Abstract
Glutathione S-transferases (GSTs) are an important enzyme family which play a critical role in detoxification system. In our study, GST was purified from muscle tissue of Chalcalburnus tarichii Pallas with 301.5-fold purification and 19.07% recovery by glutathione agarose affinity chromatography. The purity of enzyme was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, showing a two band, because of having heterodimer structure. KM values were 1.59 and 0.53 mM for 1-chloro-2,4-dinitrobenzene (CDNB) and glutathione (GSH), respectively. Vmax values for CDNB and GSH were also determined as 5.58 and 1.88 EU/mL, respectively. In addition, inhibition effects of Ag+, Cu2+, Cd2+, Fe3+, Pb2+, Cr2+, Co2+ and Zn2+ metal ions were investigated on the enzyme activity and IC50, Ki values were calculated for these metal ions.
Introduction
The glutathione S-transferases (GSTs) belong to a multigene enzyme superfamily which play a significant role in the detoxification prosesCitation1. GSTs reduce the toxic effect of endogenous and environmental chemicals with phase II system. The reaction catalyzed by this enzyme is conjugation thiol group of reduced glutathione (GSH) with varieties of electrophiles. Conjugation with GSH renders toxic substance more solubleCitation2. GSTs are divided in four classes including cytosolic, mitochondrial, microsomal and fosfomycin resistance proteins identified in bacteriaCitation3. Some GSTs use GSH not as substrate, but as cofactors. There are many functions of GSTs in metabolism. These functions are hormone biosynthesis, tyrosine degradation, peroxide breakdown, dehydroascorbate reduction and other functionsCitation4.
GSTs are classified according to the protein sequence and structure. Members of the same class of cytosolic GSTs match more than 40% amino acid sequence identity. Between classes, proteins have less than 25% sequence identity. Cytosolic GSTs are categorized as alphaCitation5, betaCitation6, deltaCitation7,Citation8, epsilonCitation9, zetaCitation10, thetaCitation11, muCitation12, nuCitation13, piCitation14, sigmaCitation15,Citation16, tauCitation17, phiCitation18 and omegaCitation19.
GSTs in fish have not been characterized as much as mammalian GSTs. There is GST catalytic activity in all fish species examined to date. Similarity to the rodent GSTs, alpha, mu, pi class GSTs have been defined in brown bullhead (Ameriurus nebulosus) and largemouth bass (Micropterus salmoides)Citation20, juvenile white sturgeon (Acipensert ransmontanus)Citation21, chinook salmon (Oncorhynchus tshawytscha)Citation22. Identified classes in fish species compose the major GST isoforms.
Up to now, fish have been proven to be useful experimental models for the evaluation of the health of aquatic ecosystems exposed to environmental pollution and the associated biochemical changesCitation23–27. It is important to understand that the detoxification mechanism of aquatic species is contaminated with metals, pesticides, herbicides and persistent pollutantsCitation28.
Van Lake Fish (Chalcalburnus tarichii Pallas) is the only fish known to live in the Lake VanCitation29. Rivers which breeding and feeding areas of C. tarichii Pallas are poured to Van Lake through areas where made intensive agricultural activities. On the other hand, it was seen that, fish getting in river to breed, enters fields through water supplies and poisoned by pesticides. Van Lake fish is called as Chalcalburnus tarichi by Pallas in 1811. Van Lake fish show endemic and anadromous behaviors in Van Lake basin belong to Cyprinidae familyCitation30.
To our understanding of toxicity mechanism and responses of C. tarichii Pallas muscle to some metal ions, a better understanding of GST is required. The present study was initiated for the purification and characterization of C. tarichii Pallas muscle GST. Here, we investigated effects of some metal ions on C. tarichii Pallas GST activity.
Materials and methods
Chemicals
GSH-agarose was obtained from Sigma-Aldrich (St. Louis, MO). Reduced GSH, 1-chloro-2,4-dinitrobenzene (CDNB), the protein assay reagent and the chemicals for electrophoresis were purchased from Sigma Chem. Company (St. Louis, MO). All other chemicals used were of analytical grade and purchased from either Sigma or Fluka (Munich, Germany).
Preparation of homogenates
All procedures were carried out at 4 °C. Muscle tissue of C. tarichii Pallas (10 g) was powdered with liquid nitrogen for 10 min to decompose cell membranes. The sample was homogenized using homogenizer for 2 min in 50 mM Tris/HCl buffer (pH 7.2) containing 1 mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 13 000 g for 1 h and the supernatant was collected.
Purification of GST
Prepared homogenate was applied to GSH-agarose affinity column equilibrated with 10 mM K-phosphate buffer (pH 7.4) containing 150 mM NaCl. The column was washed with equilibrium buffer and then enzyme was eluted with gradient of 5–10 mM GSH in 50 mM Tris/HCl (pH 9.5). 1.5 mL fractions were collected.
Protein determinations
Protein content was measured according to the Bradford methodCitation31 using bovine serum albumin as a standard.
Enzyme assay
The activity of GST was determined in accordance with a previous procedureCitation32 using CDNB as a model substrate. The assay system included a phosphate buffer (pH 6.5), GSH (20 mM) and CDNB (25 mM). A spectrophotometer (Shimadzu UV-1208, Japan) was used to estimate the changes in absorbance at 340 nm for 3 min.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
To determine the enzyme purity, SDS–PAGE was performed according to Laemmli’s methodCitation33. Gels were stained for appearance of protein band using a standard Coomassie Blue method. The electrophoretic pattern was photographed.
Enzyme kinetics
The kinetic values of GST for GSH and CDNB were determined by the procedure as follows: enzyme activity was measured at five different concentrations of GSH or CDNB with fixed saturated concentrations of other substrate. KM constant and Vmax value were calculated from Lineweaver–Burk graphs.
Inhibition effect on enzyme activity
To determine the effects of metal ions on C. tarichii Pallas muscle GST activity, the different concentrations of Ag+, Cu2+, Cd2+, Fe2+, Pb2+, Cr2+, Co2+ and Zn2+ were added into the activity medium and activity of the enzyme was measured. The absence of metal ions was used as the control (100% activity). IC50 values were calculated from activity (%)–metal ion concentration graph. In order to determine Ki constants, at five different fixed concentrations of substrate (GSH), three different inhibitor concentrations were added to the reaction medium. Lineweaver–Burk graphs were drawn by using 1/V versus 1/[GSH] values, and Ki constants and inhibition types were obtained from these graphs.
Results
Glutathione agarose affinity chromatography was used for the purification of C. tarichii Pallas GST. GST was purified with 301.5-fold and a total yield of 19.07%. The results of the purification of GST are summarized in . SDS–PAGE was used to check the purity of the enzyme. Two bands were observed after electrophoresis (). The result indicates that the native enzyme is a heterodimer.
Figure 1. Lane 2: aliquots and lane 3: aliquots of purified GST from Chalcalburnus tarichii Pallas muscle; lane 1: standard proteins: β-galactosidase (120 kDa), albumin (85 kDa), ovalbumin (50 kDa), carbonic anhydrase (35 kDa), β-lactoglobulin (25 kDa) and lysozyme (20 kDa) were used as standards (peqGOLD Protein Marker III).
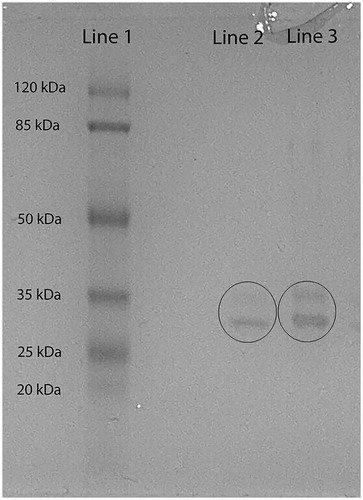
Table 1. Purification steps of Chalcalburnus tarichii Pallas muscle GST.
KM constants and Vmax values of GST for C. tarichii Pallas were calculated from the Lineweaver–Burk graphs and the results are shown in . These kinetic parameters were determined using GSH and CDNB as substrates. Vmax values and KM constant for each substrate were determined by linear regression analysis of 1/V versus 1/[S]. KM constants were calculated as 1.59 and 0.53 mM, and also Vmax values were found as 5.58 and 1.88 EU/mL for CDNB and GSH, respectively. According to KM constants results, it can be said that the affinity of GSH is better than CDNB to enzyme as a substrate.
Table 2. Vmax values, Km constant of C. tarichii Pallas muscle GST.
Inhibitory effects of Ag+, Cu2+, Cd2+, Fe2+, Pb2+, Ni2+, Co2+ and Zn2+ ions on C. tarichii Pallas GST activity were studied at various concentrations using GSH as the substrate. IC50 values and Ki constants were determined by graphs. Ki values are shown in . The results are summarized in .
Figure 2. Lineweaver–Burk graph with five different substrate (GSH) concentrations and three different metal ions (Ag+, Cu2+, Pb2+, Ni2+, Zn2+) concentrations for determination of Ki.
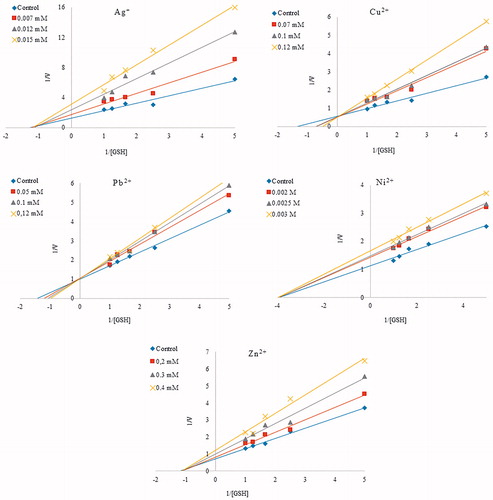
Table 3. Ki constants and IC50 values obtained from regression analysis graphs for GST from C. tarichii Pallas muscle in the presence of different metal ions.
Cd2+, Co2+, Fe2+ had no inhibitory effect on C. tarichii Pallas GST activity. It is found that purified C. tarichii Pallas GST was inhibited, in a concentration dependent way, by Ag+, Cu2+, Pb2+, Ni2+ and Zn2+. The concentrations of metal ions needed to inhibit the enzyme with 50% are named as IC50 values. The IC50 values of Ag+, Cu2+, Pb2+, Ni2+ and Zn2+ on C. tarichii Pallas GST are 0.011, 0.103, 0.113, 2.5, 0.253 mM, and also Ki values of these metals are 0.014 ± 0.004, 0.088 ± 0.033, 0.430 ± 0.092, 7.00 ± 0.001, 0.897 ± 0.429, respectively. According to results, the best inhibitor for C. tarichii Pallas GST is Ag+.
Discussion
It is well-known that the aquatic environment is contaminated with several types of organic and non-organic pollutantsCitation34. These pollutants influence the integrity of ecosystems and physiological functions of animalsCitation34 as well as of people as consumersCitation35. Pollutants water can be evaluated with chemical analysis; however, chemical monitoring is not indicated their generally negative impact on organisms. To detect and determine the effect of contamination on aquatic organisms, biochemical and molecular markers are requiredCitation36. Biochemical and molecular markers may be defined as eliminating the toxic effects of chemical substances in the organism, such as GSTsCitation37.
Fish have been used as aquatic contamination indicators for many years. One of the intensely investigated biochemical markers in fish is the GST conjugation enzyme. The GST activity assay as a response of the fish organism to aquatic contamination has been described in many papers from all over the worldCitation36,Citation38.
Metabolism of xenobiotics occurs in two stages. The first stage of metabolism, unmasking or adding reactive functional groups, involves oxidation, reduction or hydrolysisCitation39. The second stage is conjugation reactions which add water-soluble groups such as reduced GSH, glucuronic acid linked uridine diphosphate-glucuronic acid or glycine. The resulting conjugate is usually a very polar substance that cannot be resorbed, and it is therefore excreted from the organismCitation18.
In fish, GSTs are used primarily as a biomarker indicating aquatic environment pollution with wastewater of industrial and agricultural origin. GST activity has been studied in various tissues of different fish species both under laboratory and under natural conditions. To date, GSTs are purified from some fish species such as Monopterus albusCitation40, rainbow trout (Comaklı), bonito liver (Guller). In this study, C. tarichii Pallas muscle GST was purified and characterized. In this study, GST was first purified from C. tarichii Pallas muscle. The purification steps included preparation of homogenate and GSH-agarose affinity chromatography. shows a purification with a specific activity of 35 583 EU/mg protein, a yield of 19.07%, and a purification coefficient of 301.7. Huang et al. purified GST from M. albus liver using Sepharose 6B affinity column and achieved 300-fold purification with 14% recoveryCitation40. Comaklı et al. purified GST from rainbow trouterythrocytes, and they achieved 11.026-fold purification with 59% recovery. Akkemik et al. purified GST from turkey liver, and they found 252.7-fold with a yield of 45%, with a specific activity of 164.31 U/mgCitation41. KM constants and Vmax values for CDNB and GSH substrates were calculated via Lineaweaver–Burk graphs. KM constant and Vmax value for CDNB (at saturate concentration of GSH) were estimated as1.59 mM and 5.58 EU/mL, respectively. Similarly, KM constant as 0.53 mM and Vmax value as 1.88 EU/mL were obtained for GSH (at saturate concentration of CDNB). According to our result, it can be said that the affinity of GSH is better than CDNB to enzyme as a substrate. To understand toxic impacts of some metal ions on C. tarichii Pallas muscle GST, inhibition effect of them was examined. We determined that Ag+, Cu2+, Pb2+, Ni2+ and Zn2+ inhibited the GST from C. tarichii Pallas muscle, while Cd2+, Co2+and Fe2+ did not affect the enzyme activity. Since Ag+, Ni2+ and Zn2+ exhibit a non-competitive inhibition effect on the enzyme activity, Vmax values are decreased and KM values are not changed. Since Cu2+ and Pb2+ have competitive inhibition effect on the enzyme activity, these inhibitors have no effect on Vmax but raises KM. According to IC50 and Ki results, Ag+ was the most effective inhibitor with the IC50 value of 0.011 mM and Ki constants of 0.014 ± 0.004 mM. The inhibition order of metal was Ag+ > Cu2+ > Pb2+ > Zn2+ > Ni2+, and IC50 values and Ki constants are given in .
Cytosolic GSTs are dimeric proteins made up of two identical subunits or two different subunits, i.e. homodimers or heterodimersCitation42, the molecular weight of which is approximately 25 kDa. In our study, cytosolic C. tarichii Pallas muscle GST was purified. Our SDS–PAGE result is indicating that the enzyme is a heterodimer, because of exhibiting two protein bands. Similar results have showed other eukaryotic species as follows: rat liverCitation43, bovine brainCitation44, turkey liverCitation41, human liverCitation45 and kidneyCitation46. These results support our findings.
Conclusions
Consequently, we purified GST from C. tarichii Pallas muscle for the first time. Here, we determined KM constants and Vmax values of C. tarichii Pallas GST using CDNB and GSH as substrates. Also, inhibitory effects of metals on enzyme activity were reported. The results are important to understand of toxic effects metal ions on C. tarichii Pallas.
Declarations of interest
The authors report no declarations of interest.
References
- Mannervik B, Danielson UH. Glutathione transferases – structure and catalytic activity. Crit Rev Biochem Mol 1988;23:283–337
- Suzuki T, Nishio K, Tanabe S. The MRP family and anticancer drug metabolism. Curr Drug Metab 2001;2:367–77
- Atkinson HJ, Babbitt PC. Glutathione transferases are structural and functional outliers in the thioredoxin fold. Biochemistry 2009;48:11108–16
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol 2005;45:51–88
- Sinning I, Kleywegt GJ, Cowan SW, et al. Structure determination and refinement of human-alpha class glutathione transferase-A1-1, and a comparison with the mu-class and pi-class enzymes. J Mol Biol 1993;232:192–212
- Rossjohn J, Polekhina G, Feil SC, et al. A mixed disulfide bond in bacterial glutathione transferase: functional and evolutionary implications. Struct Fold Des 1998;6:721–34
- Wilce MCJ, Board PG, Feil SC, Parker MW. Crystal-structure of a theta-class glutathione transferase. EMBO J 1995;14:2133–43
- Oakley AJ, Harnnoi T, Udomsinprasert R, et al. The crystal structures of glutathione S-transferases isozymes 1–3 and 1–4 from Anopheles dirus species B. Protein Sci 2001;10:2176–85
- Sawicki R, Singh SP, Mondal AK, et al. Cloning, expression and biochemical characterization of one Epsilon-class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem J 2003;370:661–9
- Polekhina G, Board PG, Blackburn AC, Parker MW. Crystal structure of maleylacetoacetate isomerase/glutathione transferase zeta reveals the molecular basis for its remarkable catalytic promiscuity. Biochemistry 2001;40:1567–76
- Rossjohn J, McKinstry WJ, Oakley AJ, et al. Human theta class glutathione transferase: the crystal structure reveals a sulfate-binding pocket within a buried active site. Structure 1998;6:309–22
- Ji X, Armstrong RN, Gilliland GL. Snapshots along the reaction coordinate of an S(N)Ar reaction catalyzed by glutathione transferase. Biochemistry 1993;32:12949–54
- Schuller DJ, Liu Q, Kriksunov IA, et al. Crystal structure of a new class of glutathione transferase from the model human hookworm nematode Heligmosomoides polygyrus. Proteins Struct Funct Bioinform 2005;61:1024–31
- Reinemer P, Dirr HW, Ladenstein R, et al. 3-Dimensional structure of class-pi glutathione-S-transferase from human placenta in complex with S-hexylglutathione at 2.8 angstrom resolution. J Mol Biol 1992;227:214–26
- Ji XH, Vonrosenvinge EC, Johnson WW, et al. 3-Dimensional structure, catalytic properties, and evolution of a sigma-class glutathione transferase from squid, a progenitor of the lens S-crystallins of cephalopods. Biochemistry 1995;34:5317–28
- Kanaoka Y, Ago H, Inagaki E, et al. Cloning and crystal structure of hematopoietic prostaglandin D synthase. Cell 1997;90:1085–95
- Thom R, Cummins I, Dixon DP, et al. Structure of a tau class glutathione S-transferase from wheat active in herbicide detoxification. Biochemistry 2002;41:7008–20
- Reinemer P, Prade L, Hof P, et al. Three-dimensional structure of glutathione S-transferase from Arabidopsis thaliana at 2.2 angstrom resolution: structural characterization of herbicide-conjugating plant glutathione S-transferases and a novel active site architecture. J Mol Biol 1996;255:289–309
- Board PG, Coggan M, Chelvanayagam G, et al. Identification, characterization, and crystal structure of the omega class glutathione transferases. J Biol Chem 2000;275:24798–806
- Doi AM, Pham RT, Hughes EM, et al. Molecular cloning and characterization of a glutathione S-transferase from largemouth bass (Micropterus salmoides) liver that is involved in the detoxification of 4-hydroxynonenal. Biochem Pharmacol 2004;67:2129–39
- Donham RT, Morin D, Jewell WT, et al. Characterization of glutathione S-transferases in juvenile white sturgeon. Aquat Toxicol 2005;71:203–14
- Donham RT, Morin D, Jewell WT, et al. Characterization of cytosolic glutathione S-transferases in juvenile Chinook salmon (Oncorhynchus tshawytscha). Aquat Toxicol 2005;73:221–9
- Steinberg CEW, Lorenz R, Spieser OH. Effects of atrazine on swimming behavior of zebrafish, Brachydanio-Rerio. Water Res 1995;29:981–5
- Hussein SY, ElNasser MA, Ahmed SM. Comparative studies on the effects of herbicide atrazine on freshwater fish Oreochromis niloticus and Chrysichthyes auratus at Assiut, Egypt. Bull Environ Contam Toxicol 1996;57:503–10
- Wiegand C, Krause E, Steinberg C, Pflugmacher S. Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio). Ecotox Environ Safe 2001;49:199–205
- Kavitha P, Rao JV. Toxic effects of chlorpyrifos on antioxidant enzymes and target enzyme acetylcholinesterase interaction in mosquito fish, Gambusia affinis. Environ Toxicol Phar 2008;26:192–8
- De Silva PMCS, Samayawardhena LA. Effects of chlorpyrifos on reproductive performances of guppy (Poecilia reticulata). Chemosphere 2005;58:1293–9
- Hoffman RS, Capel PD, Larson SJ. Comparison of pesticides in eight US urban streams. Environ Toxicol Chem 2000;19:2249–58
- Guler M, Kivanc MR, Turkoglu V, et al. In vitro determination of 6PGD enzyme activity purified from Lake Van fish (Chalcalburnus tarichii Pallas, 1811) liver exposed to pesticides. Bull Environ Contam Toxicol 2013;91:560–4
- Danulat E, Selcuk B. Life-history and environmental-conditions of the anadromous Chalcalburnus-tarichi (Cyprinidae) in the highly alkaline Lake Van, Eastern Anatolia, Turkey. Arch Hydrobiol 1992;126:105–25
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Pabst MJ, Habig WH, Jakoby WB. Glutathione S-transferase A: a novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem 1974;249:7140–8
- Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 1970;227:680–5
- Sen A, Kirikbakan A. Biochemical characterization and distribution of glutathione S-transferases in leaping mullet (Liza saliens). Biochemistry 2004;69:993–1000
- Perez-Lopez M, Novoa-Valinas MC, Melgar-Riol MJ. Glutathione S-transferase cytosolic isoforms as biomarkers of polychlorinated biphenyl (Arochlor-1254) experimental contamination in rainbow trout. Toxicol Lett 2002;136:97–106
- Faverney CR, Devaux A, Lafaurie M, et al. Toxic effects of wastewaters collected at upstream and downstream sites of a purification station in cultures of rainbow trout hepatocytes. Arch Environ Con Toxicol 2001;41:129–41
- van der Oost R, Beyer J, Vermeulen NPE. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Phar 2003;13:57–149
- Lenartova V, Holovska K, Pedrajas JR, et al. Antioxidant and detoxifying fish enzymes as biomarkers of river pollution. Biomarkers 1997;2:247–52
- Goeptar AR, Scheerens H, Vermeulen NPE. Oxygen and xenobiotic reductase activities of cytochrome-P450. Crit Rev Toxicol 1995;25:25–65
- Huang Q, Liang L, Wei T, et al. Purification and partial characterization of glutathione transferase from the teleost Monopterus albus. Comp Biochem Phys C 2008;147:96–100
- Akkemik E, Taser P, Bayindir A, et al. Purification and characterization of glutathione S-transferase from turkey liver and inhibition effects of some metal ions on enzyme activity. Environ Toxicol Phar 2012;34:888–94
- Noble E, Barre H, Dierickx PJ. Effect of diet and beta-naphthoflavone on hepatic and renal glutathione S-transferase isoenzymes in carp (Cyprinus carpio). Fish Physiol Biochem 1998;18:203–12
- Tu CPD, Reddy CC. On the multiplicity of rat-liver glutathione S-transferases. J Biol Chem 1985;260:9961–4
- Young PR, Briedis AV. Purification and kinetic mechanism of the major glutathione S-transferase from bovine brain. Biochem J 1989;257:541–8
- Awasthi YC, Dao DD, Saneto RP. Interrelationship between anionic and cationic forms of glutathione S-transferases of human-liver. Biochem J 1980;191:1–10
- Singh SV, Leal T, Ansari GAS, Awasthi YC. Purification and characterization of glutathione S-transferases of human-kidney. Biochem J 1987;246:179–86

