compounds toward human U937, K562 and HL60 leukemia cell lines were determined by assessing their effect using the MTS reduction assay. The addition of the mixture of prodigiosin compounds obtained from the silica gel column resulted in a significant decrease of viable cells. The IC50 for the mixture of prodigiosin compounds against U937 cells was about 0.7 μM, while the IC50 was approximately 2.5 μM for K562 cells and 1.5 μM toward HL60 cells. As shown in , P-4 along with P-5 and P-6 were highly cytotoxic at 2 μM while P-2 was less toxic compared with the other compounds.
Effect of prodigiosin compounds on protein kinase activity
The mixture of prodigiosin compounds did not affect CaM kinase activity as shown in . Although DMSO used as a solvent somewhat reduced the enzyme activity, no further inhibition was observed in the presence of prodigiosin compounds. Prodigiosin compounds also showed no inhibition (data not shown) toward Src kinase activity. Consequently, both PKA and PKC activities were either not induced or inhibited by the mixture of prodigiosin compounds or the individual compounds (data not shown). Based on these results, we concluded that the prodigiosin compounds had no affinity for the tested protein kinases.
Figure 4. Effect of the prodigiosin compounds on the activity of CaM kinase. The CaM kinase assay was conducted in the presence of the indicated concentrations of the mixture of prodigiosin compounds. The values of enzyme activity assayed in duplicate were within 10% of each other and the mean values are shown. Enzyme activity at 100% (no addition) is 0.611 nmol Pi incorporated/20 min.
Inhibition of protein phosphatase activity
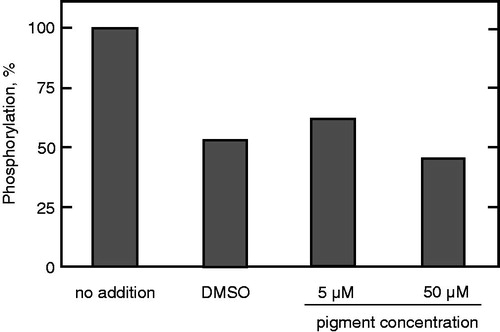
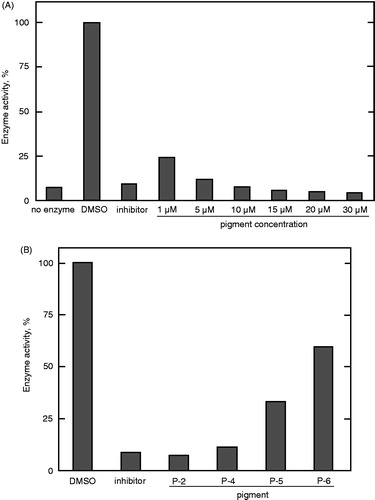
The effects of prodigiosin compounds on protein tyrosine phosphatase 1B (PTP1B) and protein phosphatase 2 A (PP2A) activities were examined. As shown in , the prodigiosin mixture gradually decreased PTP1B enzyme activity, completely inhibiting it at 10 μM. Then, each prodigiosin congener was investigated individually to determine the most potent inhibitory compound from the mixture. As shown in , P-2 was the best inhibitor at 10 μM. The inhibitory activities of the other compounds gradually decreased with an increase in the alkyl chain length at C-3.
Figure 5. Inhibition of PTP1B by the prodigiosin compounds. The assay was carried out in the presence of (A) the mixture of the compounds at different concentrations and (B) individual prodigiosin compounds at 10 μM. Data represent a typical result from two independent experiments and the mean values of enzyme activity assayed in duplicate. Values in duplicate assay were within 10% of each other.
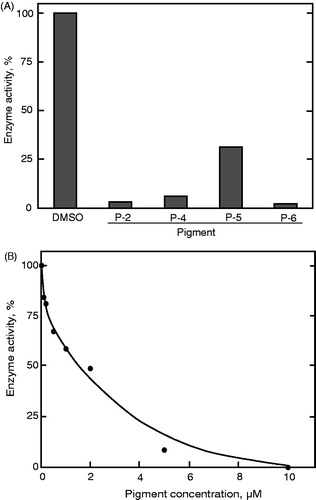
Similar to the PTP1B assay, PP2A enzyme inhibition was examined at 10 μM of each compound. Each prodigiosin compound strongly inhibited the enzyme activity (). Again, P-2 and P-6 were the most potent inhibitors of PP2A enzyme activity. The IC50 value of P-2 was approximately 2 μM ().
Figure 6. Inhibition of PP2A activity by the prodigiosin congeners. (A) The assay was conducted in the presence of the individual compounds at 10 μM. (B) Enzyme inhibition was assayed in the presence of P-2 at different concentrations. Data represent a typical result from two independent experiments and the mean values of enzyme activity assayed in duplicate. Values in duplicate assay were within 10% of each other.
Discussion
Much attention has been given to the microorganisms that inhabit the sea and their respective metabolites. As revealed in previous and the present research, the red-pigmented prodigiosin family compounds from Pseudoalteromonas sp. 1020R strain have strong anticancer activityCitation1.
The induction of apoptotic mechanisms by prodigiosins are known to involve both extrinsic and intrinsic pathways. Investigation of those apoptotic pathways revealed that prodigiosins are mainly involved in the intrinsic pathway via the mitochondriaCitation4. This may be explained by the hydrophobic nature of these compounds, which enables them to penetrate through the lipophilic membrane bilayer. However, prodigiosin-induced apoptosis via the death receptor signaling extrinsic pathway is also important, as it was reported not to involve p53 signalingCitation7. In fact, as supported by our results, prodigiosins are known to cause apoptosis both dependentlyCitation8 and independentlyCitation8–10 of p53 tumor suppressor protein. The prodigiosins were cytotoxic toward HL60, U937 and K562 leukemia cell lines with varying IC50 values. Despite the fact that HL60 and K562 lack p53 proteinCitation11,Citation12, prodigiosins could still induce apoptosis in all three types of cells. p53 protein is known to be involved in a number of cellular processes including gene transcription, DNA repair, cell cycling, genomic stability, senescence and apoptosisCitation11. It is also known that the lack of p53 protein or defective p53 due to mutations makes cancer cells insensitive to anti-cancer drugs, thus limiting their clinical efficacy as chemotherapeutic agentsCitation13. Different kinds of cellular signals resulting from DNA damage, a defective cell cycle, hypoxia, loss of cell survival factors and other types of cell stresses are known to activate p53, which in turn either mediates cell-cycle arrest to ensure further survival of the cells or initiates apoptosis through the activation of pro-apoptotic proteinsCitation4, thus accelerating the death of injured cells. This process depends on to what extent the cell compartments are damaged. However, our results clearly show that the prodigiosin family of compounds induces apoptosis in cancer cell lines regardless of the presence of p53 protein. This suggests that the apoptosis process in these cells is caused from the activation of multiple pathways. This confirms the results from our previous study where the prodigiosin compounds were determined to cause DNA fragmentation in U937 cells, which is considered to be a stimulus for the activation of p53Citation1. Our results suggest that other p53 independent pathways contributed to apoptosis in the presence of the prodigiosin compounds in the K562 and HL60 cells. This may explain the lower effect of prodigiosin compounds on these cells compared with the higher IC50 values observed for the U937 cells. In these cell lines, an extrinsic apoptosis pathway which is triggered independently of p53 proteinCitation7 may play an essential role in cell death. However, when the cell permeability of prodigiosins and their accumulation in mitochondria is taken into accountCitation4, the intrinsic apoptotic pathway may still be a critical mechanism for programmed cell death. Regardless, it is essential to investigate how prodigiosins may affect other pathways that are involved in the apoptosis process. Based on previously reported data, we investigated their effects on signal transduction pathway molecules as possible targets of apoptosis.
2-Methyl-3-pentylprodiginine (prodigiosin) had the highest apoptotic effect on the cells, followed by 2-methyl-3-hexylprodiginine and 2-methyl-3-heptylprodiginine (). 2-Methyl-3-butylprodiginine had the lowest effect compared with its congeners. This might be because of its low hydrophobicity, which may hinder its penetration through the membrane lipid bilayer. However, it had the strongest in vitro inhibitory effect on the protein phosphatases as shown in and .
Although, inhibition of selective members of the protein phosphatases by prodigiosin-like compounds has been previously studiedCitation5, the action of prodigiosin and its close congeners on this class of enzymes was not reported. Furthermore, many of the previously investigated compounds were primarily synthesised rather than naturally derivedCitation5. Additionally, there was no direct evidence of involvement of the protein kinases except for the prevention of prodigiosin-induced apoptosis by the activation of PKCCitation14.
The described results clearly show that the prodigiosins inhibit the activity of the tested phosphatases, but not the kinases. Unlike the phosphatases, the kinases do not seem to have high affinity for the prodigiosin compounds, as all the kinases tested herein were either not inhibited or not significantly inhibited, indicating selective action of prodigiosins toward phosphatases. The fact that as the length of the prodigiosin side chain increased, the extent of inhibition decreased suggested that the compound structure plays an essential role in the compound binding to the enzyme and thus, the inhibition (). Although the exact mechanism of inhibition is unknown, it is likely that a prodigiosin with a shorter alkyl chain more easily penetrates into the active site loop of the enzyme. In a review on PTP1B inhibitors, Zhang and ZhangCitation15 highlighted that increasing the compound hydrophobicity can be an effective strategy to improve PTP1B inhibitor bioavailability because of enhanced permeability through the cell membrane. Conversely, CombsCitation16 suggested that extremely hydrophobic small molecule inhibitors are not desirable agents because of their ability to nonspecifically inhibit enzyme activities. When both the hydrophobicity and membrane permeability of prodigiosin compounds are taken into account, it seems that these compounds could be specific and competitive inhibitors of PTP1BCitation4. The PTP1B is considered to be a highly challenging drug target because of its highly conserved and positively charged active-site pocketCitation15. Therefore, because of their selective affinity and relatively low IC50 values of less than 1 µM, prodigiosin and its congeners provide a good starting point as potent drug candidates against type 2 diabetes mellitus and cancerCitation17.
PP2A, an enzyme belonging to the family of serine/threonine dephosphorylating phosphatases, was also investigated. To determine which compound in the pigment had the highest influence on PP2A enzyme activity, the isolated prodigiosin congeners were tested at 10 μM. All tested compounds inhibited the enzyme activity (). Similar to the PTP1B assay (), 2-methyl-3-butylprodiginine and 2-methyl-3-heptylprodiginine showed the highest inhibitory effect toward PP2A. In a more detailed study, the IC50 value of 2-methyl-3-butylprodiginine was found to be approximately 2 μM (). When protein binding of the compound to the bovine serum albumin present in the reaction solution is taken into account, the true IC50 value may, in fact, be lower.
Overall, the present study provides insight into the molecular mechanisms of cytotoxicity of prodigiosin compounds. The inhibitory effect of these compounds toward protein phosphatases provides a basis for the design of potential drug candidates against type II diabetes and cancer.
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Wang Y, Nakajima A, Hosokawa K, et al. Cytotoxic prodigiosin family pigments from Pseudoalteromonas sp. 1020R isolated from the Pacific coast of Japan. Biosci Biotechnol Biochem 2012;76:1229–32
- Williamson NR, Fineran PC, Gristwood T, et al. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2007;2:605–18
- Montaner B, Pérez-Tomás R. The prodigiosins: a new family of anticancer drugs. Curr Cancer Drug Target 2003;3:57–65
- Pandey R, Chander R, Sainis KB. Prodigiosins as anti-cancer agents: living up to their name. Curr Pharm Des 2009;15:732–41
- Fürstner A, Reinecke K, Prinz H, Waldmann H. The core structures of roseophilin and the prodigiosin alkaloids define a new class of protein tyrosine phosphatase inhibitors. ChemBioChem 2004;5:1575–9
- Toomik R, Ek P. A potent and highly selective peptide substrate for protein kinase C assay. Biochem J 1997;322:455–60
- Ravi R, Jain AJ, Schulick RD, et al. Elimination of hepatic metastases of colon cancer cells via p53-independent cross-talk between irinotecan and Apo2 Ligand/TRAIL. Cancer Res 2004;64:9105–14
- Castillo-Ávila W, Abal M, Robine S, Pérez-Tomás R. Non-apoptotic concentrations of prodigiosin (H+/Cl− symporter) inhibit the acidification of lysosomes and induce cell cycle blockage in colon cancer cells. Life Sci 2005;78:121–7
- Montaner B, Navarro S, Piqué M, et al. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol 2000;131:585–93
- Soto-Cerrato V, Viñals F, Lambert JR, Pérez-Tomás R. The anticancer agent prodigiosin induces p21WAF1/CIP1 expression via transforming growth factor-beta receptor pathway. Biochem Pharmacol 2007;74:1340–9
- Ju JF, Banerjee D, Lenz HJ, et al. Restoration of wild-type p53 activity in p53-null HL-60 cells confers multidrug sensitivity. Clin Cancer Res 1998;4:1315–22
- Feinstein E, Gale RP, Reed J, Canaani E. Expression of the normal p53 gene induces differentiation of K562 cells. Oncogene 1992;7:1853–7
- Yu JL, Rak JW, Coomber BL,et al. Effect of p53 status on tumor response to antiangiogenic therapy. Science 2002;295:1526–8
- Ramoneda BM, Pérez-Tomás R. Activation of protein kinase C for protection of cells against apoptosis induced by the immunosuppressor prodigiosin. Biochem Pharmacol 2002;63:463–9
- Zhang S, Zhang Z-Y. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today 2007;12:373–81
- Combs AP. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J Med Chem 2010;53:2333–44
- Stuible M, Doody KM, Tremblay ML. PTP1B and TC-PTP: regulators of transformation and tumorigenesis. Cancer Metastasis Rev 2008;27:215–30