Abstract
Carbonic anhydrase IX (CAIX) is involved in pathological processes including tumorgenicity, metastases and poor survival in solid tumors. Twenty-two neuroblastoma samples of patients who were surgically treated at the University Medical Center Hamburg-Eppendorf were evaluated immunohistochemically for expression of CAIX. Results were correlated with clinical parameters and outcome. Neuroblastoma Kelly and SH-EP-Tet-21/N cells were examined for CAIX expression and inhibited with specific inhibitors, FC5-207A and FC8-325A. 32% of neuroblastoma tumors expressed CAIX. This was significantly associated with poorer survival. Kelly and SH-EP-Tet-21/N cells showed a major increase of CAIX RNA under hypoxic conditions. Proliferation of Kelly cells was significantly decreased by CAIX inhibitors, FC5-207A and FC8-325A, while proliferation of SH-EP-Tet-21/N cells was only significantly affected by FC8-325A. CAIX is a potent biomarker that predicts survival in neuroblastoma patients. CAIX-targeted therapy in neuroblastoma cell lines is highly effective and strengthens the potential of CAIX as a clinical therapeutic target in a selected patient collective.
Introduction
Neuroblastoma is the most frequent solid tumor in childhood outside the central nervous system and most often arises from sympathetic neuroblast cells in abdomen, neck and pelvisCitation1,Citation2. The tumor can combine characteristics of the cells from which it originates with extensive heterogeneity, pluripotential differentiation and migratory abilities, leading to a wide range of clinical presentation from spontaneous regression to fatal progression and dissemination to preferential sitesCitation3,Citation4. Neuroblastoma is responsible for 15% of all cancer-related deaths in childhoodCitation5. The outcome strongly correlates with clinical factors, such as age, stage, pathology and biological factors (e.g. MYCN-amplification). In general, children under 18 months or with limited disease have a good prognosis through surgical interventionCitation5, while the prognosis of high-risk neuroblastoma with disseminated disease (International Neuroblastoma Staging System stage IV) is still poorCitation6–12.
Carbonic anhydrase IX is a HIF-1α-inducible protein that regulates intra- and extracellular pH homeostasis under hypoxia. Tumor hypoxia, in a wide range of solid tumors, e.g. breast cancerCitation13, prostate cancerCitation14, gastric cancerCitation15, oral squamous cell cancerCitation16, leads to an increased malignancy with an increased metastatic rate and treatment resistance with a poor prognosis. In breast cancer, CAIX is vital for growth and metastasis of hypoxic tumors, and has been shown to be a specific and targetable biomarker for metastasisCitation13,Citation17.
In neuroblastoma, CAIX has previously been shown to be up-regulated in patients with adverse clinicopathological and biological factors, e.g. MYCN amplification and to correlate with worse survivalCitation18. CAIX messenger RNACitation19 and proteinCitation20 have been found to be up-regulated in neuroblastoma cells under hypoxic conditions in vitro.
The aim of our study was to evaluate CAIX as a potential therapeutic target for treatment of neuroblastoma by analyzing the impact of CAIX expression on clinicopathological characteristics and survival, as well as by evaluating the effect of CAIX-targeted inhibitors on cell vitality and proliferation in neuroblastoma cell lines.
Materials and methods
Patients
Samples from 22 patients with neuroblastoma, who were surgically treated at the University Medical Center Hamburg-Eppendorf between July 2005 and October 2011, were used for this study. Tumor samples were selected on the basis of availability of tissues and follow-up data.
Clinical follow-up data were obtained by reviewing the hospital records, contacting patients on an outpatient basis or by phone call. Overall survival was calculated from the date of surgery to the date of death or last follow-up. None of the patients died from a cause other than neuroblastoma. All tumors were categorized into groups according to the International Neuroblastoma Staging System (INSS)Citation1. Histological grading was determined according to HughesCitation21. None of the patients had been pre-treated. The study was approved by the Ethics Committee of the Chamber of Physicians in Hamburg, Germany. Written informed consent was obtained from all parents of the patients for the use of the resected samples and clinical data for research purposes.
Immunohistochemistry
For the immunohistochemistry, the HRP-ACE-System from R&D Systems (Minneapolis, MN) was used. Sections were counterstained with Mayer’s hematoxylin solution (Merck, Darmstadt, Germany). Tumor tissue was identified by hematoxylin-eosin (HE) staining. The CAIX staining was performed using the primary antibody M75 (BioScience Slovakia, Bratislava, Slovak Republic) at a dilution of 1:200. Control sections were incubated with antibody diluent (DAKO, Glostrup, Denmark) without primary antibody at 4 °C overnight and then treated as other samples. The immunostaining was scored by two examiners.
Cell culture
Neuroblastoma Kelly cells (Sigma-Aldrich, Munich, Germany) and SH-EP Tet-21/N cells (reported by Lutz et al.Citation22,Citation23, and provided by G. Eschenburg, Hamburg, Germany) were grown in T75 culture flasks (Sarstedt, Nürmbrecht, Germany) in RPMI 1640 medium (Gibco, Thermo Fisher Scientific Inc., Waltham, MA) with 10% FBS (Gibco, Thermo Fisher Scientific Inc., Waltham, MA) at 37 °C either in air with 5% CO2 under normoxic or with 5% CO2, 5% O2 balanced with N under hypoxic conditions.
Reverse transcription quantitative PCR (RT qPCR)
Cells were grown to confluence. Total RNA was isolated with RNeasy Mini Kit (Qiagen, Hilden, Germany) in accordance to the manufacturer’s protocol and reversely transcribed with Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany). CAIX and 18S specific primers (CAIX: Cat. No. PPH01751A; 18S: Cat. No. 330001 PPH05666E, Qiagen, Hilden, Germany) were used for amplification of cDNA, which was detected with Maxima SYBR Green (Thermo Fisher Scientific Inc., Waltham, MA) in a Lightcycler 4800 (Roche, Penzberg, Germany). Data were analyzed using the 2(−Delta Delta C(T)) method as previously describedCitation24.
Cell proliferation assay
Cells were seeded at 5000 cells/well in 96-well plates. After 24 h of incubation, CAIX inhibitors FC5-207A and FC8-325ACitation25 () were added to the cells in final concentrations of 200 µM and 500 µM. Cells were then cultured either under hypoxic or normoxic conditions. The cell viability assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega, Mannheim, Germany) was carried out in accordance to the manufacturer’s protocol at 24, 48 and 72 h. Absorbance was measured at 490 nm (FLUOStar, Omega, Offenbrug, Germany).
Statistical analysis
The statistical analysis was conducted using SPSS version 13.0 (SPSS, Chicago, IL). A p value less than 0.05 was defined as significant. Kaplan-Meier survival analysis and log-rank test were performed to compare the survival time between groups.
Results
Patient characteristics and expression of carbonic anhydrase IX
In total, 22 surgically resected pediatric neuroblastoma specimens were included in this study. The mean age of the patients at the time of operation was 667 days, with the mean follow-up time of 1179 days. Staging and grading are summarized in . Metastases had occurred in 59% of the patients of which 23% were CAIX positive.
Table 1. Patient characteristics.
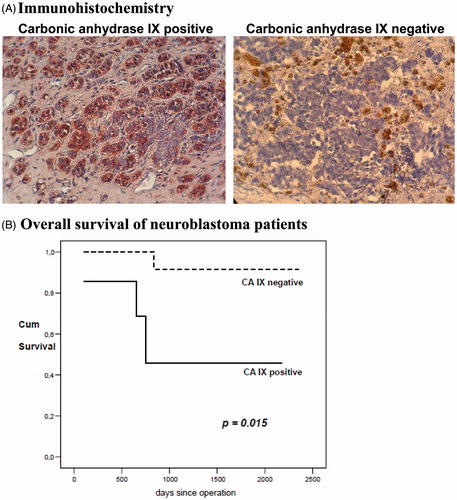
Carbonic anhydrase IX expression of the 22 neuroblastoma specimens was determined by immunohistochemistry. shows representative staining patterns for CAIX positive and negative tumor tissue. The lack of staining or weak staining of CAIX (i.e. ≤20% of tumor cells expressed CAIX) was classified as CAIX-negative expression and moderate to strong staining (i.e. >20% of tumor cells expressed CAIX) was classified as CAIX-positive expression. A total of seven (32%) out of the 22 tumors were CAIX positive and 15 (68%) samples were CAIX negative (). There was no correlation of CAIX expression with age, staging, grading or metastatic dissemination in univariate analysis (data not shown).
Figure 1. Expression and impact on survival of carbonic anhydrase IX. (A) Immunohistochemistry: Representative images of carbonic anhydrase IX positive and negative immunohistochemical staining of neuroblastoma tissue are shown (20× standard microscopic enlargement). (B) Overall survival: For the Kaplan-Meier survival analysis, patients were grouped according to positive and negative carbonic anhydrase IX expression. Overall survival of neuroblastoma patients with no carbonic anhydrase IX expression was significantly better than that of carbonic anhydrase IX positive patients (p = 0.015).
Impact of carbonic anhydrase IX expression on survival
Next, the relationship between CAIX expression and survival of patients with neuroblastoma was examined. Overall survival was analyzed by the Kaplan-Meier method, and the log-rank test was used for univariate analysis (). The mean survival was 1967 days. Expression of CAIX in the primary tumor was statistically significantly associated with poorer overall survival (p = 0.015) as compared to CAIX-negative expression (CAIX+ 2225 versus CAIX- 1295 days).
Upregulation of carbonic anhydrase IX under hypoxia
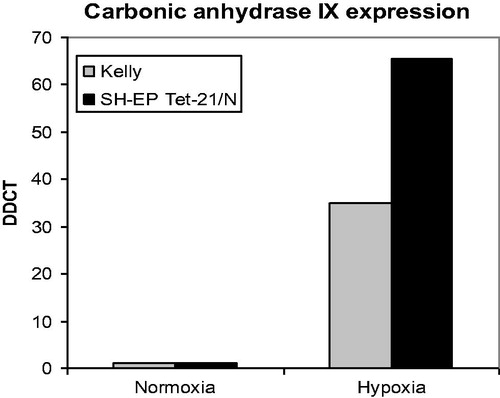
Furthermore, Kelly and SH-EP Tet-21/N neuroblastoma cell lines were examined for expression of CAIX. The levels of CAIX RNA were quantified under normoxic and hypoxic conditions. Kelly and, even more strongly, SH-EP Tet-21/N cells show a significant upregulation of CAIX levels under hypoxic conditions ().
Inhibition of carbonic anhydrase IX
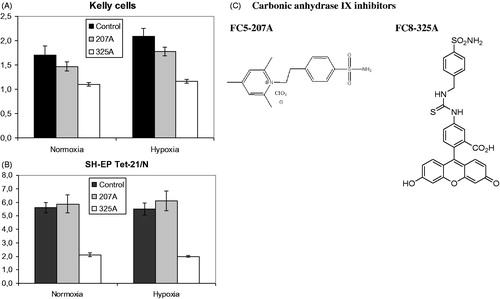
The membrane impermeable carbonic anhydrase inhibitor FC5-207A as well as a fluorescent probe FC8-325A () were evaluated in the proliferation assay under hypoxic and normoxic conditions. In Kelly cells, a significant reduction of proliferation can be observed for both inhibitors under normoxia (FC5-207A: p = 0.022; FC8-325A: p < 0.001), which is even stronger under hypoxic conditions (FC5-207A: p = 0.004; FC8-325A: p < 0.001) compared to the control (). In SH-EP Tet-21/N cells (), a significant reduction of proliferation could only be observed for substance FC8-325A (normoxia: p < 0.001, hypoxia: p < 0.001), which was especially pronounced. Differences between the control and substance FC5-207A were not significant. Overall, the potency of the inhibitors is enhanced under hypoxic conditions.
Figure 3. Proliferation of Kelly and SH-EP Tet-21/N neuroblastoma cells under treatment with carbonic anhydrase IX inhibitors FC5-207A and FC8-325A. (A) A significant reduction of proliferation can be observed for both inhibitors under normoxia (FC5-207A: p = 0.022; FC8-325A: p < 0.001) and even more strongly under hypoxic conditions (FC5-207A: p = 0.004; FC8-325A: p < 0.001) compared to the control in Kelly cells. (B) A significant reduction of proliferation could only be observed for substance FC8-325A (normoxia: p < 0.001; hypoxia: p < 0.001). Differences between the control and substance FC5-207A were not significant. (C) Chemical structures of carbonic anhydrase inhibitors FC5-207A and FC8-325A.
Discussion
In our patient cohort, a positive expression of carbonic anhydrase IX was found in about a third of neuroblastoma tumor tissues of all stages. This positive expression was significantly associated with a negative impact on survival. To further evaluate CAIX as a therapeutic target, Kelly and SH-EP Tet-21/N neuroblastoma cells were, firstly, examined for CAIX expression, showing a major increase of CAIX RNA under hypoxic conditions compared to normoxia. Secondly, cells were exposed to treatment with carbonic anhydrase IX inhibitors, FC5-207A and FC8-325A, both leading to a significant decrease in proliferation of Kelly cells, while only FC8-325A had a significant effect on SH-EP Tet-21/N.
Impact of CAIX expression and survival
A hypoxic microenvironment furthers the exploitation of both genetic and adaptive means of tumor cells to survive and proliferateCitation26,Citation27. For example, this microenviroment can lead to a natural selection of aggressive and metastasizing tumor cell clonesCitation26. Hypoxic response markers, e.g. HIF-1alpha, CAIX and GT-1 (glucose transporter-1) are well-established prognostic markers in solid cancersCitation26. The hypoxia-dependent expression of CAIX has been described for many solid tumors, including gastric cancerCitation26,Citation27, breast cancerCitation28, prostate cancerCitation14, colon cancerCitation29, oral squamous cell cancerCitation16, esophageal cancerCitation30,Citation31, where it is associated with malignant phenotypeCitation31, adverse clinicopathological factorsCitation18, tumor cell disseminationCitation29,Citation32,Citation33 and poor survivalCitation15,Citation16,Citation26,Citation30,Citation31.
Carbonic anhydrase IX has been shown to be involved in numerous pathological processes including tumorgenicityCitation16 and was suggested to be involved in malignant transformationCitation34. Its expression in gastric cancer is associated with increased invasion, supporting the hypothesis that increased CAIX expression may contribute to invasion and thus advanced disease and tumor progressionCitation34. The presence of CAIX has also been linked with poorer survival/prognosis in several other solid tumorsCitation15,Citation16,Citation26,Citation30,Citation31.
In neuroblastoma, a previous study has shown that CAIX is expressed at significantly higher levels in tumors from patients with adverse clinicopathological and biological factorsCitation18. In this study, 23% of the patients showed a strong expression of CAIX in immunostaining. Although CAIX expression, independent of high risk disease, could not be linked to significantly poorer survival, these earlier findings indicate that CAIX is a biomarker of aggressive disease in neuroblastomasCitation18. In the current study, we found a strong expression of CAIX in 32% of the tumors in our patient cohort. In contrast to the earlier study, in our study CAIX expression was significantly associated with poorer survival.
CAIX as therapeutic target
The study on neuroblastoma by Dungwa et al.Citation18 and our current findings suggest that CAIX is a biomarker of aggressive disease in neuroblastomas. Being a tumor-specific biomarker, CAIX has been proposed as a possible therapeutic target for several tumor entitiesCitation18,Citation32,Citation33 for the hypoxic primary tumor as well as for metastases. Further, CAIX had not previously been examined as a target for neuroblastoma. There are several carbonic anhydrase inhibiting substances available, which are CAIX specific, through their unique structure and membrane impermeabilityCitation35. To evaluate the effectiveness of a CAIX-targeted treatment approach for neuroblastoma, we firstly examined neuroblastoma cell lines for their response regarding CAIX expression under hypoxic conditions. Both cell lines showed a major increase of CAIX RNA during hypoxia, SH-EP Tet-21/N cells even more strongly compared to Kelly cells. When subjecting the neuroblastoma cells to carbonic anhydrase inhibitors, FC5-207A and FC8-325A, a significant decrease of cell proliferation was seen in Kelly cells. In SH-EP Tet-21/N cells, only treatment with FC8-325A had a significant anti-proliferative effect. The differences in response of SH-EP Tet-21/N cells to the two inhibitors call for further examination of the inhibitory mechanisms of these substances. The present findings with neuroblastoma cell lines strongly suggest the effectiveness of CAIX inhibitors in the treatment of this tumor.
Clinical implications
Our results show that inhibition of CAIX is a potent anti-proliferative treatment in vitro. About a third of neuroblastoma patients, in our study, showed expression of CAIX, thus making CAIX a feasible clinical target in this selected patient cohort. Further in vitro studies are necessary to shed light on the mechanisms of suppression of tumor proliferation by various CAIX inhibitors. Xenograft tumor model studies have shown that the use of CAIX inhibiting sulfonamides is feasible in vivo, which constitutes an important step towards clinical applicabilityCitation36,Citation37. There are also preclinical and clinical trials on the way to establish biological inhibitors for CAIXCitation38–40. With this, CAIX-targeted therapy could rapidly become even more attractive clinically.
Conclusions
In our study, we found CAIX to be a potent biomarker that predicts survival in neuroblastoma patients. Furthermore, CAIX-targeted therapy in neuroblastoma cell lines is highly effective and strengthens the concept of a role of CAIX as a clinical therapeutic target in a selected patient cohort.
Ackowledgements
The authors thank Beate Roth and Brigit Appl for expert technical assistance.
Declaration of interest
The authors declare that there is no other conflict of interests regarding the publication of this paper.
References
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11:1466–77
- Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Oncologist 2003;8:278–92
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003;3:203–16
- Ciccarone V, Spengler BA, Meyers MB, et al. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res 1989;49:219–25
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007;369:2106–20
- Castleberry RP, Pritchard J, Ambros P, et al. The International Neuroblastoma Risk Groups (INRG): a preliminary report. Eur J Cancer 1997;33:2113–16
- Fukuda M, Miyajima Y, Miyashita Y, Horibe K. Disease outcome may be predicted by molecular detection of minimal residual disease in bone marrow in advanced neuroblastoma: a pilot study. J Pediatr Hematol Oncol 2001;23:10–13
- Hero B, Simon T, Horz S, Berthold F. Metastatic neuroblastoma in infancy: what does the pattern of metastases contribute to prognosis? Med Pediatr Oncol 2000;35:683–7
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol 1999;17:2264–79
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med 1999;341:1165–73
- Pinkerton CR, Blanc Vincent MP, Bergeron C, et al. Induction chemotherapy in metastatic neuroblastoma – does dose influence response? A critical review of published data standards, options and recommendations (SOR) project of the National Federation of French Cancer Centres (FNCLCC). Eur J Cancer 2000;36:1808–15
- Seeger RC, Reynolds CP, Gallego R, et al. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children's Cancer Group Study. J Clin Oncol 2000;18:4067–76
- Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76
- Fiaschi T, Giannoni E, Taddei ML, et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 2013;12:1791–801
- Jung JH, Im S, Jung ES, Kang CS. Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci 2013;10:1217–23
- Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, et al. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J Oral Pathol Med 2012;41:667–74
- Lock FE, McDonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013;32:5210–19
- Dungwa JV, Hunt LP, Ramani P. Carbonic anhydrase IX up-regulation is associated with adverse clinicopathologic and biologic factors in neuroblastomas. Hum Pathol 2012;43:1651–60
- Jogi A, Vallon-Christersson J, Holmquist L, et al. Human neuroblastoma cells exposed to hypoxia: induction of genes associated with growth, survival, and aggressive behavior. Exp Cell Res 2004;295:469–87
- Hussein D, Estlin EJ, Dive C, Makin GW. Chronic hypoxia promotes hypoxia-inducible factor-1 alpha-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol Cancer Ther 2006;5:2241–50
- Hughes M, Marsden HB, Palmer MK. Histologic patterns of neuroblastoma related to prognosis and clinical staging. Cancer 1974;34:1706–11
- Lutz W, Stohr M, Schurmann J, et al. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene 1996;13:803–12
- Lutz W, Fulda S, Jeremias I, et al. MycN and IFN gamma cooperate in apoptosis of human neuroblastoma cells. Oncogene 1998;17:339–46
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8
- Cecchi A, Hulikova A, Pastorek J, et al. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem 2005;48:4834–41
- Jubb AM, Buffa FM, Harris AL. Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J Cell Mol Med 2010;14:18–29
- Kato Y, Yashiro M, Noda S, et al. Expression of a hypoxia-associated protein, carbonic anhydrase-9, correlates with malignant phenotypes of gastric carcinoma. Digestion 2010;82:246–51
- Luo Y, Cai J, Xue H, et al. Functional SDF1 alpha/CXCR4 signaling in the developing spinal cord. J Neurochem 2005;93:452–62
- Schweiger T, Kollmann D, Nikolowsky C, et al. Carbonic anhydrase IX is associated with early pulmonary spreading of primary colorectal carcinoma and tobacco smoking. Eur J Cardiothorac Surg 2014;46:92–9
- Birner P, Jesch B, Friedrich J, et al. Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol 2011;18:3330–7
- Tanaka N, Kato H, Inose T, et al. Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br J Cancer 2008;99:1468–75
- Fiaschi T, Giannoni E, Taddei ML, et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 2013;12:1791–801
- Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76
- Chen J, Rocken C, Hoffmann J, et al. Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut 2005;54:920–7
- Pastorekova S, Casini A, Scozzafava A, et al. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett 2004;14:869–73
- Dubois L, Lieuwes NG, Maresca A, et al. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol 2009;92:423–8
- Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31
- Oosterwijk E, Bander NH, Divgi CR, et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol 1993;11:738–50
- Siebels M, Rohrmann K, Oberneder R, et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX(R)) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol 2011;29:121–6
- van Dijk J, Uemura H, Beniers AJ, et al. Therapeutic effects of monoclonal antibody G250, interferons and tumor necrosis factor, in mice with renal-cell carcinoma xenografts. Int J Cancer 1994;56:262–8