Abstract
Twelve 4-benzoyl-1-dichlorobenzoylthiosemicarbazides have been tested as potential antibacterials. All the compounds had MICs between 0.49 and 15.63 µg/ml toward Micrococcus luteus, Bacillus cereus, Bacillus subtilis and Staphylococcus epidermidis indicating, in most cases, equipotent or even more effective action than cefuroxime. In order to clarify if the observed antibacterial effects are universal, further research were undertaken to test inhibitory potency of two most potent compounds 3 and 11 on clinical isolates of Staphylococcus aureus. Compound 11 inhibited the growth of methicillin-sensitive S. aureus (MSSA) at MICs of 1.95–7.81 µg/ml, methicillin-resistant S. aureus (MRSA) at MICs of 0.49–1.95 µg/ml and MDR–MRSA at MIC of 0.98 and 3.90 µg/ml, respectively. Finally, inhibitory efficacy of 3 and 11 on planktonic cells and biofilms formation in clinical isolates of S. aureus and Haemophilus parainfluenzae was tested. The majority of cells in biofilm populations of MSSA and MRSA were eradicated at low level of 3, with MBICs in the range of 7.82–15.63 µg/ml.
Introduction
As is well known and has often been described minutely in literature, the chronic misuse and overuse of antibiotics in medical and agricultural practices has fueled the rise of multi-drug resistant organisms (MDROs), limiting the action of drugs previously considered to be highly activeCitation1–3. Among them, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, extended spectrum beta-lactamase enterobacteria, Clostridium difficile as well as MDR Gram-negative bacteria Pseudomonas aeruginosa are the most important cause of concernCitation1,Citation4–7. The problem of MDROs is particularly evident in hospital settings where the antibiotic usage reaches maximum, but is also found in community-acquired infections. Furthermore, antimicrobial chemotherapy is very often ineffective for the treatment of infections with bacterial biofilm populations, a major cause of nosocomial infectionsCitation8. Indeed, microorganisms commonly attach to living tissue and non-living surfaces, including those of indwelling medical devices, and produce extracellular polymers that facilitate adhesion and provide a structural matrix. In this state, biofilm-associated microorganisms behave differently from planktonic organisms with respect to growth rates and, consequently, are highly resistant to both antibiotics and host immune defensesCitation9. Therefore, there is an urgent need to identify and develop new antibacterial agents that are able to overcome multidrug-resistance mechanisms and persistent infections caused by biofilms.
In recent worksCitation10–15, our group reported the synthesis and evaluation of antibacterial activity of thiosemicarbazide derivatives substituted at N1 position with benzoyl or hetaroyl moiety and at N4 with benzoyl, aryl or alkyl group. From these studies, we were able to conclude that presence of benzoyl nucleus at both N1 and N4 positions of thiosemicarbazide core seems to has a great impact on inhibitory activity of this class of compounds on the growth of Gram-positive bacteria. Considering these perspectives, we synthesized two series of thiosemicarbazide derivatives with the 2,3- or 2,4-dichlorobenzoyl group at N1 position and methyl-, nitro- or chlobenzoyl substituent at N4 and tested them for their antibacterial activity against a panel of Gram-positive and Gram-negative reference strains and – for representative model compounds – for their cytotoxicity against HeLa and L929 cell lines. In this article, we present results of these in vitro studies, which allowed us to confirm the validity of the aforementioned assumption. In order to clarify if the observed antibacterial effects are universal, further research were undertaken to test inhibitory effect of two the most potent compounds on 16 methicillin-sensitive and MRSA and two MDR–MRSA clinical isolates. Finally, these compounds were also tested on planktonic cells and biofilms formation in all the above MSSA and MRSA pathogens and in 13 clinical isolates of opportunistic Haemophilus parainfluenzae. Studies of these treatments have shown some promising results that we also report in this contribution.
Methods
General comments
All the reagents were purchased from Alfa–Aesar (Lancashire, United Kingdom) and Sigma–Aldrich (Steinheim, Germany), and used without further purification. Melting points were determined by using Fischer–Johns apparatus (Sanyo, Japan) and are uncorrected. The 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance instrument using dimethyl sulfoxide (DMSO)-d6 as a solvent and TMS as an internal standard. Chemical shifts are expressed as δ (ppm). MS spectra were recorded on Bruker microTOF-Q II and processed using Compass Data Analysis software. The purity of the compounds was checked by TLC on plates precoated with silica gel Si 60 F254, produced by Merck Co. (Darmstadt, Germany). The spots were detected by exposure to UV-lamp at λ = 254 nm. Elemental analyses were performed on AMZ 851 CHX analyser (PG, Gdansk, Poland) and the results were within ±0.4% of the theoretical value. The structures of compounds 7–12 are known (CAS numbers: 648863-38-1, 648880-12-0, 648867-59-8, 649541-88-8, 812687-54-0, 649542-50-7, respectively), however there are no references reporting their use, preparation or physicochemical characterization, therefore their data are included into this article.
General procedure for the synthesis of 4-benzoyl-1-dichlorobenzoyl-thiosemicarbazides (1–12)
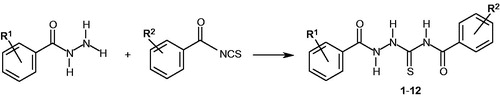
A solution of 0.01 mol of 2,3-dichloro- or 2,4-dichlorobenzoic acid hydrazide and equimolar amount of appropriate benzoyl isothiocyanate derivative in 25 ml of anhydrous ethanol was heated under reflux for 5 min. Next, the solution was cooled and the solid formed was filtered off, washed with diethyl ether, dried and crystallized from ethanol.
1-(2,3-dichlorobenzoyl)-4-(2-methylbenzoyl)-thiosemicarbazide (1)
Yield: 70%, m.p. 178–80 °C, 1H-NMR (300 MHz): 2.47 (s, 3H, CH3), 7.28–7.62 (m, 5H, Ar-H), 7.69 (dd, 1H, ArH, J = 1.4 Hz, 7.7 Hz), 7.86 (dd, 1H, Ar-H, J = 1.4 Hz, 7.9 Hz), 11.43, 11.99, 12.42 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 18.07, 124.22, 126.78, 127.11, 127.52, 129.31, 129.68, 130.91, 132.27, 132.38, 134.69, 134.86, 161.73, 168.49, 178.48. Anal. calc. for C16H13Cl2N3O2S (%): C 50.3, H 3.4, N 11.0. Found: C 50.1, H 3.7, N 11.1. HRMS (ESI): calculated for [M + H]+ = 382.018; measured: 382.013.
1-(2,3-dichlorobenzoyl)-4-(3-methylbenzoyl)-thiosemicarbazide (2)
Yield: 83%, m.p. 176–8 °C, 1H-NMR (300 MHz): 2.46 (s, 3H, CH3), 7.45–7.60 (m, 3H, Ar-H), 7.67–7.73 (m, 1H, Ar-H), 7.80–7.90 (m, 3H, Ar-H), 11.43, 11.82, 12.48 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 19.44, 124.57, 126.81, 127.06, 127.50, 127.80, 130.31, 130.39, 130.93, 132.86, 134.86, 136.54, 161.71, 166.51, 178.85. Anal. calc. for C16H13Cl2N3O2S (%): C 50.3, H 3.4, N 11.0. Found: C 50.5, H 3.7, N 11.2. HRMS (ESI): calculated for [M + H]+ = 382.018; measured: 382.018.
1-(2,3-dichlorobenzoyl)-4-(4-nitrobenzoyl)-thiosemicarbazide (3)
Yield: 67%, m.p. 222–4 °C, 1H-NMR (300 MHz): 7.57 (t, 1H, Ar-H, J = 7.7 Hz), 7.70 (dd, 1H, Ar-H, J = 1.6 Hz, 7.7 Hz), 7.86 (dd, 1H, Ar-H, J = 1.6 Hz, 7.7 Hz), 8.24 (dd, 2H, Ar-H, J = 2.0 Hz, 6.9 Hz), 8.42 (dd, 2H, Ar-H, J = 2.0 Hz, 6.9 Hz), 11.47, 12.24, 12.35 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 122.01, 126.80, 127.10, 127.50, 128.97, 130.65, 130.94, 134.83, 148.51, 161.72, 164.87, 178.25. Anal. calc. for C15H10Cl2N4O4S (%): C 43.6, H 2.4, N 13.6. Found: C 43.5, H 2.6, N 13.5. HRMS (ESI): calculated for [M + H]+ = 412.987; measured: 412.989.
1-(2,3-dichlorobenzoyl)-4-(2-chlorobenzoyl)-thiosemicarbazide (4)
Yield: 68%, m.p. 182–4 °C, 1H-NMR (300 MHz): 7.48–7.72 (m, 6H, Ar-H), 7.86 (dd, 1H, Ar-H, J = 1.6 Hz, 7.9 Hz), 11.44 (s, 1H, NH), 12.26 (s, 2H, 2 × NH). 13C-NMR (75 MHz): 125.81, 126.82, 127.13, 127.51, 127.90, 128.25, 128.59, 130.65, 130.83, 130.92, 132.69, 134.83, 161.72, 165.67, 178.13. Anal. calc. for C15H10Cl3N3O2S (%): C 44.7, H 2.5, N 10.4. Found: C 44.8, H 2.6, N 10.7. HRMS (ESI): calculated for [M + H]+ = 401.963; measured: 401.964.
1-(2,3-dichlorobenzoyl)-4-(3-chlorobenzoyl)-thiosemicarbazide (5)
Yield: 72%, m.p. 196–8 °C, 1H-NMR (300 MHz): 7.51–8.27 (m, 7H, Ar-H), 11.44, 12.02, 12.34 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 126.09, 126.78, 127.11, 127.48, 129.08, 129.36, 130.67, 130.94, 131.52, 131.81, 132.51, 134.76, 161.78, 164.99, 178.58. Anal. calc. for C15H10Cl3N3O2S (%): C 44.7, H 2.5, N 10.4. Found: C 44.5, H 2.8, N 10.1. HRMS (ESI): calculated for [M + H]+ = 401.963; measured: 401.965.
1-(2,3-dichlorobenzoyl)-4-(4-chlorobenzoyl)-thiosemicarbazide (6)
Yield: 77%, m.p. 208–10 °C, 1H-NMR (300 MHz): 7.56 (t, 1H, Ar-H, J = 7.8 Hz), 7.64–7.72 (m, 3H, Ar-H), 7.84 (dd, 1H, Ar-H, J = 1.6 Hz, 7.9 Hz), 8.04 (dd, 2H, Ar-H, J = 2.0 Hz, 6.8 Hz), 11.45, 11.97, 12.37 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 128.47, 128.82, 128.92, 131.05, 132.36, 132.66, 136.48, 138.45, 163.47, 167.06, 180.41. Anal. calc. for C15H10Cl3N3O2S (%): C 44.7, H 2.5, N 10.4. Found: C 44.4, H 2.2, N 10.4. HRMS (ESI): calculated for [M + H]+ = 401.963; measured: 401.963.
1-(2,4-dichlorobenzoyl)-4-(2-methylbenzoyl)-thiosemicarbazide (7)
Yield: 80%, m.p. 190–2 °C, 1H-NMR (300 MHz): 2.36 (s, 3H, CH3), 7.21–7.74 (m, 7H, Ar-H), 11.27, 11.87, 12.30 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 18.06, 124.22, 125.98, 126.72, 128.08, 129.31, 129.70, 130.67, 131.27, 132.28, 132.36, 134.23, 136.68, 161.54, 168.49, 178.43. Anal. calc. for C16H13Cl2N3O2S (%): C 50.3, H 3.4, N 11.0. Found: C 50.0, H 3.5, N 11.3.
1-(2,4-dichlorobenzoyl)-4-(3-methylbenzoyl)-thiosemicarbazide (8)
Yield: 85%, m.p. 204–6 °C, 1H-NMR (300 MHz): 2.35 (s, 3H, CH3), 7.33–7.47 (m, 2H, Ar-H), 7.54 (dd, 1H, Ar-H, J = 2.0 Hz, 8.2 Hz), 7.63–7.81 (m, 4H, Ar-H), 11.25, 11.71, 12.36 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 21.14, 126.26, 127.68, 128.76, 129.50, 129.81, 131.42, 132.03, 132.37, 132.97, 134.17, 135.95, 138.25, 163.28, 168.13, 180.57. Anal. calc. for C16H13Cl2N3O2S (%): C 50.3, H 3.4, N 11.0. Found: C 50.4, H 3.5, N 11.2.
1-(2,4-dichlorobenzoyl)-4-(4-nitrobenzoyl)-thiosemicarbazide (9)
Yield: 73%, m.p. 208–10 °C, 1H-NMR (300 MHz): 7.54 (dd, 1H, Ar-H, J = 2.2 Hz, 8.2 Hz), 7.62–7.73 (m, 2H, Ar-H), 8.13 (dd, 2H, Ar-H, J = 2.0 Hz, 6.9 Hz), 8.30 (dd, 2H, Ar-H, J = 2.0 Hz, 6.9 Hz), 11.30 (s, 1H, NH), 12.15 (s, 2H, 2 × NH). 13C-NMR (75 MHz): 123.72, 127.70, 129.81, 130.67, 131.42, 132.38, 132.94, 135.95, 138.12, 150.22, 163.29, 166.53, 180.11. Anal. calc. for C15H10Cl2N4O4S (%): C 43.6, H 2.4, N 13.6. Found: C 43.5, H 2.4, N 13.5.
1-(2,4-dichlorobenzoyl)-4-(2-chlorobenzoyl)-thiosemicarbazide (10)
Yield: 70%, m.p. 176–8 °C, 1H-NMR (300 MHz): 7.47–7.84 (m, 7H, Ar-H), 11.38 (s, 1H, NH), 12.25 (s, 2H, 2 × NH). 13C-NMR (75 MHz): 125.79, 125.98, 127.90, 128.10, 128.23, 128.58, 129.71, 130.68, 130.82, 131.26, 132.69, 134.24, 161.56, 165.61, 178.15. Anal. calc. for C15H10Cl3N3O2S (%): C 44.7, H 2.5, N 10.4. Found: C 44.8, H 2.7, N 10.6.
1-(2,4-dichlorobenzoyl)-4-(3-chlorobenzoyl)-thiosemicarbazide (11)
Yield: 78%, m.p. 214–6 °C, 1H-NMR (300 MHz): 7.48–7.58 (m, 2H, Ar-H), 7.62–7.73 (m, 3H, Ar-H), 7.97–8.01 (m, 1H, Ar-H), 11.29, 11.92, 12.27 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 125.97, 126.13, 127.14, 128.10, 129.07, 129.72, 130.67, 131.25, 131.49, 131.78, 132.52, 134.25, 161.56, 164.93, 178.58. Anal. calc. for C15H10Cl3N3O2S (%): C 44.7, H 2.5, N 10.4. Found: C 44.5, H 2.4, N 10.3.
1-(2,4-dichlorobenzoyl)-4-(4-chlorobenzoyl)-thiosemicarbazide (12)
Yield: 83%, m.p. 230–2 °C, 1H-NMR (300 MHz): 7.51–7.72 (m, 5H, Ar-H), 7.95 (d, 2H, Ar-H, J = 8.4 Hz), 11.27, 11.87, 12.29 (3s, 3H, 3 × NH). 13C-NMR (75 MHz): 127.68, 128.89, 129.80, 131.08, 131.41, 132.37, 132.97, 135.94, 138.43, 163.38, 168.03, 180.40. Anal. calc. for C15H10Cl3N3O2S (%): C 44.7, H 2.5, N 10.4. Found: C 44.7, H 2.3, N 10.3.
Antibacterial screening against reference strains and clinical isolates of S. aureus
The antimicrobial activity of the compounds was tested on the Gram-positive strains (S. aureus ATCC 25923, S. aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, Bacillus subtilis ATCC 6633, B. cereus ATCC 10876, Micrococcus luteus ATCC 10240) and on the Gram-negative strains (Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Proteus mirabilis ATCC 12453). Besides, 10 MSSA, 6 MRSA and 2 MDR–MRSA clinical isolates from the collection of the Department of Pharmaceutical Microbiology of Medical University in Lublin were used in our experiments. Microbial suspensions were prepared in sterile saline (0.85% NaCl) with an optical density of 0.5 McFarland standard – 150 × 106 CFU/ml (CFU – colony-forming units). All the stock solutions of the tested compounds were dissolved in DMSO. Mueller–Hinton medium was used with a series of 2-fold dilutions of the tested substances in the range of final concentrations from 3.91 to 1000 µg/ml. Cefuroxime, vancomycin and teicoplanine were used as control antimicrobial agents.
The in vitro antibacterial activity of the tested compounds were screened on the basis of MIC (minimal inhibitory concentration), defined as the lowest concentration of compound at which there was no visible growth of tested microorganisms. Determination of the MIC values was achieved by a broth microdilution method, according to CLSI recommendationCitation16. MBC (minimal bactericidal concentration), defined as the lowest concentration of compound that resulted in >99.9% reduction in CFU of the initial inoculum, was determined by a broth microdilution technique by plating out the contents of wells (20 µl), that showed no visible growth of bacteria, onto Mueller–Hinton agar plates and incubating at 35 °C for 18 h. Both MIC and MBC values are given in µg/ml, according to CLSI referenceCitation16.
Inhibitory effect of compounds 3 and 11 on planktonic cells and biofilm formation by S. aureus and H. parainfluenzae
In order to assay the influence of 3 and 11 against the planktonic cells of S. aureus and H. parainfluenzae isolates, 198 µl of TSB (for S. aureus) or TSB+HTMS (for H. parainfuenzae) medium without (control) and with a series of 2-fold dilution of the tested compound was inoculated with 2 µl of the standardized microbial suspension (total volume per each well – 200 µl), and then incubated for 18 h at 35 °C in the presence of ∼5% CO2. After incubation, spectrophotometric measurements of optical density at wavelength λ = 600 nm (OD600) of the bacterial cultures with or without the tested compound were done by using a microplate reader (ELx800 BioTek) in order to determine MIC. The MIC values were determined by comparison to the growth of a control (compound-free) medium. The experiments were performed in triplicate.
In order to assay the effect of the compounds on biofilm formation, the method based on staining with 0.1% crystal violet described previously by Kaplan and MulksCitation17 with some modificationsCitation18 was used. The activity of the tested compound against biofilm formation was determined on the basis of MBIC, defined as the lowest concentration of the compound at which the biofilm formation was inhibited. In order to assay the influence of the tested compound on the biofilm formation, 198 µl of TSB (for S. aureus) or TSB+HTMS (for H. parainfuenzae) medium without (control) and with a series of 2-fold dilution of the tested compound was inoculated with 2 µl of the standardized microbial suspension (total volume per each well – 200 µl), and then incubated at 35 °C in the presence of ∼5% CO2. After overnight incubation of bacterial culture, the medium above the culture was decanted and then the plates were washed extensively several times with distilled water to remove non-adherent or loosely adherent cells, dried in inverted position and stained with 200 µl of 0.1% crystal violet. The plates were left for 15 min to stain the cells, then washed extensively under distilled water to remove unbound dye. Next, in order to elicit a response to each of the wells, 200 µl of isopropyl alcohol (Color Gram 2 R 3-F, bioMerieux) was added and the plates were left at room temperature for 15 min to solubilize the dye. The optical density of the alcohol–dye solution in each well was read at wavelength λ = 600 nm (OD600) by using a microplate reader (BioTek ELx800). The blank control wells without or with 2-fold dilution of the tested compound added to TSB or TSB+HTMS broth without bacterial suspension were incubated under the same conditions. The experiments were performed in triplicate.
The effects of compounds 2, 8 and 9 on the viability of HeLa and L929 cell lines
The effects of compounds 2, 8 and 9 on the viability of HeLa (ATTC® Catalog No. CCL-2™, human epithelial cells) and L929 (ATTC® Catalog No. CCL-1™, mouse fibroblasts) cell lines were evaluated using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay according to international standards: ISO 10993-5:2009(E)Citation19. The cells were placed into 96-well plates (Falcon) at a density of 2.5 × 105/100 μl/well in culture medium and 100 µl of tested compounds were added to the final concentrations of: 0.50, 1.50, 3.00, 6.50, 12.00 and 25.00 µg/ml. Cells cultured in the non-supplemented medium and supplemented with 1.0% of DMSO were served as control of proliferation. Afterward, the cells were exposed to the tested compounds for 24 h. Then, 1 mg/ml MTT (50 μl/well) was added to each well, incubated at 37 °C, 10% CO2 for 2 h and developed as described in . All the experiments were performed in triplicate.
Computational details
Conformational search was performed using the Amber force field as implemented in HyperChem8.0.3.Citation20 and default convergence criteria. For the most stable conformers, population analysis was carried out using the Merz–Kollman schemeCitation21 at the HF/6-31G theory level with the use of the Gaussian packageCitation22.
Results and discussion
Chemistry
All the title thiosemicarbazide derivatives (compounds 1–12, generalized in ) were synthesized as described previously by our groupCitation11–15 using condensation reactions of 2,3-dichloro- or 2,4-dichlorobenzoic acid hydrazide with appropriate benzoyl isothiocyanate derivative. The obtained compounds were fully characterized by elemental analyses, 1H, and 13C NMR spectra.
Antibacterial screening against a panel of reference strains
Broth microdilution assay was used to evaluate the antibacterial activity of all the synthesized thiosemicarbazide derivatives 1–12 and the antimicrobial results for the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) are reported in . The cefuroxime was included as a control. The data from the assay showed that none of tested compounds showed an inhibitory effect on the growth of Gram-negative bacteria examined up to concentration 1000 µg/ml. In contrast, the title thiosemicarbazides 1–12 had potent activity against Gram-positive bacteria and interesting trends in the structure-activity relationships (SAR) were observed that can be summarized as follows:
With the exception of compounds 1, 6 and 12 with MIC at 1.95 µg/ml, all of the studied thiosemicarbazide derivatives displayed activity stronger than or equipotent to control antibiotic cefuroxime against M. luteus, with MICs in the range of 0.49–0.98 µg/ml. Among them, the best antibacterial activity was found for two 4-nitrophenyl derivatives 3 and 9 as well as for two other compounds with meta substitution, that is for 3-methylphenyl 8 and 3-chlorophenyl 11 derivatives, being independent of the position and electronic nature of the substituent;
Toward Bacillus spp. all of the studied thiosemicarbazide derivatives had a range of MIC values between 0.49 and 15.63 µg/ml, indicating a more effective action than those standard drug cefuroxime. Again, as the indicates, no effect of the position and electronic nature of the substituent on the growth inhibition of B. cereus was observed. Indeed, compounds 3 and 9 containing 4-nitrophenyl group as well as compound 11 with 3-chlorophenyl group demonstrated the most potent activity with MIC at 0.98 µg/ml while compound 12 with 4-chlorophenyl group inhibited bacterial growth at much higher concentration (MIC at 15.63 µg/ml). Toward B. subtilis the most potent agents were three compounds with meta substitution, that is 3-methylphenyl derivatives 2 and 8 and 3-chlorophenyl derivative 11, that showed inhibition at a concentration of 0.49 µg/ml;
Staphylococcus epidermidis was also inhibited by compounds 1–12, however at somewhat higher concentrations compared to M. luteus and Bacillus spp., with MICs in the range of 0.49–15.63 µg/ml. The most active compounds were 3-chlorophenyl derivative 11 and 4-nitrophenyl derivative 3, that showed inhibition at a concentration of 0.49 and 1.95 µg/ml, respectively, while 4-chlorophenyl derivative 12 had an efficient effect at a concentration of 15.63 µg/ml;
In general, the activity of studied thiosemicarbazide derivatives toward S. aureus species was much lower in comparison to remaining Gram-positive bacteria tested. In contrast to the discussion above on the inhibition of M. luteus, B. cereus, B. subtilis and S. epidermidis, SAR is very sensitive to minor structural changes in the scaffold of the investigated compounds. As seen from the data collected in , the best antibacterial agents were 3, 9 and 11 with MICs in the range of 1.95–3.91 µg/ml. Two other compounds 2 and 10 showed inhibitory action at a slightly higher concentration (MIC at 7.82 µg/ml) while bioactivity of remaining compounds was negligible; MICs at 15.63 or higher, even at 500 µg/ml.
Table 1. In vitro antibacterial screening of compounds 1–12 against panel of reference strains.
Overall, among thiosemicarbazides examined, compounds 3 with 4-nitrophenyl substitution and 11 with 3-chlorophenyl substitution were found to be the most potent antibacterial agents with MIC in the range of 0.49–1.95 µg/ml toward all the Gram-positive bacteria tested. In the case of remaining compounds, the presence and position of electron-withdrawing or electron-donating substituents on the phenyl moiety did not dramatically affect the antibacterial activity, suggesting that the observed antibacterial effect is rather species specific. Indeed, among the strains studied, the most susceptible bacterium to the effects of studied compounds were M. luteus, with MIC results in the range of 0.49–1.95 µg/ml and B. subtilis with MIC results (excluded 12 with MIC at 7.82 µg/ml) in the range of 0.49–3.91 µg/ml. Again, with the exception of 12 with MICs at 15.63 µg/ml, B. cereus and S. epidermidis were also inhibited at generally low concentrations, however at somewhat higher as compared to M. luteus and B. subtilis, with MICs in the range of 0.98–7.82 and 0.49–7.82 µg/ml, respectively. With only few exceptions observed for 2, 3, 9, 10 and 11 (MIC range 1.95–7.82 µg/ml), S. aureus bacterium was in general resistant to tested compounds, with MIC at 15.63 µg/ml or higher. The low antibacterial activity of studied thiosemicarbazides against S. aureus may reflect a number of mechanisms including, e.g. differences in their uptake by bacteria, differences in their intracellular stabilities or compartmentalization within these organisms, and/or differences in their recognition by biological target.
Subsequently, efforts were undertaken to correlate observed bioactivity with physicochemical properties of the molecule. Unfortunately, no close correlations between the geometry of molecule, the dipole moment, ESP charges, the electrostatic potential surfaces, the electronic distribution of the frontier orbitals HOMO and LUMO orbitals were observed (data not shown).
Cytotoxicity against L929 and HeLa cells
In order to test if antibacterial activity of title compounds against reference strains of Gram-positive bacteria is not due to their general toxicity, the effects of representative model compounds (2, 8 and 9) on the viability of L929 and HeLa cell lines were evaluated using the MTT assay. Toxicity, expressed as CC30, was defined as the highest dilution of test samples that causes 30% or greater destruction of cells. As evidenced by the data collected in and , compounds 2, 8 (with the exception of inhibitory efficacy on S. aureus) and 9 display antibacterial activity at non-toxic concentrations in mammalian cells. The selectivity indexes against HeLa and L929 cell lines (SI = CC30/MIC) are in the range of 3.50–58.41 and 3.66–62.82, respectively. Thus, these results make title compounds good candidates for further study.
Table 2. Metabolic activity of mouse L929 and human HeLa cell lines*.
Antibacterial screening against clinical isolates of MSSA and MRSA and MDR–MRSA
In order to clarify if the observed antibacterial effects of title compounds are universal, further research were undertaken to test inhibitory effect of 2 most potent compounds 3 and 11 on 10 MSSA, 6 MRSA and 2 MDR–MRSA pathogens isolated from hospitalized patients.
According to European Centre for Disease Prevention and Control, S. aureus has now spread worldwide and is the most frequently identified antimicrobial drug-resistant pathogen in hospitals in Europe, the Americas, North Africa and the Middle and Far EastCitation23. This pathogen has been recognized as largely responsible for both minor and serious to even life threatening acute and chronic infections, including atopic dermatitis, boils, bloodstream infections, central nervous system infections, toxic shock syndrome, pericarditis, endocarditis, pneumonia and osteomyelitisCitation24–31. Also opportunistic H. parainfluenzae, under favorable conditions, may be the etiologic agents of various and unspecified infections, including those associated with biofilm formationCitation32–34. Thus, there is an urgent need to identify and develop new antimicrobial agents effective against these bacteria that do not rapidly succumb to resistance.
As indicated from data collected in , the results of antibacterial assay for 11 are really promising; the compound inhibited the growth of MSSA at MICs of 1.95–7.81 µg/ml, MRSA at MICs of 0.49–1.95 µg/ml and MDR–MRSA at MIC of 0.98 and 3.90 µg/ml, respectively. Unfortunately, with the exception of MDR–MRSA pathogens that were inhibited at relatively low concentration of 15.6 µg/ml, all the clinical isolates in population of both MSSA and MRSA were eradicated at high level of 3, with MICs at 125 and 62.5 µg/ml, respectively.
Table 3. In vitro antibacterial screening of 3 and 11 against clinical isolates S. aureus.
Bactericidal and bacteriostatic activity
The microbiological studies were completed by the evaluation of the minimum bactericidal concentrations (MBCs) which allows for establishing of the bactericidal or bacteriostatic type of exerted inhibition. On the basis of MBC/MIC ratio, the tested compounds 1–12 are considered to have both bactericidal (MBC/MIC ≤ 4) and bacteriostatic (MBC/MIC > 4) effect on the tested strains, both reference and clinical isolates, suggesting the concept that two different targets for the inhibitory activities of thiosemicarbazide derivatives exist in these microorganisms; one of which is responsible for bacteriostatic activity and the other for bactericidal activity. These results are in line with our previous enzymatic experiments for this class of compoundsCitation13–15.
Inhibitory effects on planktonic cells and biofilms formation in clinical isolates of S. aureus and H. parainfluenzae
Finally, the inhibitory effects of compounds 3 and 11 on planktonic cells and biofilms formation in 16 clinical isolates of human pathogen S. aureus and 13 clinical isolates of opportunistic H. parainfluenzae were tested.
In general, bacterial growth is characterized by two life forms, single, independent cells (planktonic or suspended) or sessile aggregates. The latter is commonly referred to as the biofilm mode of growth. Many divergent definitions of bacterial biofilms exist in literature, but all agree that biofilms are composed of multiple bacteria that form a consortium. Also, much of the recent literature generally agrees that bacteria growing as biofilms (attached to a surface), are the major cause of sepsis and chronic infections, such as pneumonia in cystic fibrosis patients, chronic wounds, chronic otitis media and implant- and catheter-associated infections, particularly in immunocompromised patients. The important hallmarks of biofilm-associated infections are extreme resistance to antibiotics and an extreme capacity for evading the host immune defencesCitation9,Citation35,Citation36. This clinical impact of biofilms has inspired researchers to investigate the biofilm mode of growth intensively in the recent years.
As mentioned earlier, in our study, we evaluated in vitro inhibitory effect of 3 and 11 on biofilm formation in S. aureus and H. parainfluenzae and compared them with those obtained from their planktonic cell counterparts. The results from these experiments are shown in as minimal inhibitory concentrations of compounds required to inhibit planktonic cultures (MICs) and cells in biofims (MBICs). As indicated from these results, compounds 3 and 11 did not have a substantial inhibitory effect on both on planktonic cells and biofilms formation in H. parainfluenzae (MIC and MBIC in the range of 62.5–1000 µg/ml, or even higher). Unlike H. parainfluenzae, the majority of cells in biofilm populations of MSSA and MRSA were eradicated at low levels of 3, with MBICs in the range of 7.82–15.63 µg/ml. A detailed comparative examination of tolerance of biofilms versus planktonic cells indicated that in most cases 3 completely block the planktonic growth at the MIC for biofilm inhibition, suggesting that the biofilm inhibition is at least partly due to killing the planktonic bacteria before biofilms could be established. In contrast to 3, both planktonic and biofilm cultures of S. aureus were generally tolerant to 11. As depicted in , compound 11 was able to inhibit biofilm formation at low concentration (MIC range 1.95–15.63 µg/ml) only in one population of MSSA and three populations of MRSA.
Table 4. In vitro effects of 3 and 11 on planktonic cells and biofilms formation in clinical isolates of S. aureus and H. parainfluenzae.
Conclusions
In conclusion, twelve 4-benzoyl-1-dichlorobenzoylthiosemicarbazides have been tested for their antibacterial activity against a panel of Gram-positive and Gram-negative reference strains. All the compounds were effective against M. luteus, B. cereus, B. subtilis and S. epidermidis, indicating, in most cases, equipotent or even more effective action against M. luteus and Bacillus spp. than those standard drug cefuroxime. In order to clarify if the observed antibacterial effects are universal, further research were undertaken to test inhibitory potency of two most potent compounds 3 and 11 on clinical isolates of S. aureus. New lead structure 11 was identified with potent activity against MSSA, MRSA and MDR–MRSA that will direct the production of future analogues. Finally, inhibitory efficacy of 3 and 11 on planktonic cells and biofilms formation in clinical isolates of S. aureus and H. parainfluenzae was tested. The excellent activity of 3 against biofilm populations of MSSA and MRSA opens a possibility of obtaining new potential candidates against MRSA and -susceptible S. aureus biofilms. Promising results presented in this article have inspired the direction for future toxicity and mode of action studies and the design of new analogues.
Declaration of interest
The authors declare no conflict of interest. The project was funded by the National Science Centre (decision number: 2012/05/D/NZ7/02278).
References
- Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1–12
- Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008;46:155–64
- Amin R, Krammer B, Abdel-Kader N, et al. Antibacterial effect of some benzopyrone derivatives. Eur J Med Chem 2010;45:372–8
- Taubes G. The bacteria fight back. Science 2008;321:356–61
- Boucher HW, Talbot GH, Benjamin DK, et al. 10 × 20 Progress-development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1685–94
- Hardej D, Ashby CR, Khadtare NS, et al. The synthesis of phenylalanine-derived C5-substituted rhodanines and their activity against selected methicillin-resistant Staphylococcus aureus (MRSA) strains. Eur J Med Chem 2010;45:5827–32
- LaDow JE, Warnock DC, Hamill KM, et al. Bicephalic amphiphile architecture affects antibacterial activity. Eur J Med Chem 2011;46:4219–26
- Rice LB. Unmet medical needs in antibacterial therapy. Biochem Pharmacol 2006;71:991–5
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22
- Siwek A, Stefańska J, Wawrzycka-Gorczyca I, Wujec M. Synthesis and in vitro antibacterial evaluation of 1-substituted-4-ethoxycarbonylmethylthiosemicarbazides and products of their dehydrocyclization. Heteroatom Chem 2010;21:131–8
- Plech T, Wujec M, Siwek A, et al. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur J Med Chem 2011;46:241–8
- Siwek A, Stefańska J. Antimicrobial activity and SAR study of some novel thiosemicarbazide derivatives bearing piperidine moiety. Med Chem 2011;7:690–6
- Siwek A, Stączek P, Wujec M, et al. Biological and docking studies of topoisomerase IV inhibition by thiosemicarbazides. J Mol Model 2011;17:2297–303
- Siwek A, Stączek P, Stefańska J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur J Med Chem 2011;46:5717–26
- Siwek A, Stączek P, Kosikowska U, et al. Does dehydrocyclization of 4-benzoylthiosemicarbazides in acetic acid lead to s-triazoles or thiadiazoles? Struct Chem 2012;23:1441–8
- CLSI, Performance Standards for Antimicrobial Susceptibility Testing; Eighteenth International Supplement. CLSI Document M7-MIC. Wayne, PA: Clinical Laboratory Standards Institute; 2008
- Kaplan JB, Mulks MH. Biofilm formation is prevalent among field isolates of Actinobacillus pleuropneumoniae. Vet Microbiol 2005;108:89–94
- Kosikowska U, Malm A. The preliminary analysis of the ability of biofilm formation in vitro under stationary conditions by Haemophilus parainfluenzae isolates from throat of healthy people. Sepsis 2009;2:203–6
- American National Standard. ANSI/AAMI/ISO 10993-5: 2009/(R)2014. Biological evaluation of medical devices, Part 5: Tests for in vitro cytotoxicity
- Hyperchem 8.0.3. HyperCube Inc., USA, Gainsville, FL, 2007
- Singh UCh, Kollman PA. An approach to computing electrostatic charges for molecules. J Comp Chem 1984;5:129–45
- Frisch MJ. Gaussian 09, revision A.02. Wallingford: Gaussian Inc.; 2009
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2009. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) ECDC, Stockholm; 2010
- Henocq E, Hewitt B, Guerin B. Staphylococcal and human dander IgE antibodies in superinfected atopic dermatitis. Clin Allergy 1982;12:113–20
- Projan SJ, Novick RP. The molecular basis of pathogenicity. In: Crossley KB, Archer GL, eds. The staphylococci in human disease. New York: Churchill Livingstone; 1997:55–82
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 2000;13:16–34
- Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol 2009;20:456–70
- Bronner S, Monteil H, Prevost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 2004;28:183–200
- Cheung AL, Bayer AS, Zhang G, et al. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol 2004;40:1–9
- Miller M, Dreisbach A, Otto A, et al. Mapping of interactions between human macrophages and Staphylococcus aureus reveals an involvement of map kinase signaling in the host defense. J Proteome Res 2011;10:4018–32
- Jarvest RL, Berge JM, Berry V, et al. Nanomolar inhibitors of Staphylococcus aureus methionyl trna synthetase with potent antibacterial activity against Gram-positive pathogens. J Med Chem 2002;45:1959–62
- Pillai A, Mitchell JL, Hill SL, Stockley RA. A case of Haemophilus parainfluenzae pneumonia. Thorax 2000;55:623–4
- Frankard J, Rodriguez-Villalobos H, Struelens MJ, Jacobs F. Haemophilus parainfluenzae: an underdiagnosed pathogen of biliary tract infections? Eur J Clin Microbiol Infect Dis 2004;23:46–8
- Cardines R, Giufre M, Atti ML, et al. Haemophilus parainfluenzae meningitis in an adult associated with acute otitis media. New Microbiol 2009;2:213–15
- Bjarnsholt T. The role of bacterial biofilms in chronic infections. Acta Pathol Mic Sc 2013;121:1–58
- Saginur R, StDenis M, Ferris W, et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother 2006;50:55–61