Abstract
Carbonic anhydrases (CAs, EC 4.2.1.1), which are involved in a variety of physiological and pathological processes, are ubiquitous metalloenzymes mainly catalyzing the reversible hydration of carbon dioxide (CO2) to bicarbonate () and proton (H+). In this study, a dozen of bromophenol derivatives (1–12) were evaluated as metalloenzyme CA (EC 4.2.1.1) inhibitors against the human carbonic anhydrase isoenzymes I and II (hCA I and II). Cytosolic hCA I and II isoenzymes were effectively inhibited by bromophenol derivatives (1–12) with Kis in the low nanomolar range of 1.85 ± 0.58 to 5.04 ± 1.46 nM against hCA I and in the range of 2.01 ± 0.52 to 2.94 ± 1.31 nM against hCA II, respectively.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are biological catalysts for the interconversion of carbon dioxide (CO2) and water to bicarbonate () and a proton (H+)Citation1–3:
CAs are polifunctional enzymes which play a crucial role in different physiological and biochemical processes such as acid–base homeostasis, respiratory gas exchange, ionic transport, electrolytes secretion, muscular contraction in vertebrates, photosynthesis in cyanobacteria, plants and algae, synthesis of fatty acids, biosynthetic reactions including ureagenesis and gluconeogenesisCitation4–7. Recently, many studies revealed that CAs are also widely distributed in prokaryotesCitation8. Indeed, six distinct CA classes are known to date: α-, β-, γ-, δ-, ζ- and η-CAsCitation9–11. α-CAs have normally monomer structures and rarely dimer form. β-CAs are dimers, tetramers or octamers, γ-CAs are trimers, whereas the δ- and ζ-CAs are less well understood up to nowCitation8. In humans, 15 different α-CA isoforms were described so far, which differ by molecular features, oligomeric arrangement, cellular localization, distribution in organs and tissues, expression levels and kinetic propertiesCitation12–14. These CAs comprise CA I, II, III, IV, VA, VB, VI, VII, IX, XII, XIII and XIV, all of which contain a zinc ion (Zn2+) in their active site, coordinated to the imidazole groups of three histidine residues and to a water molecule (H2O)/hydroxide ion (–OH) the substrate that reacts with CO2Citation15–18. There are five cytosolic forms (CA I, II, III, VII and XIII), five membrane associated isozymes (CA IV, IX, XII, XIV and XV), two mitochondrial forms (CA VA and VB) and a secreted CA isoenzyme (CA VI). There are three additional non-catalytic CA isoforms (CA VIII, X and XI) whose functions remain unclearCitation19–21.
CA inhibitors (CAIs) are clinically used as diuretics and anti-glaucoma drugs. In addition, CAIs could have potential as anti-obesity, anti-cancer and anti-infective drugsCitation22–25. Naturally occurring bromophenols are important in synthetic organic chemistry and medicinal chemistry. These compounds are mostly isolated in marine life, especially from marine algaeCitation26–28. As it can be seen from the brief description given above, bromophenols show useful biological activities. In our previous studies, we synthesized bromophenols containing polybromides in their structures. In this study, we determined the effects of a dozen of bromophenol derivatives (1–12) on cytosolic hCA I, and II isoenzymes.
Experimental
Affinity chromatography is a purification method of separating biochemical mixtures based on a highly specific interaction such as that between enzyme and substrateCitation29. In this study, both CA isoenzymes were purified by Sepharose-4B-l-tyrosine-sulfanilamide affinity chromatography in a single stepCitation30–32. The column material including Sepharose-4B-l-tyrosine-sulfanilamide was prepared according to a previous methodCitation33–35. Thus, homogenate solution acidity was adjusted and supernatant was transferred to the previously prepared Sepharose-4B-l-tyrosine-sulphanilamide affinity columnCitation36–38. The proteins flow in the column eluates was spectrophotometrically determined at 280 nm. For determination of both isoenzymes purity, sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) for both isoenzymes was performed after purification step. This technique widely used in biochemistry, forensics, genetics, molecular biology and biotechnology for the separation of biological macromolecules including proteins according to their electrophoretic mobility. The presence and purity of both isoenzymes were visualized by SDS–PAGE. After this process, a single band was observed for each isoenzymeCitation39. This protein imaging method was previously describedCitation40–42. In this application, the imaging method was performed out in 10% and 3% acrylamide for the running and the stacking gel, respectively, with 0.1% SDSCitation43,Citation44.
Both CA isoenzymes activities were determined according to the method of Verpoorte et al.Citation45 and described previouslyCitation46,Citation47. The protein quantity was spectrophotometrically measured at 595 nm during the purification steps according to the Bradford methodCitation48. Bovine serum albumin was used as the standard proteinCitation49. For determining the inhibition effect of each bromophenol derivative, an activity (%)–[Bromophenols] graph was drawn. To determine Ki values, three different bromophenols concentrations were tested. In these experiments, different substrate concentration was used and Lineweaver–Burk curves were drawnCitation50,Citation51 as previously describedCitation52.
Results and discussion
Naturally occurring bromophenols compounds are abundantly found in marine life. They were frequently isolated from red algae of the family Rhodomelaceae and had some important biological activitiesCitation53–55. It was reported that the bromophenol derivatives coordinate to the active site Zn2+ and to block the reaction catalysis. For example, (4,5-dihydroxy-2-methylphenyl)(3,4-dihydroxyphenyl)methanone was found much more effective inhibitors against hCA I and IICitation38. In another study, it was found that hCA I, II and VI were inhibited by a series of bisphenol and bromophenol derivativesCitation27. Recently, Balaydın et al. determined CA I, II, IV and VI inhibition effects of novel cyclohexanonyl bromophenol derivatives including naturally occurring novel cyclohexanonyl bromophenol 2(R)-2-(2,3,6-tribromo-4,5-dihydroxybenzyl)cyclohexanone and some of them showed interesting inhibitory profileCitation28. In a recent study, it was demonstrated that dimethoxy-bromophenol derivatives incorporating cyclopropane moieties have shown picomolar inhibition against cytosolic CA I, II and tumor-associated CA IX, and XIICitation9. The relationship between anti-oxidant molecules and CA isoenzyme inhibition is well establishedCitation56–58. It is well known that bromophenol derivatives had some biological activities including anti-oxidant and radical scavengingCitation55, acetylcholine esterase inhibition propertiesCitation55,Citation59. The clinical usage of CAIs has been established as anti-glaucoma agents, diuretics and anti-epileptic. CAIs were also used in the treatment of mountain sickness, osteoporosis, gastric and duodenal ulcers and neurological disordersCitation60–62.
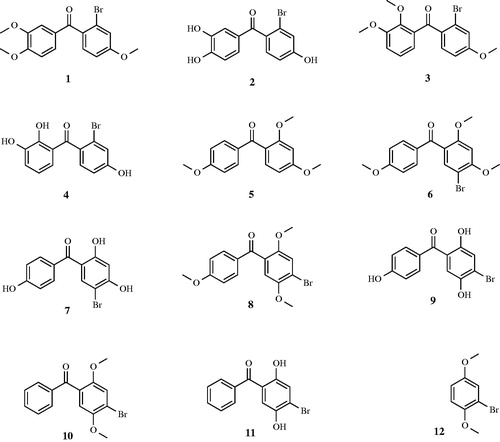
Both physiologically relevant hCA I, and II isoforms were included in our study. A dozen of bromophenol derivatives (1–12) were evaluated for their inhibition properties against hCA I and II isoenzymes, showing generally an efficient inhibition. The chemical structures of bromophenol derivatives (1–12) are given in . Also, CA I and II inhibiting effects of a dozen of bromophenol derivatives (1–12) are shown in . It was well known that developing isoenzyme-specific CAIs should be highly beneficial in obtaining novel classes of drugs devoid of various undesired side-effectsCitation25. We declare here the first study on the inhibitory effects of a dozen of bromophenol derivatives (1–12) against hCA I and II using esterase activity.
Table 1. Human carbonic anhydrase isoenzymes I and II inhibition profile of bromophenol derivatives (1–12).
Low cytosolic isoenzyme hCA I is found in many tissues, however, it was demonstrated that this isoenzyme is involved in retinal and cerebral edema, and its inhibition may be a valuable tool for fighting these conditions. Also, it was reported that if Ki value of a dozen of bromophenol derivatives (1–12) was less than 10 nM (Kis < 10 nM). The results obtained from this study clearly indicate that a dozen of bromophenol derivatives (1–12) had effective inhibition profile against slow cytosolic isoform hCA I, and cytosolic dominant rapid isozymes hCA II with low nanomolar range (Kis < 10 nM). These compounds bind to hCA I in the nanomolar range. Ki values are ranging in 1.85 ± 0.58 to 5.04 ± 1.46 nM for hCA I isoenzyme. However, acetazolamide (AZA) considered being a broad-specificity CAI owing to its widespread inhibition of CAs, demonstrated showed Ki value of 6.76 ± 2.55 nM against hCA I. Bromophenol (3), possessing three methoxy (–OCH3) and a bromine (–Br) group, was the best hCA II inhibitor (Ki: 1.79 ± 0.22 nM). It is well known that the molecules, which had three –OCH3 and a –Br group, demonstrated effective CA isoenzyme inhibition propertiesCitation9,Citation10. The inhibition effects of all bromophenol derivatives (1–12) are higher than that of acetazolamide (AZA; Ki: 6.76 ± 2.55 nM). AZA, 5-acetamido-1,3,4-thiadiazole-2-sulfonamide, is considered the good CAI and is approved for the treatment of a range of conditions including glaucoma, epilepsy and altitude sicknessCitation5.
CA II is involved in several diseases including epilepsy, edema, altitude sickness and glaucoma. Against the physiologically dominant isoform hCA II, all bromophenol derivatives (1–12) demonstrated Kis of 2.01 ± 0.52 to 2.94 ± 1.31 nM (). The bromophenol 11, which had two methoxy (–OH) and a bromine (–Br) groups, was the best hCA II inhibitor (Ki: 2.01 ± 0.52 nM). Phenolic compounds have a lot of nutritional and pharmacological properties including anti-oxidant propertiesCitation62–81 and enzymes inhibitionCitation82–86. However, all bromophenol derivatives (1–12) have shown similar hCA II inhibition properties. These results showed that all bromophenol derivatives (1–12) had higher affinity toward hCA II than that of hCA I isoform. Also, AZA, which may interact with the distinct hydrophobic and hydrophilic halves of the CA II active site, showed Ki value of 5.85 ± 2.56 nM. This standard demonstrated less inhibition activity than that of all bromophenol derivatives (1–12).
Conclusion
In conclusion, a dozen of bromophenol derivatives (1–12) were evaluated against cytosolic hCA I and II. These bromophenols have shown nanomolar inhibition against cytosolic CA I and II. Novel bromophenols were found to be effective hCA I and II inhibitors. Both isoenzymes were potently inhibited by bromophenol derivatives (1–12) with Kis in the range of 1.85 ± 0.58 to 5.04 ± 1.46 nM against hCA I and in the range of 2.01 ± 0.52 to 2.94 ± 1.31 nM against hCA II, respectively.
Acknowledgement
I.G. and S.H.A. would like to extend their sincere appreciation to the Research Chairs Program at King Saud University for funding this research.
Declaration of interest
The authors report there is no conflict of interests.
References
- Hisar O, Beydemir Ş, Gülçin İ, et al. Effect of low molecular weight plasma inhibitors of rainbow trout (Oncorhyncytes mykiss) on human erythrocytes carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:35–9
- Aras Hisar Ş, Hisar O, Beydemir Ş, et al. Effect of vitamin E on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Acta Vet Hung 2004;52:413–22
- Hisar O, Beydemir Ş, Gülçin İ, et al. The effect of melatonin hormone on carbonic anhydrase enzyme activity in rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Turk J Vet Anim Sci 2005;29:841–5
- Ward C, Langdon SP, Mullen P, et al. New strategies for targeting the hypoxic tumour microenvironment in breast cancer. Cancer Treat Rev 2013;39:171–9
- Tanpure RP, Ren B, Peat TS, et al. Carbonic anhydrase inhibitors with dual-tail moieties to match the hydrophobic and hydrophilic halves of the carbonic anhydrase active site. J Med Chem 2015;58:1494–501
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: in vivo and in vitro studies. J Enzyme Inhib Med Chem 2008;23:266–70
- Vullo D, De Luca V, Del Prete S, et al. Sulfonamide inhibition studies of the c-carbonic anhydrase from the Antarctic cyanobacterium Nostoc commune. Bioorg Med Chem 2015;23:1728–34
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50
- Yıldırım A, Atmaca U, Keskin A, et al. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605
- Carta F, Mannelli LDC, Pinard M, et al. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem 2015;23:1828–40
- Şentürk M, Gülçin İ, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11
- Arabaci B, Gülçin İ, Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014;19:10103–14
- Güney M, Coşkun A, Topal F, et al. Oxidation of cyanobenzocycloheptatrienes: synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg Med Chem 2014;22:3537–43
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Coban TA, Beydemir S, Gücin İ, et al. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I–XIV. Bioorg Med Chem 2009;17:5791–5
- Akbaba Y, Akıncıoğlu A, Göçer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42
- Göksu S, Naderi A, Akbaba Y, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82
- Sethi KK, Verma SM, Tanç M, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of the cytosolic mammalian carbonic anhydrase isoforms I, II and VII with benzene sulfonamides incorporating 4,5,6,7-tetrachlorophthalimide moiety. Bioorg Med Chem 2013;21:5168–74
- Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902
- Sharma A, Tiwari M, Supuran CT. Novel coumarins and benzocoumarins acting as isoform-selective inhibitors against the tumor-associated carbonic anhydrase IX. J Enzyme Inhib Med Chem 2014;29:292–6
- Joseph P, Turtaut F, Ouahrani-Bettache S, et al. Cloning, characterization, and inhibition studies of a b-carbonic anhydrase from Brucella suis. J Med Chem 2010;53:2277–85
- Burghout P, Vullo D, Scozzafava A, et al. Inhibition of the b-carbonic anhydrase from Streptococcus pneumoniae by inorganic anions and small molecules: toward innovative drug design of antiinfectives? Bioorg Med Chem 2011;19:243–8
- Aksu K, Nar M, Tanc M, et al. The synthesis of sulfamide analogues of dopamine related compounds and their carbonic anhydrase inhibitory properties. Bioorg Med Chem 2013;21:2925–31
- Akbaba Y, Balaydın HT, Menzek A, et al. Synthesis and biological evaluation of novel bromophenolderivatives as carbonic anhydrase inhibitors. Arch Pharm 2013;346:447–54
- Balaydin HT, Durdagi S, Ekinci D, et al. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, methoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2012;27:467–75
- Balaydin HT, Senturk M, Menzek A. Synthesis and carbonic anhydrase inhibitory properties of novel cyclohexanonyl bromophenol derivatives. Bioorg Med Chem Lett 2012;22:1352–7
- Atasever A, Ozdemir H, Gulcin I, Kufrevioglu OI. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70
- Gribble GW. The diversity of naturally occurring organobromine compounds. Chem Soc Rev 1999;28:335–46
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61
- Gocer H, Aslan A, Gülçin İ, Supuran CT. Spirobisnaphthalenes effectively inhibit carbonic anhydrase. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. http://dx.doi.org/10.3109/14756366.2015.1047359
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602
- Uhlen M. Affinity as a tool in life science. Biotechniques 2008;44:649–54
- Akbaba Y, Bastem E, Topal F, et al. Synthesis and carbonic anhydrase inhibitory effects of novel sulfamides derived from 1-aminoindanes and anilines. Arch Pharm 2014;347:950–7
- Gül Hİ, Kucukoglu K, Yamali C, et al. Synthesis of 4-(2-substitutedhydrazinyl)benzenesulfonamides and their carbonic anhydrase inhibitory effects. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. http://dx.doi.org/10.3109/14756366.2015.1047359
- Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9
- Öztürk Sarıkaya SB, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62
- Nar M, Çetinkaya Y, Gülçin İ, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Gülçin İ, Küfrevioğlu Öİ, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibition effects of some chemicals on the enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302
- Şişecioğlu M, Gülçin İ, Çankaya M, et al. Purification and characterization of peroxidase from Turkish black radish (Raphanus sativus L.). J Med Plants Res 2010;4:1187–96
- Aksu K, Topal F, Gülçin I, et al. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch Pharm 2015;348:446–55
- Gülçin İ, Beydemir Ş, Çoban TA, Ekinci D. The inhibitory effect of dantrolene sodium and propofol on 6-phosphogluconate dehydrogenase from rat erythrocyte. Fresen Environ Bull 2008;17:1283–7
- Şentürk M, Gülçin İ, Çiftci M, Küfrevioğlu Öİ. Dantrolene inhibits human erythrocyte glutathione reductase. Biol Pharm Bull 2008;31:2036–9
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Akıncıoğlu A, Akbaba Y, Göçer H, et al. Novel sulfamides as potential carbonic anhydrase isoenzymes inhibitors. Bioorg Med Chem 2013;21:379–85
- Göçer H, Akıncıoğlu A, Öztaşkın N, et al. Synthesis, antioxidant and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine related compounds. Arch Pharm 2013;346:783–92
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Köksal E, Gülçin İ. Purification and characterization of peroxidase from cauliflower (Brassica oleracea L.) buds. Protein Peptide Lett 2008;15:320–6
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66
- Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi.org/10.3109/14756366.2015.1036051
- Köksal E, Ağgül AG, Bursal E, Gülçin İ. Purification and characterization of peroxidase from sweet gourd (Cucurbita moschata Lam. Poiret). Int J Food Prop 2012;15:1110–19
- Balaydın HT, Gülçin İ, Menzek A, et al. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–95
- Öztaşkın N, Çetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57
- Gülçin İ, Beydemir Ş, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–16
- Beydemir Ş, Gülçin İ. Effect of melatonin on carbonic anhydrase from human erythrocyte in vitro and from rat erythrocyte in vivo. J Enzyme Inhib Med Chem 2004;19:193–7
- Gülçin İ, Beydemir Ş, Hisar O. The effect of α-tocopherol on the antioxidant enzymes activities and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Acta Vet Hung 2005;53:425–33
- Innocenti A, Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I–XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64
- Çetinkaya Y, Göçer H, Gülçin İ, Menzek A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch Pharm 2014;347:354–9
- Akıncıoğlu A, Topal M, Gülçin İ, Göksu S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm 2014;347:68–76
- Taslimi P, Gulcin İ, Ozgeris B, et al. The human carbonic anhydrase isoenzymes I and II (hCA I and II) inhibition effects of trimethoxyindane derivatives. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi:10.3109/14756366.2015.1014476
- Büyükokuroğlu ME, Gülçin İ, Oktay M, Küfrevioğlu Öİ. In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 2001;44:491–5
- Gülçin İ, Beydemir Ş, Alici HA, et al. In vitro antioxidant properties of morphine. Pharmacol Res 2004;49:59–66
- Gülçin İ, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005;53:281–5
- Gülçin İ, Berashvili D, Gepdiremen A. Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J Ethnopharmacol 2005;101:287–93
- Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-d-glucopyranosyl)-hederagenin. Phytother Res 2006;20:130–4
- Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213–20
- Gülçin İ, Elias R, Gepdiremen A, Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 2006;223:759–67
- Gülçin İ. Comparison of in vitro antioxidant and antiradical activities of l-tyrosine and l-dopa. Amino Acids 2007;32:431–8
- Gülçin İ. Daştan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzyme Inhib Med Chem 2007;22:685–95
- Gülçin İ. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J Enzyme Inhib Med Chem 2008;23:871–6
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37
- Köksal E, Gülçin İ, Öztürk Sarıkaya SB, Bursal E. On the in vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395–405
- Gülçin İ, Elias R, Gepdiremen A, et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 2009;43:195–212
- Gülçin İ. Antioxidant activity of l-adrenaline: an activity–structure insight. Chem Biol Interact 2009;179:71–80
- Gülçin İ. Antioxidant properties of resveratrol: a structure–activity insight. Innov Food Sci Emerg 2010;11:210–18
- Gülçin İ, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 2010;3:43–53
- Gülçin İ. Antioxidant activity of eugenol – a structure and activity relationship study. J Med Food 2011;14:975–85
- Göçer H, Gülçin İ. Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int J Food Sci Nutr 2011;62:821–5
- Şişecioğlu M, Çankaya M, Gülçin İ, Özdemir M. The inhibitory effect of propofol on lactoperoxidase. Protein Peptide Lett 2009;16:46–9
- Gülçin İ, Beydemir Ş, Hisar O, et al. Melatonin administration increases antioxidant enzymes activities and reduce lipid peroxidation in the rainbow trout (Oncorhynchus mykiss, Walbaum) erythrocytes. Turk J Vet Anim Sci 2009;33:241–5
- Şişecioğlu M, Çankaya M, Gülçin İ, Özdemir M. Interactions of melatonin and serotonin to lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2010;25:779–83
- Gülçin İ, Beydemir S. Phenolic compounds as antioxidants: carbonic anhydrase isoenzymes inhibitors. Mini Rev Med Chem 2013;13:408–30
- Gülçin İ, Beydemir Ş, Hisar O. The effect of α-tocopherol on the antioxidant enzymes activities and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Acta Vet Hung 2005;53:425–33
- Şentürk M, Gülçin İ, Çiftci M, Küfrevioğlu Öİ. Dantrolene inhibits human erythrocyte glutathione reductase. Biol Pharmacol Bull 2008;31:2036–9