Abstract
A new series of compounds derived from thiophene-2-carboxamide were synthesized and characterized by IR, 1H-NMR and 13C-NMR, mass spectrometry and elemental analysis. These compounds were further used to prepare their Co(II), Ni(II), Cu(II) and Zn(II) metal complexes. All metal(II) complexes were air and moisture stable. Physical, spectral and analytical data have shown the Ni(II) and Cu(II) complexes to exhibit distorted square-planar and Co(II) and Zn(II) complexes tetrahedral geometries. The ligand (L1) and its Cu(II) complex were characterized by the single-crystal X-ray diffraction method. All the ligands and their metal(II) complexes were screened for their in-vitro antimicrobial activity. The antibacterial and antifungal bioactivity data showed that the metal(II) complexes were found to be more potent than the parent ligands against one or more bacterial and fungal strains.
Introduction
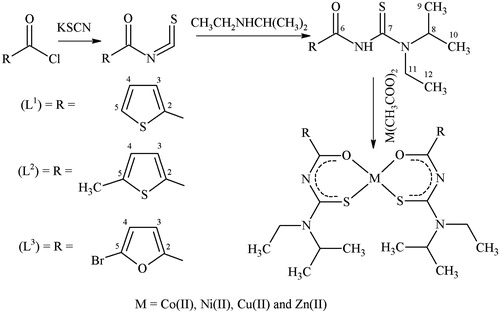
Many biologically active thiourea-based (carboxamide) compounds have been reported possessing antibacterialCitation1–3, antifungalCitation4,Citation5, anticancerCitation6,Citation7, antiviralCitation8, pesticidalCitation9, cytotoxicCitation10, herbicidalCitation11 and insecticidalCitation12 activities. Thiourea derivatives have been effectively used in organocatalysisCitation13 and as curing agents for epoxy resins. They also act as versatile ligands, which can coordinate with the metal ions either in mono anionic bi-dentate or in neutral formCitation14 via oxygen and sulfur atoms of carbonyl (C=O) and thiocarbonyl (C=S) groupsCitation15. The process of chelation/coordination plays an important role in biological systemsCitation16. It has also been suggested that biological activity of many compounds is enhancedCitation17,Citation18 upon coordination with the metal(II) atoms. In view of the significant structural and biological applications of thiourea, we wish to report new thiourea (carboxamide) derivatives, N-[ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1), N-[ethyl(propan-2-yl)carbamothioyl]-5-methylthiophene-2-carboxamide (L2) and 5-bromo-N-[ethyl(propan-2-yl)carbamothioyl]furan-2-carboxamide (L3), and their Co(II), Ni(II), Cu(II) and Zn(II) complexes by incorporating aliphatic moieties in thiophenyl and furanyl nucleous. In achieving the task to evaluate the effect of metal ions on antimicrobial activity, we have examined the synthesized compounds for their in-vitro antibacterial activity against four Gram-negative (Escherichia coli, Shigella sonnei, Pseudomonas aeruginosa and Salmonella typhi) and two Gram-positive (Staphylococcus aureus and Bacillus subtilis) bacterial strains, and for antifungal activity against six fungal strains (Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata). We also report herein the crystal structure of one of the ligand, N-[ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1) and its Cu(L1)2 complex.
Materials and methods
Experimental
All chemicals used were of analytical grade. All metallic salts were used as acetate. Melting points were recorded on Fisher Johns melting point apparatus. Infrared spectra were recorded on Shimadzu FT-IR spectrometer. The C, H and N analysis was carried out using a Perkin Elmer, USA, model. The 1H- and Citation13C-NMR spectra were recorded in DMSO-d6 using TMS as internal standard on a Bruker Spectrospin Avance DPX-500 spectrometer. Electron impact mass spectra (EIMS) were recorded on JEOL MS Route Instrument. In vitro antibacterial and antifungal properties were studied at HEJ Research Institute of Chemistry, International Centre for Chemical Sciences, University of Karachi, Pakistan and Department of Chemistry, The Islamia University, Bahawalpur, Pakistan.
General procedure for the synthesis of ligands (L1–L3)
N-[Ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1)
The ligand, N-[ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1) was synthesized by following a reported methodCitation19, in which a solution of thiophenyl carbonyl chloride (20 mmol, 2.14 mL) in dry acetone (30 mL) was added dropwise to a solution of KSCN (20 mmol, 1.94 g) in acetone (30 mL). The reaction mixture was refluxed for 1 h and then cooled to room temperature. A solution of N-ethyl-isopropyl amine (20 mmol, 2.4 mL) in acetone (20 mL) was added dropwise to the mixture in 20 min and refluxing continued for 2 h. Then KCl was removed by filtration and light yellow filtrate was placed into water (250 mL) and added few drops of conc. HCl under constant agitation. The wax-like material was formed which was separated by filtration, washed several times with cold water and then with diethyl ether. The crude product was purified by recrystallization from a solution mixture of ethanol-dichloromethane (1:1). As a result of recrystallization, fine shiny colorless crystals of (L1) were obtained, which were suitable for X-rays analysis. The same procedure was used for the preparation of other ligands.
Yield: 81%. Color (white crystalline solid); m.p. 133–135 °C. IR (KBr, cm−1): 3265 (NH), 3070–3030 (CH), 1680 (C=O), 1575 (aromatic C=C), 1235 (C=S), 885 (C–S). 1H-NMR (DMSO–d6, δ, ppm): 11.53 (s, 1H, CONH), 8.19 (d, 1H, J = 4.7 Hz, thienyl C5–H), 8.05 (d, 1H, J = 4.10 Hz, thienyl C3–H), 7.45 (dd, 1H, J = 4.5, 4.1 Hz, thienyl C4–H), 2.55 (m, 2H, C11–H), 2.30 (m, 1H, C8–H),1.0 (d, 6H, C9–H, C10–H), 0.96 (t, 3H, C12–H). 13C-NMR (DMSO–d6, δ, ppm): 178.90 (C=S), 172.0 (C=O), 137.93 (C2), 136.73 (C3), 134.33 (C5), 131.10 (C4), 52 (C8), 45 (C11), 20 (C9, C10), 15.0 (C12). EIMS (70 eV) m/z (%): 256.39. Anal. Calcd. for C11H16N2OS2 (256.39): C: 51.53; H: 6.29; N: 10.93; S: 25.01; Found: C: 51.61; H: 6.31; N: 10.88; S: 25.10%.
N-[Ethyl(propan-2-yl)carbamothioyl]-5-methylthiophene-2-carboxamide (L2)
Yield: 79%. Color (white crystalline solid); m.p. 129–131 °C. IR (KBr, cm−1): 3270 (NH), 3065–3025 (CH), 1675 (C=O), 1580 (aromatic C=C), 1240 (C=S), 880 (C–S). 1H-NMR (DMSO–d6, δ, ppm): 11.60 (s, 1H, CONH), 7.80 (d, 1H, J = 3.6 Hz, thienyl C3–H), 7.10 (d, 1H, J = 3.30 Hz, thienyl C4–H), 2.58 (m, 2H, C11–H), 2.45 (s,3H, thienyl-CH3), 2.30 (m, 1H, C8–H), 1.0 (d, 6H, C9–H, C10–H), 0.93 (t, 3H, C12–H). 13C-NMR (DMSO–d6, δ, ppm): 179.10 (C=S), 172.12 (C=O), 141.33 (C5), 136.90 (C2), 135.63 (C3), 131.10 (C4), 53 (C8), 45 (C11), 20 (C9, C10), 16.5 (thienyl-CH3), 15.0 (C12). EIMS (70 eV) m/z (%): 270.41. Anal. Calcd. for C12H18N2OS2 (270.41): C: 53.30; H: 6.71; N: 10.36; S: 23.72; Found: C: 53.43; H: 6.74; N: 10.42; S: 23.59%.
5-Bromo-N-[ethyl(propan-2-yl)carbamothioyl]furan-2-carboxamide (L3)
Yield: 78%. Color (off-white crystalline solid); m.p. 123–125 oC. IR (KBr, cm−1): 3280 (NH), 3075–3030 (CH), 1680 (C=O), 1575 (aromatic C=C), 1250 (C=S), 1160 (C-O), 690 (C–Br). 1H-NMR (DMSO–d6, δ, ppm): 11.62 (s, 1H, CONH), 7.70 (d, 1H, J = 3.7 Hz, furanyl C3–H), 7.40 (d, 1H, J = 3.67 Hz, furanyl C4–H), 2.60 (m, 2H, C11–H), 2.32 (m, 1H, C8–H),1.05 (d, 6H, C9–H, C10–H), 0.97 (t, 3H, C12–H). 13C-NMR (DMSO–d6, δ, ppm): 179.20 (C=S), 172.1 (C=O), 139.83 (C2), 133.93 (C5), 130.13 (C3), 127.28 (C4), 52 (C8), 46 (C11), 21 (C9, C10), 15.5 (C12). EIMS (70 eV) m/z (%): 319.22. Anal. Calcd. for C11H15BrN2O2S (319.22): C: 41.39; H: 4.74; N: 8.78; S: 10.04; Found: C: 41.30; H: 4.79; N: 8.69; S: 10.11%.
General procedure for the synthesis of metal(II) complexes
The solution of Cu(CH3COO)2.H2O (0.5 mmol) in methanol (15 mL) was added dropwise to a stirred solution of ligand N-[ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1) (1 mmol) in methanol (20 mL). The reaction mixture was refluxed for 2 h. The precipitates of dark green color were formed during refluxing. The precipitated product thus formed was filtered, washed with methanol and dried under vacuum. The precipitates were dissolved in a mixture of ethanol and dichloromethane (1:1) and a clear solution was kept in a refrigerator for one week. Suitable dark green color crystals for X-ray studies were obtained for Cu(L1)2 complex. The same method was used for the preparation of all other complexes.
1H- and 13C-NMR data of Zn(II) complexes
[Zn (L1-H)2] (4)
1H-NMR (DMSO–d6, δ, ppm): 8.30 (d, 2H, thienyl C5–H), 8.20 (d, 2H, thienyl C3–H), 7.57 (dd, 2H, thienyl C4–H), 2.60 (m, 4H, C11–H), 2.35 (m, 2H, C8–H), 1.06 (d, 12H, C9–H, C10–H), 1.01 (t, 6H, C12–H). 13C-NMR (DMSO–d6, δ, ppm): 180.30 (C=S), 173.5 (C=O), 138.30 (C2), 137.03 (C3), 134.50 (C5), 131.35 (C4), 52.2 (C8), 45.15 (C11), 20.20 (C9, C10), 15.15 (C12).
[Zn (L2-H)2] (8)
1H-NMR (DMSO–d6, δ, ppm): 7.95 (d, 2H, thienyl C3–H), 7.20 (d, 2H, thienyl C4–H), 2.63 (m, 4H, C11–H), 2.50 (s,6H, thienyl-CH3), 2.35 (m, 2H, C8–H), 1.06 (d, 12H, C9–H, C10–H), 0.98 (t, 6H, C12–H). 13C-NMR (DMSO–d6, δ, ppm): 180.50 (C=S), 173.42 (C=O), 141.48 (C5), 137.30 (C2), 135.95 (C3), 131.30 (C4), 53.19 (C8), 45.16 (C11), 20.20 (C9, C10), 16.65 (thienyl-CH3), 15.15 (C12).
[Zn (L3-H)2] (12)
1H-NMR (DMSO–d6, δ, ppm): 7.85 (d, 2H, furanyl C3–H), 7.52 (d, 2H, furanyl C4–H), 2.67 (m, 4H, C11–H), 2.38 (m, 2H, C8–H),1.10 (d, 12H, C9–H, C10–H), 1.03 (t, 6H, C12–H). 13C-NMR (DMSO–d6, δ, ppm): 180.60 (C=S), 173.5 (C=O), 140.23 (C2), 134.13 (C5), 130.39 (C3), 127.47 (C4), 52.17 (C8), 46.15 (C11), 21.2 (C9, C10), 15.65 (C12).
Biological activity
Antibacterial studies (in-vitro)
The synthesized ligands (L1–L3) and their respective metal(II) complexes were tested against four Gram-negative (E. coli, S. sonnei, P. aeruginosa and S. typhi) and two Gram-positive (St. aureus and B. subtilis) bacterial strains by the disc diffusion methodCitation20,Citation21. The test compounds (ligand/complex) were dissolved (10 mg/mL) in DMSO. A known volume (10 µL) of the solution was applied with the help of a micropipette onto the sterilized filter paper discs. The discs were dried at room temperature over night and stored in sterilized dry containers. Discs soaked with 10 µL of DMSO and dried in air at room temperature were used as the negative control. The standard antibiotic discs used as positive control were prepared as mention above in the laboratory by applying a known concentration of the standard antibiotic solution. Ampicillin was used as a standard antibiotic. Bacterial culture was grown in nutrient broth medium at 37 oC overnight and spread on to solidified nutrient agar medium in Petri plates using sterilized cotton swabs. Test and control disks were then applied to the medium surface with the help of sterilized forceps. The plates were incubated at 37 oC for 24–48 h. The results were recorded by measuring the zone of inhibition in mm against each compoundCitation21. The experiments were carried out in triplicate and the values obtained were statistically analyzed.
Antifungal activity (in-vitro)
Antifungal activity of all the compounds was studied against six fungal strains (T. longifusus, C. albicans, A. flavus, M. canis, F. solani and C. glabrata) according to literature protocolCitation21,Citation22. Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 (cfu) mL−1 fungal spore suspensions and transferred to petri dishes. Discs soaked in 20 mL (200 µg/mL in DMSO) of test compounds were placed at different positions on the agar surface. The plates were incubated at 32 oC for 7 days. The results were recorded as percentage of inhibition and compared with the standard drugs miconazole and amphotericin B.
Results and discussion
Chemistry
The ligands (L1–L3) were synthesized by the reaction of potassium thiocyanate with thiophene-2-carbonyl chloride, 5-methyl-thiophene-2-carbonyl chloride and 5-bromo-furan-2-carbonyl chloride, respectively, in dry acetone followed by condensation of the resulting product with N-ethyl-isopropyl amine (Scheme 1). The ligands (L1–L3) thus formed were soluble in ethanol, ethyl acetate, DMF and DMSO; however, slightly soluble in tetrahydrofuran, diethyl ether and insoluble in aliphatic and aromatic hydrocarbons. The metal(II) complexes were obtained by stoichiometric reaction of the corresponding ligands with metals [Co(II), Ni(II), Cu(II) and Zn(II)] as acetate in a molar ratio of metal:ligand (M:L) as 1:2 (Scheme 1). All metal(II) complexes were air and moisture stable. They were soluble in mixture of ethanol and dichloromethane (1:1), DMF and DMSO. The ligands and their metal(II) complexes were characterized by their physical, spectral and analytical data. The structure of ligand, N-[ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1) and its Cu(L1)2 complex was determined from single crystal X-ray diffraction data. Physical measurements and analytical data of the metal(II) complexes are given in Supplementary Tables 1 and 2.
Spectral characterization of ligands (L1–L3) and their metal(II) complexes
IR spectra
The main IR vibrational bands of the synthesized ligands (L1–L3) and their metal(II) complexes were found in their expected region. The characteristic IR bands, are given in “Experimental” section and . The IR spectra of newly synthesized ligands showedCitation23 the strong peak at 3265–3280 cm−1 due to N–H vibrations and medium peaks at 1675–1680 and 1235–1250 cm−1, respectively, due to carbonyl (C=O) and thiocarbonyl (C=S) vibrations, which strongly support the preparation of desired compounds. The ligands (L1) and (L2) showed bands at 880–885 due to (C–S) vibrations assignedCitation24 to thiophene and (L3) displayed band at 1160 cm−1 due to (C–O) vibrations assigned to furane moiety. A weaker peak at 690 cm−1 was also observed by ligand (L3), which was dueCitation25 to v(C-Br) vibrations. The IR spectra of all the metal(II) complexes exhibited major changes in comparison to the spectra of the subsequent ligands. The most prominent change observed was, the carbonyl (C=O) and thiocarbonyl (C=S) bands originally appearing at 1675–1680 and 1235–1250 cm−1 in the spectra of the ligand, shifted to lower frequency by 13–15 cm−1 at 1660–1670 and 1220–1237 cm−1, respectively, in the spectra of metal(II) complexes indicatingCitation1,Citation26 the involvement in coordination with the metal(II) ions. The decrease in frequency is due to delocalization of electronsCitation27. The N–H vibrations appearing in the ligands at 3265–3280 cm−1 were also disappeared in the metal complexes giving a clue of deprotonation, which may undergo through tautomerism. However, keto form was identified as a stable product. Coordination of carbonyl-O and thiocarbonyl-S is further justified by the appearance of new bands at 450–460 and 525–536 cm−1 corresponding to M–S and M–O linkages, respectively. This linkage is also supported by X-ray structure of the Cu(L1)2 complex as shown in and .
Table 1. Conductivity, magnetic and spectral data of metal(II) complexes.
1H-NMR spectra
The spectra of all the ligands (L1–L3) showed a broad singlet peak at 11.53–11.62 ppm due to N–H protonCitation1,Citation28 and the protons of ethyl and isopropyl groups (C8–H, C9–H, C10–H, C11–H and C12–H) were observed as doublet to multiplet at 0.93–2.60 ppm. The methyl (CH3) protons of (L2) and C3–H to C5–H protons of all ligands were observed at 2.45 and 7.10–8.19 ppm as a singlet and doublet, respectively. On comparison of the spectra of ligand with those of Zn(II) complexes, the N–H protons disappeared which is also supported by the IR spectra and X-rays technique and all other remaining protons underwent downfield shift by 0.05–0.15 ppm due to coordination and increased conjugationCitation30.
13C-NMR spectra
The thiophene, furane, carbonyl and thiocarbonyl carbons of all the ligands were observedCitation30 at 131.1–141.3, 127.3–139.8, 172.0–172.12 and 179.90–179.20 ppm, respectively. The comparison of the spectra of Zn(II) complexes showed downfield shifting of carbonyl-C and thiocarbonyl-C from 172.0–172.1 and 179.9–179.2 ppm in free ligands to 173.42–173.5 and 180.3–180.6 ppm in the zinc(II) complexes, respectively, revealing the coordination of carbonyl-O and thiocarbonyl-S with the Zn(II) metal ion. Furthermore, all other carbons in the spectra of the Zn(II) complexes underwent downfield shifting by 0.15–0.4 ppm due to increased conjugation and coordination.
Electronic spectra
The electronic spectral values of Co(II), Ni(II), Cu(II) and Zn(II) complexes in DMF are recorded in . The spectra of Co(II) complexes showed only one absorption band at 17 995–18 380 cm−1 assigned to the transition 4A2 (F) → 4T1(F)Citation31. This in turn, propose tetrahedral geometry for the Co(II) complexes. This is also supported by magnetic moment values (3.78–3.94 B.M) of Co(II) complexesCitation31. The Ni(II) complexes exhibited two absorption bands at 13 390–13 515 cm−1 and at 18 770–19 230 cm−1 assigned to the transitions 1A1g → 1B2g and 1A1g → 1A2g, respectively, which give clue for square-planar geometryCitation32. The electronic spectra of Cu(II) complexes showed low-energy absorption bands at 15 190–15 660 cm−1 assigned to the transitions,2B1g → 2E1g. The high-energy bands at 21 160–21 640 cm−1 was assigned to the transition,2B1g → 2A1g. These transitions as well as the measured magnetic moment values (1.83–190 B.M) propose a square-planar geometry for Cu(II) complexCitation33. The diamagnetic Zn(II) complexes did not show any d–d transitions and their spectra were dominatedCitation32 only by the charge transfer band at 27 190–27 360 cm−1 proposing a tetrahedral geometry for the Zn(II) complexes.
Molar conductivity and magnetic properties of the metal(II) complexes
Molar conductance was carried out in DMF solution and the results reported in indicate the values in a lower range (10.9–18.2 Ω−1 cm2 mol−1), thus showing their non-electrolytic natureCitation34. The magnetic moment values of Co(II) complexes were found in the range of 3.78–3.94 B.M, expected for three unpaired electrons and are in agreement with their tetrahedral geometryCitation35. The Cu(II) and Ni(II) complexes showed µeff values compatible for their square-planer geometry. The Zn(II) complexes exhibited diamagnetic natureCitation36.
X-Ray crystallographic studies of ligand (L1) and Cu(L1)2 complex
Single crystal X-ray structure of ligand (L1)
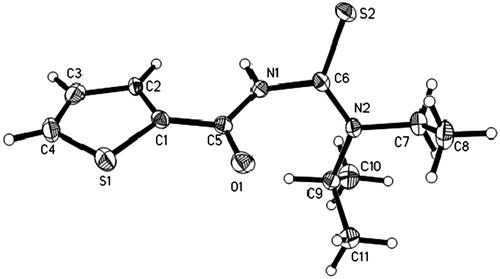
The molecular structure of N-[ethyl(propan-2-yl)carbamothioyl]thiophene-2-carboxamide (L1) () showed their expected bond lengths and anglesCitation37,Citation38. Data collection and refinement of (L1) are listed in Supplementary Table 3. The bond lengths of the carbonyl C5–O1 and thiocarbonyl C6–S2 groups were, C5–O1 = 1.221(2) Å, C6–S2 = 1.6792(16) Å, respectively, for double bonds. All C–N bonds, C7–N2 = 1.475(2) Å and C9–N2 = 1.493(2) Å represented single bond and C6–N2 = 1.323(2) Å, C6–N1 = 1.417(2) Å and C5–N1 = 1.374(2) Å showing a partial double bond character (Supplementary Table 4). This information indicated partial electron delocalization within the C5–N1–C6–N2 fragment. The C6–N2 bond, adjacent to the alkyl group, was slightly shorter than C6–N1. These bond distances were in good agreement with those observed in literature, as reported in the Cambridge Structural DatabaseCitation37,Citation38.
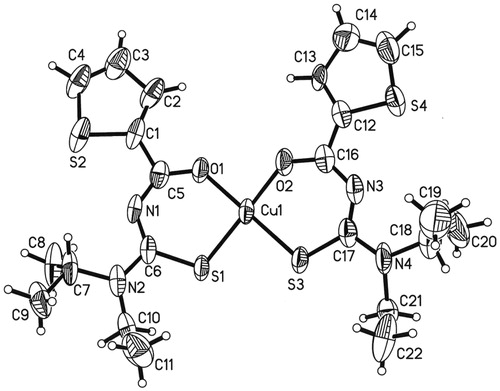
Molecular structure of Cu(L1)2 complex
The molecular structure of Cu(L1)2 complex () was obtained from X-ray single crystal studies and, the data collection and crystal refinement parameters are given in Supplementary Table 3 and selected bond lengths and bond angles are given in Supplementary Tables 7 and 8. The structure of Cu(L1)2 complex showed the bond lengths and anglesCitation15 as expected. The Cu(L1)2 complex existed as a monomer unit in which each copper Cu1 atom was coordinated with each oxygen and each sulfur of two ligands () in a cis-fashion, with slightly distorted square planar geometryCitation15. In both chelate rings, the distances of N1–C6 (1.333(6) Å) and N3–C17 (1.337(6) Å) in the thiourea fragment were almost same, but the distances of N1–C5 (1.310(6) Å) and N3–C16 (1.325(6) Å) (Supplementary Table 8) were slightly different, which supported the distorted square planar geometry of the complex. The bond lengths of the carbonyl O1–C5 = 1.273(5) Å; O2–C16 = 1.267(5) Å and thiocarbonyl S1–C6 = 1.746(4) Å; S3–C17 = 1.716(5) Å groups were in between those for double and single bonds (Supplementary Table 7). The investigated complex observed same behavior for C-N–C-N bond lengths, which is shorter than the average single C–N bond length of 1.48 Å and greater than average double C = N bond length 1.16 Å, being C5–N1 = 1.310(6) Å, C6–N1 = 1.333(6) Å, C17–N3 = 1.337(6) Å, C16–N3 = 1.325(6) Å, C17–N4 = 1.344(6) Å, C6–N2 = 1.336(5) Å, thus showing variable degree of double and single bond characterCitation15.
Impact of metal/ligand coordination on bioactivity
In vitro antibacterial bioassay
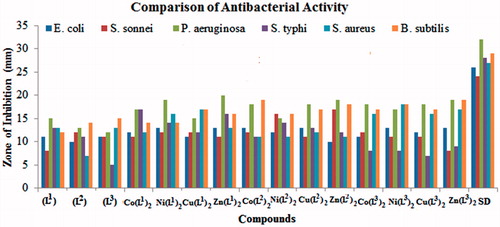
Antibacterial activity of newly synthesized ligands (L1–L3) and their metal(II) complexes was determined against four Gram-negative (E. coli, S. sonnei, P. aeruginosa and S. typhi) and two Gram-positive (S. aureus and B. subtilis) bacterial strains and obtained data are recorded in . The antibacterial activity of prepared compounds was compared with the activity of standard drug (ampicillin). The synthesized compounds showed varying degree of inhibitory effects: low (up to 10 mm), moderate (up to 11–15 mm) and significant (above 15 mm). The obtained data indicated that all the ligands (L1–L3) showed moderate (11–15 mm) activity against all the tested bacterial strains except, (b) and (e) which are weakly inhibited (05–10 mm) by (L1) and (L3), respectively. All Co(L1)2 complexes possessed significant (17 mm) activity against (c) and (d), and moderate (11–14 mm) against (a), (b), (e) and (f) strains. Similarly, the Ni(L1)2 complexes displayed significant (16–19 mm) activity against (c) and (e), and moderate (12–14 mm) against (a), (b), (d) and (f) bacterial strains. The Cu(L1)2 complexes also showed significant (17 mm) activity against (e) and (f) and moderate (11–15) against (a)–(d) strains. The metal(II) complexes, Co(L2)2 and Cu(L2)2 possessed significant (16–19 mm) activity against (c) and (f), and moderate (11–15 mm) against (a), (b), (d) and (e) bacterial strains. In the same way, the Co(L3)2, Ni(L3)2, Cu(L3)2 and Zn(L3)2 complexes also showed significant (16–20 mm) activity against (c), (e) and (f), and moderate (11–15 mm) against (a), (b) and (d) strains. Moreover, the Zn(L1)2, Ni(L2)2 and Zn(L2)2 complexes showed significant (16–19 mm) activity against (c), (d) and (f) strains, respectively. The same complexes, showed moderate (16–20 mm) activity against all the remaining tested bacterial strains. Conclusively, the ligands (L1–L3) possessed smaller average (11.5 mm) activity against different strains than the average (14.3 mm) activity of the metal(II) complexes, which showed that the activity is enhancedCitation39,Citation40 upon coordination. The comparative and average activity data of the ligands and their metal(II) complexes is reproduced in .
Table 2. Antibacterial bioassay (concentration used 1 mg/mL of DMSO) of ligands (L1–L3) and their metal(II) complexes.
In vitro antifungal bioassay
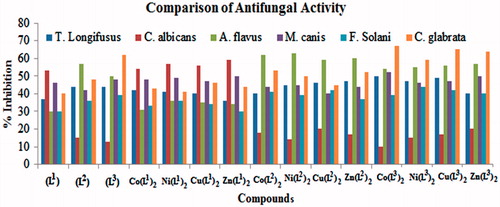
The newly synthesized ligands (L1–L3) and their metal(II) complexes were subjected to screening for their antifungal activity against, T. longifucus, C. albican, A. flavus, M. canis, F. solani and C. glaberata fungal strains and results are recorded in . The obtained results were compared with the standard drugs Miconazole and Amphotericin B. The ligand (L1) showed moderate (37–53%) activity against (a), (b), (d) and (f) and weak (30%) against (c) and (e) fungal strains. The ligand (L2) displayed significant activity (57%) against (c), moderate (36–48%) against (a) and (d)–(f) and weak (15%) against (b) fungal strains. The activity of ligand (L3) was found to be significant (62%) against (f), moderate (39–50%) against (a) and (c)–(f) and, weak (13%) against (b) fungal strains. The results of antifungal activity exhibited that the metal(II) complexes Co(L1)2, Ni(L1)2, Cu(L1)2 and Zn(L1)2 showed significant activity (54–59%) against (b), moderate (34–50%) against (a), (c), (d) and (f) and weak activity (30–33%) against (e) fungal strains. The Co(L2)2, Ni(L2)2, Cu(L2)2 and Zn(L2)2 complexes also, showed significant activity (59–63%) against (c), moderate (37–53%) against (a) and (d)–(f) and weak (14–20%) against (b) fungal strains. Similarly, the Co(L3)2, Ni(L3)2, Cu(L3)2 and Zn(L3)2 complexes showed significant activity (54–67%) against (c) and (f), moderate (39–52%) against (a), (d) and (e), and weak (10–20%) against (b) fungal strains. The average activity data showed that the metal(II) complexes exhibited greater average activity (43.6%) than the average activity of the ligands (40.8%). It is therefore, concluded that the antifungal activity of the ligands increasedCitation41,Citation42 upon chelation/coordination with the metal ions. The comparative activity data of the ligands and their metal(II) complexes is presented in .
Table 3. Antifungal bioassay (concentration used 200 µg/mL) of ligands (L1–L3) and metal(II) complexes.
Conclusions
All the newly synthesized ligands (L1–L3) act as bidentate and coordinate through carbonyl-O and thiocarbonyl-S to the metal(II) ions. The bonding of ligands to the metal(II) ion is supported by their physical, analytical and spectral data. These observations are further supported by the X-ray crystallographic data of Cu(L1)2 complex. In vitro antibacterial and antifungal studies of the ligands and their metal(II) complexes against representative bacterial and fungal strains showed that the ligands (L1–L3) and their Co(II), Ni(II), Cu(II) and Zn(II) complexes were found to have moderate to significant activity. However, against one or more bacterial strains the bioactivity of the ligands enhanced upon coordination with the metal ions.
Supplementary material available online
Supplementary Tables S1-S8.
IENZ_1050011_Supplementary_Index.pdf
Download PDF (52.8 KB)Acknowledgements
The authors are thankful to HEJ research Institute of Chemistry, University of Karachi, Pakistan, for providing their help in taking NMR, mass spectral and antibacterial/antifungal data.
Declaration of interest
This work was supported by Collaboration between University of Manchester, UK and Bahauddin Zakariya University, Multan (Pakistan) on Indigenous Fellowship program sponsored by the Higher Education Commission, Government of Pakistan to one of the authors (MH).
References
- Binzet G, Arslan H, Florke U, et al. Synthesis, characterization and antimicrobial activities of transition metal complexes of N,N-dialkyl-N′-(2-chlorobenzoyl)thiourea derivatives. J Coord Chem 2006;59:1395–406
- Arslan H, Duran N, Borekci G, et al. Antimicrobial activity of some thiourea derivatives and their nickel and copper complexes. Molecules 2009;14:519–27
- Saeed S, Rashid N, Ali M, Hussain R. Synthesis, characterization and antibacterial activity of nickel (II) and copper (II) complexes of N-(alkyl(aryl)carbamothioyl)-4-nitrobenzamide. Eur J Chem 2010;1:200–5
- Saeed S, Rashid N, Hussain R, et al. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur J Med Chem 2010;45:1323–31
- Campo RD, Criado JJ, Garcia E, et al. Thiourea derivatives and their nickel(II) and platinum(II) complexes: antifungal activity. J Inorg Biochem 2002;89:74–82
- Saeed S, Rashid N, Hussain R, et al. Synthesis, spectroscopic characterization, crystal structure and antifungal activity of thiourea derivatives containing a thiazole moiety. Central Eur J Chem 2010;8:550–8
- Sacht C, Datt MS, Otto S, Roodt A. Synthesis, characterisation and coordination chemistry of novel chiral N,N-dialkyl-N-menthyloxycarbonylthioureas. Crystal and molecular structures of N,N-diethyl-N-(-)-(3R)-menthyloxycarbonylthiourea and cis-(S,S)-[Pt(L)Cl(DMSO)] [where HL = N-(+)-(3R)-menthyloxycarbonyl-N′-morpholinothiourea or N-benzoyl-N′,N′-diethylthiourea]. J Chem Soc Dalton Trans 2000;24:4579–86
- Abdel HMH, Gad-Elkareen MAMGE, El-Adasy ABAEAAM, Othman IM. N-1-Naphthyl-3-oxobutanamide in heterocyclic synthesis: a facile synthesis of nicotinamide, thieno[2,3-b]pyridine, and bi- or tricyclic annulated pyridine derivatives containing naphthyl moiety. Phosph Sulf Silicon Relat Elem 2009;184:2263–80
- Khan MH, Bano Q, Nızamuddin. Pesticidal activities of some 3-aryl-4-methylpyrazolo[3,4-b][1,5]benzodiazepines and 4-aryl-2-imino-5-methyl-1,3-thiazino[4,5-b][1,5]benzodiazepines. J Agr Food Chem 1995;43:2719–21
- Fuks L, Anuszewska E, Kruszewska H, et al. Platinum(II) complexes with thiourea derivatives containing oxygen, sulfur orselenium in a heterocyclic ring: computational studies and cytotoxic properties. Trans Metal Chem 2010;35:639–47
- Ke SY, Xue SJ. Synthesis and herbicidal activity of N-(o-Fluoro phenoxyacetyl)thioureas derivatives and related fused heterocyclic compounds. Arkivoc 2006;10:63–8
- Zhang YM, Wei TB, Xian L, Gao LM. An efficient synthesis of polymethylene-bis-aroyl thioure derivatives under the condition of phase-transfer catalysis. Phosphorus Sulfur Silicon Relat Elem 2004;179:2007–13
- Jung SH, Kim DY. Catalytic enantioselective electrophilic α-hydrazination of β-ketoesters using bifunctional organocatalysts. Tetrahedron Lett 2008;49:5527–30
- Koch KR. New chemistry with old ligands: N-alkyl- and N,N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord Chem Rev 2001;216:473
- Binzet G, Florke U, Kulcu N, Arslan H. Crystal and molecular structure of bis(4-bromo-N-(di-n-butylcarbamothioyl)benzamido) copper(II) complex. Eur J Chem 2012;3:211–13
- Kharodawala MJ, Rana AK. Synthesis, characterization, and biological activity of some transition metal chelates of 4-acyloxime-2-pyrazolin-5-ones. Synth React Inorg Metal-Org Chem 2003;33:1483–504
- Bagihalli GB, Patil SA. Synthesis, spectral characterization, in vitro biological and DNA cleavage studies of Co(II), Ni(II), Cu(II), and Zn(II) complexes with 1,2,4-triazole Schiff bases. J Coord Chem 2009;62:1690–700
- Banerjee P, Pandey OP, Sengupta SK. Microwave assisted synthesis, spectroscopic and antibacterial studies of titanocene chelates of Schiff bases derived from 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles. Trans Met Chem 2008;33:1047–52
- Hernandez W, Spodine E, Vega A, et al. Cis-trans isomerism in copper(II) complexes with N-acyl thiourea ligands. Z Anorg Allg Chem 2004;630:1381–6
- Chohan ZH, Praveen M, Ghaffar A. Synthesis, characterisation and biological role of anions (nitrate, sulphate, oxalate and acetate) in Co(II), Cu(II) and Ni(II) metal chelates of some Schiff base derived amino acids. Synth React Inorg Met-Org Chem 1998;28:1673–87
- Rahman AU, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. The Netherlands: Harwood Academic Publishers; 2005
- Chohan ZH, Youssoufi MH, Jarrahpour A, Ben Hadda T. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: indolenyl sulfonamide derivatives. Eur J Med Chem 2010;45:1189–99
- Halli MB, Sumathi RB. Synthesis, spectroscopic, antimicrobial and DNA cleavage studies of new Co(II), Ni(II), Cu(II), Cd(II), Zn(II) and Hg(II) complexes with naphthofuran-2-carbohydrazide Schiff base. J Mol Str 2012;1022:130–8
- Prashanthi Y, Raj S. Synthesis and characterization of transition metal complexes with N,O; N,N and S,N-donor Schifff base ligands. J Sci Res 2010;2:114–26
- Patel IJ, Parmar SJ. Synthesis and studies of novel optically active Schiff's Base derivatives and their antimicrobial activities. E-J Chem 2010;7:617–23
- Prakash A, Singh BK, Bhojak N, Adhikari D. Synthesis and characterization of bioactive zinc(II) and cadmium(II) complexes with new Schiff bases derived from 4-nitrobenzaldehyde and acetophenone with ethylenediamine. Spectrochim Acta A 2010;76:356–62
- Mikami N, Nakagawa I, Shimanouch T. Far infra-red spectra and metal-ligand force constants of acetylacetonates of transition metals. Spectrochim Acta A 1967;23:1037–42
- Freeman RA. Handbook of nuclear magnetic resonance. 2nd ed. Harlow, UK: Longman Publishing; 1997
- Grevy JM, Tellez F, Bernes S, et al. Coordination compounds of thiabendazole with main group and transition metal ions. Inorg Chim Acta 2002;339:532–42
- Pasto DJ. Organic structure determination. London: Prentice Hall International; 1969
- Nair MS, Joseyphus RS. Synthesis and characterization of Co(II), Ni(II), Cu(II) and Zn(II) complexes of tridentate Schiff base derived from vanillin and dl-α-aminobutyric acid. Spectrochim Acta A 2008;70:749–53
- Lever ABP. Inorganic electronic spectroscopy. Amsterdam: Elsevier; 1984
- Larabi L, Hared Y, Reguig A, Mostafa M. Synthesis, structural study and electrochemical properties of copper (II) complexes derived from benzene and p-toluenesulphonylhydrazone. J Serb Chem Soc 2003;68:85–95
- Badawy MA, Mohamed GG, Omar MM, et al. Synthesis, spectroscopic and thermal characterization of quinoxaline metal complexes. Eur J Chem 2010;1:282–8
- Cotton FA, Wilkinson G. Advanced inorganic chemistry. 5th ed. New York: Wiley Interscience Publication; 1988
- Chohan ZH. Metal-based antibacterial and antifungal sulfonamides: synthesis, characterization, and biological properties. Trans Met Chem 2009;34:153–61
- Saeed S, Rashid N, Jasinski JP, et al. N-(Diethylcarbamothioyl)-4-nitrobenzamide. Acta Cryst 2010;E66:o2589
- Mansuroglu DS, Arslan H, Derveer DV, Binzet G. Synthesis and characterization of N-(2,2-Diphenylacetyl)-N′-substituted thiourea derivatives: the crystal structure of N-(2,2-Diphenylacetyl)-N′-(4-chloro phenyl)-thiourea. Phosphorus, Sulfur and Silicon 2009;184:3221–30
- Rehman SU, Chohan ZH, Naz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enz Inhib Med Chem 2005;20:333–40
- Sumrra SH, Chohan ZH. Synthesis, characterization and biological properties of thienyl derived triazole Schiff bases and their oxovanadium(IV) complexes. J Enz Inhib Med Chem 2012;27:187–93
- Chohan ZH, Hanif M. Design, synthesis, and biological properties of triazole derived compounds and their transition metal complexes. J Enzyme Inhib Med Chem 2010;25:737–49
- Sumrra SH, Chohan ZH. In vitro antibacterial, antifungal and cytotoxic activities of some triazole Schiff bases and their vanadyl(IV) complexes. J Enz Inhib Med Chem 2013;28:1291–9