Abstract
A phenoloxidase was extracted and purified from hemocytes of Ephestia kuehniella by using ammonium sulfate, Sepharyl G-100 and DEAE-Cellulose fast flow chromatographies. At the final stage of purification, a protein was purified by molecular mass of 78.5 kDa, specific activity of 1.17 U/mg protein, recovery of 20.48% and purification fold of 16.71. The purified PO showed the highest activity at pH 4–5 and temperatures of 35–40 °C. Na+, K+, Mn+, Zn2+ and Mg2+ decreased activity of the purified PO but Ca2+ and Cu2+ increased the enzymatic activity. EDTA (General chelating agent), DTC (Copper chelating agent) and EGTA (Calcium chelating agent) significantly decreased PO activity but TTHA (Magnesium chelating agent) showed no statistically significant effects. Kinetic parameters of the purified enzyme showed the highest Vmax when L-DOPA was used as substrate but no significant differences were observed in case of Km for used L-DOPA, pyrocatechol and hydroquinone. In vitro inhibition of the purified PO by using two insect growth regulators, Hexaflumuron and Pyriproxyfen, revealed IC50 of 96.41 and 38.59 µg/ml for these compounds, respectively. Kinetic studies using different concentrations of L-DOPA and IC50 concentrations of the two IGRs revealed the increase of Km value versus control and competitive inhibition. Finally, column chromatography of hemolymph revealed peak III showing endogenous inhibitors of phenoloxidase by molecular weight of 27.3 that showed competitive inhibition on the PO.
Introduction
Immune systems of insects utilize phagocytosis, nodule formation and encapsulation as cellular responses and antimicrobial peptides as humeral responses against non-self-agentsCitation1. In all mentioned responses, phenoloxidases (POs) have crucial roles in final stages to disable invading agents completely. In fact, invading agents are recognized by pattern recognition proteins (PGRPs) leading to serine protease cascade in hemolymph. The protease changes prophenoloxidase (Inactive zymogen) to phenoloxidaseCitation2. On the other hand, the enzyme is activated by hemolymph serine proteases at Arg-Phe bond by the removal of a N-terminal fragment including 50 amino acid residuesCitation3,Citation4. Activated phenoloxidase catalyzes conversion of phenols to quinones. Finally, melanin is synthesized from polymerization of quinone to be deposited in formed nodule or capsule around invading agentCitation1.
It has now been known that there are two types of phenoloxidases in insects. The first is laccase-type enzymes (EC 1.10.3.2) that oxidize o- or p-diphenols to quinonesCitation5,Citation6. The second is tyrosine-like enzymes that hydroxylate tyrosine (EC 1.14.18.1) and oxidize o-diphenols to quinones (EC 1.10.3.1)Citation6. The first type involves sclerotization and tanning of cuticle but the second type refers to PO involved in immune responses of insects. Insect prophenoloxidases (Inactive form of PO) structurally are homologous with hemocyanins of arthropods and hexamerin proteins of insects. The enzyme is a polypeptide of approximately higher than 50 kDa molecular weight containing 0.14–0.22% of copper, two atoms of copper per protein moleculeCitation6.
Study on insect phenoloxidases is important due to not only determining the molecular and physiological impacts of POs on insect immunity but also targeting the enzyme to decrease population of agricultural pests. In fact, any disruptions in PO activities may lead to death of host or increase efficiency of microbial agents in biocontrol programs. Although the process might be of interest, a few studies have been carried out to purify and screen PO inhibitors in insects. The processes need careful attention since the enzyme is unstable and rapid loss of enzymatic activity may occur during purification process. So far, proPO has been purified and characterized from some insect species including Hemiptera, Lepidopteran, Dipteran, Blattodea and OrthopteraCitation7. Hence, objectives of the current study are (i) extraction and purification of PO from hemocytes of E. kuehniella larvae, (ii) determination of enzymatic parameters by using different substrates, pH, temperature and chelating agents, (iii) determining the effect of two insect growth regulators and (iv) extraction of an endogenous inhibitor from hemolynph of the larvae.
Materials and methods
Insect rearing
E. kuehniella was reared on artificial diet containing wheat bleached flour (43 g), yeast, Saccharomyces cerevisiae (6 g) and glycerine (20 ml) in plastic containers (17 × 9 × 5 cm) at 25 ± 1 °C, 70% of humidity and 16L:8D conditionsCitation8.
Collection of hemolymph
Hemolymph of E. kuehniella was collected from the first abdominal proleg of larvae (20 µl of hemolymph per larva) by a 50 μl sterile glass capillary tube (Sigma-Aldrich Co., London, UK). The hemolymph was immediately diluted with an anticoagulant solution (0.01 M ethylenediamine tetraacetic acid, 0.1 M glucose, 0.062 M NaCl, and 0.026 M citric acid, pH 4.6)Citation9.
PO preparation and assay
The collected samples were centrifuged at 13 000 rpm for 5 min; the supernatant was discarded and the pellet was washed gently twice with a universal buffer (20 mM, pH 7, containing succinate, glycine and 2-orpholino ethansulforic acid; FrugoniCitation10. Cells were homogenized in 500 μl of the buffer, centrifuged at 13 000 rpm for 15 min, and the hemocyte lysate supernatant (HLS) was used to assess PO.
Samples were pre-incubated with buffer at 30 °C for 30 min before the addition of 50 μl of 10 mM aqueous solution of substrate L-dihydroxyphenylalanin (L-DOPA). The mixture was incubated for 5 min at 30 °C and PO activity was measured at 492 nm. One unit of PO activity represents the amount of enzyme required to produce an increase in OD490 of 0.01 per minCitation11.
Purification of PO
The method of Pang et al.Citation12 was used to purify PO of E. kuehniella in a three-step procedure as follows:
Ammonium sulfate treatments
Samples were first subjected to ammonium sulfate precipitation by using fractions of 30% and 70% concentrations. The two different ammonium sulfate treatments were collected by centrifugation at 5000 rpm and the pellets obtained in each treatment were suspended in a minimal volume of universal buffer (pH 7). Precipitated samples were transferred to dialyze tube, put in 200 ml of the buffer for 20 h.
Sepharyl G-100 gel filtration
The ammonium sulfate fractions were subjected to gel filtration containing Sepharyl G-100 column (2 cm × 100 cm) equilibrated with universal buffer pH 7 containing 0.05% (v/v) Triton X-100. Fractions of 1.5 ml were collected at a flow rate of 20 ml/h with the same buffer.
DEAE-Cellulose fast flow separation
Fractions showing the highest PO activity of sepharyl G-100 column were applied to a DEAE-Cellulose fast flow column (3 × 30 cm) equilibrated with universal buffer pH 6.0. After washing the column with the same buffer, bound proteins were eluted with a linear gradient of sodium chloride (0–0.5 M) in the equilibrating buffer. Fractions (1.5 ml each) were collected at a flow rate of 60 ml/h. Fractions with PO activity were pooled and stored at −20 °C for further analysis.
Polyacrylamide gel electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE) was used to determine the purity and molecular mass of the enzyme using a 4% (w/v) stacking gel and a 10% (w/v) separating gel. The molecular mass of the enzyme was estimated by using standards: β-galactosidase (116 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45 kDa), lactate dehydrogenase (35.5 kDa), restriction endonuclease Bsp 981 (25 kDa), β-lactoglobulin (18.4 kDa) and lysozyme (14.4 kDa). After SDS-PAGE, proteins on the polyacrylamide gel were stained with 0.2% Coomassie brilliant blue R-250.
Effect of pH and temperature on the purified PO
The effects of temperature and pH on the purified PO of E. kuehniella were examined by using 10 mM solution of L-dihydroxyphenylalanine (L-DOPA) as substrate and various ranges of pH and temperature. Optimal pH was determined using universal buffer at a range of 3–10. The effect of temperature on PO activity was determined by incubating the reaction mixture at 20, 25, 30, 35, 40, 45, 50, 60 and 70 °C for 5 min. Reaction mixture was 50 μl of buffer, 30 μl of L-DOPA and 20 μl of the enzyme. pH of reaction and temperature were changed as described earlier. Incubation time was continued for 5 min and absorbance was read at 492 nm.
Kinetic parameters of PO
Kinetic parameters of the purified PO were determined using different substrates L-DOPA, catechol and hydroquinone. Concentrations of the substrates were 2, 4, 6, 8 and 10 mM. Reaction mixture was 50 μl of universal buffer, 30 μl of substrates (mentioned concentrations) and 20 μl of the purified PO. Incubation time was continued for 5 min and absorbance was read at 492 nm. Obtained data were inserted in Sigma-Plot software (version 6, San Jose, CA) to calculate Vmax and Km values.
Effect of ions and chelating agents on the purified PO
The effects of mono- and di-valent cations (concentrations of 0.5, 3 and 5 mM) on the purified PO of E. kuehniella were determined by using Na+, K+, Ca2+, Cu2+, Mg2+, Zn2+ and Mn+. Reaction mixture was 50 μl of universal buffer, 30 μl of substrates (mentioned concentrations), 30 μl of the cations in given concentration and 20 μl of the purified PO. Incubation time was continued for 5 min and absorbance was read at 492 nm. The effect of enzyme inhibitors on PO activity was studied using different concentrations (2, 4, 6, 8 and 10 mM) of ethylenediaminetetraacetic acid (EDTA), diethyldithiocarbamate (DTC), N,N,N′,N′-tetraacetic acid (EGTA) and triethylenetetramine hexaacetic acid (TTHA). Reaction mixture was 50 μl of universal buffer, 30 μl of substrates (mentioned concentrations), 30 μl of the cations in given concentration and 20 μl of the purified PO. Incubation time was continued for 5 min and absorbance was read at 492 nm.
In vitro effects of hexaflumuron and pyriproxuyfen on the purified PO
Different concentrations (30 μl) of hexaflumuron and pyriproxyfen were prepared (5–100 µg/ml) and pre-incubated with 30 μl of L-DOPA (10 mM) in 50 μl of universal buffer for 5 min. Then, 20 μl of the purified PO was added and reaction was continued for additional 5 min prior to read absorbance at at 492 nm. OD values was changed to relative activity (%), inserted in POLO-PC software to calculate IC values (Inhibitory concentration).
Effects of hexaflumuron and pyriproxyfen on kinetic parameters of the Purified PO
IC50 concentrations (30 μl) of hexaflumuron and pyriproxyfen were preincubated with 50 μl of universal buffer and different concentrations of L-DOPA (30 μl, 2–10 mM) for 5 min. Then, 20 μl of the purified PO was added and reaction was continued for additional 5 min prior to read absorbance at 492 nm. Data were inserted to Sigma Plot software (Version 6) to calculate kinetic parameters and type of enzymatic inhibition.
Extraction of PO endogenous inhibitor from hemolymph of E. kuehniella
The method described by Sugumaran and NellaiappanCitation13 was adopted by a slight modification to extract and purify endogenous inhibitor of PO from the hemolymph of E. kuehniella. The collected hemolymph was centrifuged at 13 000 rpm for 30 min at 4 °C. Obtained supernatant was mixed with phosphate buffer (20 mM, pH 7) in 1:1 ration. In addition, it was incubated for 30 min at 5 °C. The mixture was subjected to 0 to 60% ammonium sulfate fractionation. The precipitate was collected by centrifugation at 5000 rpm for 30 min, dissolved in minimum amount of phosphate buffer (6 ml) and dialyzed against 300 µl of buffer for 20 h. The protein precipitated inside the dialysis bag was collected by centrifugation at 5000 rpm for 30 min, dissolved in 5 ml of a buffer containing 100 mM sodium acetate, pH 6.0 containing 0.5 M NaCl, 1 mM CaCl2, 1 mM MgCl2 and 1 mM MnCl2 and chromatographed on Sepharyl G-100 column pre-equilibrated with the same buffer. Fractionation was carried out taking 37 fractions. Five peaks were obtained after measuring protein in each fraction. PO activity was determined by using 50 μl of universal buffer, 30 μl of L-DOPA, 30 μl of sample from each peak and 20 μl of the purified Enzyme. Incubation was continued for 5 min prior to read absorbance at 492 nm. SDS-PAGE was carried out showing molecular mass and purity of the peak caused PO inhibition.
Protein assay
Protein concentrations were assayed according to the method described by Lowry et al.Citation14. The method recruits reaction of Cu2+, produced by the oxidation of peptide bonds with Folin–Ciocalteu reagent. In the assay, 20 µl of sample was added to 100 µl of reagent, and incubation was made for 30 min prior to read absorbance at 545 nm (Recommended by Ziest Chem. Co., Tehran, Iran).
Statistical analysis
The experiments were designed based on a complete randomized scheme. All data were compared by one-way analysis followed by Tukey test at p ≤ 0.05).
Results
Purification of PO from E. kuehniella
The enzyme was purified by a three-step procedure from hemocytes of E. kuehniella (). Precipitated samples from 70% ammonium sulfate were subjected to Sepharyl G-100 column leading to collection of 73 fractions. Fractions 25–42 showed the highest PO activity, and were pooled and subjected to DEAE-cellulose fast flow column (Supplementary 1). In ion-exchange chromatography, 30 fractions were collected that fractions 11–17 showed the highest enzymatic activity. These fractions were pooled and loaded into SDS-PAGE to find purity and molecular mass of the purified protein (Supplementary 1). SDS-PAGE revealed a single band of purified protein in comparison with crude and sepharose G-100 samples by molecular mass of 78.5 kDa (Supplementary 2). At the end of purification process, a molecule was purified by 78.5 kDa of molecular weight, specific activity of 1.17 U/mg protein, recovery of 20.48% and purification fold of 16.71 ().
Table 1. Purification of PO from the hemolymph of E. kuehniella.
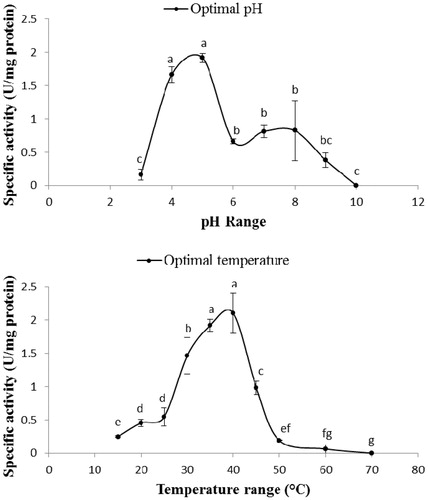
Effect of pH and temperature on the purified PO
Although the highest activity of the purified PO was obtained at pH 5, no statistical differences were observed between pHs 4 and 5 (). The PO activity decreased from pH 6 and reached zero at pH 10 (). Analysis of PO activity versus temperature revealed that peak activity was found at 35 and 40 °C although the enzymatic activity sharply increased from 25–40 °C and no activity was observed at 70 °C ().
Kinetic parameters of the purified PO
Kinetic parameters of the purified PO are shown in and by using L-DOPA, pyrocatechol and hydroquinone. Linweaver–Burk analysis revealed that Km of the enzyme had no statistical differences among the used substrates but the highest and the lowest Vmax values were observed in case of L-DOPA and Pyrocatechol ().
Figure 2. Double reciprocal plot to show the kinetic parameters of the purified PO from E. kuehniella by using L-DOPA, pyrocatechol and hydroquinone as substrates. (1/Vmax = intercept on the 1/V0 ordinate, −1/Km = intercept on the negative side of the 1/[S] abscissa).
![Figure 2. Double reciprocal plot to show the kinetic parameters of the purified PO from E. kuehniella by using L-DOPA, pyrocatechol and hydroquinone as substrates. (1/Vmax = intercept on the 1/V0 ordinate, −1/Km = intercept on the negative side of the 1/[S] abscissa).](/cms/asset/e0000678-57bd-4ff9-831f-4bcf30ff6809/ienz_a_954107_f0002_b.jpg)
Table 2. Kinetic parameters of the purified PO from E. kuehniella.
Effects of ions and chelating agents on the purified PO
Different concentrations of cations induced significant effects on activity of the purified PO from E. kuehniella (). Different concentrations of Na+, K+, Mn+, Zn2+ and Mg2+ significantly decreased the enzymatic activity in a dose-dependent manner that the highest negative effect was observed in case of Mn+ (). Ca2+ and Cu2+ significantly increased the activity of the purified PO although the highest increase was obtained by using 5 mM concentration of Cu2+ (). EDTA (General chelating agent), EGTA (Calcium chelating agent) and DTC (Copper chelating agent) significantly decreased the activity of the purified PO that DTC had the highest inhibitory effect (). No statistical differences were observed in case of TTHA (Magnesium chelating agent; ).
Table 3. Effects of mono- and divalent cations on the purified PO activities of E. kkuehniella.
Table 4. Effects of chelating agents on the purified PO activities of E. kkuehniella.
Effects of hexaflumuron and pyriproxyfen on the purified PO
show dose-response line regressions and IC50 values of hexaflumuron and pyriproxyfen on the purified PO of E. kuehniella (Supplementary 3). IC50 of 96.41 and 38.59 µ/ml of hexaflumuron and pyriproxyfen against the purified PO were found respectively (). Additionally, effects of these two IGRs on kinetic parameters of the enzyme revealed higher Km value of the treated enzyme versus control (Supplementary 4; ). Meanwhile, no effects were found on Vmax value of treated and control samples ().
Table 5. Inhibition parameters of hexaflumuron and pyriproxyfen on the purified PO of E. kuehniella.
Table 6. Kinetic parameters of the purified PO from E. kuehniella in control and treated conditions of hexaflumuron and pyriproxyfen.
Extraction of PO endogenous inhibitor from hemolymph of E. kuehniella
Fractionation of samples for extraction of PO endogenous inhibitor revealed five peaks on Sepharyl G-100 column. Fractions regarding each peak were carefully collected and their effects were assayed on the purified PO by using L-DOPA as substrates. It was revealed that Peak III significantly decreased the enzymatic activity to lower than 50% in comparison with other fractions (Supplementary 5a, b). SDS-PAGE of the sample in peak III revealed a single band 27.3 kDa of molecular weight (Supplementary 5c). Meanwhile, kinetic studies on the enzyme inhibited by purified inhibitor revealed competitive inhibitor since Km value of the treatment and control were 14.94 mM and 6.87 mM. No statistical differences were found in case of Vmax (Supplementary 5d).
Discussion
Phenoloxidases are one of the main components of insect immunity. Numerous attempts have been made to purify the enzyme, find its molecular mass and tissue allocationCitation2,Citation15. The enzyme have now been purified and characterized in some insect arthropods. In the current study, one isoform of PO was purified from hemocytes of E. kuehniella by molecular mass of 78.5 kDa. Majority of the relevant studies have purified a PO by one isoform but two or three isoforms have been purified in some studiesCitation2,Citation3. Meanwhile, PO was measured to be 78.5 kDa of molecular mass that corresponds with reports on POs of other insectsCitation2. Liu et al.Citation16 believed that different biochemical properties of POs like pH, response to xenobiotic and isoforms could be attributed to species and its survival conditions that appeared in in vitro studies. PO is one of the sensitive enzymes in insects. Several studies reported that the enzymes were affected whenever an insect was treated by chemicals or even host plants. Also, number of isoforms depends on species and its immune capability.
Temperature and pH are the two key factors in the biochemical media that crucially affect enzymatic reactions. Any changes of these factors could alter enzymatic activity leading to affect on physiological process. Although purified PO from hemocytes of E. kuehniella had optimal pH of 5 and temperature of 40 °C but no statistical differences have been observed by pH of 4 and 35 °C. Majority of studies have been reported acidic or neutral pHs for activities of POs but alkaline PO have been reported in Helicoverpa virescens Fabricius (Lepidoptera: Noctuidae) and H. cuneaCitation17,Citation18. These differences could be correlated with pH of hemolymph that indicate type of species and survival conditionsCitation16,Citation19. Enzymes have temperature-dependent activities that are correlated with enzymatic stability and temperature of biochemical media. Hence, biological reactions occur faster with increasing temperature upto the point of enzyme denaturation, above which the enzyme activity and the rate of reaction decrease sharply.
It was found that the purified PO from hemocytes of E. kuehniella could oxidize L-DOPA, pyrocatechol and hydroquinone although the higher Vmax was ordinary observed in case of L-DOPA, hydroquinone and pyrocatechol. Although the lower Km was observed in case of L-DOPA but there was no statistical differences among used substrates. The results of substrate specificity showed that the purified PO from E. kuehniella was probably a kind of tyrosinase not a laccase or a cathecol oxidase. Type of PO isoforms and evolutionary processes may affect the amount of Km and of course the efficiency of the enzyme to act on the substrateCitation20. Meanwhile, binding affinity of PO could be affected by the nature of the active site of PO. More precisely, differences in substrate-protein contact points or differences in the size of the substrate-binding pocket can affect PO binding affinity in different insectsCitation20.
Metal ions are other factors that affect enzymatic activity via several possible mechanisms, e.g. they can activate electrophile and nucleophile binding and releasing electronsCitation20. Although different cations significantly affected activity of the purified PO from hemocytes of E. kuehniella but the highest effects were observed by using Cu2+ and its specific chelating agent (DTC). Meanwhile, similar effects of Cu2+ and its specific chelating agent (EGTA) on increasing of the purified PO activity could be attributed to possible substitution between the metals during enzyme activationCitation20. It was determined that proPO are polypeptides which contain 0.14–0.22% copper, indicating two atoms of copper per protein moleculeCitation8. Increased activity of the PO from hemocytes of E. kuehniella demonstrate that the enzyme is a metalloprotein that and small changes at the metal center can amplify its biological role. Moreover, metal ions (Mainly di-valent cations) increase PO activity by changing its protein foldingCitation16,Citation20. In fact, binding of metal ions significantly affect secondary structure of some peptides depending on their natureCitation20. It has now been determined that secondary structures of the purified PO have the lowin β-turn (14.33%) and the α-helix content (37.28%)Citation20. Involvements of the metal ions to media containing purified PO cause augmentation of the β-sheet and increase activity of the enzymeCitation20.
Majority of the studies dealing with effect of hormones and IGRs indicate changes of PO activity in vivo. Since, hormones and IGRs affect number of circulating hemocytes interpretation of their direct effects on PO activity could not be reliable. Hence, the current study determined direct effects of hexaflumuron and pyriproxyfen on the purified PO activity by determing IC50 values and kinetic parameters of the enzyme. IC50 calculation revealed that pyriproxyfen is more effective than hexaflumuron because of the lower IC50. Moreover, Linewever–Burk analysis revealed increase of Km value when the enzyme was incubated with two IGRs showing competitive inhibition. In case, inhibitor binds to enzyme so that substrate and inhibitor compete to engage active siteCitation21. In such a way, increasing of substrate concentration could lead to natural activity of enzymeCitation21. In a similar study, Mirhaghparast and ZibaeeCitation22 showed that different concentrations of hexaflumuron and pyriproxyfen resulted IC50 values of 0.047 and 0.11 μg/ml on the purified PO of C. suppressalis. Yan-Yan et al.Citation23 reported that concentrations of hexaflumuron up to 153 mg/L increased PO activity of S. littura then the enzymatic activity decreased along with higher used concentrations.
In the present study, a low molecular weight inhibitor was extracted and purified from hemolymph of E. kuehniella by measuring PO activity in various fractions. The inhibitor showed competitive inhibition by increasing of Km value versus control. PO inhibitors of insects have been extracted and purified from cuticle. Tsukamoto et al.Citation24 purified three low molecular weight inhibitors from pupal extracts of house flies. These inhibitors showed competitive inhibition of endogenous phenoloxidase activity. The endogenous inhibitor of PO regulates activity of the enzyme during infection, wounding and/or metamorphosis and prevents undesired melanization and sclerotization reactions somehow showing protecting role of intrinsic organs. In case, more detailed studies are required to sequence the protein and find its the structure–function relationship with the enzyme.
Conclusions
Purification and characterization of insect’s POs are mandatory since those are the unique enzymes that play critical roles in melanization and sclerotization of cuticle, wound healing and immune reactions. Moreover, several studies have shown that these enzymes could be a target to control insect pests. Currently, several compounds like IGRs, cantharidin, 4-Hexylresorcinol, Kojic acid and Quercetin that are safe for non-target organisms and environment. These researches will lead to a basic tool for the development of new insecticides in integration with microbial agents. As a future project, protein and genomic sequence of the enzyme must be elucidated and its expression must be determined in intact and immune challenge conditions.
Supplemental Material.pdf
Download PDF (277.9 KB)Acknowledgements
This study was supported by research deputy in university of Guilan.
Declaration of interest
The authors report no declarations of interest.
References
- Klowden MJ. Physiological systems in insects. 2nd ed. New York (NY): Elsevier; 2007
- Park JW, Lee BL. Insect immunology. In: Gilbert LI, ed. Insect molecular biology and biochemistry. San Diego (CA): Elsevier; 2012:480
- Fujimoto K, Okino N, Kawabata S, et al. Nucleotide sequence of the cDNA encoding the proenzyme of phenol oxidase A1 of Drosophila melanogaster. Proc Natl Acad Sci USA 1995;92:7769–73
- Kim MS, Baek MJ, Lee MH, et al. A new easter-type serine protease cleaves a masquerade-like protein during prophenoloxidase activation in Holotrichia diomphalia larvae. J Biol Chem 2002;277:39999–40004
- Arakane Y, Muthukrishnan S, Beeman RW, et al. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA 2005;102:11337–42
- Dittmer NT, Suderman RJ, Jiang H, et al. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol 2004;34:29–41
- Zibaee A, Bandani AR, Malagoli D. Purification and characterization of phenoloxidase from the hemocytes of Eurygaster integriceps (Hemiptera: Scutelleridae). Comp Biochem Physiol Part B 2011;158:117–23
- Lima FM, Favero S, Lima JOG. Production of the Mediterranean flour moth, Anagasta kuehniella (Zeller) (Lepidoptera: Pyralidae), on an artificial diet containing corn meal. Neot Entomol 2001;30:37–42
- Azambuja P, Garcia ES, Ratcliffe NA. Aspects of classification of Hemiptera hemocytes from six triatomine species. Mem Inst Oswaldo Cruz 1991;86:1–10
- Frugoni JAC. Tampone universale di Britton e Robinson a forza ionica costante. Gazz Chim Italiana 1957;87:403–7
- Leonard C, Kenneth S, Ratcliffe NA. Studies on prophenoloxidase and protease activity of Blaberua craniifer hemocytes. Insect Biochem 1985;15:803–10
- Pang Q, Zhanga S, Shi Z, et al. Purification and characterisation of phenoloxidase from amphioxus Branchiostoma belcheri tsingtauense. Fish Shell Immunol 2005;19:139–48
- Sugumaran M, Nellaiappan K. Characterization of a new phenoloxidase inhibitor from the cuticle of Manduca sexta. Biochem Biophys Res Commun 2000;268:379–83
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75
- Genta FA, Souza RS, Garcia ES, Azambuja P. Phenoloxidases from Rhodnius prolixus: temporal and tissue expression pattern and regulation by ecdysone. J Insect Physiol 2010;56:1253–9
- Liu G, Yang L, Fan T, et al. Purification and characterization of phenoloxidase from crab Charybdis japonica. Fish Shellfish Immunol 2006;20:47–57
- Lockey TD, Ourth DD. Isolation and characterization of hemolymph phenoloxidase from Heliothis virescens larvae. Comp Biochem Physiol Part B 1992;102:891–6
- Ajamhassani M, Sendi JJ, Farsi MJ, Zibaee A. Purification and characterization of phenoloxidase from the hemolymph of Hyphantria cunea (Lepidoptera: Arctiidae). Invert Surv J 2012;9:64–71
- Harrison JF. Insect acid–base physiology. Ann Rev Entomol 2001;46:221–50
- Feng C, Song Q, Lü W, Lu J. Purification and characterization of hemolymph prophenoloxidase from Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae. Comp Biochem Physiol B 2008;151:139–46
- Palmer T. Enzymes. Chichester: Harwood Publishing; 2001
- Mirhaghparast SK, Zibaee A. Effects of hexaflumuron and pyriproxyfen on the purified phenoloxidase of Chilo suppressalis Walker (Lepidoptera: Crambidae). Arch Phytopathol Plant Prot 2013;46:1775–84
- Yan-Yan J, Xiu-Cui Q, Hui L. Effect of hexaflumuron on phenoloxidase activity in Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Acta Entomol Sinica 2010;53:517–24
- Tsukamoto T, Ichimaru Y, Kanegae N, et al. Identification and isolation of endogenous insect phenoloxidase inhibitors. Biochem Biophys Res Commun 1992;184:86–92