Abstract
Fourteen new naphthalene-based thiosemicarbazone derivatives were designed as anticancer agents against LNCaP human prostate cancer cells and synthesized. MTT assay indicated that compounds 6, 8 and 11 exhibited inhibitory effect on LNCaP cells. Among these compounds, 4-(naphthalen-1-yl)-1-[1-(4-hydroxyphenyl)ethylidene)thiosemicarbazide (6), which caused more than 50% death on LNCaP cells, was chosen for flow cytometric analysis of apoptosis. Flow cytometric analysis pointed out that compound 6 also showed apoptotic effect on LNCaP cells. Compound 6 can be considered as a promising anticancer agent against LNCaP cells owing to its potent cytotoxic activity and apoptotic effect.
Introduction
Cancer is a very common condition which affects people all over the world. Lots of diseases or alterations can cause cancer so it is not a single disease. A change occurs in the body then cells start to grow and multiply uncontrollablyCitation1.
There are many types of cancer and prostate cancer is one of the most important cancer types. Prostate cancer is the second most constantly diagnosed cancer in male population, accounting for ∼14% of all male cancers worldwideCitation2.
Prostate cancer is a kind of neoplasm which is regulated by androgenic hormones. Androgens regulate biological pathways which are important for proliferation, differentiation and survival of benign and malignant prostate tissue. Correspondingly, androgen activity is mediated by androgen receptors so these receptors are considered to have a great importance in the progression of prostate cancer. First step for dealing with androgen receptors originated from a research on LNCaP cell line which is derived from a metastatic lesion of the lymph nodes of a prostate cancer patient. LNCaP cells growth is stimulated in vitro by androgens, estrogens, progestogens and several antiandrogens. After it was found that the growth of prostate cancer had been androgen-sensitive, hormonal therapy was used to treat metastatic disease. At first all patients with prostate cancer responded to hormonal therapy but then the majority gradually developed resistance so it has turned out an obligation to find out new ways for the treatment of prostate cancerCitation2–8.
Thiosemicarbazones possess a wide range of pharmacological effects including antineoplastic, antibacterial, antifungal and antiviral activity and they give rise to a great variety of coordination modes depending on their chemical structure. Since the 1950s, their anticancer activity was investigated and some of them were explored as antileukemic agents. Thiosemicarbazones have an important biological role mostly related to their inhibition of ribonucleotide reductase; similar to potent anticancer drugs such as triapine and methisazone. The ability of thiosemicarbazones to chelate metal ions has been defined as a major factor in their antiproliferative activity. The redox activity of Fe–thiosemicarbazone complexes is crucial in their anticancer activity inducing oxidative damage and the inhibition of ribonucleotide reductase. This enzyme plays an important role in the synthesis and repair of DNA. For this purpose, a promising potent molecule called Triapine™ was designed. The compound is a thiosemicarbazone that affects tyrosyl radical in R2/p53R2 by forming a redox active complex with iron, producing reactive oxygen species. Some studies have revealed that triapine shows considerably higher efficacy than N-hydroxyurea in both enzyme and cell assays. Recent studies also show that some thiosemicarbazone derivatives inhibit topoisomerase II α enzymeCitation9–12. However, some naphthalene derivatives have also been reported as potent apoptosis inducers, P-glycoprotein inhibitors, microtubule inhibitors or glutamamide analogsCitation13–21.
On the basis of afore-mentioned findings, herein we reported the synthesis and evaluation of a new series of naphthalene-based thiosemicarbazone derivatives as anticancer agents against LNCaP cells.
Methods
Chemistry
All reagents were purchased from commercial suppliers and were used without further purification. Melting points were determined on an Electrothermal 9100 melting point apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. The 1H NMR and 13C NMR spectra of the compounds were recorded on a Bruker spectrometer (Bruker, Billerica, MA). Mass spectra were recorded on an Agilent LC-MSD-Trap-SL Mass spectrometer (Agilent Technologies, Palo Alto, CA). Elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyzer (Perkin-Elmer, Norwalk, CT). Thin Layer Chromatography (TLC) was performed on TLC Silica gel 60 F254 aluminum sheets (Merck, Darmstadt, Germany) to check the purity of the compounds.
General procedure for the synthesis of the compounds
4-(Naphthalen-1-yl)thiosemicarbazide (A)
A mixture of 1-naphtyl isothiocyanate (0.1 mol) and hydrazine hydrate (0.2 mol) in ethanol (30 mL) was stirred at room temperature for 4 h and then filtered. The residue was crystallized from ethanol.
4-(Naphthalen-1-yl)-1-(1-phenylethylidene)thiosemicarbazides (1–14)
A mixture of 4-(naphthalen-1-yl)thiosemicarbazide (A) (0.01 mol) and aromatic ketone (0.01 mol) was refluxed in ethanol for 8 h, filtered and crystallized from ethanol.
4-(Naphthalen-1-yl)-1-(1-phenylethylidene)thiosemicarbazide (1)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.43 (3H, s, CH3), 7.34–7.41 (3H, m, aromatic), 7.51–7.61 (5H, m, aromatic), 7.96–7.99 (4H, m, aromatic), 10.33 (1H, s, N–H), 10.71 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.22 (CH3), 123.17 (CH), 125.32 (CH), 125.88 (CH), 125.96 (C), 126.22 (CH), 126.48 (CH), 126.77 (CH), 127.91 (CH), 128.09 (CH), 128.30 (CH), 129.23 (CH), 129.46 (CH), 133.59 (CH), 135.77 (C), 137.30 (C), 137.45 (C), 148.52 (C), 178.80 (C).
MS (ESI) (m/z): [M + 1]+ 320.
Anal. Calcd. for C19H17N3S: C, 71.44; H, 5.36; N, 13.16. Found: C, 71.43; H, 5.34; N, 13.15.
4-(Naphthalen-1-yl)-1-[1-(4-fluorophenyl)ethylidene)thiosemicarbazide (2)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.33 (3H, s, CH3), 7.18–7.30 (4H, m, aromatic), 7.47–7.61 (5H, m, aromatic), 7.81–7.86 (1H, m, aromatic), 7.93–7.99 (1H, m, aromatic), 10.44 (1H, s, N–H), 10.86 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.22 (CH3), 114.87 (CH), 115.08 (CH), 115.21 (CH), 123.33 (CH), 124.59 (CH), 125.43 (C), 126.30 (CH), 126.61 (CH), 126.88 (CH), 127.99 (CH), 128.99 (CH), 130.57 (CH), 133.69 (C), 133.94 (C), 134.04 (C), 147.65 (C), 164.17 (C), 178.90 (C).
MS (ESI) (m/z): [M + 1]+ 338.
Anal. Calcd. for C19H16FN3S: C, 67.63; H, 4.78; N, 12.45. Found: C, 67.65; H, 4.77; N, 12.46.
4-(Naphthalen-1-yl)-1-[1-(4-chlorophenyl)ethylidene)thiosemicarbazide (3)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.41 (3H, s, CH3), 7.32–7.43 (2H, m, aromatic), 7.45–7.60 (5H, m, aromatic), 7.89–7.93 (2H, m, aromatic), 8.11–8.12 (2H, m, aromatic), 10.37 (1H, s, N–H), 10.75 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.64 (CH3), 114.96 (CH), 121.89 (CH), 122.38 (CH), 123.33 (C), 125.41 (CH), 126.06 (CH), 126.9 (CH), 128.11 (CH), 128.28 (CH), 128.45 (CH), 128.69 (CH), 130.56 (CH), 134.59 (C), 135.89 (C), 136.38 (C), 136.53 (C), 147.31 (C), 178.95 (C).
MS (ESI) (m/z): [M + 1]+ 354.
Anal. Calcd. for C19H16ClN3S: C, 64.49; H, 4.56; N, 11.87. Found: C, 64.50; H, 4.55; N, 11.88.
4-(Naphthalen-1-yl)-1-[1-(4-bromophenyl)ethylidene)thiosemicarbazide (4)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.41 (3H, s, CH3), 7.35–7.44 (1H, m, aromatic), 7.53–7.58 (7H, m, aromatic), 7.84–7.86 (1H, m, aromatic), 8.06–8.08 (2H, m, aromatic), 10.38 (1H, s, N–H), 10.76 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.14 (CH3), 122.87 (CH), 123.32 (CH), 123.43 (CH), 125.42 (C), 125.98 (CH), 126.07 (C), 126.40 (CH), 126.91 (CH), 127.98 (CH), 128.53 (CH), 128.95 (CH), 131.04 (CH), 131.38 (CH), 133.67 (C), 135.88 (C), 136.76 (C), 147.39 (C), 178.94 (C).
MS (ESI) (m/z): [M+ + 2] 400.
Anal. Calcd. for C19H16BrN3S: C, 57.29; H, 4.05; N, 10.55. Found: C, 57.28; H, 4.06; N, 10.56.
4-(Naphthalen-1-yl)-1-[1-(4-nitrophenyl)ethylidene)thiosemicarbazide (5)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.48 (3H, s, CH3), 7.48–7.59 (4H, m, aromatic), 7.82–7.85 (1H, m, aromatic), 7.91–7.92 (1H, m, aromatic), 7.97–8.00 (1H, m, aromatic), 8.16–8.24 (3H, m, aromatic), 8.32–8.37 (1H, m, aromatic), 10.49 (1H, s, N–H), 10.94 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.27 (CH3), 123.18 (CH), 123.33 (CH), 123.64 (CH), 125.45 (C), 126.04 (CH), 126.14 (CH), 126.48 (CH), 127.05 (CH), 127.86 (CH), 128.01 (CH), 130.52 (CH), 133.7 (CH), 135.81 (C), 143.79 (C), 146.07 (C), 147.46 (2C), 179.2 (C).
MS (ESI) (m/z): [M + 1]+ 365.
Anal. Calcd. for C19H16N4O2S: C, 62.62; H, 4.43; N, 15.37. Found: C, 62.63; H, 4.42; N, 15.38.
4-(Naphthalen-1-yl)-1-[1-(4-hydroxyphenyl)ethylidene)thiosemicarbazide (6)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.37 (3H, s, CH3), 6.76–6.80 (2H, m, aromatic), 7.47–7.61 (4H, m, aromatic), 7.82–8.04 (5H, m, aromatic), 9.76 (1H, s, O–H), 10.23 (1H, s, N–H), 10.57 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.10 (CH3), 114.95 (2CH), 123.28 (CH), 125.40 (CH), 125.95 (C), 126.04 (CH), 126.27 (CH), 126.74 (CH), 127.98 (CH), 128.37 (CH), 128.54 (2CH), 130.53 (C), 133.67 (C), 135.91 (C), 149.02 (C), 158. 84 (C), 178.48 (C).
MS (ESI) (m/z): [M + 1]+ 336.
Anal. Calcd. for C19H17N3OS: C, 68.04; H, 5.11; N, 12.53. Found: C, 68.05; H, 5.12; N, 12.54.
4-(Naphthalen-1-yl)-1-[1-(4-methoxyphenyl)ethylidene)thiosemicarbazide (7)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.40 (3H, s, CH3), 3.78 (3H, s, O-CH3), 6.92–6.95 (2H, m, aromatic), 7.49–7.60 (4H, m, aromatic), 7.82–7.92 (3H, m, aromatic), 7.99–8.05 (2H, m, aromatic), 10.29 (1H, s, N–H), 10.62 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.18 (CH3), 55.20 (CH3), 113.52 (CH), 113.72 (CH), 113.78 (CH), 123.25 (CH), 125.39 (CH), 125.95 (C), 126.04 (CH), 126.26 (CH), 126.76 (CH), 127.99 (CH), 128.45 (CH), 129.96 (CH), 130.51 (C), 133.68 (C), 135.89 (C), 148.63 (C), 160.35 (C), 178.59 (C).
MS (ESI) (m/z): [M + 1]+ 350.
Anal. Calcd. for C20H19N3OS: C, 68.74; H, 5.48; N, 12.02. Found: C, 68.75; H, 5.49; N, 12.03.
4-(Naphthalen-1-yl)-1-[1-(4-methylphenyl)ethylidene)thiosemicarbazide (8)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.32–2.34 (3H, m, CH3), 2.40 (3H, s, CH3), 7.19–7.26 (2H, m, aromatic), 7.49–7.58 (4H, m, aromatic), 7.82–8.02 (5H, m, aromatic), 10.31 (1H, s, N–H), 10.66 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.24 (CH3), 20.87 (CH3), 123.26 (CH), 125.41 (CH), 125.96 (CH), 126.05 (C), 126.29 (CH), 126.82 (2CH), 128.00 (CH), 128.79 (2CH), 130.51 (CH), 133.68 (CH), 134.77 (C), 135.88 (C), 138.98 (2C), 148.73 (C), 178.73 (C).
MS (ESI) (m/z): [M + 1]+ 334.
Anal. Calcd. for C20H19N3S: C, 72.04; H, 5.74; N, 12.60. Found: C, 72.05; H, 5.75; N, 12.61.
4-(Naphthalen-1-yl)-1-[1-(4-trifluoromethylphenyl)ethylidene)thiosemicarbazide (9)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.37 (3H, s, CH3), 7.47–7.61 (6H, m, aromatic), 7.71–7.78 (4H, m, aromatic), 7.90–7.91 (1H, m, aromatic), 10.44 (1H, s, N–H), 10.86 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.30 (CH3), 122.86 (CH), 123.31 (CH), 124.91 (CH), 125.43 (C), 125.57 (C), 126.01 (CH), 126.10 (CH), 126.45 (CH), 126.63 (CH), 127.60 (2CH), 128.00 (CH), 130.53 (CH), 133.69 (C), 135.83 (C), 141.40 (C), 141.51 (C), 146.86 (C), 179.13 (C).
MS (ESI) (m/z): [M + 1]+ 388.
Anal. Calcd. for C20H16F3N3S: C, 62.00; H, 4.16; N, 10.85. Found: C, 62.01; H, 4.17; N, 10.86.
4-(Naphthalen-1-yl)-1-[1-(4-methylsulphonylphenyl)ethylidene)thiosemicarbazide (10)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.47 (3H, s, CH3), 3.23 (3H, s, CH3), 7.51–7.58 (6H, m, aromatic), 7.89–7.91 (4H, m, aromatic), 8.15–8.16 (1H, m, aromatic), 10.46 (1H, s, N–H), 10.88 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.35 (CH3), 43.47 (CH3), 123.31 (CH), 125.45 (CH), 126.04 (CH), 126.13 (C), 126.48 (CH), 126.74 (CH), 127.18 (CH), 127.45 (CH), 127.68 (CH), 128.02 (CH), 130.53 (CH), 133.70 (CH), 135.82 (C), 140.80 (C), 141.57 (C), 142.37 (C), 146.67 (C), 179.14 (C).
MS (ESI) (m/z): [M + 1]+ 398.
Anal. Calcd. for C20H16F3N3S: C, 60.43; H, 4.82; N, 10.57. Found: C, 60.44; H, 4.83; N, 10.58.
4-(Naphthalen-1-yl)-1-[1-(4-cyanophenyl)ethylidene)thiosemicarbazide (11)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.44 (3H, s, CH3), 7.49–7.58 (4H, m, aromatic), 7.83–7.99 (5H, m, aromatic), 8.26–8.30 (2H, m, aromatic), 10.47 (1H, s, N–H), 10.87 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.10 (CH3), 111.31 (CH), 111.36 (C), 118.83 (C), 123.33 (CH), 125.45 (CH), 126.07 (C), 126.11 (CH), 126.48 (CH), 127.01 (CH), 127.61 (CH), 127.98 (CH), 130.55 (CH), 132.04 (2CH), 133.69 (C), 135.86 (C), 141.87 (C), 146.46 (C), 179.14 (C).
MS (ESI) (m/z): [M + 1]+ 345.
Anal. Calcd. for C20H16N4S: C, 69.74; H, 4.68; N, 16.27. Found: C, 69.75; H, 4.69; N, 16.28.
4-(Naphthalen-1-yl)-1-[1-(4-trifluoromethoxyphenyl)ethylidene)thiosemicarbazide (12)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.34 and 2.37 (3H, 2 s, CH3), 7.36–7.41 (4H, m, aromatic), 7.48–7.49 (2H, m, aromatic), 7.81–7.91 (2H, m, aromatic), 8.09–8.10 (2H, m, aromatic), 8.18–8.22 (1H, m, aromatic), 10.58 (1H, s, N–H), 10.95 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.22 (CH3), 118.74 (CH), 120.50 (CH), 120.71 (CH), 121.29 (CH), 123.84 (CH), 124.57 (C), 125.28 (CH), 125.88 (CH), 126.61 (CH), 127.61 (CH), 128.69 (2CH), 128.95 (C), 133.69 (CF3), 136.70 (2C), 147.82 (C), 149.03 (C), 176.79 (C).
MS (ESI) (m/z): [M + 1]+ 404.
Anal. Calcd. for C20H16F3N3OS: C, 59.55; H, 4.00; N, 10.42. Found: C, 59.56; H, 4.01; N, 10.43.
4-(Naphthalen-1-yl)-1-[1-(4-(4-morpholinyl)phenyl)ethylidene)thiosemicarbazide (13)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 2.28 (3H, s, CH3), 3.17 (4H, s, morpholine), 3.72 (4H, s, morpholine), 6.91–6.92 (3H, m, aromatic), 7.50–7.56 (6H, m, aromatic), 8.26–8.30 (2H, m, aromatic), 10.25 (1H, s, N–H), 10.56 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 14.02 (CH3), 47.63 (2CH2), 65.95 (2CH2), 113.79 (CH), 113.88 (2CH), 123.19 (CH), 125.39 (CH), 125.95 (C), 126.04 (CH), 126.16 (CH), 126.70 (C), 127.72 (CH), 127.92 (CH), 128.01 (CH), 130.46 (CH), 133.67 (C), 135.86 (C), 149.03 (C), 151.75 (C), 178.31 (C).
MS (ESI) (m/z): [M + 1]+ 405.
Anal. Calcd. for C23H24N4OS: C, 68.29; H, 5.98; N, 13.85. Found: C, 68.28; H, 5.97; N, 13.86.
4-(Naphthalen-1-yl)-1-[1-(4-(1-piperidinyl)phenyl)ethylidene)thiosemicarbazide (14)
1H NMR (500 MHz) (DMSO-d6) δ (ppm): 1.54–1.58 (6H, m, piperidine), 2.36 (3H, s, CH3), 3.20–3.31 (4H, m, piperidine), 6.81–6.90 (6H, m, aromatic), 7.49–7.63 (5H, m, aromatic), 10.22 (1H, s, N–H), 10.55 (1H, s, N–H).
13C NMR (100 MHz) (DMSO-d6) δ (ppm): 13.97 (CH3), 23.91 (CH2), 24.94 (2CH2), 48.44 (CH2), 48.59 (CH2), 114.55 (CH), 114.21 (2CH), 123.14 (CH), 125.37 (CH), 125.93 (C), 126.02 (CH), 126.59 (CH), 126.64 (C), 127.59 (CH), 127.93 (CH), 128.00 (CH), 130.41 (CH), 133.66 (C), 135.83 (C), 149.17 (C), 151.98 (C), 178.19 (C).
MS (ESI) (m/z): [M + 1]+ 403.
Anal. Calcd. for C24H26N4S: C, 66.22; H, 6.79; N, 17.16 Found: C, 66.23; H, 6.78; N, 17.17.
Cytotoxicity
Drug preparation
Compounds 1–14 were dissolved in dimethyl sulfoxide (DMSO, Sigma, St Louis, MO) and diluted further with Dulbecco’s modified Eagle’s medium (DMEM, Sigma) to obtain the required final concentrations (5, 10, 25, 50 and 75 µM).
Cell culture
Human prostate cancer cell line (LNCaP) was obtained from the American Type Culture Collection and grown in complete medium containing DMEM supplemented with 10% fetal calf serum (Sigma) and 1% penicillin (10 000 unit)–streptomycin (10 mg/mL) solution (Sigma) in a humidified atmosphere of 95% O2 and 5% CO2 in air at 37 °C. When confluence was achieved, the cells were detached with 0.25% trypsin-EDTA (Sigma), centrifuged at 1200 rpm, 4 °C for 5 min and counted with a cell counter (CEDEX, Roche, Mannheim, Germany).
Experimental groups
Control: Complete medium only.
DMSO group: Complete medium with a final concentration of 0.1% DMSO (solvent).
Compounds 1–14: Treated with 5, 10, 25, 50 and 75 µM for 24 h.
MTT assay
LNCaP cells were first incubated until confluence and then inoculated into 96-well plates (1 × 104/well). The drugs were applied after 24 h and the cells incubated further for 24 h. Cellular survival in the presence or absence of drugs was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) colorimetric assayCitation22. When applicable, MTT test was applied and cell survival was determined by measuring the formazan absorbance at 550 nm with a microplate reader (BioTek, Winooski, VT). Since cell number in each well is proportional to the absorbance of solubilized formazan, the optical density read from the drug-treated wells was converted to a percentage of living cells against the control using the following formula:
Flow cytometric analysis
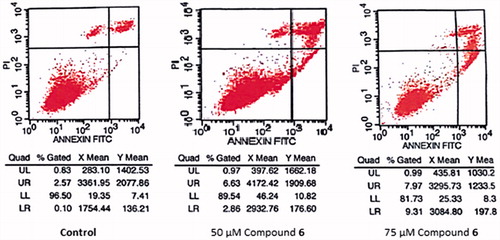
Since the half maximal inhibitory concentration (IC50) value of compound 6 was calculated as 75 µM for 24 h treatment, 50 and 75 µM doses of the compound were used in our subsequent flow cytometric analysis for the detection of type of cell death. Apoptotic cells were determined by staining with fluorescein isothiocyanate (FITC)-labeled Annexin V (Invitrogen, Camarillo, CA) and subsequent flow cytometric analysis. The cells were seeded in 25 cm2 flasks at a density of 1 × 106 cells/flask, incubated with or without compound for 24 h and then stained according to the manufacturer’s instructions. Cell suspensions were centrifuged at 1200 rpm and 4 °C for 5 min twice in cold phosphate buffered saline (Sigma) and then resuspended in binding buffer (1 × 106 in 100 µL). After adding 5 µL Annexin V with FITC and 10 µL propidium iodide (PI), the cells were incubated in the dark at room temperature for 15 min. The samples were then diluted with 400 µL binding buffer, analyzed by a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) and discriminated by CellQuest data acquisition programme (San Jose, CA). Quadrant settings were based on the control samples and at least 15 × 103 cells were analyzed per sample. Annexin V assay is based on detecting of phosphatidylserine that translocates from the inner to the outer lipid layer of plasma membrane during early apoptosis. Alive cells are negative for both PI and Annexin V, early apoptotic cells are PI negative but Annexin V positive while dead/late apoptotic cells are positive for both PI and Annexin VCitation23.
Statistical analysis
Data were expressed as the mean percent fraction of control ± standard error of mean. Statistical significance ascertained by one way analysis of variance followed by Tukey’s multiple comparison test. The apoptotic results were depicted as percentage of cells. All results are the mean of at least three independent assays and the p value less than 0.05 was considered to be significant.
Results and discussion
The synthesis of thiosemicarbazone derivatives (1–14) was carried out according to the steps depicted in Scheme 1. In the initial step, 4-(naphthalen-1-yl)thiosemicarbazide (A) was synthesized via the reaction of 1-naphtyl isothiocyanate with hydrazine hydrate. The reaction of 4-(naphthalen-1-yl)thiosemicarbazide (A) with aromatic ketones afforded 4-(naphthalen-1-yl)-1-(1-phenylethylidene)thiosemicarbazides (1–14). Some properties of the compounds are given in . The 1H NMR, 13C NMR, mass spectral data and elemental analyses were in agreement with the proposed structures of the compounds (1–14).
Scheme 1. The synthetic route for the preparation of the thiosemicarbazone derivatives (1–14). Reagents and conditions: (i) NH2NH2·H2O, ethanol, r.t., 4 h; (ii) ArCOCH3, ethanol, reflux, 8 h.
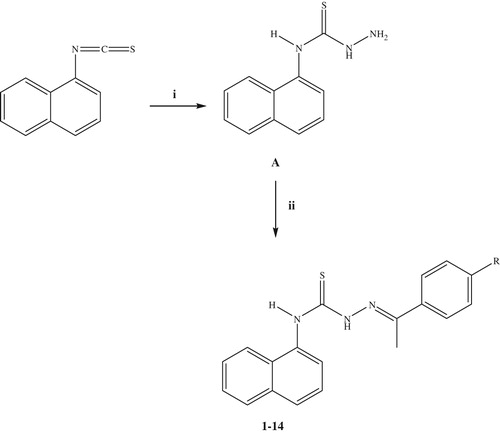
Table 1. Some properties of the thiosemicarbazone derivatives (1–14).
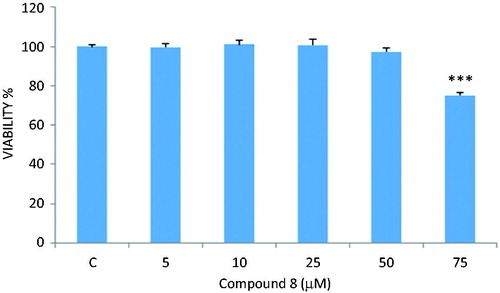
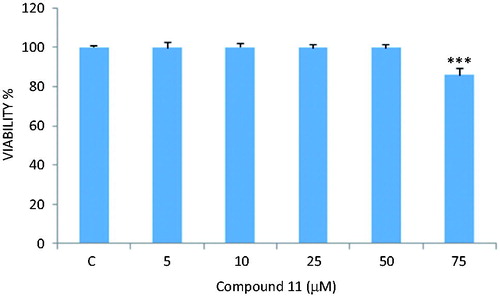
MTT assay was carried out to determine the cytotoxic effects of the compounds (1–14) on LNCaP cells. Compound 6 did not exhibit any cytotoxic activity against LNCaP cells at the concentrations of 5, 10 and 25 µM, whereas the compound decreased the viability percentages to 83% and 45% at the concentrations of 50 and 75 µM, respectively (). Compounds 8 and 11 decreased significantly the viability percentages to ∼75% and 85% at the concentration of 75 µM, respectively ( and ). Other compounds did not cause a significant change on cell viability rates at the concentrations of 10, 25, 50 and 75 µM.
Figure 1. Reducing effect of compound 6 on the number of living LNCaP cells. *p < 0.05, ***p < 0.001.
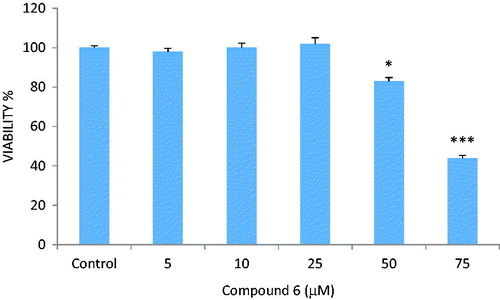
Figure 3. MTT analysis results of LNCaP treated with different concentrations of compound 11. Cell viability inhibiting effect was found with only 75 µM. ***p < 0.001.
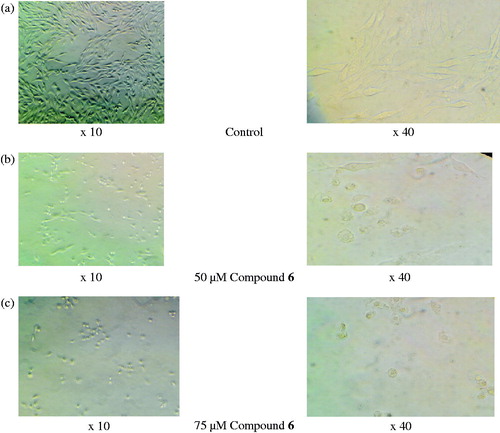
When compared with control groups, compound 6 decreased the cell viability rate more than 50% (50 and 75 µM) and therefore flow cytometric analysis was carried out to determine the apoptotic effects of compound 6 ( and ). The assay revealed that the rate of apoptosis increased dose-dependently for 24 h when compared with control groups (). The increased activity can be attributed to the presence of p-hydroxyl group due to its −σ and −π effects and ability to form readily hydrogen bonds.
Table 2. Apoptotic percentages of the control and drug groups (n = 3).
Conclusion
In the present article, new naphthalene-based thiosemicarbazone derivatives were synthesized and evaluated for their cytotoxicity against LNCaP cancer cell line. According to MTT assay, compounds 6, 8 and 11 exhibited inhibitory effect on LNCaP cells. Among these compounds, compound 6, which caused more than 50% death on LNCaP cells, was chosen for flow cytometric analysis of apoptosis. Flow cytometric analysis indicated that compound 6 also showed apoptotic effect on LNCaP cells.
Declaration of interest
The authors report no conflicts of interest.
References
- Silverstein A, Silverstein V, Nunn Silverstein L. Cancer. Minneapolis (MN): Twenty-First Century Books; 2006
- Gerritsen WR. The evolving role of immunotherapy in prostate cancer. Ann Oncol 2012;23:22–7
- Culig Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol 2014;2:231–8
- Kung HJ, Evans CP. Oncogenic activation of androgen receptor. Urol Oncol 2009;27:48–52
- Suzuki H, Ueda T, Ichikawa T, Ito H. Androgen receptor involvement in the progression of prostate cancer. Endocr Relat Cancer 2003;10:209–16
- Chuu CP, Kokontis JM, Hiipakka RA, et al. Androgens as therapy for androgen receptor positive castration-resistant prostate cancer. J Biomed Sci 2011;18:1–11
- Culig Z, Klocker H, Bartsch G, Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer 2002;9:155–70
- Nouri M, Ratther E, Stylianou N, et al. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: an opportunity for intervention. Front Oncol 2014;4:371–6
- Yu Y, Kalinowski DS, Kovacevic Z, et al. Thiosemicarbazones from the old to new: iron chelators that are more than just ribonucleotide reductas inhibitors. J Med Chem 2009;52:5271–94
- Chapman TR, Kinsella TJ. Ribonucleotide reductase inhibitor s: a new look at an old target for radiosensitization. Front Oncol 2012;1:1–6
- Moorthy NS, Cerqueira NM, Ramos MJ, Fernandes PA. Aryl- and heteroaryl-thiosemicarbazone derivatives and their metal complexes: a pharmacological template. Rec Pat Anticancer Drug Discov 2013;8:168–82
- Kalinowski DS, Quach P, Richardson DR. Thiosemicarbazones: the new wave in cancer treatment. Future Med Chem 2009;1:1143–51
- Álvarez C, Álvarez R, Corchete P, et al. Synthesis and biological activity of naphthalene analogues of phenstatins: naphthylphenstatins. Bioorg Med Chem Lett 2007;17:3417–20
- Jiang S, Crogan-Grundy C, Drewe J, et al. Discovery of (naphthalen-4-yl)(phenyl)methanones and N-methyl-N-phenylnaphthalen-1-amines as new apoptosis inducers using a cell- and caspase-based HTS assay. Bioorg Med Chem Lett 2008;18:5725–8
- Yuan JW, Wang SF, Luo ZL, et al. Synthesis and biological evaluation of compounds which contain pyrazole, thiazole and naphthalene ring as antitumor agents. Bioorg Med Chem Lett 2014;24:2324–8
- Halder AK, Adhikary N, Maity MK, Jha T. Synthesis, pharmacological activity and comparative QSAR modeling of 1,5-N,N′-substituted-2-(substituted naphthalenesulphonyl)glutamamides as possible anticancer agents. Eur J Med Chem 2010;45:1760–71
- Rasolofonjatovo E, Provot O, Hamze A, et al. Conformationnally restricted naphthalene derivatives type isocombretastatin A-4 and isoerianin analogues: synthesis, cytotoxicity and antitubulin activity. Eur J Med Chem 2012;52:22–32
- Lokhande TN, Viswanathan CL, Joshia A, Juvekarb A. Design, synthesis and evaluation of naphthalene-2-carboxamides as reversal agents in MDR cancer. Bioorg Med Chem 2006;14:6022–6
- Rajabi M, Khalilzadeh MA, Tavakolinia F, et al. Naphthalene-fused (α-alkoxycarbonyl)methylene-γ-butyrolactones: antiproliferative activity and binding to bovine serum albumin and DNA. DNA Cell Biol 2012;31:783–9
- Medarde M, Maya ABS, Ferez-Melero C. Naphthalene combretastatin analogues: synthesis, cytotoxicity and antitubulin activity. J Enzyme Inhib Med Chem 2004;19:521–40
- Reddy GR, Kuo CC, Tan UK, et al. Synthesis and structure–activity relationships of 2-amino-1-aroylnaphthalene and 2-hydroxy-1-aroylnaphthalenes as potent antitubulin agents. J Med Chem 2008;51:8163–7
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;16:55––63
- van Engeland M, Nieland LJ, Ramaekers FC, et al. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998;31:1–9