Abstract
In this study, we investigated the effects of antibacterial drugs (moxifloxacin hydrochloride, levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium) on human serum paraoxonase-1 (hPON1) enzyme activity from human serum in vitro conditions. For this purpose, hPON1 enzyme was purified from human serum using simple chromatographic methods. The antibacterial drugs exhibited inhibitory effects on hPON1 at low concentrations. Ki constants were calculated to be 2.641 ± 0.040 mM, 5.525 ± 0.817 mM, 35.092 ± 1.093 mM, 252.762 ± 5.749 mM and 499.244 ± 10.149 mM, respectively. The inhibition mechanism of moxifloxacin hydrochloride was competitive, whereas levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium were noncompetitive inhibitors.
Introduction
Moxifloxacin (MOX) (1S,6S)-1-cyclopropyl-7-[2,8-diazobicyclo(4.3.0)non-8-yl]-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinolone-3-carboxylic acid is a fourth generation new 8-methoxyquinolone derivate of fluoroquinolonesCitation1. The fluoroquinolone derivative drug is anti-infective compound. The compound has a broad antibacterial spectrum against gram-positive and gram-negative organisms including anaerobic bacteriaCitation2. The bactericidal activity of MOX is mediated by the inhibition of DNA gyrase (topoisomerase II) and topoisomerase IV, which are essential enzymes in bacterial DNA replication, transcription, repair and recombinationCitation1.
Levofloxacin (LV), (−)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid is one of the third generation of fluoroquinolones antimicrobial drug which exhibits activity against both gram-positive and gram-negative bacteria through inhibition of their DNA gyraseCitation3. Levofloxacin is a new derivative from the fluoroquinolone groupCitation4.
Cefepime is a semi-synthetic fourth generation cephalosporin with in vitro activity against to gram-positive and gram-negative bacteria, including Pseudomonas aeruginosa. It is more effective against to gram-negative pathogens because of its rapid penetration of the bacterial cell membraneCitation5.
Cefotaxime sodium is a drug of the third generation cephalosporin family. It is widely used against to gram-negative bacteriaCitation6.
Ceftizoxime sodium (6R,7R)-7-[(Z)-2-(2-aminothiazol-4-yl)-2-meth oxyiminoacetamido]-3-cephem-4-carboxylate is a third generation cephalosporin antibiotic. It is used to reduce the infection caused by both gram-negative and gram-positive bacteriaCitation7.
The human serum paraoxonase-1 (hPON1) enzyme (arylesterase, EC 3.1.8.1, hPON1), which is synthesized in the liver, is an ester hydrolase. The calcium-dependent enzyme is associated with high-density lipoprotein (HDL) and has a molecular weight is known as 43–45 kDaCitation8–10. Due to its physiological effects, paraoxonase was previously thought to be a lactonase. Recently, it is reported its several substrates in the literatureCitation11,Citation12. However, the physiological substrate and the biological function of this enzyme have not yet been elucidated.
hPON1 is critical to neutralize the free oxygen radicals, including hydrogen peroxideCitation13 and inhibit lipid peroxidation through the hydrolysis of cholesteryl linoleate hydroperoxides. In addition, hPON1 inactivates the formation of oxidized phospholipids in oxidized low-density lipoprotein (LDL) and neutralizes the effects of atherogenic lipid peroxides. It indicates the protective effect of cell membranesCitation10,Citation14,Citation15. Therefore, hPON1 exerts an anti-oxidative stress effect through the hydrolysis of lipid peroxides and the prevention of the oxidation of LDL. In addition, the enzyme inhibits the oxidation of HDLCitation16. In this way, paraoxonase contributes to function of reverse cholesterol transport. Thus, the formation of atherosclerosis may be slow down or stopped by preventing the accumulation of cholesterol in macrophagesCitation17.
It is well known that oxidative stress increases in the cardiovascular diseases and some other vital disorders such as diabetes mellitus, chronic renal failure, hyperthyroidism and age-related macular degeneration. Increased oxidative stress causes the formation of reactive oxygen species (ROS) and accordingly leads the decreasing HDL quantitiy in the metabolic system. Thus, it results the decrease in hPON1 activity associated with HDL in the serumCitation18–22. Hence, HDL (good cholesterol) quantitiy in the blood is important. Because, hPON1 serves as an antioxidant enzyme by protecting LDL and HDL from oxidative stressCitation8.
hPON1 enzyme have been purified from various sources using different methods so far. For example, hPON1 from rat liver was purified using hydroxyapatite adsorption, DEAE-Sepharose CL-6B chromatography, Cibacron Blue 3GA non-specific affinity chromatography, anion exchange on Mono Q HR 5/5, DEAE-cellulose and a final affinity chromatography on Concovalin A-Sepharose by Pla and colleaguesCitation23. In a study, hPON1 from human serum was purified with ammonium sulfate fractionation and hydrophobic interaction chromatographyCitation24. Ekinci and Beydemir purified hPON1 with ammonium sulfate precipitation, DEAE-Sephadex anion exchange chromatography and Sepharose 4B-L-tyrozine-1-napthylamine hydrophobic interaction chromatographyCitation25. Isgor and Beydemir also purified hPON1 with ammonium sulphate fractionation (60–80%), DEAE-Sephadex anion exchange chromatography and Sephadex G-200 gel filtration chromatographyCitation26. Particularly some enzymes such as carbonic anhydrase, glucose 6-phosphate dehydrogenase and sorbitol dehydrogenase including paraoxonase are studied as drug target enzymes by scientistCitation25–38.
Because drugs and other chemical compounds such as insecticides, herbicides and even some gases as sarin, soman used chemical weapons show binding to the enzyme structures their effects. Changes in the enzymatic activities in the metabolism can be critical for human life. Particularly deficiency or inhibition of the hPON1 stimulates many metabolic disorders including atherosclerosis as mentioned above. The studies on hPON1-drug interaction are few in the literature. Due to optimize for the enzyme-drug interaction studies of our laboratory equipment, many studies in this subject are performedCitation25–38.
Consequently, PON1 enzyme was purified from human serum by using a simple and rapid procedure. In vitro inhibition effects of moxifloxacin hydrochloride, levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium on this vital enzyme were investigated in this study.
Materials and methods
Materials
The materials used in this study, including DEAE-Sephadex A50, Sepharose 4B, 1-naphthylamine, paraoxon, protein assay reagents and chemicals for electrophoresis, were obtained from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). All of the other chemicals used were of analytical grade and were obtained from either Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany) or Merck KGaA (Darmstadt, Germany). Moxifloxacin hydrochloride, levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium were obtained from local pharmaceutical manufacturing companies. For the enzyme activity assays, we used a Chebios UV–VIS spectrophotometer. The peristaltic pump used for enzyme purification was obtained from Ismatec (ISM833) (IDEX Health & Science, Wertheim, Germany), the centrifuge machine was purchased from Hermle Labotechnic GmbH (Wehingen, Germany) and the electrophoresis system was a BioRad Mini Protean system (Bio-Rad Laboratories GmbH, München, Germany).
Paraoxonase activity assay
Human serum samples were supplied from the Research Hospital at Ataturk University. hPON1 activity was determined at 25 °C with paraoxon (diethyl p-nitrophenyl phosphate) (1 mM) in 50 mM glycine/NaOH (pH 10.5) containing 1 mM CaCl2. hPON1 assay was based on the estimation of p-nitrophenol at 412 nm. The molar extinction coefficient of p-nitrophenol (ɛ = 18.290 M−1cm−1 at pH 10.5) was used to calculate hPON1 activityCitation39. One enzyme unit was defined as the amount of enzyme that catalyses the hydrolysis of 1 µmol of substrate at 25 °CCitation40. Assays were performed using a spectrophotometer (CHEBIOS UV–VIS).
Ammonium sulphate precipitation
Human serum precipitated with 60–80% ammonium sulphate was carried out in our previous studies. The precipitate was obtained after centrifugation at 15 000 × g for 20 min and redissolved in a 100 mM Na-phosphate buffer (pH 7.0)Citation25,Citation26,Citation30–33,Citation35–38.
DEAE-Sephadex A50 anion exchange chromatography
At first, the anion exchange column was equilibrated with a 100 mM Na-phosphate buffer (pH 7.0). Then, the enzyme solution, which had been dialyzed in the presence of 1 mM Na-phosphate buffer (pH 7.0) for 2 h, was loaded onto the DEAE-Sephadex A50 anion exchange column (3 cm× 30 cm). Later, the chromatography column was washed with a 100 mM Na-phosphate buffer (pH 7.0), and then elution was carried out by an increasing linear gradient of 0–1.5 M NaCl. The elution fractions which were collected were checked for enzyme activity at 412 nm. Tubes which displayed the same enzyme activity were combined. All these procedures were performed at 4 °C.
Sephadex G-200 gel filtration chromatography
In the first process, the sephadex G-200 column (60 cm× 2 cm) was equilibrated with a 100 mM Na-phosphate buffer (pH 7.0). The fractions obtained from the DEAE-Sephadex A50 anion exchange column were the mixed with glycerol and loaded onto the gel filtration column with the same buffer. Finally, the enzyme solutions were eluated from the sephadex G-200 column. The protein amount (280 nm) and enzyme activity (412 nm) for all tubes was recorded. The tubes which is showed enzyme activity were combined for other kinetic studies.
Protein determination
In previous studies that were also performed in our laboratory it was found spectrophotometrically at 595 nm according to the Bradford method to quantitative protein assay during the purification stepsCitation25,Citation26,Citation30–33,Citation35–38.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was applied to check that the enzyme was purified according to the Laemmli's procedure as in previous studies which were conducted in our. The obtained single band was photographed after electrophoresisCitation25,Citation26,Citation30–33,Citation35–38.
In vitro drugs studies
We examined the inhibitory effects of five antibacterial drugs: moxifloxacin hydrochloride, levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium. All compounds were tested in triplicate for each concentration used. hPON1 activities were measured in the presence of different drug concentrations. Control activity was assumed to be 100% in the absence of an inhibitor. For each drug, a percent activity versus drug concentration graph was drawn. For determination of Ki values, three different inhibitor concentrations were tested for each drug. In these experiments, paraoxone was used as a substrate at five different concentrations (0.15, 0.30, 0.45, 0.60 and 0.75 mM). Lineweaver-Burk curves were used for determination of Ki and the inhibitor typeCitation41.
Results and discussion
A calcium-dependent enzyme, hPON1, is known as ester hydrolyser for organophosphates (OPs) and it has lactonase activityCitation39,Citation42. hPON1 is a glycosylated protein with a molecular weight of approximately 43 kDaCitation8–10,Citation43.
In recent years, this enzyme has gained importance due to its antioxidant effects. Together with the physiological role of paraoxonase, which is not completely elucidated, hPON1 plays an important protective role against cellular damage induced by toxic agents, such as toxic organophosphatesCitation44. hPON1 is one of the endogenous antioxidants in the human body. There are many endogenous free oxygen radical cleaning systems, including paraoxonases, in the human bodyCitation9. The antioxidant effects of hPON1 have been reported by numerous studies in the literature. hPON1 protects LDL, HDL and macrophages from oxidative stress by cleaning free oxygen radicals in the living metabolism. Thus, hPON1 is critical for cardiovascular diseases such as atherosclerosis and other vascular diseasesCitation25,Citation26,Citation30–32,Citation45,Citation46. The hPON1 activity decreases in cardiovascular diseases, hypercholesterolemia, diabetes, gastric and pancreatic cancer, rheumatoid arthritis, smoking habit, aging, obesity, menopause and acute renal failureCitation47–51.
Due to the physiological importance of this enzyme, many rearchers have recently carried out different studies on the three dimension structure of the hPON1 and the drug-enzyme interactions. For example, statins are the most widely pharmacological molecules and HMG-CoA reductase inhibitors. The drugs of this group play an important role to lower cholesterol levels by inhibiting the enzyme HMG-CoA reductase. Because, high cholesterol levels may result to the cardiovascular diseasesCitation52. The hypocholesterolemic drugs such as spironolactone, mevastatin, lovastatin, pravastatin, prulifloxacin, simvastatin and atorvastatin have a positive effect on hPON1 activity. These drugs prevent the inactivation of hPON1 via their anti-oxidative propertiesCitation53–56. In addition, aspirin, a salicylate drug, is used extensively for the treatment and prevention of vascular disease. It has been reported that aspirin significantly increases hPON1 activity in patients with coronary artery diseaseCitation57. However, valsartan and barnidipine, which are antihypertensive drugs, have not a positive effect on hPON1 activityCitation58,Citation59.
The effects on enzymes of drugs reveals the importance of enzyme studiesCitation60,Citation61. More than ten enzymes are purified and investigated their kinetic properties in our laboratory. Moreover, our research group has studied on enzyme-drug interactions, metal and pesticide toxicity on various enzyme structures and activities including paraoxonase. Given these reports by our laboratory are used by many researchers as a referenceCitation26–38,Citation62–65. For instance, Ekinci and Beydemir (2008) examined the in vitro effects of some analgesic drugs, lornoxicam(1), indomethacin(2), tenoxicam(3), diclofenac sodium(4), ketoprofen(5) and lincomycine(6), on hPON1 activity from human serumCitation25. They founded that lornoxicam inhibits the enzyme activity significantly, compared to the other analgesics. The inhibition order of the drugs determined as 1 > 2 > 3 > 4 > 5 > 6. In another study, the in vitro impacts of some commonly used antibiotics as teicoplanin, rifamycin, tobramycin, ceftriaxone sodium, cefuroxime sodium, ceftazidime, ornidazole and amikacin sulfate were investigated on human serum hPON1 activity. The major inhibitor was teicoplanin and its inhibition type was determined as noncompetitiveCitation32.
In this study, we purified hPON1 from human serum using only a simple procedure, including ammonium sulphate fractionation (60–80%), DEAE-Sephadex anion exchange chromatography and Sephadex G-200 gel filtration chromatography. The enzyme was obtained with a specific activity of 4612.4 EUxmg−1 proteins, with ∼231-fold purification and a yield of 34.2% (). shows a protein band and molecular weight (Mw) of hPON1 as 43 kDa. This result is in agreement with other studiesCitation23–26,Citation30–33,Citation35–38,Citation66. In this study, we investigated the in vitro effects of antibacterial drugs, such as (a) moxifloxacin hydrochloride, (b) levofloxacin hemihidrate, (c) cefepime hydrochloride, (d) cefotaxime sodium and (e) ceftizoxime sodium on hPON1 activity (). It is of crucial importance that the antibacterial drugs are potent inhibitors for human serum hPON1 (). The compounds (a) moxifloxacin hydrochloride, (b) levofloxacin hemihidrate, (c) cefepime hydrochloride, (d) cefotaxime sodium and (e) ceftizoxime sodium have inhibitory effects ( and ). According to the results, the order of inhibitors from the strongest to the weakest is as follows; (a) moxifloxacin hydrochloride >(b) levofloxacin hemihydrate >(c) cefepime hydrochloride >(d) cefotaxime sodium >(e) ceftizoxime sodium. For these drugs, Ki values were determined from the Lineweaver–Burk plots using different paraoxon concentrations ( and ). We found that moxifloxacin hydrochloride showed competitive inhibition, while others inhibited in a noncompetitive manner. Since moxifloxacin hydrochloride inhibit in a competitive manner, it could be considered to have a connection with the amino acids of the active site ().
Figure 1. SDS-PAGE analysis of purified hPON1. Lane (A) is standard proteins (kD): Bovine serum albumin (66.000), aldolase (47.500), triosephosphate isomerase (32.000) and soy bean trypsin inhibitor (24.000). Lane B contains a human serum sample.
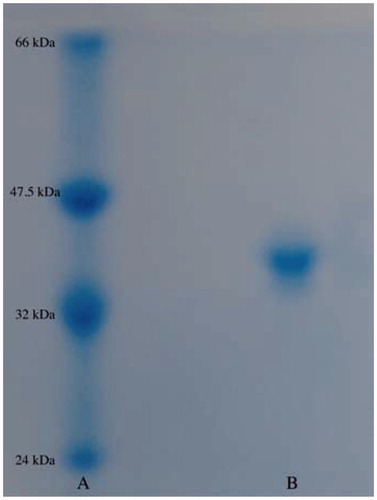
Figure 2. In vitro effect of antibacterial drugs: (a) moxifloxacin hydrochloride, (b) levofloxacin hemihidrate, (c) cefepime hydrochloride, (d) cefotaxime sodium and (e) ceftizoxime sodium at five different concentrations on hPON1 activity.
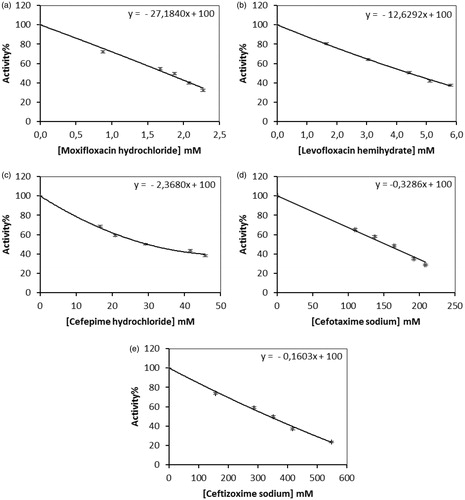
Figure 3. Ki graphs for paraoxonase from human serum. (a), (b), (c) and (d) Lineweaver–Burk graphs in five different substrate (paraoxon) concentrations and three different (a) moxifloxacin hydrochloride, (b) levofloxacin hemihidrate, (c) cefepime hydrochloride, (d) cefotaxime sodium and (e) ceftizoxime sodium concentrations for determination of Ki.
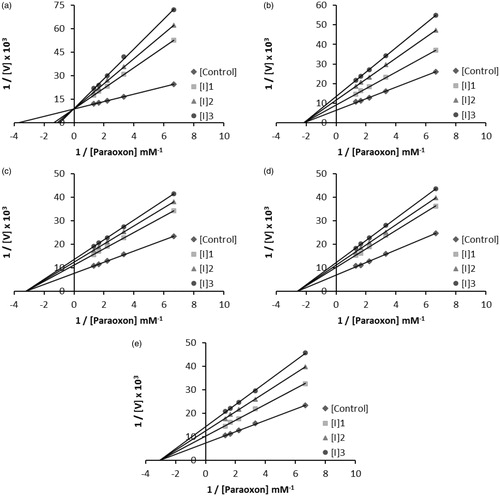
Figure 4. Schematic representation for the interaction of moxifloxacin hydrochloride (a) with the hPON1 active site.
Table 1. Summary of the hPON1 purification procedure.
Table 2. IC50, Ki values and inhibition types.
The functional groups of moxifloxacin hydrochloride may interact with amino acids of the enzyme's active site. Harel and colleagues reported the crystal structure of hPON1 and observed amino acid residues of the active siteCitation67. According to the above information, we have designed a possible scheme for moxifloxacin hydrochloride (). In the crystal structure of the enzyme's active site, His 115 and His 134 bind to catalytic Ca2+. According to our intended model, we found that the calcium ion of hPON1 interact with the chlorine electronegative atom in (a) moxifloxacin hydrochloride. Hydrogen atom of hydroxyl group of (a) moxifloxacin hydrochloride interact with the chlorine electronegative atom as shown in .
It is known that an adult human has approximately 5 l of the blood in total. Accordingly, blood concentrations of (c) cefepime hydrochloride, (d) cefotaxime sodium and (e) ceftizoxime sodium were calculated as 0.175 mM, 0.418 mM and 0.493 mM, respectively. These values are observed under IC50 values. However, the blood concentrations of (a) moxifloxacin hydrochloride and (b) levofloxacin hemihidrate were determined to be 0.183 mM and 0.269 mM, respectively, which are similar this IC50 values. This mechanism can be clarified by in vivo studies.
In conclusion, we purified hPON1 enzyme using three simple purification steps and investigated the in vitro effects of antibacterial drugs on the enzyme activity. Then, the possible drug binding scheme is proposed for moxifloxacin hydrochloride with competitive inhibition. Later, we compared to their blood concentrations with IC50 values of drugs.
Declaration of interest
The authors report no conflicts of interest
References
- Kamruzzamana M, Alam AM, Lee SH, et al. Spectrofluorimetric study of the interaction between europium(III) and moxifloxacin in micellar solution and its analytical application. Spectrochim Acta A 2012;86:375–80
- Cruz LA, Hall R. Enantiomeric purity assay of moxifloxacin hydrochloride by capillary electrophoresis. J Pharmaceut Biomed 2005;38:8–13
- Ocana JA, Callejon M, Barragan FJ. Terbium-sensitized luminescence determination of levofloxacin in tablets and human urine and serum. Analyst 2000;125:1851–4
- Lacy CF, Armstrong LL, Goldman MP, Lance L. Drug information handbook with international trade names index. 18th ed. Lexi-Comp; 2010.22:1134–47
- Cheathama SC, Sheab KM, Healyc DP, et al. Steady-state pharmacokinetics and pharmacodynamics of cefepime administered by prolonged infusion in hospitalised patients. Int J Antimicrob Agents 2011;37:46–50
- Chena D, Wanga H, Zhangb Z, et al. Chemiluminescence determination of cefotaxime sodium with flow-injection analysis of cerium (IV)–rhodamine 6G system and its application to the binding study of cefotaxime sodium to protein with on-line microdialysis sampling. Spectrochim Acta A 2011;78:553–7
- Bharath C, Prasad CS, Bharathi DV, et al. Structural identification and characterization of impurities in ceftizoxime sodium. J Pharm Biomed Anal 2007;43:733–40
- Aviram M, Rosenblat M, Billecke S, et al. Human serum paraoxonase (PON1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radical Bio Med 1999;26:892–04
- Mackness B, Durrington PN, Mackness MI. Human serum paraoxonase. Gen Pharmacol 1998;31:329–36
- Mackness MI, Mackness B, Arrol S, et al. Presence of paraoxonase in human interstitial fluid. FEBS Lett 1997;416:377–80
- Aharoni A, Gaidukov L, Khersonsky O, et al. The evolvability of promiscuous protein functions. Nat Genet 2005;37:73–6
- Khersonsky O, Tawfik DS. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005;44:6371–82
- Ates O, Azizi S, Alp HH, et al. Decreased serum paraoxonase 1 activity and increased homocysteine and malondialdehyde levels in age-related macular degeneration. Tohoku J Exp Med 2009;217:17–22
- Aviram M, Hardak E, Vaya J, et al. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions. Circulation 2000;101:2510–7
- Watson AD, Berliner JA, Hama SY, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidised low densitylipoprotein. J Clin Invest 1995;96:2882–91
- Aviram M, Rosenblat M, Bisgair CL. Paraoxonase inhibits high density lipoprotein (HDL) oxidation and preserves its functions: a possible peroxidative role for paraoxonase. J Clin Invest 1998;101:1581–90
- Ekmekci O, Donma O, Ekmekci H. Paraoxonase. Cerrahpaşa J Med 2004;35:78–82
- Abbott CA, Mackness MI, Kumar S, et al. Serum paraoxonase activity, concentration and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscl Throm Vas Biol 1995;15:1812–18
- Ayub A, Mackness MI, Arrol S, et al. Serum paraoxonase after myocardial infarction. Arterioscl Throm Vas Biol 1999;19:330–5
- Baskol G, Karakucuk S, Oner AO, et al. Serum paraoxonase 1 activity and peroxidation levels in patients with age-related macular degeneration. Ophthalmologica 2006;220:12–16
- Dantoine TF, Depord J, Charmes JP, et al. Decrease of serum paraoxonase activity in chronic renal failure. J Am Soc Nephrol 1998;9:2082–8
- Mackness B, Mackness MI, Arrol S, et al. Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity, concentration in non-insulin dependent diabetes mellitus. Atherosclerosis 1998;139:341–9
- Pla A, Rodrigo L, Hernandez AF, Gil Lopez FO. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem-Biol Interact 2007;167:63–70
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–74
- Ekinci D, Beydemir S. Effect of some analgesics on paraoxonase-1 purified from human serum. J Enzyme Inhib Med Chem 2009;24:1034–9
- Isgor MM, Beydemir S. Some cardiovascular therapeutics inhibit paraoxonase 1 (PON1) from human serum. Eur J Pharmacol 2010;645:135–42
- Beydemir S, Ciftci M, Kufrevioglu OI. Purification and characterization of glucose 6-phosphate dehydrogenase from sheep erythrocytes and inhibitory effects of some antibiotics on enzyme activity. J Enzyme Inhib Med Chem 2002;17:271–7
- Ciftci M, Beydemir S, Ekinci D. Effects of some drugs on enzymatic activity of glucose 6-phosphate dehydrogenase from chicken erythrocytes in vitro. Asian J Chem 2008;20:2189–96
- Ekinci D, Beydemir S, Alim Z. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–7
- Senturk M, Ekinci D, Alici HA, Beydemir S. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: an in vitro and in vivo insight. Pest Biochem Physiol 2011;101:206–11
- Alici HA, Ekinci D, Beydemir S. Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Bioch 2008;41:1384–90
- Ekinci D, Beydemir S. Evaluation of the impacts of antibiotic drugs on PON 1; a major bioscavenger against cardiovascular diseases. Eur J Pharmacol 2009;617:84–9
- Dilek EB, Kufrevioglu OI, Beydemir S. Impacts of some antibiotics on human serum paraoxonase 1 activity. J Enzyme Inhib Med Chem 2013;28:758–64
- Alim Z, Beydemir S. Effects of some anti-neoplastic drugs on sheep liver sorbitol dehydrogenase. Arch Physiol Biochem 2012;118:244–52
- Soyut H. In vitro inhibitory effects of ofloxacin hydrochloride, ampicillin sodium, cefotaxime sodium, and ceftizoxime sodium on purified paraoxonase-1 (hPON1) from human serum. Scholarly J Phys Appl Chem 2013;1:10–16
- Soyut H. In vitro inhibitory effects of clarithromycin, cefepime hydrochloride, acycloviron, and cefazolin sodium on purified paraoxonase-1 from human serum. Int J Appl Chem 2013;1:9–20
- Turkes C, Soyut H, Beydemir S. Inhibition effects of gemcitabine hydrochloride, acyclovir, and 5-fluorouracil on human serum paraoxonase-1 (hPON1): in vitro. Open J Biochem 2013;1:10–15
- Turkes C, Soyut H, Beydemir S. Effect of calcium channel blockers on paraoxonase-1 (PON1) activity and oxidative stress. Pharmacol Rep 2014;66:74–8
- Renault F, Chabriere E, Andrieu JP, et al. Tandem purification of two HDL-associated partner proteins in human plasma, paraoxonase (PON1) and phosphate binding protein (HPBP) using hydroxyapatite chromatograpy. J Chromatogr B 2006;836:15–21
- Mackness MI, Durrington PN. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis 1995;115:243–53
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–66
- Gaidukov L, Tawfik DS. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry 2005;44:11843–54
- Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscl Throm Vas Biol 2001;21:473–80
- La Du BN, Aviram M, Billecke S, et al. On the physiological role(s) of the paraoxonases. Chem Biol Interact 1999;119:379–88
- Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 2004;37:1304–16
- Rozenberg O, Shih SD, Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 2005;181:9–18
- Tomas M, Senti M, Garcia-Faria F, et al. Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscl Throm Vas Biol 2000;20:2113–9
- Mackness B, Durrington PN, Boulton AJM, Hine D. Serum paraoxonase activity in patients with type 1 diabetes compared to healthy controls. Eur Clin Invest 2002;32:259–64
- Akcay MN, Polat MF, Yilmaz I, Akcay G. Serum paraoxonase levels in pancreatic cancer. Hepatogastroenterol 2003;50:225–7
- Akcay MN, Yilmaz I, Polat MF, Akcay G. Serum paraoxonase levels in gastric cancer. Hepatogastroenterol 2003;50:273–5
- Ferretti G, Bacchetti T, Moroni C, et al. Paraoxonase activity in high density lipoproteins: a comparison between healthy and obese females. J Clin Endocrinol Metab 2005;90:1728–33
- Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet 2007;370:1829–39
- Kumar A. Effects of simvastatin on paraoxonase 1 (PON1) activity and oxidative stress. Asian Pac J Trop Med 2010;3:310–14
- Nagila A, Permpongpaiboon T, Tantrarongroj S, et al. Effect of atorvastatin on paraoxonase1 (PON1) and oxidative status. Pharmacol Rep 2009;61:892–8
- Malin R, Laaksonen R, Knuuti J, et al. Paraoxonase genotype modifies the effect of pravastatin on high-density lipoprotein cholesterol. Pharmacogenetics 2001;11:625–33
- Leviev I, James R. Simvastatin increases plasma levels of the antioxidant enzyme paraoxonase by PON1 gene activation. Atheroslerosis 2000;151:41
- Blatter-Garin MC, Kalix B, De PS. Aspirin use is associated with higher serum concentrations of the anti-oxidant enzyme, paraoxonase-1. Diabetologia 2003;46:593–4
- Saisho Y, Komiya N, Hirose H. Effect of valsartan, an angiotensin II receptor blocker, on markers of oxidation and glycation in Japanese type 2 diabetic subjects: blood pressure-independent effect of valsartan. Diabetes Res Clin Pract 2006;74:201–3
- Spirou A, Rizos E, Liberopoulos EN. Effect of barnidipine on blood pressure and serum metabolic parameters in patients with essential hypertension: a pilot study. J Cardiovasc Pharmacol Ther 2006;11:256–61
- Senturk M, Alici HA, Beydemir S, Kufrevioglu OI. In vitro and in vivo effects of some benzodiazepine drugs on human and rabbit erythrocyte carbonic anhydrase enzymes. J Enzyme Inhib Med Chem 2012;27:680–4
- Balaydin H, Soyut H, Ekinci D, et al. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzyme Inhib Med Chem 2012;27:43–50
- Gulcin I, Beydemir S, Coban TA, Ekinci D. The inhibitory effect of dantrolene sodium and propofol on 6-phosphogluconate dehydrogenase from rat erythrocyte. Fresenius Environ Bull 2008;17:1283–7
- Gulcin I, Beydemir S, Coban TA, Ekinci D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: an in vivo and in vitro study. J Enzyme Inhib Med Chem 2008;23:266–70
- Kaya ED, Soyut H, Beydemir S. Carbonic anhydrase activity from the gilthead sea bream (Sparus aurata) liver: the toxicological effects of heavy metals. Environ Toxicol Pharmacol 2013;36:514–21
- Ekinci D, Beydemir S. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–20
- Rodrigo L, Gil F, Herdanez AF, et al. Identification of paraoxonase 3 in rat liver microsomes: purification and biochemical properties. Biochem J 2003;376:261–8
- Harel M, Aharoni AI, Gaidukov L, et al. Structure and evolution of the serum paraoxonase family of detoxifying and antiatherosclerotic enymes. Nat Struct Mol Biol 2004;11:412–19

