Abstract
Tyrosinase is a copper-containing enzyme widely distributed in nature, involved in the biosynthesis of melanin whose role is to protect the skin from ultraviolet damage. A great interest has been shown on the melanin involvement in malignant melanoma and other carcinogenetic processes. These phenomena have encouraged the research of tyrosinase inhibitors useful in therapeutic field as well as in foods and cosmetics to prevent browning. The idea was to screen our “in house” database to select suitable lead compounds for the discovery of potential drug-inhibiting enzyme. The obtained biological results demonstrated that compounds containing 4-fluorobenzyl moiety at N − 1 position of indole system showed the best activity. In addition, the role of the portion linked to the carbonyl group at C − 3 was discussed. A Lineweaver–Burk kinetic analysis of the most active indoles, CHI 1043 and derivative 4, showed a mixed-type inhibition in the presence of l-3,4-dihydroxyphenylalanine (l-DOPA) as substrate.
Introduction
Tyrosinase (EC1.14.18.1), also known as polyphenoloxidase, is a copper-containing enzyme that catalyses two distinct reactions of melanin synthesis: the hydroxylation of tyrosine by monophenolase action (monophenolase activity) and the oxidation of l-3,4-dihydroxyphenylalanine (l-DOPA) to o-dopaquinone (diphenolase activity)Citation1. Furthermore, in humans dopaquinone is converted by a series of complex reactions involving cyclization and oxidative polymerizations which finally results in the formation of melaninCitation2,Citation3. The production of abnormal pigmentation is a serious aesthetic problem in human beingsCitation4. Exogenous causes, particularly ultraviolet light exposure, are a common factor in pigmentary abnormalities such as melasma, solar lentigines and ephelides. In addition, tyrosinase is responsible for the undesired enzymatic browning of fruits and vegetablesCitation5. Therefore, tyrosinase inhibitors have become increasingly important in food industry as well as in the medicinal and cosmetic productsCitation6,Citation7. The architecture of catalytic site of tyrosinase consists of two copper ions bound to six histidines. Three types of tyrosinase (met-, oxy- and deoxy-tyrosinase), exhibiting different binuclear copper structures of the active site, are involved in the formation of melanin pigments. Based on the interaction between the inhibitor and the enzyme, tyrosinase inhibitors can be classified into four types, namely, competitive, uncompetitive, non-competitive and mixedCitation4,Citation8–13.
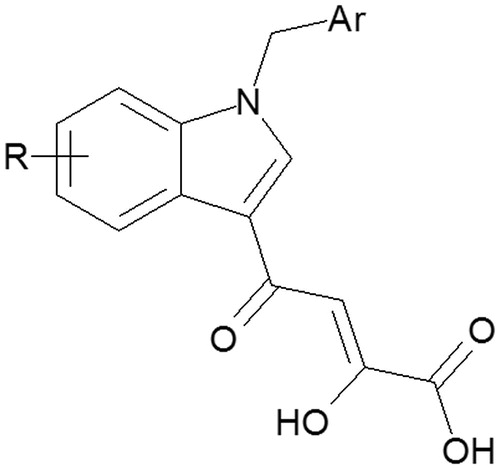
In previous articlesCitation14–18, we reported the biological activity of numerous indole diketo acid derivatives as potent Integrase Strand Transfer Inhibitors (INSTIs) () in the HIV-1 life cycle. The mechanism of their antiviral action was due to the ability to coordinate, into the catalytic site, the magnesium ions required for the integration process, thus blocking the IN function.
On these bases, the idea was to screen our “in house database” to select suitable leads for the future discovery of potential enzyme tyrosinase inhibitors.
The main goal was the identification of molecular structures able to efficiently block the two copper cofactors of enzyme, similarly to what observed in the case of chelating effect of indole diketo acid derivatives on Mg ions.
Our research group is from many years engaged in the study of the indole nucleus, representing one of the most important structural motifs in the drug discovery, and usually described as “privileged scaffolds”. As reported in literature, indole derivatives have the unique property of mimicking the structure of peptides and to bind reversibly to enzymes, providing in this way the opportunities to discover novel drugs acting on several biological targets with different mode of actionCitation19–23.
In the light of these considerations and considering the perspectives on how the indole scaffolds might be exploited in the future, herein, we report the synthesis and the biological activity of some compounds containing 4-methoxy-1H-indole scaffold as potential tyrosinase inhibitors. For the most promising inhibitors, the Lineweaver–Burk kinetic analysis was performed in order to clarify if their mechanism of inhibition is competitive, uncompetitive, non-competitive or mixed.
Material and methods
Chemistry
All the commercially available reagents and solvents were used without any further purification. The microwave-assisted reactions were carried out in a CEM Focused Microwave Synthesis System, Model Discover, working at the power necessary for refluxing under atmospheric conditions. Melting points were determined on a BUCHI Melting Point B-545 apparatus and are uncorrected. Elemental analyses (C, H and N) were carried out on a Carlo Erba Model 1106 Elemental Analyzer and the results are within ±0.4% of the theoretical values. Merck silica gel 60 F254 plates were used for analytical TLC; column chromatography was performed on Merck silica gel 60 (230–400 mesh) and Flash Chromatography (FC) on a Biotage SP1 EXP. 1H-NMR spectra were recorded in CDCl3 with TMS as internal standard or [D6]DMSO on a Varian Gemini-300 spectrometer. Chemical shifts were expressed in δ (ppm) and coupling constants (J) in hertz (Hz). All the exchangeable protons were confirmed by addition of D2O. Mushroom tyrosinase, l-DOPA, Kojic acid, dimethyl sulfoxide (DMSO) and sodium acetate were purchased from Sigma–Aldrich (St. Louis, MO).
General procedures for the synthesis of indoles CHI 1043 and 1–7
Derivatives CHI 1043 and 1–7 were prepared following the synthetic procedures previously reported by us and all the spectral data are in accordance with the literatureCitation14,Citation24.
Tyrosinase inhibition assay
Tyrosinase inhibition was assayed according to the method of MasamotoCitation25 with minor modificationsCitation26. Briefly, aliquots (0.05 ml) of sample at various concentrations (125–500 μM) were mixed with 0.5 ml of l-DOPA solution (1.25 mM), 0.9 ml of sodium acetate buffer solution (0.05 M, pH 6.8) and preincubated at 25 °C for 10 min. Then, 0.05 ml of an aqueous solution of mushroom tyrosinase (333 U/ml) was added last to the mixture. The reaction mixture was immediately monitored for the formation of dopachrome by measuring the linear increase in adsorbance (Abs) at 475 nm.
The extent of inhibition by the addition of samples is expressed as inhibition percentage and calculated as follows:
where A: Abs acetate buffer and enzyme; B: Abs acetate buffer; C: Abs acetate buffer, test sample and enzyme; D: Abs acetate buffer and test sample
The concentrations leading to 50% activity lost (IC50) were also calculated by interpolation of the dose-response curves. Kojic acid [5-hydroxy-2 -(hydroxymethyl)-4H-pyran-4-one], a fungal secondary metabolite used as skin-whitening agent, was employed as a positive standard (8–35 μM).
Kinetic analysis
The inhibition kinetics of the most active compounds on the tyrosinase were studied using Lineweaver–Burk plotsCitation27. For the test, the reaction mixture consisted of four different concentrations of l-DOPA (0.6–5 mM) as substrate and mushroom tyrosinase in acetate buffer (0.05 M, pH 6.8). Test samples at various concentrations (125–500 μM) were added to the reaction mixture.
Statistical analysis
All the experiments were performed at least 3 times in order to ensure the reproducibility of the results. Values are given as means ± SEM and they were analysed statistically by means of analysis of variance (ANOVA) followed by Student’s t-test. Value of p > 0.05 are regarded as significant.
Results and discussion
Our previous studies led to the discovery of potent INSTIs belonging to the class of diketo acid derivativesCitation14–17.
Following the synthetic procedure reported by us (Scheme 1)Citation16, 4-methoxy-substituted indole (a) was converted into the corresponding 3-acetyl derivative (b) under Vilsmeier–Haack conditions, using phosphoryl chloride and an excess of N,N-dimethylacetamide.
Scheme 1. Reagents and conditions: (i) POCl3, CH3CON(CH3)2, RT, 12 h; (ii) benzyl bromide or chloride, NaH, DMF, mw: 10 min at continuous temperature (50 °C), 100 W; (iii) diethyl oxalate, dry CH3ONa, THF, two separate steps under the same conditions mw: 2 min, at continuous temperature (50 °C), 250 W and (iv) 2 N NaOH, MeOH, RT, 1.5 h.
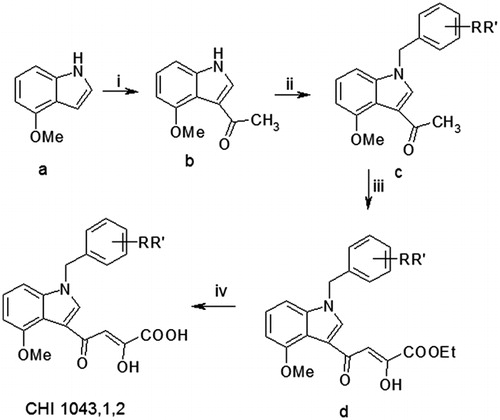
The obtained intermediate was N-alkylated by reaction with the suitable benzyl bromide under microwave (MW) irradiation conditions, thus reducing reaction time, usual thermal reagents degradation and increasing the yields.
Reaction of benzyl derivatives (c) with diethyl oxalate under the same conditions provided key diketoester intermediates (d) which were converted into the corresponding diketoacids (CHI 1043, 1 and 2) by hydrolysis in basic medium.
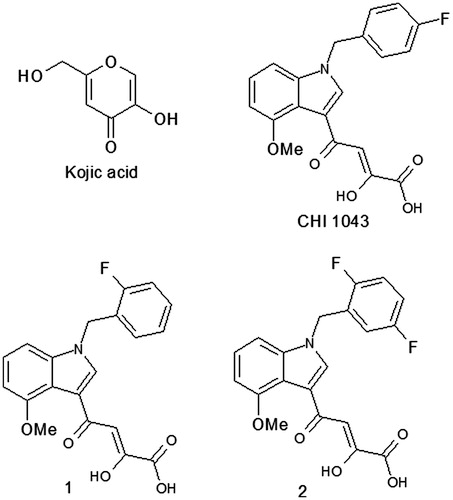
For the initial screening, we focused on the compounds that is depicted in (CHI 1043, 1 and 2) containing one or two fluorine atoms on the benzyl moiety, taking into account the role of the “privileged indole scaffolds” drug discovery and the results of a previous article by Liu et al.Citation28 which demonstrated the influence of halogenated substituents on the pharmacokinetic effects.
The inhibitory effects of these derivatives were investigated on the diphenolase activity of mushroom tyrosinase using l-DOPA as substrate and compared with kojic acid (), which is a widely used skin-whitening material in cosmetic industry.
Results of the above-reported preliminary screening () showed that the diketo acid portion was not crucial for the activity and confirmed the pivotal role of halogen substitution. Particularly, only the 4-fluoro-substituted CHI 1043 showed tyrosinase inhibition with an IC50 value of 312 µM; on the contrary the presence of the same substituent in a different position (1) and in higher number (2) afforded less active compounds.
Table 1. Inhibitory activity against mushroom tyrosinase of indole derivatives.
With the aim of improving our knowledge about the enzymatic activity of this class of potential indole tyrosinase inhibitors (ITIs), new 4-methoxy-1H-indoles were designed and synthesized.
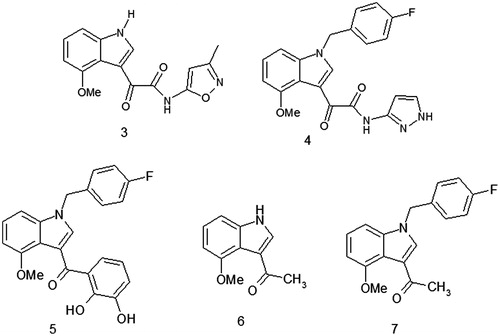
Particularly, we focused our attention on compounds in which the diketo acid portion has been replaced with other functionalities capable of performing the chelating effect on the metal ions into the catalytic site of enzyme (3, 4 and 5) maintaining in two of them (4 and 5) the typical 4-fluoro substitution ().
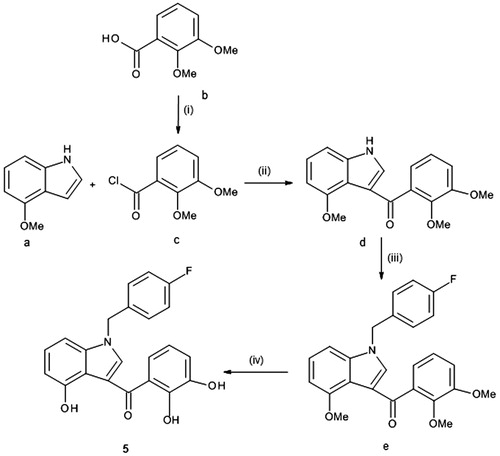
Derivatives 3–5 were prepared according to a standard synthetic chemistryCitation24 that is reported in Schemes 2 and 3.
Scheme 2. Reagents and conditions: (i) oxalyl chloride, dry Et2O, N2, 0 °C, 3 h; (ii) NH2-Het, dry THF, N2, rt, 1.5 h and (iii) 4-fluorobenzyl bromide, t-BuOK, THF, rt, 2 h.
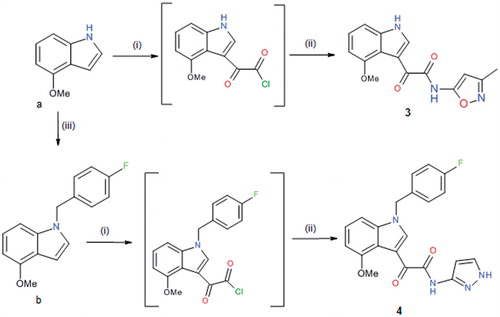
Scheme 3. Reagents and conditions: (i) thionyl chloride, Δ, 2 h; (ii) Et2AlCl, CH2Cl2, 0 °C, 2 h; (iii) 4-fluorobenzyl bromide, t-BuOK, THF, rt, 2 h and (iv) BBr3 1 M sol. in CH2Cl2, CH2Cl2, rt, 16 h.
Treatment of commercially available 4-methoxy-1H-indole with oxalyl chloride at 0 °C produced 2 -(1H-3-indolyl)-2-oxoacetyl chloride as an inseparable intermediate which gave the compound 3 after the reaction with the appropriate arylamine. To prepare the N-alkylated indole 4, the starting material firstly was treated with 4-fluorobenzyl bromide and a catalytic amount of potassium t-butoxide and then, the obtained intermediate b, was converted into the desired compound by reaction with the arylamine (Scheme 2).
Scheme 3 shows the pathway to synthesize derivative 5. By 3-acylation of 4-methoxy-1H-indole (a) with 2,3-dimethoxybenzoyl chloride (c) that had been obtained from the corresponding acid (b) and thionyl chloride, the 4-methoxy-1H-indol-3-yl (2,3-dimethoxyphenyl)methanone (d) was prepared. It was then alkylated by treatment with 4-fluorobenzyl bromide using a catalytic amount of potassium t-butoxide. Finally, the desired indole derivative 5 was obtained by reaction of N-alkylated intermediate (d) with BBr3 in CH2Cl2.
In addition, on the basis of a molecular simplification approach we also tested some fragments (6 and 7) isolated during the synthetic pathway employed for obtaining the tyrosinase inhibitor CHI 104314 ().
The obtained results () showed that derivatives 4 and 7 produced inhibitory effects on mushroom tyrosinase activity with IC50 values of 224 and 372 µM, respectively, while compounds 3, 5 and 6 did not affect enzyme activity.
It was recently reported that the fluoro derivatives showed higher activity than chloro and bromo analogues and better pharmacobiological properties. In particular, it was observed that the position of the substituent plays an important role influencing the biological activityCitation10. Accordingly, the results of tyrosinase inhibition assay demonstrated that compounds 3 and 6, in which the 4-fluorobenzyl moiety was removed, were completely inactive.
On the contrary, derivative 7 structurally related to derivative 6 but characterized by the 4-fluorobenzyl substitution showed anti tyrosinase activity.
Among the 4-fluoro-substituted derivatives, only compound 5 was completely inactive thus suggesting that not only the substituent at N − 1 position of the indole system but also the portion linked to the carbonyl group at C − 3 plays a key role in the tyrosinase inhibition.
Finally, the better biological activity of derivative 4 suggested that an improvement of enzymatic inhibition can be achieved by modulating the coordinating properties, also according to the specificity of the catalytic site of tyrosinase.
For this reason, molecular modelling studies are in progress to shed new important information useful in the further development of this new class of compounds as potential tyrosinase inhibitors.
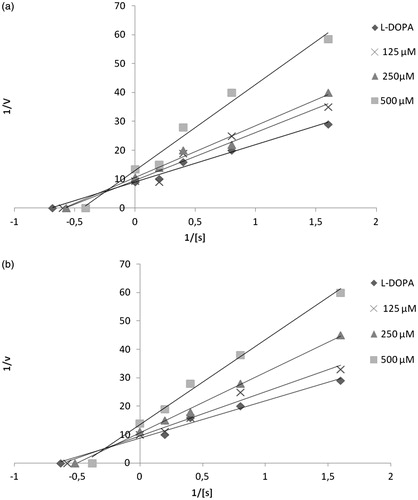
Kinetics of mushroom tyrosinase inhibition by derivatives CHI 1043 and 4
The inhibition kinetics of derivatives CHI 1043 and 4 were studied using Lineweaver–Burk plots. As shown in , the results of the Lineweaver–Burk double-reciprocal plots yielded a group of lines that intercepted at the second quadrant. These results indicated that our most promising ITIs (CHI 1043 and 4) were mixed-type diphenolase inhibitors of mushroom tyrosinase. The mixed-type inhibition implies that compounds can bind to a free enzyme as well as to an enzyme–substrate complex.
Conclusions
In summary, novel indole derivatives have been designed, synthesized and tested as tyrosinase inhibitors. The biological results confirmed the positive influence of the 4-fluorophenyl moiety as substituent at N − 1 position of indole nucleus and suggested the key role of the substituent at C − 3 position of the same scaffold on tyrosinase inhibition.
Declaration of interest
The authors declare no conflict of interest.
References
- Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 2005;62:1707–23
- Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res 2007;21:805–16
- Sanchez-Ferrer A, Rodriguez-Lopez JN, Garcia-Canovas F, Garcia-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1995;1247:1–11
- Khan KM, Maharvi GM, Khan MT, et al. Tetraketones: a new class of tyrosinase inhibitors. Bioorg Med Chem 2006;14:344–51
- Martinez MV, Whitaker J.R. The biochemistry and control of enzymatic browning. Trends Food Sci Technol 1995;6:195–200
- Casanola-Martin GM, Le-Thi-Thu H, Marrero-Ponce Y, et al. Tyrosinase enzyme: 1. An overview on a pharmacological target. Curr Top Med Chem 2014;14:1494–501
- Mendes E, Perry Mde J, Francisco AP. Design and discovery of mushroom tyrosinase inhibitors and their therapeutic applications. Exp Opin Drug Discov 2014;9:533–54
- Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci 2009;10:2440–75
- Mojzych M, Dolashki A, Voelter W. Synthesis of pyrazolo[4,3-e][1,2,4]triazine sulfonamides, novel Sildenafil analogs with tyrosinase inhibitory activity. Bioorg Med Chem 2014;22:6616–24
- Hamidian H. Synthesis of novel compounds as new potent tyrosinase inhibitors. BioMed Res Int 2013;2013:207181
- Casanola-Martin GM, Marrero-Ponce Y, Khan MT, et al. Dragon method for finding novel tyrosinase inhibitors: Biosilico identification and experimental in vitro assays. Eur J Med Chem 2007;42:1370–81
- Akhtar T, Hameed S, Khan KM, et al. Design, synthesis, and urease inhibition studies of some 1,3,4-oxadiazoles and 1,2,4-triazoles derived from mandelic acid. J Enzyme Inhib Med Chem 2010;25:572–6
- Khan KM, Maharvi GM, Khan MT, et al. A facile and improved synthesis of sildenafil (Viagra) analogs through solid support microwave irradiation possessing tyrosinase inhibitory potential, their conformational analysis and molecular dynamics simulation studies. Mol Divers 2005;9:15–26
- De Luca L, Barreca ML, Ferro S, et al. A refined pharmacophore model for HIV-1 integrase inhibitors: Optimization of potency in the 1H-benzylindole series. Bioorg Med Chem Lett 2008;18:2891–5
- Ferro S, De Luca L, Barreca ML, et al. Docking studies on a new human immunodeficiency virus integrase-Mg-DNA complex: phenyl ring exploration and synthesis of 1H-benzylindole derivatives through fluorine substitutions. J Med Chem 2009;52:569–73
- Ferro S, De Luca L, Barreca ML, et al. New chloro,fluorobenzylindole derivatives as integrase strand-transfer inhibitors (INSTIs) and their mode of action. Bioorg Med Chem 2010;18:5510–18
- De Luca L, De Grazia S, Ferro S, et al. HIV-1 integrase strand-transfer inhibitors: design, synthesis and molecular modeling investigation. Eur J Med Chem 2011;46:756–64
- De Luca L, Gitto R, Christ F, et al. 4-[1-(4-Fluorobenzyl)-4-hydroxy-1H-indol-3-yl]-2-hydroxy-4-oxobut-2-enoic acid as a prototype to develop dual inhibitors of HIV-1 integration process. Antivir Res 2011;92:102–7
- Ubeid AA, Do S, Nye C, Hantash BM. Potent low toxicity inhibition of human melanogenesis by novel indole-containing octapeptides. Biochim Biophys Acta 2012;1820:1481–9
- Kaushik NK, Kaushik N, Attri P, et al. Biomedical importance of indoles. Molecules 2013;18:6620–62
- Dolle RE, Nelson KH Jr. Comprehensive survey of combinatorial library synthesis: 1998. J Comb Chem 1999;1:235–82
- Franzen RG. Recent advances in the preparation of heterocycles on solid support: a review of the literature. J Comb Chem 2000;2:195–214
- Dolle RE. Comprehensive survey of combinatorial library synthesis: 2000. J Comb Chem 2001;3:477–517
- Ferro S, De Grazia S, De Luca L, et al. Structural modification of diketo acid portion in 1H-benzylindole derivatives HIV-1 integrase inhibitors. Heterocycles 2009;78:947–59
- Masamoto Y, Ando H, Murata Y, et al. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem 2003;67:631–4
- Germano MP, Cacciola F, Donato P, et al. Betula pendula leaves: polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012;83:877–82
- Huang KF, Chen YW, Chang C, Chou ST. Walther extracts on mushroom tyrosinase. Food Chem 2005;89:583–7
- Liu X, Zhang F, Liu H, et al. Effect of halogenated substituents on the metabolism and estrogenic effects of the equine estrogen, equilenin. Chem Res Toxicol 2003;16:741–9