Abstract
A new series of indolylhydrazones (6) and indole-based 4-thiazolidinones (7, 8) have been designed, synthesized and screened for in vitro antitubercular activity against Mycobacterium tuberculosis H37Rv. 4-Thiazolidinone derivatives 7g–7j, 8g, 8h and 8j displayed notable antituberculosis (anti-TB) activity showing 99% inhibition at MIC values ranging from 6.25 to 25.0 µg/ml. Compounds 7g, 7h, 7i, 8h and 8j demonstrated anti-TB activity at concentrations 10-fold lower than those cytotoxic for the mammalian cell lines. The indolylhydrazone derivative 6b has also been evaluated for antiproliferative activity against human cancer cell lines at the National Cancer Institute (USA). Compound 6b showed an interesting anticancer profile against different human tumor-derived cell lines at sub-micromolar concentrations with obvious selectivity toward colon cancer cell line COLO 205.
Introduction
Tuberculosis (TB) represents an enduring deadly disease and appears as the second leading cause of infectious disease mortality worldwide, after the acquired immunodeficiency syndrome, caused by the human immunodeficiency virus (HIV). In 2011, WHO reported about 8.7 million new cases of TB (13% co-infected with HIV) with 1.4 million deaths including almost 1 million HIV-negative and 430 000 HIV-positive individualsCitation1. The rise in the incidence is partly due to the poverty and inequity and partly to coinfection with HIV, which greatly increases the risk of progressing new or latent TB infections to active diseases. Incomplete drug treatments in third-world countries fuel the appearance of multi-drug-resistant (MDR) and extensively-drug-resistant (XDR) strains of the pathogenCitation2. In addition, patient non-compliance resulting from the long-term therapy of the infection lasting between 6 and 9 months has contributed to the emergence of MDR-TBCitation3. Therefore, there is an urgent need to develop new antitubercular drugs to combat the spread of TB, particularly its hard-to-kill MDR and latent forms.
The synthetically accessible and pharmacologically active 4-thiazolidinone scaffold has provided the impetus for the discovery of a number of novel antitubercular agents in recent years. An early representative actithiazic acid, (−)-2-(5-carboxypentyl)thiazolidin-4-one (), isolated from the culture broth of a strain of streptomyces shows highly specific in vitro activity against MTBCitation4,Citation5. 2-Arylhydrazono-4-oxo-3-phenyl-5-thiazolidinone acetic acidsCitation6, piperidone based 1,3-thiazolidin-4-onesCitation7, isonicotinyl hydrazide derivatives containing the 4-thiazolidinone nucleusCitation8 and numerous different 2,3-disubstituted or 2,3,5-trisubstituted 4-thiazolidinonesCitation9–11 have been reported to possess appreciable in vitro antitubercular activity. In a recent report by Pathak et al., 3-(4-chlorophenyl)-N-[4-oxo-2-(substitutedphenyl)-1,3-thiazolidin-3-yl]-1H-pyrazole-5-carboxamides have been described as promising antitubercular agents with MIC values in the range of 0.4–4 µg/mlCitation12. Likewise, spirothiazolidinone featured molecules, N-alkyl substituted spirothiazolidinonesCitation13, spirothiazolidinones bearing the imidazo[2,1-b]thiazole residueCitation14 and indole-2-carboxamides with a spirothiazolidinone moietyCitation15, have been found to be effective as growth inhibitors of MTB. Besides, several small synthetic molecules with an indole nucleusCitation16–18 or acyl/aroyl hydrazone moietiesCitation19,Citation20 have been reported to possess antitubercular potential. We have recently reported on the synthesis of novel spirothiazolidinone derivatives of the 5-fluoro-3-phenyl-1H-indole scaffold with promising in vitro antitubercular properties against the Mycobacterium tuberculosis H37Rv strainCitation21. Studies on the identification of the molecular target for indole-2-carboxamides suggest that they may inhibit M. tuberculosis growth by acting on the MmpL3 protein, which belongs to the family of membrane transportersCitation22.
Several studies have been devoted to the antiproliferative activity of hydrazide–hydrazone (–CO–NH–N=CH–) based compounds that have structural similarity to our target indolylhydrazones (6). In an early report, Germain et al. identified a series of acyl hydrazones to be selectively lethal to breast cancer stem cell enriched populationsCitation23. Many acyl/aroyl hydrazones with a variety of heterocyclic spacers were reported for their antiproliferative propertiesCitation24–27. More relevant to the present study was the identification of 5-chloro-3-methyl/phenyl-indole-2-carboxylic acid benzylidenehydrazides as anticancer agents by Zhang et al. (). These compounds were found to be potent inducers of apoptosis and inhibited tubulin polimerization in G2/M phase in breast cancer cell line T47DCitation28. Moreover, some of the indolyhydrazones synthesized as precursors of 4-thiazolidinone derivatives in the present study (6a, 6d–h) were previously patented by Bamaung et al. as angiogenesis inhibitorsCitation29.
Encouraged by the above data and in continuation of an ongoing program aiming at the discovery of new leads with antitubercular activity, here we report the synthesis, structural identification and in vitro antitubercular activity of a new series of 5-fluoro-N-[5-(non)substituted-2-(non)substitutedphenyl-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamides (7a–7l, 8a–8l) and their precursors 5-fluoro-N2-[(non)substitutedbenzylidene]-3-phenyl-1H-indole-2-carbohydrazides (6a–6l) against M. tuberculosis H37Rv. In view of the utility of hydrazide–hydrazone-based compounds for the discovery of novel antiproliferative agents, we also report on the preliminary antitumor screening results of 5-fluoro-N2-(4-methylbenzylidene)-3-phenyl-1H-indole-2-carbohydrazide (6b), selected by the National Cancer Institute (NCI) as a prototype.
Experimental
Chemistry
Melting points were determined in open capillary tubes with a Buchi B-540 melting point apparatus (Büchi Labortechnik AG, Flawil, Switzerland) and are uncorrected. Microanalyses were performed on a Thermo Finnigan Flash EA 1112 elemental analyzer (Thermo Scientific, Waltham, MA). IR spectra were recorded in KBr discs (νmax in cm−1) on a Perkin-Elmer 1600 FTIR and Shimadzu IRAffinity-1 FTIR (Perkin-Elmer, Waltham, MA) spectrophotometer (Shimadzu Corporation, Kyoto, Japan). 1H NMR (DMSO-d6),13C NMR (proton decoupled, DEPT-135) (DMSO-d6) and heteronuclearcorrelation 1H–13C (HSQC, HMBC) (DMSO-d6) spectra were run on Bruker AC 200 (200 MHz; Bruker BioSpin GmbH, Rheinstetten/Karlsruhe, Germany) and VarianUNITYINOVA (500 MHz) instruments. Chemical shifts are reported as δ (ppm) relative to TMS as internal standard and coupling constants (J) are given in hertz (Hz). MS (ESI-) were determined on a Finnigan LCQ Advantage Max mass spectrometer (Thermo Scientific, Waltham, MA). Analytical HPLC was performed on a Shimadzu LC-10AD VP Liquid Chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with Shimadzu SCL-10A VP System Controller, Shimadzu DGU-14A Degasser, Shimadzu CTO-10AS VP Column Oven, Shimadzu SIL-10AD VP Auto Injector and Shimadzu SPD-M10A VP Diode Array Detector set at 304 nm. The data were collected and analyzed by using LC Workstation (Class VP System, Shimadzu). Chromatographic resolutions were carried out at room temperature on a Kromasil 100-5SIL microporous silica column (25 cm × 4.6 mm). Hexane–ethyl acetate (80:20) was used as the mobile phase at a flow rate of 1.2 mL/min. All solvents were HPLC grade (br.: broad, bnz.: benzylidene, ind.: indole, thz.: thiazolidinone).
Ethyl 2-benzyl-2-(4-fluorophenylhydrazono)acetate (3)
To a solution of 1 (0.02 mol) in ethanol (10 mL), water (10 mL) and conc. HCl (6 mL), 7% aqueous NaNO2 solution (10 mL) was added dropwise at 0 °C with stirring. The resulting solution of diazonium salt (2) was poured into a cooled (0 °C) mixture of ethyl 2-benzyl-3-oxo-butanoate (0.02 mol), ethanol (10 mL), water (10 mL) and KOH (5.4 g) while stirring. The resulting mixture was refrigerated overnight. The red oily residue thus obtained was separated, washed with water and used without further purification.
Ethyl 5-fluoro-3-phenyl-1H-indole-2-carboxylate (4)
A solution of 3 (0.02 mol) in conc. HCl (20 mL) was heated under reflux for 4 h. The crude product was filtered off, washed with water until tested neutral to litmus and used without further purification.
5-Fluoro-3-phenyl-1H-indole-2-carbohydrazide (5)
A mixture of 4 (0.02 mol), ethanol (20 mL) and H2NNH2·H2O (98%, 8 mL) was heated under reflux for 6 h. The resulting brown crystals were filtered off and recrystallized from ethanol–chloroform. m.p. 222–225 °C; IR(KBr): νmax 3279 (N–H), 1624 (C=O); 1H NMR (DMSO-d6/200 MHz): δ 4.48 (s, 2H, NH2), 7.10 (td, 1H, J = 9.1, 2.2, H6-ind.), 7.21 (dd, 1H, J = 9.8, 2.0, H4-ind.), 7.34–7.38 (m, 1H, H7-ind.), 7.42–7.61 (m, 5H, 3-C6H5-ind.), 8.86 (s, 1H, CONH), 11.82 (s, 1H, NH); 13C NMR (proton decoupled, DMSO-d6/125 MHz): δ 104.65 (d, J = 23.7, C4-ind.), 112.78 (d, J = 26.1, C6-ind.), 114.24 (d, J = 10.0, C7-ind.), 117.50 (d, J = 4.3, C3-ind.), 127.36 (d, J = 8.7, C3a-ind.), 129.21 (3-C6H5(C4)-ind.), 129.94 (3-C6H5(C3,C5)-ind.), 130.26 (C2-ind.), 130.41 (3-C6H5(C2,C6)-ind.), 132.82 (C7a-ind.), 134.27 (3-C6H5(C1)-ind.), 158.28 (d, J = 232.2, C5-ind.), 162.23 (C=O).
General procedure for the synthesis of 5-fluoro-N2-[(non) substitutedbenzylidene]-3-phenyl-1H-indole-2-carbohydrazides (6a–6l)
A mixture of 5 (0.005 mol) and an appropriate benzaldehyde (0.006 mol) was refluxed in 15 mL abs. ethanol for 3 h. The precipitate obtained was purified either by recrystallization from ethanol–chloroform or by washing with hot ethanol.
5-Fluoro-N2-(4-methylbenzylidene)-3-phenyl-1H-indole-2-carbohydrazide (6b). Brown crystals (91%); m.p. 250–252 °C; IR(KBr): νmax 3314, 3280 (N–H), 1651 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 2.32 (s, 3H, 4-CH3), 7.14 (br. t, 1H, J = 8.3 Hz, H6-ind.), 7.24 (br. d, 2H, J = 4.4 Hz, H3,H5-bnz.), 7.32 (br. d, 2H, J = 9.3 Hz, H4,3-C6H5(H4)-ind.), 7.46–7.56 (m, 7H, H2,H6-bnz. and H7,3-C6H5(H2,H3,H5,H6)-ind.), 8.04 (s, 1H, N=CH), 11.33 (s, 1H, CONH), 12.04 (s, 1H, NH); 13C NMR (HSQC, HMBC) (DMSO-d6/125 MHz): δ 21.70 (4-CH3), 104.85 (d, J = 23.5 Hz, C4-ind.), 113.28 (d, J = 25.1 Hz, C6-ind.), 114.38 (d, J = 10.0 Hz, C7-ind.), 118.67 (C3-ind.), 127.08 (d, C3a-ind.), 127.42 (3-C6H5(C4)-ind.), 127.77 (C2, 6-bnz.), 129.15 (3-C6H5(C3,5)-ind.), 129.95 (C3,5-bnz. and C2-ind.), 130.13 (3-C6H5(C2,6)-ind.), 131.98 (C1-bnz.), 133.01 (C7a-ind.), 134.01 (3-C6H5(C1)-ind.), 140.72 (C4-bnz.), 148.13 (C=N), 158.39 (d, J = 233.9 Hz, C5-ind.), 158.89 (CONH); MS (ESI-) m/z (%): 370 (M−H−, 100). Anal calcd for C23H18FN3O (371.41): C, 74.38; H, 4.88; N, 11.31. Found: C, 74.39; H, 5.09; N, 11.15.
(E)-5-Fluoro-N2-(4-methoxybenzylidene)-3-phenyl-1H-indole-2-carbohydrazide (6c). See RefCitation30.
5-Fluoro-3-phenyl-N2-[4-(trifluoromethyl)benzylidene]-1H-indole-2-carbohydrazide(6i). Yellow needles (92%); m.p. 254–255 °C; IR(KBr): νmax 3270 (N–H), 1638 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 7.15 (td, 1H, J = 8.8; 2.0 Hz, H6-ind.), 7.32–7.53 (m, 7H, H4, H7,3-C6H5-ind.), 7.78 (br. s, 2H, H2,H6-bnz.), 7.90 (br. s, 2H, H3,H5-bnz.), 8.16 (s, 1H, N=CH), 11.61 (s, 1H, CONH), 12.05 (s, 1H, NH). Anal calcd for C23H15F4N3O (425.38): C, 64.74; H, 3.55; N, 9.88. Found: C, 64.44; H, 3.47; N, 9.63.
5-Fluoro-N2-[4-(methoxycarbonyl)benzylidene]-3-phenyl-1H-indole-2-carbohydrazide (6j). Yellow crystals (94%); m.p. 256–258.5 °C; IR(KBr): νmax 3263 (N–H), 1703 (C=O), 1649 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.85 (s, 3H, 4-COOCH3), 7.15 (td, 1H, J = 9.0; 2.0 Hz, H6-ind.), 7.33–7.53 (m, 7H, H4,H7,3-C6H5-ind.), 7.82 (br. s, 2H, H2,H6-bnz.), 8.00 (br. s, 2H, H3,H5-bnz.), 8.14 (s, 1H, N=CH), 11.58 (s, 1H, CONH), 12.07 (s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 52.91 (4-COOCH3), 104.91 (d, J = 23.5 Hz, C4-ind.), 113.31 (C6-ind.), 114.44 (d, J = 8.6 Hz, C7-ind.), 119.13 (C3-ind.), 126.95 (C3a-ind.), 127.48 (3-C6H5(C4)-ind.), 127.90 (C2,6-bnz.), 129.15 (3-C6H5(C3,5)-ind.), 129.57 (C4-bnz. and C2-ind.), 130.26 (C3,5-bnz. and 3-C6H5(C2,6)-ind.), 131.18 (C7a-ind.), 133.18 (3-C6H5(C1)-ind.), 139.10 (C1-bnz.), 146.58 (C=N), 154.96 (CONH), 158.41 (d, J = 233.9 Hz, C5-ind.), 166.50 (4-COOCH3); MS (ESI-) m/z (%): 414 (M−H−, 100). Anal calcd for C24H18FN3O3 (415.42): C, 69.39; H, 4.37; N, 10.12. Found: C, 69.34; H, 4.63; N, 9.76.
N2-(2,6-dichlorobenzylidene)-5-fluoro-3-phenyl-1H-indole-2-carbohydrazide (6k). Yellow needles (89%); m.p. 205–206 °C; IR(KBr): νmax 3309 (N–H), 1673 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 7.15 (br. t, 1H, H6-ind.), 7.30–7.51 (m, 10H, H3-5-bnz. and H4,H7,3-C6H5-ind.), 8.27 (s, 1H, N=CH), 11.71 (s, 1H, CONH), 12.07 (s, 1H, NH). Anal calcd for C22H14Cl2FN3O (426.27): C, 61.99; H, 3.81; N, 9.86. Found: C, 62.12; H, 4.03; N, 9.89.
N2-(2-chloro-6-fluorobenzylidene)-5-fluoro-3-phenyl-1H-indole-2-carbohydrazide (6l). Yellow needles (87%); m.p. 223–225 °C; IR(KBr): νmax 3324, 3169 (N–H), 1654 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 7.15 (br. t, 1H, J = 8.8 Hz, H6-ind.), 7.30–7.52 (m, 10H, H3-5-bnz. and H4,H7,3-C6H5-ind.), 8.32 (s, 1H, N=CH), 11.67 (s, 1H, CONH), 12.05 (s, 1H, NH); MS (ESI-) m/z (%): 410/408 (M−H−, 28/100). Anal calcd for C22H14ClF2N3O (409.82): C, 64.48; H, 3.84; N, 10.25. Found: C, 64.43; H, 4.10; N, 10.16.
General procedure for the synthesis of 5-fluoro-N-[5-(non) substituted-2-(non)substitutedphenyl-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamides (7a–7l, 8a–8l)
A mixture of 6a–6l (0.0025 mol) and mercaptoacetic acid or 2-mercaptopropionic acid (0.01 mol) was refluxed in 30 mL dry benzene for 5–6 h using a Dean-Stark water separator. Excess benzene was evaporated in vacuo. The resulting residue was triturated with saturated NaHCO3 solution until CO2 evolution ceased and was allowed to stand overnight or in some cases refregerated until solidification. The solid thus obtained was washed with water, dried and recrystallized from ethanol or ethanol–chloroform.
5-Fluoro-N-(4-oxo-2-phenyl-1,3-thiazolidin-3-yl)-3-phenyl-1H-indole-2-carboxamide (7a). White powder (62%); m.p. 219–220.5 °C; IR(KBr): νmax 3364 (N–H), 1708, 1678 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.75 (d, 1H, J = 15.6 Hz, H5-thz.), 3.89 (dd, 1H, J = 15.9; 1.5 Hz, H5-thz.), 5.84 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.18 (dd, 1H, J = 9.8; 2.4 Hz, H4-ind.), 7.25 (br. s, 5H, 3-C6H5-ind.), 7.37–7.39 (m, 3H, 2-C6H5(H3-5)-thz.), 7.44–7.48 (m, 3H, 2-C6H5(H2,H6)-thz. and H7-ind.), 9.96 (s, 1H, CONH), 11.84 (s, 1H, NH). Anal calcd for C24H18FN3O2S (431.48): C, 66.81; H, 4.20; N, 9.74. Found: C, 67.15; H, 3.90; N, 9.69.
5-Fluoro-N-[2-(4-methylphenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (7b). White needles (61%); m.p. 221–222 °C; IR(KBr): νmax 3357, 3286 (N–H), 1704, 1676 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 2.32 (s, 3H, 4-CH3), 3.74 (d, 1H, J = 16.1 Hz, H5-thz.), 3.87 (dd, 1H, J = 16.1; 1.5 Hz, H5-thz.), 5.80 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.16–7.19 (m, 3H, 2-C6H5(H3,H5)-thz. and H4-ind.), 7.26 (br. s, 5H, 3-C6H5-ind.), 7.32 (d, 2H, J = 8.3 Hz, 2-C6H5(H2,H6)-thz.), 7.47 (dd, 1H, J = 8.8; 4.4 Hz, H7-ind.), 9.92 (s, 1H, CONH), 11.83 (s, 1H, NH); 13C NMR (HSQC, HMBC) (DMSO-d6/125 MHz): δ 21.49 (4-CH3), 30.02 (C5-thz.), 62.44 (C2-thz.), 104.92 (d, J = 24.0 Hz, C4-ind.), 113.81 (d, J = 26.8 Hz, C6-ind.), 114.62 (d, J = 9.1 Hz, C7-ind.), 119.79 (d, J = 4.8 Hz, C3-ind.), 127.08 (d, J = 10.1 Hz, C3a-ind.), 127.26 (C2/3-C6H5(C4)-ind.), 127.38 (C2/3-C6H5(C4)-ind.), 128.32 (2-C6H5 (C2,6)-thz.), 129.03 (3-C6H5(C3,5)-ind.), 129.83 (2-C6H5(C3,5)-thz.), 130.20 (3-C6H5(C2,6)-ind.), 133.19 (C7a-ind.), 133.37 (3-C6H5(C1)-ind.), 135.83 (2-C6H5(C1)-thz.), 139.11 (2-C6H5(C4)-thz.), 158.36 (d, J = 233.9 Hz, C5-ind.), 160.90 (CONH), 169.65 (CO-thz.); MS (ESI-) m/z (%): 444 (M−H−, 100). Anal calcd for C25H20FN3O2S (445.51): C, 67.40; H, 4.22; N, 9.43. Found: C, 67.24; H, 3.92; N, 9.66.
5-Fluoro-N-[2-(4-methoxyphenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (7c). White powder (65%); m.p. 184–185.5 °C; IR(KBr): νmax 3352, 3292 (N–H), 1697, 1670 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.74 (d, 1H, J = 16.1 Hz, H5-thz.), 3.77 (s, 3H, 4-OCH3), 3.86 (dd, 1H, J = 16.1; 1.5 Hz, H5-thz.), 5.80 (s, 1H, H2-thz.), 6.92 (d, 2H, J = 8.8 Hz, 2-C6H5(H3,H5)-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.18 (dd, 1H, J = 9.8; 2.4 Hz, H4-ind.), 7.26–7.30 (m, 5H, 3-C6H5-ind.), 7.37 (d, 2H, J = 8.8 Hz, 2-C6H5(H2,H6)-thz.), 7.48 (dd, 1H, J = 9.0; 4.4 Hz, H7-ind.), 9.88 (s, 1H, CONH), 11.83 (s, 1H, NH). Anal calcd for C25H20FN3O3S (461.51): C, 65.06; H, 4.37; N, 9.10. Found: C, 65.18; H, 4.32; N, 9.13.
N-[2-(4-chlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (7d). Beige crystals (89%); m.p. 234–236 °C; IR(KBr): νmax 3291 (N–H), 1713, 1658 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.75 (d, 1H, J = 15.6 Hz, H5-thz.), 3.91 (dd, 1H, J = 16.0; 1.8 Hz, H5-thz.), 5.85 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.0; 2.3 Hz, H6-ind.), 7.18 (dd, 1H, J = 9.8; 2.5 Hz, H4-ind.), 7.24–7.27 (m, 5H, 3-C6H5-ind.), 7.43 (d, 2H, J = 8.2 Hz, 2-C6H5(H3,H5)-thz.), 7.46–7.49 (m, 3H, 2-C6H5(H2,H6)-thz. and H7-ind.), 10.01 (s, 1H, CONH), 11.85 (s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 29.94 (C5-thz.), 61.77 (C2-thz.), 104.93 (d, J = 24.0 Hz, C4-ind.), 113.80 (d, J = 26.4 Hz, C6-ind.), 114.61 (d, J = 9.6 Hz, C7-ind.), 119.72 (d, J = 5.3 Hz, C3-ind.), 127.06 (d, J = 10.1 Hz, C3a-ind.), 127.33 (C2/3-C6H5(C4)-ind.), 127.35 (C2/3-C6H5(C4)-ind.),128.98 (3-C6H5(C3,5)-ind.), 129.29 (2-C6H5(C3,5)-thz.), 130.18 (3-C6H5(C2,6)-ind.), 130.24 (2-C6H5(C2,6)-thz.), 133.22 (C7a-ind.), 133.37 (3-C6H5(C1)-ind.), 134.20 (2-C6H5(C1/C4)-thz.), 138.20 (2-C6H5(C1/C4)-thz.), 158.37 (d, J = 233.9 Hz, C5-ind.), 161.01 (CONH), 169.51 (CO-thz.). Anal calcd for C24H17ClFN3O2S (465.93): C, 61.87; H, 3.88; N, 9.02. Found: C, 62.21; H, 4.21; N, 8.92.
N-[2-(4-bromophenyl)-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (7e). Beige powder (84%); m.p. 228–229 °C; IR(KBr): νmax 3295 (N–H), 1713, 1656 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.75 (d, 1H, J = 16.0 Hz, H5-thz.), 3.91 (dd, 1H, J = 16.0; 1.4 Hz, H5-thz.), 5.84 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.2; 2.3 Hz, H6-ind.), 7.18 (dd, 1H, J = 9.6; 2.3 Hz, H4-ind.), 7.26 (br. s, 5H, 3-C6H5-ind.), 7.41 (d, 2H, J = 8.7 Hz, 2-C6H5(H2,H6)-thz.), 7.47 (dd, 1H, J = 9.2; 4.6 Hz, H7-ind.), 7.57 (d, 2H, J = 8.7 Hz, 2-C6H5(H3,H5)-thz.), 10.01 (s, 1H, CONH), 11.85 (s, 1H, NH); MS (ESI-) m/z (%): 510/508 (M-H-, 100/87). Anal calcd for C24H17BrFN3O2S (510.38): C, 56.58; H, 3.56; N, 8.23. Found: C, 56.94; H, 3.79; N, 8.12.
5-Fluoro-N-[2-(4-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (7f). Beige crystals (89%); m.p. 217–219 °C; IR(KBr): νmax 3284 (N–H), 1704, 1657 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.75 (d, 1H, J = 16.0 Hz, H5-thz.), 3.90 (dd, 1H, J = 16.0; 1.8 Hz, H5-thz.), 5.85 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.2; 2.7 Hz, H6-ind.), 7.17–7.20 (m, 3H, 2-C6H5(H3,H5)-thz. and H4-ind.), 7.27 (br. s, 5H, 3-C6H5-ind.), 7.46–7.52 (m, 3H, 2-C6H5 (H2,H6)-thz. and H7-ind.), 9.98 (s, 1H, CONH), 11.84 (s, 1H, NH). Anal calcd for C24H17F2N3O2S (449.47): C, 64.13; H, 3.91; N, 9.35. Found: C, 64.48; H, 4.17; N, 9.18.
N-[2-(4-cyanophenyl)-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (7g). White crystals (80%); m.p. 253–255 °C; IR(KBr): νmax 3299 (N–H), 2231 (C≡N), 1713, 1655 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.77 (d, 1H, J = 15.6 Hz, H5-thz.), 3.95 (dd, 1H, J = 16.0; 1.5 Hz, H5-thz.), 5.93 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.19 (dd, 1H, J = 9.8; 2.4 Hz, H4-ind.), 7.22–7.28 (m, 5H, 3-C6H5-ind.), 7.47 (dd, 1H, J = 9.0; 4.4 Hz, H7-ind.), 7.64 (d, 2H, J = 8.3 Hz, 2-C6H5(H2,H6)-thz.), 7.84 (d, 2H, J = 8.3 Hz, 2-C6H5 (H3,H5)-thz.), 10.11 (s, 1H, CONH), 11.87 (s, 1H, NH); MS (ESI-) m/z (%): 455 (M−H−, 100). Anal calcd for C25H17FN4O2S (456.49): C, 65.78; H, 3.75; N, 12.27. Found: C, 65.83; H, 3.70; N, 12.07.
5-Fluoro-N-[2-(4-nitrophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (7h). Yellow crystals (78%); m.p. 258–260 °C; IR(KBr): νmax 3300 (N–H), 1716, 1654 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.79 (d, 1H, J = 16.1 Hz, H5-thz.), 3.97 (dd, 1H, J = 16.0; 1.5 Hz, H5-thz.), 6.00 (d, 1H, J = 1.0 Hz, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.17–7.23 (m, 4H, H4-ind. and 3-C6H5(H3-5)-ind.), 7.27 (dd, 2H, J = 8.1; 1.5 Hz, 3-C6H5(H2,H6)-ind.), 7.47 (dd, 1H, J = 8.8; 4.4 Hz, H7-ind.), 7.72 (d, 2H, J = 8.8 Hz, 2-C6H5(H2,H6)-thz.), 8.21 (d, 2H, J = 8.8 Hz, 2-C6H5(H3,H5)-thz.), 10.16 (s, 1H, CONH), 11.88 (s, 1H, NH). Anal calcd for C24H17FN4O4S (476.48): C, 60.50; H, 3.60; N, 11.76. Found: C, 60.58; H, 3.62; N, 11.77.
5-Fluoro-N-{4-oxo-2-[4-(trifluoromethyl)phenyl]-1,3-thiazolidin-3-yl}-3-phenyl-1H-indole-2-carboxamide (7i). Beige crystals (73%); m.p. 211–212.5 °C; IR(KBr): νmax 3332, 3176 (N–H), 1726, 1647 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.78 (d, 1H, J = 16.0 Hz, H5-thz.), 3.94 (dd, 1H, J = 16.0; 1.8 Hz, H5-thz.), 5.95 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.1; 2.3 Hz, H6-ind.), 7.17–7.20 (m, 4H, H4,3-C6H5(H3-5)-ind.), 7.24–7.27 (m, 2H, 3-C6H5(H2,H6)-ind.), 7.47 (dd, 1H, J = 9.2; 4.6 Hz, H7-ind.), 7.66 (d, 2H, J = 8.5 Hz, 2-C6H5(H2,H6)-thz.), 7.73 (d, 2H, J = 8.7 Hz, 2-C6H5(H3,H5)-thz.), 10.01 (s, 1H, CONH), 11.88 (s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 29.89 (C5-thz.), 61.65 (C2-thz.), 104.92 (d, J = 24.0 Hz, C4-ind.), 113.79 (d, J = 26.4 Hz, C6-ind.), 114.60 (d, J = 9.6 Hz, C7-ind.), 119.68 (d, J = 5.2 Hz, C3-ind.), 124.83 (d, J = 272.2 Hz, 4-CF3), 126.23 (d, J = 3.8 Hz, 2-C6H5(C3,5)-thz.), 127.02 (d, J = 9.6 Hz, C3a-ind.), 127.23 (C2/3-C6H5(C4)-ind.), 127.41 (C2/3-C6H5(C4)-ind.), 128.88 (2-C6H5 (C2,6)-thz./3-C6H5(C3,5)-ind.), 128.93 (2-C6H5(C2,6)-thz./3-C6H5(C3,5)-ind.), 129.92 (d, J = 31.6 Hz, 2-C6H5(C4)-thz.), 130.13 (3-C6H5(C2,6)-ind.), 133.24 (C7a-ind.), 133.33 (3-C6H5(C1)-ind.), 144,20 (2-C6H5(C1)-thz.), 158.37 (d, J = 233.9 Hz, C5-ind.), 161.25 (CONH), 169.66 (CO-thz.). Anal calcd for C25H17F4N3O2S (499.48): C, 60.12; H, 3.43; N, 8.41. Found: C, 60.34; H, 3.76; N, 8.40.
5-Fluoro-N-{2-[4-(methoxycarbonyl)phenyl]-4-oxo-1,3-thiazolidin-3-yl}-3-phenyl-1H-indole-2-carboxamide (7j). White powder (65%); m.p. 141–143 °C; IR(KBr): νmax 3244 (N–H), 1718, 1695, 1653 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.78 (d, 1H, J = 16.0 Hz, H5-thz.), 3.87 (s, 3H, 4-COOCH3), 3.93 (dd, 1H, J = 15.8; 1.8 Hz, H5-thz.), 5.92 (s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.2; 2.8 Hz, H6-ind.), 7.18 (dd, 1H, J = 9.8; 2.3 Hz, H4-ind.), 7.19–7.27 (m, 5H, 3-C6H5-ind.), 7.46 (dd, 1H, J = 9.2; 4.6 Hz, H7-ind.), 7.59 (d, 2H, J = 8.7 Hz, 2-C6H5(H2,H6)-thz.), 7.94 (d, 2H, J = 8.2 Hz, 2-C6H5(H3,H5)-thz.), 10.08 (s, 1H, CONH), 11.85 (s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 29.91 (C5-thz.), 52.95 (4-COOCH3), 61.87 (C2-thz.), 104.91 (d, J = 23.5 Hz, C4-ind.), 113.79 (d, J = 26.8 Hz, C6-ind.), 114.60 (d, J = 9.6 Hz, C7-ind.), 119.74 (d, J = 4.8 Hz, C3-ind.), 127.03 (d, J = 9.6 Hz, C3a-ind.), 127.31 (C2/3-C6H5(C4)-ind.), 127.34 (C2/3-C6H5(C4)-ind.), 128.51 (2-C6H5(C2,6)-thz.), 128.94 (3-C6H5(C2,6)-ind.), 130.14 (2-C6H5(C3,5)-thz./3-C6H5(C3,5)-ind.), 130.16 (2-C6H5(C3,5)-thz./3-C6H5(C3,5)-ind.), 130.70 (2-C6H5(C4)-thz.), 133.20 (C7a-ind.), 133.34 (3-C6H5(C1)-ind.), 144.59 (2-C6H5(C1)-thz.), 158.36 (d, J = 233.9 Hz, C5-ind.), 161.09 (CONH), 166.60 (4-COOCH3), 169.60 (CO-thz.); MS (ESI-) m/z (%): 488 (M-H-, 100). Anal calcd for C26H20FN3O4S.1/2H2O (498.52): C, 62.64; H, 4.25; N, 8.43. Found: C, 62.88; H, 4.48; N, 8.26.
N-[2-(2,6-dichlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (7k). White needles (67%); m.p. 215–217 °C; IR(KBr): νmax 3298, 3219 (N–H), 1720, 1638 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.87 (d, 1H, J = 16.1 Hz, H5-thz.), 3.91 (dd, 1H, J = 15.9; 2.0 Hz, H5-thz.), 6.73 (d, 1H, J = 1.5 Hz, H2-thz.), 7.12–7.17 (m, 2H, H4,H6-ind.), 7.26–7.36 (m, 5H, 3-C6H5-ind.), 7.42 (t, 1H, J = 8.3 Hz, 2-C6H5(H4)-thz.), 7.47–7.53 (m, 3H, 2-C6H5(H3,H5)-thz. and H7-ind.), 9.97 (s, 1H, CONH), 11.86 (s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 30.91 (C5-thz.), 57.01 (C2-thz.), 104.95 (d, J = 24.0 Hz, C4-ind.), 114.08 (d, J = 26.8 Hz, C6-ind.), 114.72 (d, J = 9.6 Hz, C7-ind.), 120.46 (d, J = 5.3 Hz, C3-ind.), 126.79 (C2-ind.), 127.13 (d, J = 10.7 Hz, C3a-ind.), 127.59 (3-C6H5(C4)-ind.), 129.08 (3-C6H5(C3,5)-ind.), 129.62 (2-C6H5(C3/5)-thz.), 130.21 (3-C6H5(C2,6)-ind.), 131.88 (2-C6H5(C3/5)-thz.), 131.92 (2-C6H5(C4)-thz.), 133.22 (C7a-ind.), 133.48 (3-C6H5(C1)-ind.), 135.71 (2-C6H5(C2,6/C1)-thz.), 135.99 (2-C6H5(C2,6/C1)-thz.), 158.38 (d, J = 233.9 Hz, C5-ind.), 161.41 (CONH), 169.12 (CO-thz.). Anal calcd for C24H16Cl2FN3O2S (500.37): C, 57.61; H, 3.22; N, 8.40. Found: C, 57.53; H, 3.51; N, 8.24.
N-[2-(2-chloro-6-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (7l). White powder (67%); m.p. 174–175.5 °C; IR(KBr): νmax 3290, 3209 (N–H), 1722, 1641 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 3.81 (d, 1H, J = 15.6 Hz, H5-thz.), 3.87 (br. d, 1H, J = 15.1 Hz; H5-thz.), 6.40 (s, 1H, H2-thz.), 7.13 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.18 (br. d, 1H, J = 9.8 Hz, H4-ind.), 7.23–7.38 (m, 7H, 2-C6H5(H3,H5)-thz. and 3-C6H5-ind.), 7.44–7.50 (m, 2H, 2-C6H5(H4)-thz. and H7-ind.), 10.21 (s, 1H, CONH), 11.86 (s, 1H, NH); 13C NMR (HSQC, HMBC) (DMSO-d6/125 MHz): δ 30.05 (C5-thz.), 55.75 (C2-thz.), 104.94 (d, J = 23.5 Hz, C4-ind.), 113.96 (d, J = 26.4 Hz, C6-ind.), 114.69 (d, J = 9.6 Hz, C7-ind.), 116.89 (d, J = 23.0 Hz, 2-C6H5(C5)-thz.), 120.17 (br. d, C3-ind.), 124.78 (d, J = 11.5 Hz, 2-C6H5(C1)-thz.), 126.37 (2-C6H5(C3)-thz.), 127.03 (d, J = 7.2 Hz, C3a-ind.), 127.14 (C2-ind.), 127.49 (3-C6H5(C4)-ind.), 129.04 (3-C6H5(C3,5)-ind.), 130.18 (3-C6H5(C2,6)-ind.), 132.28 (d, J = 10.5 Hz, 2-C6H5(C4)-thz.), 133.23 (C7a-ind.), 133.47 (3-C6H5(C1)-ind.), 134.15 (br. d, 2-C6H5(C2)-thz.), 158.38 (d, J = 233.9 Hz, C5-ind.), 162.43 (d, J = 256.4 Hz, 2-C6H5(C6)-thz.), 168.77 (CO-thz.); MS (ESI-) m/z (%): 484.5/482.5 (M-H-, 40/100). Anal calcd for C24H16ClF2N3O2S.H2O (501.93): C, 57.23; H, 3.22; N, 8.37. Found: C, 56.90; H, 3.40; N, 8.19.
5-Fluoro-N-(5-methyl-4-oxo-2-phenyl-1,3-thiazolidin-3-yl)-3-phenyl-1H-indole-2-carboxamide (8a). White powder (68%); m.p. 233–236 °C; IR(KBr): νmax 3368 (N–H), 1708, 1677 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51 (d, 3H, J = 6.7 Hz, 5-CH3-thz.), 4.05 and 4.14 (q and br. q, 1H, J = 6.8 and J = 6.8 Hz, H5-thz.), 5.82 and 5.85 (2s, 1H, H2-thz.), 7.10–7.19 (m, 2H, H4,H6-ind.); 7.24 (br. s, 5H, 3-C6H5-ind.), 7.39–7.47 (m, 6H, 2-C6H5-thz. and H7-ind.), 10.01 (br. s, 1H, CONH), 11.84 (br. s, 1H, NH). Anal calcd for C25H20FN3O2S (445.51): C, 67.40; H, 4.92; N, 9.43. Found: C, 67.63; H, 5.31; N, 9.51.
5-Fluoro-N-[5-methyl-2-(4-methylphenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (8b). Beige needles (60%); m.p. 206–210 °C; IR(KBr): νmax 3234 (N–H), 1700, 1654 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.50–1.53 (m, 3H, 5-CH3-thz.), 2.32 (s, 3H, 4-CH3), 4.04 and 4.12 (q and qd, 1H, J = 6.8 and J = 6.8; 1.5 Hz, H5-thz.), 5.79 and 5.81 (s and br. s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.15–7.19 (m, 3H, 2-C6H5(H3,H5)-thz. and H4-ind.), 7.24–7.27 (m, 5H, 3-C6H5-ind.), 7.32 (d, 2H, J = 8.0 Hz, 2-C6H5(H2,H6)-thz.), 7.45–7.49 (m, 1H, H7-ind.), 9.94 and 9.98 (s, 1H, CONH), 11.81 and 11.88 (s, 1H, NH); MS (ESI-) m/z (%): 458 (M−H−, 100). Anal calcd for C26H22FN3O2S (459.54): C, 67.96; H, 4.83; N, 9.14. Found: C, 67.89; H, 4.94; N, 8.97.
5-Fluoro-N-[2-(4-methoxyphenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (8c). White powder (70%); m.p. 188–189 °C; IR(KBr): νmax 3293 (N–H), 1714, 1639 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.50–1.53 (m, 3H, 5-CH3-thz.), 3.78 (s, 3H, 4-OCH3), 4.03 and 4.11 (q and qd, 1H, J = 6.8 and J = 7.1; 1.5 Hz, H5-thz.), 5.78 and 5.81 (2s, 1H, H2-thz.), 6.91–6.94 (m, 2H, 2-C6H5(H3,H5)-thz.), 7.12 (td, 1H, J = 9.0; 2.4 Hz, H6-ind.), 7.15–7.19 (m, 1H, H4-ind.), 7.23–7.29 (m, 5H, 3-C6H5-ind.), 7.36 (d, 2H, J = 8.8 Hz, 2-C6H5(H2,H6)-thz.), 7.45–7.49 (m, 1H, H7-ind.), 9.90 and 9.94 (2s, 1H, CONH), 11.81 and 11.88 (2s, 1H, NH); 13C NMR (HSQC, HMBC) (DMSO-d6/125 MHz): δ 19.97, 20.71 (5-CH3-thz.), 38.93, 39.25 (C5-thz.), 55.94 (4-OCH3), 60.95, 61.21 (C2-thz.), 104.92 (d, J = 24.0 Hz, C4-ind.), 113.77, 113.84 (2d, J = 26.8, J = 26.4 Hz, C6-ind.), 114.55, 114.69 (2-C6H5(C3,5)-thz.), 114.62 (d, J = 8.1 Hz, C7-ind.), 119.58, 119.82 (2d, C3-ind.), 127.05 (d, C3a-ind.), 127.09, 127.19 (3-C6H5(C4)-ind.), 127.38, 127.41 (C2-ind.), 129.05 (3-C6H5(C3,5)-ind.), 129.66, 129.84 (2-C6H5(C2,6)-thz.), 130.19, 130.22 (3-C6H5(C2,6)-ind.), 130.47 (2-C6H5(C1)-thz.), 133.17 (C7a-ind.), 133.36 (3-C6H5(C1)-ind.), 158.35 (d, J = 233.9 Hz, C5-ind.), 160.46, 160.59 (2-C6H5(C4)-thz.), 160.88, 160.94 (CONH), 172.39, 172.43 (CO-thz.). Anal calcd for C26H22FN3O3S (475.54): C, 65.57; H, 4.66; N, 8.54. Found: C, 65.23; H, 4.83; N, 8.21.
N-[2-(4-chlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (8d). White needles (71%); m.p. 211–214 °C; IR(KBr): νmax 3245 (N–H), 1700, 1655 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51 and 1.52 (2d, 3H, J = 6.8 Hz, 5-CH3-thz.), 4.06 and 4.16 (q and qd, 1H, J = 6.8 and J = 7.1; 1.5 Hz, H5-thz.), 5.83 and 5.86 (s and d, 1H, J = 1.5 Hz, H2-thz.), 7.12 (td, 1H, J = 9.0; 2.4 Hz, H6-ind.), 7.16–7.20 (m, 1H, H4-ind.), 7.22–7.26 (m, 5H, 3-C6H5-ind.), 7.41–7.49 (m, 5H, 2-C6H5-thz. and H7-ind.), 10.03 (br. s, 1H, CONH), 11.84 and 11.91 (2s, 1H, NH). Anal calcd for C25H19ClFN3O2S (479.95): C, 62.56; H, 3.99; N, 8.76. Found: C, 62.75; H, 4.21; N, 8.85.
N-[2-(4-bromophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (8e). Beige powder (57%); m.p. 198–200 °C; IR(KBr): νmax 3244 (N–H), 1700, 1654 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51 and 1.52 (2d, 3H, J = 6.8 Hz, 5-CH3-thz.), 4.06 and 4.15 (q and qd, 1H, J = 6.8 and J = 6.8; 1.5 Hz, H5-thz.), 5.82 and 5.84 (s and d, 1H, J = 1.5 Hz, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.15–7.20 (m, 1H, H4-ind.), 7.23–7.26 (m, 5H, 3-C6H5-ind.), 7.39 (d, 2H, J = 8.8 Hz, 2-C6H5(H2,H6)-thz.), 7.45–7.48 (m, 1H, H7-ind.), 7.54–7.58 (m, 2H, 2-C6H5(H3,H5)-thz.), 10.06 (br. s, 1H, CONH), 11.86 (br. s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 20.20, 20.53 (5-CH3-thz.), 38.81, 39.33 (C5-thz.), 60.49, 60.82 (C2-thz.), 104.91 (d, J = 23.5 Hz, C4-ind.), 113.81, 113.85 (2d, J = 26.4 Hz, C6-ind.), 114.62 (d, J = 9.6 Hz, C7-ind.), 119.76 (d, J = 4.8 Hz, C3-ind.), 122.70, 122.93 (2-C6H5(C4)-thz.), 127.11 (d, J = 9.6 Hz, C3a-ind.), 127.25, 127.29 (3-C6H5(C4)-ind.), 127.31, 127.49 (C2-ind.), 128.97 (3-C6H5(C3,5)-ind.), 130.16, 130.18 (3-C6H5(C2,6)-ind.), 130.44, 130.77 (2-C6H5(C2,6)-thz.), 132.20, 132.27 (2-C6H5(C3,5)-thz.), 133.22 (C7a-ind.), 133.34 (3-C6H5(C1)-ind.), 137.93, 138.70 (2-C6H5(C1)-thz.), 158.35 (d, J = 233.9 Hz, C5-ind.), 161.10, 161.14 (CONH), 172.34, 172.46 (CO-thz.); MS (ESI-) m/z (%): 524/522 (M−H−, 100/92). Anal calcd for C25H19BrFN3O2S (524.41): C, 57.26; H, 3.95; N, 8.01. Found: C, 57.22; H, 4.22; N, 8.30.
5-Fluoro-N-[2-(4-fluorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (8f). Beige powder (67%); m.p. 219–220 °C; IR(KBr): νmax 3242 (N–H), 1700, 1661 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51 and 1.52 (2d, 3H, J = 6.8 Hz, 5-CH3-thz.), 4.05 and 4.15 (q and qd, 1H, J = 6.8 and J = 7.3; 1.5 Hz, H5-thz.), 5.83 and 5.85 (s and d, 1H, J = 1.5 Hz, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.16–7.18 (m, 1H, H4-ind.), 7.20 (d, 2H, J = 8.8 Hz, 2-C6H5(H3,H5)-thz.), 7.23–7.26 (m, 5H, 3-C6H5-ind.), 7.48–7.52 (m, 3H, 2-C6H5(H2,H6)-thz. and H7-ind.), 9.99 and 10.03 (2s, 1H, CONH), 11.83 and 11.90 (2s, 1H, NH). Anal calcd for C25H19F2N3O2S (463.50): C, 64.88; H, 4.13; N, 9.07. Found: C, 65.05; H, 4.47; N, 8.97.
N-[2-(4-cyanophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (8g). White powder (73%); m.p. 199–200 °C; IR(KBr): νmax 3314 (N–H), 2228 (C≡N), 1716, 1636 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51 and 1.52 (2d, 3H, J = 6.8 Hz, 5-CH3-thz.), 4.09 and 4.20 (q and qd, 1H, J = 6.8 and J = 7.1; 1.5 Hz, H5-thz.), 5.92 and 5.93 (s and d, 1H, J = 1.5 Hz, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.16–7.27 (m, 6H, 3-C6H5,H4-ind.), 7.44–7.48 (m, 1H, H7-ind.), 7.62 (d, 2H, J = 8.3 Hz, 2-C6H5(H2,H6)-thz.), 7.82–7.85 (m, 2H, 2-C6H5(H3,H5)-thz.), 10.13 and 10.17 (2s, 1H, CONH), 11.86 and 11.93 (2s, 1H, NH); 13C NMR (HSQC,DEPT) (DMSO-d6/125 MHz): δ 20.34, 20.45 (5-CH3-thz.), 38.69, 39.44 (C5-thz.), 60.30, 60.68 (C2-thz.), 104.93 (d, J = 24.0 Hz, C4-ind.), 112.04, 112.25 (2-C6H5(C4)-thz.), 113.76 (d, J = 26.8 Hz, C6-ind.), 114.56 (d, J = 10.1 Hz, C7-ind.), 119.23 (4-CN), 119.58, 119.75 (2d, J = 5.0 Hz, C3-ind.), 127.04 (d, C3a-ind.), 127.24, 127.30 (3-C6H5(C4)-ind.), 127.50 (C2-ind.), 128.91, 128.98 (3-C6H5(C3,5)-ind.), 129.27 (2-C6H5(C2,6)-thz.), 130.13 (3-C6H5(C2,6)-ind.), 133.24 (C7a-ind./3-C6H5(C1)-ind.), 133.27, 133.33 (2-C6H5(C3,5)-thz.), 144.41, 145.12 (2-C6H5(C1)-thz.), 158.36 (d, J = 234.4 Hz, C5-ind.), 161.26 (CONH), 172.37, 172.56 (CO-thz.); MS (ESI-) m/z (%): 469 (M−H−, 100). Anal calcd for C26H19FN4O2S (470.52): C, 66.37; H, 4.07; N, 11.81. Found: C, 66.35; H, 4.37; N, 11.53.
5-Fluoro-N-[5-methyl-2-(4-nitrophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-phenyl-1H-indole-2-carboxamide (8h). Yellow crystals (66%); m.p. 193–195 °C; IR(KBr): νmax 3285 (N–H), 1716, 1638 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.52 and 1.53 (2d, 3H, J = 6.8 Hz, 5-CH3-thz.), 4.11 and 4.21 (q and qd, 1H, J = 6.8 and J = 7.1; 1.5 Hz, H5-thz.), 5.98 and 5.99 (s and d, 1H, J = 1.0 Hz, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.15–7.27 (m, 6H, 3-C6H5,H4-ind.), 7.44–7.48 (m, 1H, H7-ind.), 7.71 (d, 2H, J = 8.3 Hz, 2-C6H5(H2,H6)-thz.), 8.18–8.22 (m, 2H, 2-C6H5(H3,H5)-thz.), 10.16 and 10.20 (2s, 1H, CONH), 11.85 and 11.93 (2s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 20.32, 20.44 (5-CH3-thz.), 38.72, 39.48 (C5-thz.), 60.00, 60.38 (C2-thz.), 104.92 (d, J = 23.5 Hz, C4-ind.), 113.76, 113.83 (2d, J = 26.8, J = 26.4 Hz, C6-ind.), 114.55, 114.60 (2d, J = 9.6 Hz, C7-ind.), 119.61, 119.74 (2d, J = 4.8 Hz, C3-ind.), 124.42, 124.50 (2-C6H5(C3,5)-thz.), 126.98, 127.08 (2d, J = 9.6 Hz, C3a-ind.), 127.19, 127.24 (3-C6H5(C4)-ind.), 127.31, 127.50 (C2-ind.), 128.88, 128.89 (3-C6H5(C3,5)-ind.), 129.37, 129.66 (2-C6H5(C2,6)-thz.), 130.15, 130.18 (3-C6H5(C2,6)-ind.), 133.23, 133.24 (C7a-ind.), 133.30, 133.33 (3-C6H5(C1)-ind.), 146.36, 147.09 (2-C6H5(C1)-thz.), 148,30, 148.43 (2-C6H5(C4)-thz.), 158.36 (d, J = 233.9 Hz, C5-ind.), 161.27 (CONH), 172.32, 172.53 (CO-thz.). Anal calcd for C25H19FN4O4S.1/2H2O (499.51): C, 60.11; H, 4.08; N, 11.22. Found: C, 60.29; H, 4.25; N, 11.25.
5-Fluoro-N-{5-methyl-4-oxo-2-[4-(trifluoromethyl)phenyl]-1,3-thiazolidin-3-yl}-3-phenyl-1H-indole-2-carboxamide (8i). White needles (53%); m.p. 217–219 °C; IR(KBr): νmax 3303 (N–H), 1716, 1636 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51–1.54 (m, 3H, 5-CH3-thz.), 4.09 and 4.18 (q and qd, 1H, J = 6.8 and J = 6.8; 1.5 Hz, H5-thz.), 5.94 and 5.96 (s and d, 1H, J = 1.5 Hz, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.14–7.20 (m, 4H, 3-C6H5(H3-5),H4-ind.), 7.22–7.25 (m, 2H, 3-C6H5(H2,H6)-ind.), 7.44–7.48 (m, 1H, H7-ind.), 7.65 (d, 2H, J = 7.8 Hz, 2-C6H5(H2,H6)-thz.), 7.71–7.74 (m, 2H, 2-C6H5(H3,H5)-thz.), 10.15 (br. s, 1H, CONH), 11.93 (br. s, 1H, NH). Anal calcd for C26H19F4N3O2S (513.51): C, 60.81; H, 3.93; N, 8.18. Found: C, 60.70; H, 4.25; N, 8.22.
5-Fluoro-N-{2-[4-(methoxycarbonyl)phenyl]-5-methyl-4-oxo-1,3-thiazolidin-3-yl}-3-phenyl-1H-indole-2-carboxamide (8j). White needles (72%); m.p. 129–131 °C; IR(KBr): νmax 3300 (N–H), 1713, 1672, 1638 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51 and 1.52 (2d, 3H, J = 6.8 Hz, 5-CH3-thz.), 3.87 (s, 3H, 4-COOCH3), 4.08 and 4.18 (q and qd, 1H, J = 6.8 and J = 6.8; 1.5 Hz, H5-thz.), 5.91 and 5.92 (s and br. s, 1H, H2-thz.), 7.12 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.15–7.26 (m, 6H, 3-C6H5,H4-ind.), 7.44–7.48 (m, 1H, H7-ind.), 7.58 (d, 2H, J = 8.3 Hz, 2-C6H5(H2,H6)-thz.), 7.92–7.96 (m, 2H, 2-C6H5(H3,H5)-thz.), 10.11 (br. s, 1H, CONH), 11.83 and 11.90 (2s, 1H, NH); MS (ESI-) m/z (%): 502 (M−H−, 100). Anal calcd for C27H22FN3O4S.1/2H2O (512.55): C, 63.27; H, 4.54; N, 8.20. Found: C, 63.15; H, 4.73; N, 8.08.
N-[2-(2,6-dichlorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (8k). White powder (78%); m.p. 205.5–207 °C; IR(KBr): νmax 3295, 3212 (N–H), 1715, 1639 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.50 (d, 3H, J = 7.3 Hz, 5-CH3-thz.), 4.17 (qd, 1H, J = 7.1; 2.0 Hz, H5-thz.), 6.69 (d, 1H, J = 2.0 Hz, H2-thz.), 7.14 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.17 (dd, 1H, J = 9.2; 2.4 Hz, H4-ind.), 7.23–7.31 (m, 3H, 3-C6H5(H3-5)-ind.), 7.35 (dd, 2H, J = 8.1; 2.0 Hz, 3-C6H5(H2,H6)-ind.), 7.42 (t, 1H, J = 8.3 Hz, 2-C6H5(H4)-thz.), 7.46–7.50 (m, 2H, 2-C6H5(H3/H5)-thz. and H7-ind.), 7.53 (dd, 1H, J = 8.1; 1.5 Hz; 2-C6H5(H3/H5)-thz.), 10.11 (s, 1H, CONH), 11.91 (s, 1H, NH); 13C NMR (HSQC) (DMSO-d6/125 MHz): δ 20.70 (5-CH3-thz.), 39.76 (C5-thz.), 55.79 (C2-thz.), 104.96 (d, J = 24.0 Hz, C4-ind.), 114.02 (d, J = 26.8 Hz, C6-ind.), 114.67 (d, J = 9.6 Hz, C7-ind.), 120.36 (d, J = 5.8 Hz, C3-ind.), 126.90 (C2-ind.), 127.34 (d, J = 9.6 Hz, C3a-ind.), 127.52 (3-C6H5(C4)ind.), 129.02 (3-C6H5(C3,5)-ind.), 129.64 (2-C6H5(C3/5)-thz.), 130.20 (3-C6H5(C2,6)-ind.), 131.88 (2-C6H5(C3/5)-thz.), 131.93 (2-C6H5(C4)-thz.), 133.23 (C7a/3-C6H5(C1)-ind.), 133.50 (C7a/3-C6H5(C1)-ind.), 135.50 (2-C6H5(C2,6/C1)-thz.), 136.06 (2-C6H5(C2,6/C1)-thz.), 158.37 (d, J = 233.9 Hz, C5-ind.), 161.50 (CONH), 171.96 (CO-thz.). Anal calcd for C25H18Cl2FN3O2S (514.40): C, 58.37; H, 3.53; N, 8.17. Found: C, 58.38; H, 3.43; N, 8.01.
N-[2-(2-chloro-6-fluorophenyl)-5-methyl-4-oxo-1,3-thiazolidin-3-yl]-5-fluoro-3-phenyl-1H-indole-2-carboxamide (8l). White powder (74%); m.p. 234–236 °C; IR(KBr): νmax 3287, 3214 (N–H), 1716, 1642 (C=O); 1H NMR (DMSO-d6/500 MHz): δ 1.51–1.53 (m, 3H, 5-CH3-thz.), 4.14–4.17 (m, 1H, H5-thz.), 6.37 and 6.41 (s and br. s, 1H, H2-thz.), 7.13 (td, 1H, J = 9.3; 2.4 Hz, H6-ind.), 7.18 (br. d, 1H, J = 9.8 Hz, H4-ind.), 7.24–7.38 (m, 7H, 2-C6H5(H3,H5)-thz. and 3-C6H5-ind.), 7.44–7.49 (m, 2H, 2-C6H5(H4)-thz. ve H7-ind.), 10.23 and 10.29 (2s, 1H, CONH), 11.87 and 11.90 (2s, 1H, NH); MS (ESI-) m/z (%): 498.5/496.5 (M−H−, 45/100). Anal calcd for C25H18ClF2N3O2S (497.95): C, 60.30; H, 3.84; N, 8.44. Found: C, 60.14; H, 4.08; N, 8.50.
Biological assays
BACTEC 460TB assay
The new compounds were evaluated for in vitro antitubercular activity against M. tuberculosis H37Rv (ATCC 27294) by the BACTEC 460TB (Becton Dickinson, Sparks, MD) system. This rapid and reliable radiometric method is based on the fact that mycobacteria metabolize fatty acids to CO2. If these fatty acids are radioactively labeled with 14C, the 14CO2 end product can be quantitatively determined by the Bactec instrument.
All test compounds were dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 4000 µg/ml and kept at −20 °C until used. The final concentrations were prepared with 7H9 broth medium for mycobacteria. The final DMSO concentration was adjusted to 1% and the final concentration of the tested compounds was adjusted to 25 µg/ml in the BACTEC 12B medium. Inoculum for susceptibility testing was used from a positive BACTEC isolation vial with a growth index (GI) of 500 or more. The BACTEC culture was well mixed and 0.1 ml of culture was added to each of the vials containing the test compounds (25 µg/ml). A control vial was inoculated with a 1:100 dilution of the culture. The standard vials contained rifampicin (0.25 µg/ml). Vials were incubated at 37 °C and each vial was checked daily with a BACTEC 460 instrument. When the control GI reading was at least 30, the results were interpreted by calculating the increase in GI from the previous day. If the daily increase of GI of the control was greater than the daily GI increase of the test compound vial, the mycobacteria were reported as susceptible. If the daily increase of GI of the control was equal to or less than that of the test compound vial, the organisms were reported as resistant. The compounds demonstrating antitubercular activity at 25 µg/ml were further tested at twofold dilutions to determine the actual MICCitation31–33.
MTT proliferation assay
The cytotoxicity of the active compounds was determined by the MTT assay using the rat kidney epithelial cell line (NRK-52E)Citation34,Citation35. The cell line was obtained from American Type Culture Collection (ATCC, USA). Cells were incubated in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and antibiotics (1% penicillin, 1% streptomycin) in 95% O2/5% CO2 at 37 °C. Culture medium was changed every 2 days. The monolayer cells grown to 75–85% confluency were detached with trypsin-ethylenediaminetetraacetic acid to make single cell suspensions. Viable cells were determined using the trypan blue exclusion test and diluted with medium to give a final density of 105 cells/ml. The passage number range for the cell line was maintained between 20 and 25. One hundred microliters of cell suspension was seeded into 96-well plate at a plating density of 104 cells/well and incubated to allow for cell attachment at 37 °C in a humidified 5% CO2 atmosphere for 24 h. After 24 h, the cells were treated with serial concentrations of the test compounds. Test compounds were initially dissolved in DMSO) and further diluted in distilled water to produce the end concentrations. Ten microliters of each concentration was added to plates to obtain final concentrations of 8–250 µg/ml. The final volume in each well was 100 µl and the plates were incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. Each concentration was tested in triplicate and each test was repeated. The medium containing no sample (growth control) and 0.5% DMSO (solvent control) served as the controls. There was no difference in the cell count between the solvent and growth controls.
The growth inhibitory activities of the tested compounds were determined using the standard colorometric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Twenty microliters of MTT solution (5 mg/mL in phosphate buffered saline) was added to each well and incubated at 37 °C for 4 h. The medium with MTT was removed, the formed formazan crystals were solubilized in 100 µl of DMSO and the absorbance was measured at 570 nm Epoch microplate spectrophotometer system (BioTek Instruments, Winooski, VT). The percentage cell viability was calculated with respect to the solvent control as follows:
Anticancer screening
Primary anticancer assay was performed in accordance with the protocol of the Drug Evaluation Branch of the National Cancer Institute (Bethesda, MD). The procedure utilized basicly measured the cellular protein content of cultures stained with the protein dye sulforhodamine B spectrophotometricallyCitation36.
Results and discussion
Chemistry
The synthetic approach utilized for the target compounds is outlined in . The key intermediate 5 and indolylhydrazones 6a and 6d–6h were previously synthesized and patented by Bamaung et al. as angiogenesis inhibitorsCitation29.
The structures of the new compounds were characterized by microanalysis, IR, 1H NMR, 13C NMR (proton decoupled and DEPT-135), 2D NMR (HSQC and HMBC), electrospray ionization mass spectrometry (ESI-MS) and HPLC studies. The absolute stereochemistry of 6c was determined by an X-ray diffraction studyCitation30. The absence of the N–H2 resonance of the intermediate hydrazide (5) at δ 4.48 ppm together with the new signals of the N=CH protons at δ 8.00–8.32 ppm in the 1H NMR spectra of 6 supported the structure of the new adducts. Observation of new lactam C=O bands (1697–1726 cm−1) besides C=O amide bands (1636–1678 cm−1) in the IR spectra of 7 and 8 proved the aimed cyclization. The 4-thiazolidinone C2-H of 7 was observed at about δ 5.80–6.73 ppm as a singlet or a doublet (J = 1.0–1.5 Hz) and the C5-H2 resonated at about δ 3.74–3.97 ppm as a double doublet with coupling constants in the range of 15.6–16.1 and 1.4–2.0 Hz. Large couplings about 15 Hz characteristic of geminal interactions supported the chiral nature of the 4-thiazolidinone C2 carbon and small splittings indicated a long-range interaction between the cis C2-H and C5-H protons of the rigid 4-thiazolidinone system. These four-bond couplings (W-coupling) across the C2-H and C5-H protons were also observed in the 1H NMR spectra of 8 with coupling constants of about 1.0–1.5 Hz. Peaks associated with the indole subunit were observed in the expected regions and were assigned on the basis of 1H–1H and 1H–19F couplings.
13C NMR experiments (proton decoupled and DEPT-135) run on 5, 8g and 2D NMR experiments (HSQC and HMBC) run on 6b, 6j, 7b, 7d, 7i–7l, 8c, 8e, 8g, 8h, 8k allowed unambiguous assingment of the proton and carbon chemical shifts. The carbocyclic indole carbons which explicitly showed the 13C–19F couplings of the 5-fluoro indole core were observed as separate doublets with characteristic coupling constants related to the ipso, ortho, meta and para positions and allowed definite positional assignment of the C3, C3a, C4–7 and C7a carbons of 6–8. The transformation of 6 to 7 or 8 proceeds via the nucleophilic addition of the SH function to the C=N double bond. Upfield shifts observed in the 13C NMR resonances of the C=N carbons of 6b and 6g (δ 148.13 and 146.58 ppm) supported the expected addition as the resulting typical sp3 hybridized 4-thiazolidinone C5 absorbed at δ 29.89–39.76 ppm. Resonances assigned to the 4-thiazolidinone C2 (δ 55.75–62.44 ppm) and endocyclic C=O (δ 168.77–172.56 ppm) further confirmed the aimed conversion. Cross peaks observed between the 4-thiazolidinone 5-CH3, C5-H protons and the lactam C=O in the HMBC spectrum of 7a, 7l and 8c enabled definite assignment of the lactam C=O (δ 168.77–172.56 ppm) and the CONH (δ 160.88–161.50 ppm) carbons.
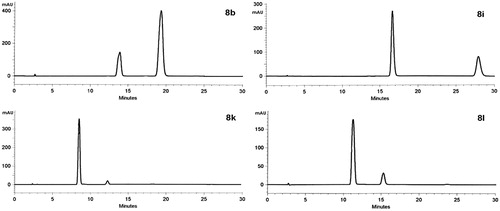
The 1H NMR spectra of 8 displayed two sets of signals for most of the protons, except for the 2,6-dichloro substituted derivative, 8k. The 4-thiazolidinone C2-H protons resonated as a singlet and a broad singlet/doublet and C5-H protons resonated as a quartet and a doublet of quartets. Indole N–H and CONH protons were observed as two separate singlets or one broad singlet. Most of the proton resonances appeared as duplicate signals or distorted multiplets (see Experimental section for details). The novel 2,3,5-trisubstituted-4-thiazolidinones (8a–8l) bear chiral C-2 and C-5 atoms and were possibly obtained as mixtures of cis and trans diastereomers. The stereoisomeric content and the purity of selected entries 8b, 8i, 8k and 8e were confirmed by analytical HPLC studies. A simple and rapid isocratic HPLC method was developed for the determination of the diastereomers of compounds 8b, 8i, 8k and 8l chosen as examples. The resolution of the diastereomers was achieved by normal-phase HPLC on a conventional silica column (Kromasil 100-5SIL microporous silica column) with hexane–ethyl acetate (80:20) as the mobil phase (). The retention times and peak-area percentages are presented in . Compound 8k, which did not display duplicate signals in 1H NMR and 13C NMR spectra, displayed two peaks on the chromatogram in an approximate ratio of 95:5 with a large excess of one of the diastereomers. The 5-unsubstituted derivatives 7b, 7i, 7k and 7l having a single chiral center at C-2 were also analyzed for comparison. As expected, the chromatograms showed only single peaks (for representative NMR spectra, see Supplementary materials).
Figure 2. Normal-phase HPLC diastereomeric resolution of compounds 8b, 8i, 8k and 8l. Column: Kromasil 100-5SIL microporous silica column (25 cm × 4.6 mm); eluent: hexane–ethyl acetate (80:20); flow rate:1.2 mL/min; detection: 304 nm.
Scheme 1. Synthesis of 6–8. Reagents and conditions: (i) 7% NaNO2, EtOH, conc. HCl, 0 °C; (ii) ethyl 2-benzyl-3-oxo-butanoate, KOH, EtOH, 0 °C; (iii) conc. HCl, reflux, 4 h; (iv) H2NNH2·H2O, EtOH, reflux, 6 h; (v) (non)substituted benzaldehyde, abs. EtOH, reflux, 5–6 h; (vi) mercaptoacetic acid, dry benzene, reflux, 5–6 h and (vii) 2-mercaptopropionic acid, dry benzene, reflux, 5–6 h.
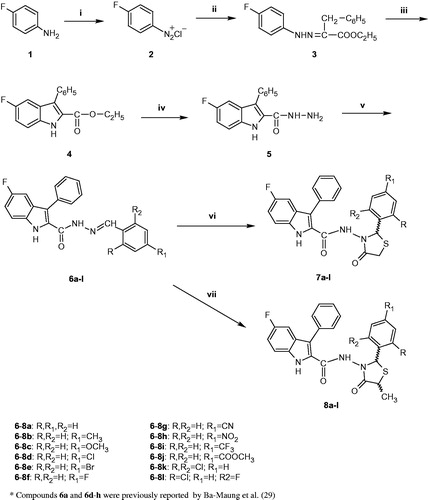
Table 1. Retention times and peak area percentages of compounds 7–8b, 8i, 8k, 8l in NP-HPLC.a
ESI-MS was used to verify the molecular weights of compounds 6b, 6j, 6l, 7–8b, e, g, j and l. All compounds were analyzed under negative-ion ESI conditions since none of the compounds were responsive to the positive-ion mode. Deprotonated [M−H]− ions observed in the ESI-MS, confirmed the calculated molecular weight of compounds 6b, 6j, 6l, 7–8b, 8c, 8g and 8l.
Biological activity
The novel indolylhydrazones (6) and indole-based 4-thiazolidinones (7, 8) were tested for in vitro antitubercular activity against M. tuberculosis H37Rv using the BACTEC 460TB systemCitation31–33. The antituberculosis (anti-TB) drug, rifampicin, was used as the positive control. The minimum concentration of compound required to hinder 99% of bacterial growth in the culture was referred as the MIC. Compounds 7g–7j, 8g, 8h and 8j were assayed using twofold dilutions starting at 25.0 µg/ml. As reported in , compounds 7g–7j, 8g, 8h and 8j exhibited significant anti-TB activity showing 99% inhibition at MIC values ranging from 25.0 to 6.25 µg/ml. The most potent entries were the CN an NO2 substituted derivatives (7g and 7h) with MIC values of 6.25 µg/ml.
Table 2. In vitro antitubercular activity and cytotoxicity screening results of compounds 6–8.
The screening results showed that the 4-thiazolidinone scaffold had an essential role in anti-TB activity since precursors (6) were inactive at the tested concentrations. When the structures of the active molecules were examined, it seemed that substitutions on the benzene ring had a profound effect on the activity. Introduction of electron-withdrawing substituents CN, NO2, CF3 and COOCH3 at the para-position of the phenyl ring, as in 7g–7j, 8g, 8h and 8j, enhanced the biological activity in the majority of the compounds. The screening results also showed that, substitution at the 5-position of the 4-thiazolidinone system had a negative effect. The 5-nonsubstituted-4-thiazolidinone derivatives (7g–7i) were found to be more active in comparison with their 5-methyl substituted congeners (8g–8i), except for compounds 7j and 8j which displayed similar MIC values ().
The active compounds, 7g–7j, 8g, 8h and 8j, were further examined for cytotoxicity using the rat kidney epithelial cell line (NRK-52E) up to 125.0 or 250.0 µg/ml. Higher concentrations could not tested due to solubility problems. The cytotoxicity results presented in are expressed as the concentrations inhibiting 50% of the cell growth (IC50). The tested compounds were found not to be cytotoxic at concentrations up to 125.0 or 250.0 µg/ml, except for 4-CN substituted derivatives 7g and 8g which exhibited low cytotoxicity at 113.4 and 60.0 µg/ml, respectively. All the tested compounds showed a differential between active and cytotoxic doses. Compounds 7g, 7h, 7i, 8h and 8j may be considered for further development as potential anti-TB agents, as they demonstrated anti-TB activity at concentrations 10-fold lower than those cytotoxic for the mammalian cell linesCitation37.
5-Fluoro-N2-(4-methylbenzylidene)-3-phenyl-1H-indole-2-carbohydrazide (6b, NSC 752358) was selected by the NCI (USA) as a prototype for evaluation in the in vitro preclinical antitumor screening program against 60 human tumor cell lines derived from nine different types of cancer, namely, leukemia, melanoma and cancers of the lung, colon, brain, ovary, breast, prostate and kidneyCitation36. This in vitro screen was subdivided into a pretest and a main test. Within the one-dose pretest consisting of approximately 60 tumor cell lines, compound 6b was added at a single dose of 10 µM to each cell line. The results were reported as the percentage of growth of the treated cells in comparison with that of the untreated control cells. Compound 6b which satisfied predetermined threshold inhibition criteria was passed on for evaluation in the main test, consisting of 60 cell lines over a five-dose range (0.01–100 µM).
shows the anticancer data for compound 6b. The NCI standard anticancer agent 5-fluorouracil (NSC 19893) was used as the reference compoundCitation38. As can be seen from , 6b exhibited significant anticancer activity at sub-micromolar concentrations against most of the tested cell lines. The GI50 values were between 0.01– and 0.1 µM with a full panel mean value of 0.039 µM, except for SF-268 (CNS Cancer), SK-MEL-28 (melanoma) and T-47D (breast cancer) cell lines. Compound 6b demostrated higher 50% growth inhibition activity than the standard 5-fluorouracil (NSC 19893) did against all of the cell lines tested except for SR (leukemia), MALME 3M (melanoma), OVCAR-3 (ovarian cancer) and T-47D (breast cancer). It is especially noteable that 6b had an obvious selectivity toward colon cancer COLO 205 cell line at both the GI50 (0.018 µM), Molar concentration leading to total growth inhibition (TGI) (0.034 µM) and LC50 (0.063 µM) levels. Compound 6b (NSC 752358) was retested using the five-dose screen and the reproducibility of its cellular actions was confirmed.
Table 3. In vitro anticancer activity of compound 6b against 60 human tumor cell lines at five-dose levels in comparison with data of NCI's standard 5-fluorouracil.a
Conclusion
In the search for effective and selective antitubercular agents, we achieved the synthesis of novel hydrazone (6) and 4-thiazolidinone (7, 8) derivatives of the 5-fluoro-3-phenyl-1H-indole scaffold. All the newly synthesized compounds (6–8) were evaluated for in vitro anti-TB activity against M. tuberculosis H37Rv. The 4-thiazolidinone derivatives 7g–7j, 8g, 8h and 8j displayed appreciable anti-TB activity showing 99% inhibition at MIC values ranging from 25.0 to 6.25 µg/ml along with rather low cytotoxicity against mammalian cell lines. Compounds 7g, 7h, 7i, 8h and 8j demonstrated anti-TB activity at concentrations 10-fold lower than those cytotoxic for the mammalian cell lines. The screening results warrant further investigation to evaluate in vivo anti-TB activity of promising analogs and elucidate the mechanism of the anti-TB potential of this class of compounds.
The antitumor potential of compound 6b, which was selected by the NCI as a prototype, has been also investigated. Although the biochemical targets of the molecule have not been identified yet, it showed an interesting anticancer profile against different human tumor-derived cell lines at sub-micromolar concentrations with GI50 values between 0.01 and 0.1 µM with a full panel mean value of 0.039 µM, except for SF-268 (CNS cancer), SK-MEL-28 (melanoma) and T-47D (breast cancer) cell lines. Compound 6b demonstrated an obvious selectivity toward colon cancer COLO 205 cell line at both the GI50 (0.018 µM), TGI (0.034 µM) and LC50 (0.063 µM) levels. These encouraging preliminary results make relevant structures an interesting avenue toward the discovery of a new class of anticancer agents worthy of further examination and scientific scrutiny.
Investigations directed toward the modification of the indole substituents as well as the substitution on the indole-2-carboxamide/carbohydrazide moiety are underway for enhanced antitubercular and anticancer activity.
Declaration of interest
This work was supported by The Research Fund of Istanbul University (Project Number T-2827) and The Scientific and Technological Research Council of Turkey (TÜBİTAK, BİDEB-2211). The authors declare no conflict of interest.
Supplementary material available online
Acknowledgements
We thank the National Cancer Institute (NCI) (Bethesda, MD, USA) for anticancer screening.
References
- World Health Organization (WHO). Global tuberculosis report (2012). Available from: http://www.who.int/tb/publications/global_report/en/ [last accessed 24 Nov 2013]
- Heym B, Philipp W, Cole ST. Mechanisms of drug resistance in Mycobacterium tuberculosis. Curr Top Microbiol Immunol 1996;215:49–69
- Mckinney JD, Jacobs WRJ, Bloom BR. Persisting problems in tuberculosis. In: Krause RM, Galin JI, Fauci AS, eds. Emerging infections. New York: Academic Press; 1998:51–146
- Sobin BA. A new Streptomyces antibiotic. J Am Chem Soc 1952;74:2947–8
- Grundy WE, Whitman AI, Rdzok EJ, et al. Actithiazic acid. I: microbiological studies. Antibiot Chemother 1952;2:399–408
- Aquino TM, Liesen AP, Silva REA, et al. Synthesis, anti-Toxoplasma gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenylmethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidineacetic acids. Bioorg Med Chem 2008;16:446–56
- Aridoss G, Amirthaganesan S, Kim MS, et al. Synthesis, spectral and biological evaluation of some new thiazolidinones and thiazoles based on t-3-alkyl-r-2,c-6-diarylpiperidin-4-ones. Eur J Med Chem 2009;44:4199–210
- Jaju S, Paklar M, Maddi V, et al. Synthesis and antimycobacterial activity of a novel series of isonicotinylhydrazide derivatives. Arch Pharm Chem Life Sci 2009;342:723–31
- Trivedi GS, Desai NC. Synthesis and antimicrobial activity of some 4-thiazolidinones. Indian J Chem 1992;31B:366–9
- Küçükgüzel ŞG, Oruç EE, Rollas S, et al. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur J Med Chem 2002;37:197–206
- Babaoğlu K, Page MA, Jones VC, et al. Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg Med Chem Lett 2003;13:3227–30
- Pathak RB, Chovatia PT, Parekh HH. Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg Med Chem Lett 2012;22:5129–33
- Srivastava T, Gaikwad AK, Haq W, et al. Synthesis and biological evaluation of 4-thiazolidinone derivatives as potential antimycobacterial agents. Arkivoc 2005;ii:120–30
- Ulusoy N. Synthesis and antituberculosis activity of cycloalkylidenehydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of imidazo[2,1-b]thiazole. Arzneim-Forsch 2002;52:565–71
- Güzel Ö, Terzioğlu N, Çapan G, Salman A. Synthesis and biological evaluation of new 5-methy-N-(3-oxo-1-thia-4-azaspiro[4.5]-dec-4-yl)-3-phenyl-1H-indole-2-carboxamide derivatives. Arkivoc 2006;xii:98–110
- Mathada BSD, Mathada MBH. Synthesis and antimicrobial activity of some 5-substituted-3-phenyl-Nβ-(substituted-2-oxo-2H-pyrano[2,3-b]quinoline-3-carbonyl)-1H-indole-2-carboxyhydrazide. Chem Pharm Bull 2009;57:557–60
- Sonar VN, Crooks PA. Synthesis and antitubercular activity of a series of hydrazone and nitrovinyl analogs derived from heterocyclic aldehydes. J Enzym Inhib Med Chem 2009;24:117–24
- Zampieri D, Mamolo MG, Laurini E, et al. 2-Aryl-3-(1H-azol-1-yl)-1H-indole derivatives: a new class of antimycobacterial compounds-conventional heating in comparison with MW-assisted synthesis. Arch Pharm Chem Life Sci 2009;342:716–22
- Negi VJ, Sharma AK, Negi JS. Biological activities of hydrazone derivatives in the new millenium. Int J Pharm Chem 2012;4:100–9
- Verma G, Marella A, Shaquiquzzaman M, et al. A review exploring biological activities of hydrazones. J Pharm Bioallied Sci 2014;2:69–80
- Cihan-Üstündağ G, Çapan G. Synthesis and evaluation of functionalized indoles as antimycobacterial and anticancer agents. Mol Divers 2012;16:525–39
- Onajole OK, Pieroni M, Tipparaju SK, et al. Preliminary structure–activity relationships and biological evaluation of novel antitubercular indolecarboxamide derivatives against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. J Med Chem 2013;56:4093–103
- Germain AR, Carmody LC, Morgan B, et al. Identification of a selective small molecule inhibitor of breast cancer stem cells. Bioorg Med Chem Lett 2012;22:3571–4
- Gangarapu K, Manda S, Thota S, et al. Microwave assisted synthesis, characterization of some new ısatin and thiophene derivatives as cytotoxic and chemopreventive agents. Lett Drug Des Discov 2012;9:934–41
- Cui Z, Li Y, Ling Y, et al. New class of potent antitumor acylhydrazone derivatives containing furan. Eur J Med Chem 2010;45:5576–84
- Kumar H, Malhotra D, Sharma R, et al. Synthesis, characterization and evaluation of isoniazid analogues as potent anticancer agents. Pharmacologyonline 2011;3:337–43
- Kumar D, Kumar NM, Ghosh S, Shah K. Novel bis(indolyl)hydrazide-hydrazones as potent cytotoxic agents. Bioorg Med Chem Lett 2012;22:212–15
- Zhang HZ, Drewe J, Tseng B, et al. Discovery and SAR of indole-2-carboxylic acid benzylidene-hydrazides as a new series of potent apoptosis inducers using a cell-based HTS assay. Bioorg Med Chem 2004;12:3649–55
- Bamaung NY, Craig RA, Kawai M, Wang J. Preparation of 3-substituted indole angiogenesis inhibitors. US patent No. 6323228 B1 20011127. 2001
- Akkurt M, Çelik İ, Cihan G, et al. 5-Fluoro-N′-[(E)-4-methoxybenzylidene]-3-phenyl-1H-indole-2-carbohydrazide. Acta Cryst 2010;E66:830
- Siddiqi SH, Libonati JP, Middlebrook G. Evaluation of a rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 1981;13:908–13
- Collins LA, Franzblau SG. Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 1997;41:1004–9
- Inderlied CB, Nash KA. Antimycobacterial agents: in vitro susceptibility testing and mechanisms of action and resistance. In: Lorian W, ed. Antibiotics in laboratory medicine. Baltimore (MD): Williams and Wilkins; 2005:155–225
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;16:55–63
- Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988;48:589–601
- National Cancer Institute, Bethesda, MD. http://dtp.nci.nih.gov/branches/btb/ivclsp.html [last accessed 10 Dec 2013]
- Tuberculosis Antimicrobial Acquisition and Coordinating Facility, Southern Research Institute, Birmingham, AL. http://www.taacf.org/Process-text.html [last accessed 10 Dec 2013]
- National Cancer Institute, Bethesda, MD. http://dtp.nci.nih.gov/docs/cancer/searches/standard_agent_table.html [last accessed 10 Dec 2013]