Abstract
The aim of this study is to search for soluble epoxide hydrolase (sEH) inhibitors from natural plants, bioassay-guided fractionation of lipophilic n-hexane and chloroform layers of an extract of the aerial parts of Glycosmis stenocarpa led to the isolation of 12 compounds (1–12) including murrayafoline-A (1), isomahanine (2), bisisomahanine (3), saropeptate (4), (24 S)-ergost-4-en-3,6-dione (5), stigmasta-4-en-3,6-dion (6), stigmast-4-en-3-one (7), β-sitosterol (8), 24-methylpollinastanol (9), trans-phytol (10), neosarmentol III (11) and (+)-epiloliolide (12). Their structures were elucidated on the basis of spectroscopic data. Among them, neosarmentol III (11) was isolated from nature for the first time. All the isolated compounds were evaluated for their inhibitory activity against sEH. Among isolated carbazole-type compounds, isomahanine (2) and bisisomahanine (3) were identified as a potent inhibitor of sEH, with IC50 values of 22.5 ± 1.7 and 7.7 ± 1.2 µM, respectively. Moreover, the inhibitory action of 2 and 3 represented mixed-type enzyme inhibition.
Introduction
Cytochrome P450 (CYP) epoxygenase in human can convert arachidonic acid to four biologically active compounds, which are cis-epoxyeicosatrienoic acid (EET) regiosiomers (5,6-, 8,9-, 11,12- and 14,15-EET)Citation1. In the signaling of tissue, EETs as endothelium-derived hyperpolarizing factors promote cell hyperpolarization by stimulating Ca2+-activated K+ channels in peripheral arteries and airway smooth muscle cellsCitation2. The interaction of EETs with G-protein-coupled receptor induces activation of BKCa channels and tissue plasminogen activator expressionCitation3,Citation4. Furthermore, EETs decrease the apoptosis induced by tumor necrosis factor-α (TNF-α) of human carcinoma cellsCitation5. Among them, 11,12-EET suppresses the NF-κB activation in human umbilical vein endothelial cells and VCAM-1 expression in human saphenous vein endothelial cells induced by TNF-α, whereas 14,15-EET inhibits IκBα degradation and increases their protein levelCitation6. Therefore, a decrease in EETs leads to the development of cardiovascular diseaseCitation3. Recently, soluble epoxide hydrolase (sEH) was identified as a hydrolase that controls EET levels by metabolizing EETs to dihydroxyeicosatrienoic acids. This enzyme has become a target for the treatment of cardiovascular diseaseCitation7.
Glycosmis stenocarpa (Rutaceae), or “com rudo trai hep”, grows to a height of 75–100 cm and is distributed in north VietnamCitation8. Previous phytochemical studies of G. stenocarpa revealed the presence of carbazole derivates, such as murrayafoline-A, murrayanine and bisisomahanineCitation7. Murrayafoline-A has been reported to show potent inhibitory activity against glycogen synthase kinase-3βCitation9 and weak inhibitory activity against sEHCitation10. In addition, murrayafoline A was found to have positive inotropic activity and increase l-type Ca2+ currents in rat ventricular myocytes by concentration-dependence modeCitation11. A deep study on effects of Murrayafoline A on focal and global Ca2+ signaling revealed the involvement of protein kinase C blockadeCitation12.
Screening of crude extracts from the aerial parts of G. stenocarpa for the development of sEH inhibitors indicated that 25 µg/ml methanol extract, n-hexane, chloroform and ethyl acetate extracts contained inhibitory activity ranging from 37.8 ± 0.9 to 72.2 ± 1.9% in vitro. Lipophilic n-hexane and chloroform extracts, which showed high inhibition rates, were subjected to column chromatography; 12 compounds were isolated. All the isolated compounds were evaluated for their inhibitory activity against sEH. Among the isolated carbazole-type compounds, isomahanine (2) and bisisomahanine (3), were identified as potent inhibitors of sEH, with IC50 values of 22.5 ± 1.7 and 7.7 ± 1.2 µM, respectively.
Materials and methods
General experimental procedures
Column chromatography was performed using silica gel (Kieselgel 60, 70–230 and 230–400 mesh, Merck, Darmstadt, Germany), Sephadex LH-20 (40–70 µm) (Amersham Biosciences, Piscataway, NJ) and YMC RP-18 resins (YMC America, Allentown, PA). Thin layer chromatography (TLC) was performed using pre-coated silica-gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck, Darmstadt, Germany). Spots were visualized by spraying with 10% aqueous H2SO4 solution followed by heating. NMR spectra were recorded using a JEOL ECA 600 spectrometer (1H, 600 MHz; 13C, 150 MHz) (Tokyo, Japan) and Bruker Advance 300 spectrometer (1H, 300 MHz; 13C, 75 MHz) (Bruker, Rheinstetten, Germany), using chloroform-d and methanol-d4 as solvents. Mass spectra were measured by Bruker Daltonics MicroQ-TOF III mass spectrometer (Bruker, Rheinstetten, Germany). Optical rotations were determined using a JASCO P-2000 automatic digital polarimeter. sEHs (10011669) and PHOME (10009134) were purchased from Cayman (Cayman, Ann Arbor, MI).
Plant materials
Dried aerial parts of G. stenocarpa were collected at Hoang Hoa Tham commune, Chi Linh district, Hai Duong province, Viet Nam, in February 2011 and identified by the botanist, Dr Ngo Van Trai at the Institute of Medicinal Materials (Ministry of Health, Hanoi, Vietnam). A voucher specimen (C450L) was deposited at the Herbarium of Institute of Natural Products Chemistry.
Extraction and isolation
The dried aerial parts (2 kg) were extracted with 95% methanol (12 l × 3 times) under reflux After removal of the solvent under reduced pressure, the crude extract (150 g) was dissolved in 1.0 l of H2O to form a suspension that was successively partitioned with n-hexane, chloroform, ethyl acetate and H2O to yield a hexane fraction (40 g), chloroform (16 g) fraction, ethyl acetate (4 g) and distilled water fraction (86 g). n-Hexane layer (40 g) was separated with silica gel column chromatography using a gradient system of n-hexane/ethyl acetate (10:1 → 0.5:1) to yield eight fractions (H1–H8). Fraction H3 was subjected to C-18 column chromatography with gradient elution of water/methanol (1:1 → 1:5) to afford compounds 4 (7 mg), 7 (5 mg) and four fractions (H31–H34). Fraction H32 was subjected to C-18 column chromatography using 70% methanol to yield compounds 1 (8 mg), 8 (6 mg) and 9 (7 mg). Fraction H34 was separated using C-18 column chromatography with 70% methanol to obtain compound 5 (5 mg). Fraction H5 was purified by C-18 column chromatography using a gradient system of water/methanol (1:1 → 0.2:1) to yield compounds 6 (11 mg) and eight fractions (H51–H58). Fraction H54 was subjected to C-18 column chromatography using 60% methanol to achieve compound 3 (13 mg). Chloroform layer (16 g) was separated with silica gel column chromatography using a gradient system of n-hexane/ethyl acetate (10:1 → 0.5:1) to obtained seven fractions (C1–C7). Fraction C3 was subjected to C-18 column chromatography using isocratic system of 80% methanol to yield compounds 2 (32 mg), 10 (9 mg) and 12 (7 mg). Fraction C6 was loaded on Sephadex LH-20 column chromatography and eluted with methanol to afford four fractions (C61–C64). Fraction C63 was subjected to C-18 column chromatography using 80 % methanol to achieve compound 11 (7 mg).
Neosarmentol III (11): colorless oil. : −22.4° (c = 0.1, in MeOH); 1H (CD3OD, 600 MHz) and 13C-NMR (CD3OD, 150 MHz) see ; HR-ESI-MS m/z 207.1364 [M + H]+(calcd. for C20H24O7: 207.1380).
Table 1. 1H and 13C NMR data for compound 11.
Enzyme inhibition assay
Enzyme assay was performed as described previouslyCitation10. Briefly, each 50 µl of sEH in 25 mM bis–Tris–HCl buffer (pH 7.0) containing 0.1% BSA and 20 µl of the respective compounds diluted from 1 to 0.031 mM dissolved in MeOH were mixed in 96-well plates. Finally, 50 µl of 40 µM PHOME were added as a substrate. After starting the enzyme reaction at room temperature, 6-methoxy-2-naphthaldehyde intensity (RFU) was measured using fluorometric determination (excitation wavelength, 330 nm; emission wavelength, 465 nm) for 60 min. The inhibition ratio was calculated using the equation:
AUDA was used as the positive control.
Statistical analysis
All the tests in the presence of inhibitors were performed in triplicate and results are presented as the means ± standard error of the mean (SEM). The results were subjected to analysis using Sigma Plot (SPP Inc., Chicago, IL).
Results and discussion
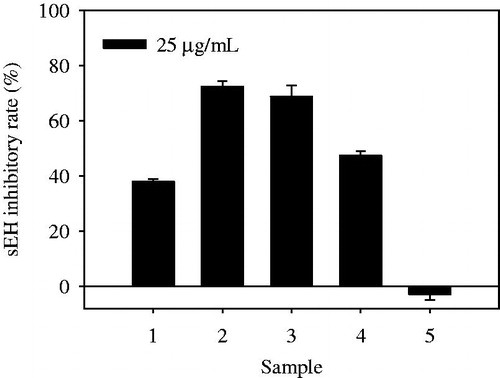
Methanol extract of the aerial parts of G. stenocarpa was suspended in distilled water and then partitioned with n-hexane, chloroform and ethyl acetate. Methanol extract, n-hexane, chloroform and ethyl acetate extracts exhibited sEH inhibitory rates of 37.8 ± 0.9, 72.2 ± 1.9, 68.8 ± 3.9 and 47.3 ± 1.5% at 25 µg/ml, respectively (). The lipophilic n-hexane and chloroform extracts obtained by activity-guided fractionation were further separated by chromatography over silica gel and C-18 columns to isolate 12 compounds, identified as: murrayafoline A (1)Citation8, isomahanine (2)Citation8, bisisomahanine (3)Citation8, saropeptate (4)Citation13, (24 S)-ergost-4-en-3,6-dione (5)Citation14,Citation15, stigmasta-4-en-3,6-dion (6)Citation15, stigmast-4-en-3-one (7)Citation16, β-sitosterol (8)Citation17, 24-methylpollinastanol (9)Citation18, trans-phytol (10)Citation19, neosarmentol III (11)Citation20 and (+)-epiloliolide (12)Citation21 (). Their structures were elucidated by a comparison of their spectroscopic data with those reported previously.
Figure 1. Effects of extract on sEH inhibitory activity determined using the fluorometric method. (1) Methanol extract, (2) n-Hexane extract, (3) Chloroform extract, (4) Ethyl acetate extract and (5) Water extract.
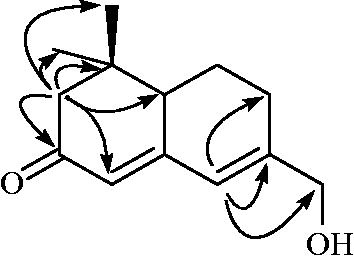
Compound 11 was obtained as colorless oil, ]: −22.4° (c = 0.1, in MeOH), and the molecular formula was suggested to be C13H18O2 based on a pseudomolecular ion peak [M + H]+ at m/z 207.1364 (calcd. 207.1380) by high-resolution electrospray ionization mass spectrometry (HR-ESI-MS). The 1H NMR spectrum revealed two double-bond proton signals δ 6.32 (1H, s) and 5.79 (1H, s), as well as four signals at δ 4.14 (2H,s), 2.33/2.07 (2H, m), 2.21/2.15 (2H, m) and 1.95/1.33 (2H, m) were assigned to methylene protons. The 13C NMR spectrum contained 13 carbon signals, including 1 ketone (δ 201.4), 4 double bond (δ 158.5, 153.9, 122.2 and 121.9), 4 methylene (δ 64.3, 53.2, 26.5 and 22.0) and 2 methyl (δ 27.5 and 18.8) carbon signals. The HMBC spectrum displayed the correlation between H-10 (δ 4.14) and C-9 (δ 153.9)/C-13 (δ 121.9)/C-8 (δ 26.5). Furthermore, the HMBC correlation indicated the cross-peak between H-2 (δ 2.33/2.07) and C-3 (δ 201.4)/C-4 (δ 122.2)/C-6 (δ 45.6)/C-1 (δ 35.8)/C-12 (δ 27.5)/C-11 (δ 18.8) ( and ). Compound 11 was identified as neosarmentol IIICitation20, and isolated from natural plants for the first time.
The isolated compounds 1–12 were evaluated for their ability to inhibit the enzymatic activity, which converts 3-phenyl-cyano (6-methoxy-2-naphthalenyl)methyl ester-2-oxiraneacetic acid (PHOME) to 6-methoxy-2-naphthaldehyde, using a fluorescence photometer at excitation and emission wavelengths of 330 and 465 nm. As listed in , compounds (1–12) exhibited inhibitory ratios ranging from 4.7 ± 1.7 to 98.9 ± 0.7% of the control value at 100 µM. Among them, compounds 2, 3 and 5–7 showed >60% inhibition in a dose-dependent manner, with IC50 values ranging from 7.7 ± 1.2 to 61.5 ± 3.4 µM. 12-(3-Adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA) was used as a positive control (IC50 = 12.2 ± 4.1 nM). Consistent with another reportCitation10, murrayafoline-A (1) did not display inhibitory activity against sEH, but the two compounds combining carbazole with a chromene ring, isomahanine (2), bisisomahanine (3), displayed inhibitory activity greater than that of compound 1. The most potent inhibitors (2 and 3) exhibited IC50 values of 22.5 ± 1.7 and 7.7 ± 1.2 µM, respectively, toward sEH. Additionally, compounds 5–7 exhibited IC50 values of 30.0 ± 1.9, 61.5 ± 3.4 and 59.5 ± 1.2, respectively ().
Table 2. The sEH inhibitory activity of compounds 1–12.
To investigate the binding mechanism of the two inhibitors (2 and 3) to the enzyme, kinetic analyses were carried out in the presence of inhibitor (0–12.5 µM) at various substrate concentrations (1.5–25.0 µM). The results were used to construct Lineweaver–Burk plots for each inhibitor, which yielded a family of straight lines with different slopes and a common intercept above the abscissa; in this case-independent binding of the substrate and inhibitor with the enzyme is designated mixed inhibition. Furthermore, the secondary plot of the slopes and ordinate intercepts of the respective Lineweaver–Burk data represented straight lines with the abscissa intercepts −Kic and −Kiu, respectively (Kic, binding constant of inhibitor with free enzyme and Kiu, binding constant of inhibitor with enzyme–substrate complex). As presented in and , the Kic and Kiu values for inhibition of sEH were 4.7 and 16.9 µM for compound 2, respectively; and 1.0 and 7.0 µM for compound 3, respectively ( and ).
Figure 4. Lineweaver–Burk plots were constructed for the inhibition of sEH by compounds 2 (A) and 3 (B). Inserts (I) and (II) represent the secondary plot of the slope and the intercept of compounds (2 and 3).
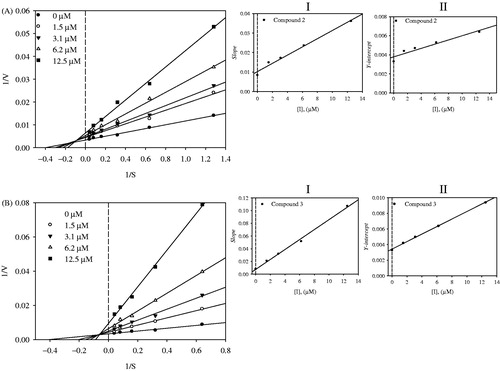
Table 3. In vitro sEH kinetic study of compounds.
Conclusions
In this study, 12 compounds (1–12) were isolated from aerial parts of G. stenocarpa. Neosarmentol III (11), which has been reported to be produced by enzymatic hydrolysis of neosedumoside IIICitation20, was isolated from a natural plant for the first time.
Extracts of the aerial parts of G. stenocarpa and their chemical constituents were tested for sEH inhibitory activity. Through activity-guided fractionation, compounds 2 and 3 from chloroform and n-hexane fractions were isolated and confirmed to be potential inhibitors, with IC50 values of 22.5 ± 1.7 and 7.7 ± 1.2 µM, respectively, and exhibited mixed-type inhibition (interaction with the free enzyme or the enzyme–substrate complex at allosteric sites). According to enzyme kinetic results, the two inhibitors bind the free enzyme with greater affinity than does the enzyme–substrate complex. Isomahanine (2) and bisisomahanine (3), with chromene ring on pyranocarbazole possessed higher sEH inhibitory activities than those of murrayafoline A (1)Citation10. This result suggests that the existence of a chromene ring might be important for the sEH inhibitory activity. This study represents the first report of a potential carbazole-type alkaloid inhibitor of sEH from a natural source.
Supplementary material available online Supplementary Figures S1-S13
IENZ_1057719_Supplementary_data.pdf
Download PDF (2.7 MB)Declaration of interest
This study was supported by the Priority Research Center Program (2009–0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea.
References
- Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate mono-oxygenase. J Lipid Res 2000;41:163–81
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 1996;78:415–23
- Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases andmetabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Res 2011;63:597–609
- Yang W, Holmes BB, Gopal VR, et al. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther 2007;321:1023–31
- Jiang JG, Chen CL, Card JW, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 2005;65:4707–15
- Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999;285:1276–9
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for, cardiovascular diseases. Nat Rev Drug Discov 2009;8:794–805
- Cuong NM, Hung TQ, Sung TV, Taylor WC. A new dimeric carbazole alkaloid from Glycsmis stenocarpa roots. Chem Pharm Bull 2004;52:1175–8
- Choi H, Gwak JS, Cho M, et al. Murrayafoline A attenuates the Wnt/β-catenin pathway by promoting the degradation of intracellular β-catenin proteins. Biochem biophys Res Commun 2010;391:915–20
- Lee GH, Oh SJ, Lee SY, et al. Discovery of soluble epoxide hydrolase inhibitors from natural products. Food Chem Toxicol 2014;64:225–30
- Son MJ, Chidipi B, Kim JC, et al. Alterations of contractions and L-type Ca2+ currents by murrayafoline-A in rat ventricular myocytes. Eur J Pharmacol 2014;740:81–7
- Kim JC, Wang J, Son MJ, et al. Sensitization of cardiac Ca(2+) release sites by protein kinase C signaling: evidence from action of murrayafoline A. Pflugers Arch 2014;7:1–15
- Wahidulla S, D’Souza L. Identity of saropeptate, aurantiamide acetate and seperglaucide. Orient J Chem 1998;14:461–2
- Eshbakova KA, Tashkhodzhaev B, Tursunov ZhI, et al. Structure of a new steroid 24S-ergost-4-en-3,6-dione from Aconitum septentrionale. Chem Nat Compd 2011;47:73–5
- Thao NP, Nam NH, Cuong NX, et al. Steroidal constituents from the soft coral sinularia dissecta and their inhibitory effects on lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Bull Korean Chem Soc 2013;34:949–52
- Alexander-Lindo RL, Morrison EY, St A, Nair MGN. Hypoglycaemic effect of stigmast-4-en-3-oneand its corresponding alcohol from the bark of Anacardium occidentale (Cashew). Phytother Res 2004;18:403–7
- Viswanadh GS, Ramaiah PA, Latsch H, Maskey R. Chemical constituents of the heartwood and bark of Homonoia riparia. J Trop Med Plants 2006;7:267–73
- Bȍhme F, Schmidt J, Sung TV, Adam G. 24-Methylpollinastanone, related triterpenoids and sterols from Costus tonkinesis. Phytochemistry 1997;4:1041–4
- Yang P, Liu D-Q, Lee T-J, et al. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg Med Chem 2015;23:38–45
- Muraoka O, Morikawa T, Zhang Y, et al. Novel megastigmanes with lipid accumulation inhibitory and lipid metabolism-promoting activities in HepG2 cells from Sedum sarmentosum. Tetrahedron 2009;65:4142–8
- Ma Q-G, Wang Y-G, Liu W-M, et al. Hepatoprotective sesquiterpenes and rutinosides from Murraya koeigii (L.) spreng. J Agric Food Chem 2014;62:4145–51