Abstract
Objective. Women with a fetal death at the time of diagnosis have higher maternal plasma concentrations of the anti-angiogenic factor, soluble vascular endothelial growth factor receptor (sVEGFR)-1, than women with a normal pregnancy. An important question is whether these changes are the cause or consequence of fetal death. To address this issue, we conducted a longitudinal study and measured the maternal plasma concentrations of selective angiogenic and anti-angiogenic factors before the diagnosis of a fetal death. The anti-angiogenic factors studied were sVEGFR-1 and soluble endoglin (sEng), and the angiogenic factor, placental growth factor (PlGF).
Methods. This retrospective longitudinal nested case–control study included 143 singleton pregnancies in the following groups: (1) patients with uncomplicated pregnancies who delivered a term infant with an appropriate weight for gestational age (n = 124); and (2) patients who had a fetal death (n = 19). Blood samples were collected at each prenatal visit, scheduled at 4-week intervals from the first trimester until delivery. Plasma concentrations of sVEGFR-1, sEng, and PlGF were determined by specific and sensitive ELISA. A linear mixed-effects model was used for analysis.
Results. (1) The average profiles of analyte concentrations as a function of gestational age for sVEGFR-1, sEng and PlGF were different between women destined to have a fetal death and those with a normal pregnancy after adjusting for covariates (p < 0.05); (2) Plasma sVEGFR-1 concentrations in patients destined to have a fetal death were significantly lower between 7 and 11 weeks of gestation and became significantly higher than those of women with a normal pregnancy between 20 and 37 weeks of gestation (p < 0.05); (3) Similarly, plasma sEng concentrations of women destined to have a fetal death were lower at 7 weeks of gestation (p = 0.04) and became higher than those of controls between 20 and 40 weeks of gestation (p < 0.05); (4) In contrast, plasma PlGF concentrations were higher among patients destined to develop a fetal death between 7 and 14 weeks of gestation and became significantly lower than those in the control group between 22 and 39 weeks of gestation (p < 0.05); (5) The ratio of PlGF/(sVEGFR-1 × sEng) was significantly higher in women destined to have a fetal death between 7 and 13 weeks of gestation (94–781%) and significantly lower (44–75%) than those in normal pregnant women between 20 and 40 weeks of gestation (p < 0.05); (6) Similar results were obtained when patients with a fetal death were stratified into those who were diagnosed before or after 37 weeks of gestation.
Conclusions. Fetal death is characterised by higher maternal plasma concentrations of PlGF during the first trimester compared to normal pregnancy. This profile changes into an anti-angiogenic one during the second and third trimesters.
Introduction
Fetal death is one of the ‘great obstetrical syndromes’ [Citation1–3]. As such, it may be expected to have: (1) multiple aetiologies; (2) a preclinical stage; and (3) genetic and environmental predisposing factors (which, alone or in combination, may modify the risk of its occurrence) [Citation1]. In addition, fetal death may be considered, in some cases, to be adaptive in nature. Indeed, fetal death of one twin may result in the improvement of preeclampsia [Citation4–7]. Thus far, most research about the causes of fetal death has been based on epidemiologic studies [Citation8–17].
The ‘Tulip’ classification system suggests that the leading causes of fetal death are: (1) pathology of the placenta and the placental bed (64.3%); (2) congenital anomalies (5.8%); (3) infection (1.7%); (4) others (4.9%); and (5) unknown causes (23.3%) [Citation18]. It is important to recognise that the term ‘cause’ is used when referring to an association between fetal death and a clinical or pathologic condition, and that the postulates for causation are not often met. Another limitation of the current classifications of the factors associated with fetal death is that subclinical pathologic processes have not been studied. For example, intra-amniotic infection/inflammation is rigorously excluded only in a minority of cases [Citation19–22]. Inadequate fetal growth is often considered a cause of fetal death [Citation9,Citation23–25], but many cases of intrauterine growth restriction result in a live birth. Thus, the causal link remains open to question. We propose that progress in understanding the causes of fetal death has been hampered by the cross-sectional nature of the studies. Indeed, most reports examine conditions present at the time of fetal death or after (but not before). Therefore, longitudinal studies are required to gain insight into pathogensis.
Pregnancy is a unique state in which both vasculogenesis and extensive angiogenesis are required for fetal and placental development [Citation26,Citation27]. The balance between angiogenesis and anti-angiogenesis is important for successful reproduction [Citation28–30]. Indeed, increased circulating concentrations of anti-angiogenic factors -soluble vascular endothelial growth factor receptor (sVEGFR)-1 and soluble endoglin (sEng)- and decreased concentrations of an angiogenic factor -placental growth factor (PlGF)- have been reported in patients with preeclampsia [Citation31–65], a small for gestational age (SGA) fetus [Citation38,Citation46,Citation49,Citation57,Citation66–70], placental abruption [Citation71], ‘mirror syndrome’ [Citation72–74], and twin-to-twin transfusion syndrome [Citation75].
The overlapping clinical features, as well as placental pathology among fetal death, preeclampsia and fetal growth restriction [Citation9,Citation23,Citation76–81], suggest that pregnancies with a fetal death may have an anti-angiogenic state as reflected by abnormal profiles of the maternal plasma concentrations of angiogenic and anti-angiogenic factors. A cross-sectional study also demonstrated a higher median delta maternal plasma sVEGFR-1 concentration among patients presenting with a fetal death than those with a normal pregnancy [Citation82]. Moreover, spontaneous resolution of early-onset preeclampsia accompanied by an improvement of the anti-angiogenic state after fetal demise in a twin pregnancy has recently been reported [Citation83].
A longitudinal study reported by our group demonstrated that patients with an SGA neonate and those who developed preeclampsia differ in the maternal plasma concentration of specific angiogenic and anti-angiogenic factors [Citation84]. This observation suggests that each obstetrical syndrome may have a unique angiogenic and anti-angiogenic profile, and that these differences may reflect the underlying mechanisms of disease, as well as the timing and magnitude of the insult responsible for the clinical phenotype. To test this hypothesis, we conducted a longitudinal study to determine whether patients who subsequently had a fetal death have a different profile in maternal plasma concentrations of sVEGFR-1, sEng, and PlGF as a function of gestational age than those with normal pregnancy outcome.
Material and methods
This retrospective, longitudinal nested case–control study included 143 women with singleton pregnancies in the following groups: (1) uncomplicated pregnancies who delivered an appropriate for gestational age neonate (controls; n = 124); and (2) patients who had a fetal death (n = 19).
Plasma samples were obtained at each prenatal visit, scheduled at 4-week intervals from the first or early second trimester until delivery. In cases in which more than one sample from the same patient in a specific gestational age group was available, the earliest sample was chosen. Fetal death was defined as death of the fetus after 20 weeks of gestation diagnosed by ultrasound examination. Fetuses with known congenital and/or chromosomal abnormalities, as well as pregnancies complicated by preeclampsia were excluded. SGA was diagnosed as a birth weight below the 10th percentile for gestational age [Citation85].
The collection and utilisation of the samples was approved by both the Human Investigation Committee of the Sotero del Rio Hospital, Santiago, Chile (a major affiliate of the Catholic University of Santiago) and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples were used in previous studies.
Doppler velocimetry
Pulsed-wave and colour Doppler ultrasound examination of the uterine and umbilical arteries was performed in some patients. The pulsatility index (PI) of the right and left uterine arteries was measured and the mean PI of the two vessels was calculated. Uterine artery Doppler velocimetry was defined as abnormal if the mean PI was above the 95th percentile for gestational age using the reference range proposed by Gomez et al. [Citation86]. The Doppler signal of the umbilical artery was obtained from a free floating loop of the umbilical cord during the absence of fetal breathing and body movement. The PI was measured. Umbilical artery Doppler velocimetry was defined as abnormal if the PI was above the 95th percentile for gestational age using the reference range proposed by Aruduini and Rizzo [Citation87].
Sample collection
Blood samples were collected into tubes containing EDTA. The samples were centrifuged for 10 min at 4°C and stored at −70°C. Laboratory personnel were blinded to the clinical diagnosis.
Human sVEGFR-1, sEng, and PlGF immunoassays
Maternal plasma concentrations of sVEGFR-1, sEng and PlGF were determined by sensitive and specific immunoassays (R&D Systems. Minneapolis, MN, USA). All immunoassays utilised a sandwich enzyme based technique and had been validated for plasma determinations of the analytes. The inter- and intra-assay coefficients of variation (CV) were: sVEGFR-1: 1.4% and 3.9%, sEng: 2.3% and 4.6% respectively; and PlGF: 6.02% and 4.8%, respectively. The sensitivity of the assays was: sVEGFR-1: 16.97 pg/ml, sEng: 0.08 ng/ml and PlGF: 9.52 pg/ml.
Statistical analysis
Cross-sectional analysis of demographic and clinical characteristic data
The Kolmogrov–Smirnov and the Shapiro–Wilk tests were used to assess the distribution of the data. Since the data were not normally distributed, we used the Kruskal–Wallis test for comparisons among groups, and the Mann–Whitney U test for comparisons between groups for continuous variables. Chi-square or Fisher's exact tests were used for comparisons of categorical variables.
Longitudinal analysis of plasma sVEGFR-1, sEng and PlGF concentrations
Changes in the plasma concentrations of angiogenic-related factors over time and between groups were determined using a linear mixed-effects model [fixed effects + random effects]. The fixed effects were the diagnosis (a factor with two levels: normal pregnancy and fetal death), the linear and quadratic effects of gestational age on the concentration of the analytes, and several covariates including maternal age, body mass index (BMI), smoking, nulliparity and duration of sample storage. The interaction terms of the linear and quadratic effects of gestational age with the diagnosis were included in the model. This allowed testing the difference in analyte concentrations between the fetal death and control groups at specific gestational ages. The random effect used in the mixed-effects model was the intercept of each individual patient (i.e. the baseline concentration at 7 weeks of gestation). The model was fitted to the natural log (loge) transformed plasma concentration after replacement of zero concentration (below the detection limit) with 99% of the smallest non-zero concentration observed in the entire dataset for a given analyte (there were 16 zero values for PIGF in the control group and they were replaced with 6.33 pg/ml, and there was 1 zero value in the control group for sVEGFR-1 which was replaced with 384.6 pg/ml).
A natural logarithmic transformation was employed to stabilise the variance across the entire gestational age range. Statistical significance of the fixed effects model was assessed using t-tests, and a p < 0.05 was considered significant. The R statistical environment (www.r-project.org) and the specialised nlme package [Jose Pinheiro, Douglas Bates, Saikat DebRoy, Deepayan Sarkar and the R Core team (2008). nlme: Linear and Non-linear Mixed Effects Models. R package version 3.1–90] were used for all longitudinal analyses.
Results
The demographic and clinical characteristics of the study population
The demographic and clinical characteristics of the study groups are displayed in . There was no significant difference in the frequency of nulliparity, smoking, the median maternal age and the median gestational age at enrollment between patients destined to have a fetal death and those who had a normal pregnancy.
Table I. Demographic and clinical characteristics of the study groups.
The median gestational age at delivery and the median birthweight were lower in patients with a fetal death than that of those in the control group (p < 0.001; ). Two patients (10.5%) with a fetal demise had clinical placental abruption, and six patients (33%) delivered neonates whose birthweights were <10th percentile for gestational age. The median gestational age at diagnosis of fetal death was 35.9 weeks (range 20–40). Fetal death was diagnosed before 24 weeks in three patients (15.7%) and at term gestation (37 weeks or more) in eight patients (42%).
This study included a total of 973 samples with 867 samples from the control group and 106 samples from patients with a fetal death. All patients except one in the control group had seven blood samples, while patients in the fetal death group had a median of six blood samples, with a range of 2–10.
Plasma concentrations of sVEGFR-1, sEng and PlGF
Plasma concentrations of sVEGFR-1, sEng, and PlGF in normal pregnant women and those of patients with fetal death for each gestational age interval are displayed in . The plasma concentration of PlGF in the first trimester (6–13 weeks) was above the detection limit of the assay in 83% (72/87) of the control group, and all samples (n = 12) obtained in the first trimester (6–13 weeks) from women destined to have a fetal death had PlGF concentrations above the sensitivity of the assay.
Table II. Median (interquartile range) plasma concentrations of sVEGFR-1, sEng, and PlGF in each gestational age interval.
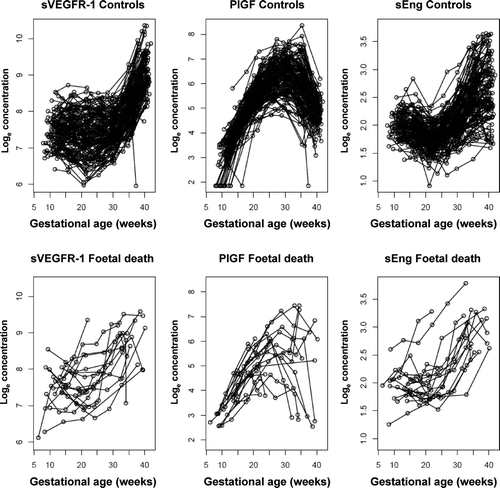
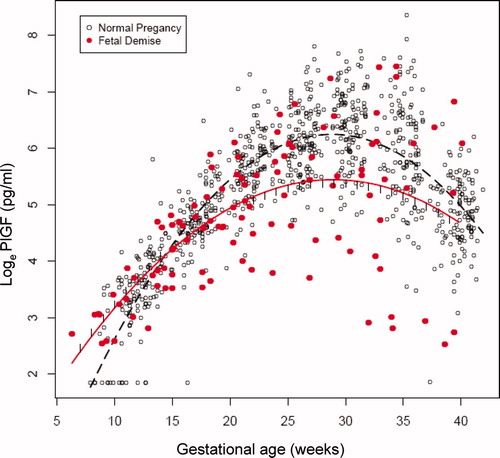
The changes in plasma concentrations of sVEGFR-1, sEng and PlGF in patients destined to have a fetal death and those who had a normal pregnancy across all gestational ages are displayed in , and , respectively. The curves in the figures represent a quadratic fit of the analyte concentration based on the gestational age (without adjusting for covariates). By examining these curves, an overview of the relationship between plasma concentrations of sVEGFR-1, sEng, PlGF and gestational age in patients who subsequently had a fetal death and those with a normal pregnancy outcome can be surmised. The mean maternal plasma concentration of sVEGFR-1 in the fetal death group was lower during the first trimester, and became higher than that of the control group during the second and third trimesters (). Similar changes were observed for sEng (). In contrast, the mean maternal plasma PlGF concentration in patients destined to have a fetal death was higher in the first trimester, but lower than that of the control group until term (). Individual profiles of the maternal plasma concentrations of sVEGFR-1, sEng and PlGF in women destined to have a normal pregnancy (controls) and those destined to have a fetal death are displayed in .
Figure 1. Maternal plasma concentrations (actual data on a loge scale) of soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) in women with a normal pregnancy (ˆ) and patients destined to have a fetal death (•). Each curve represents a quadratic model fit of the concentrations as a function of gestational age in normal pregnant women (dashed line) and those with a fetal death (solid line) without adjusting for covariates. The short vertical lines on the solid curve denote statistical significance between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates.
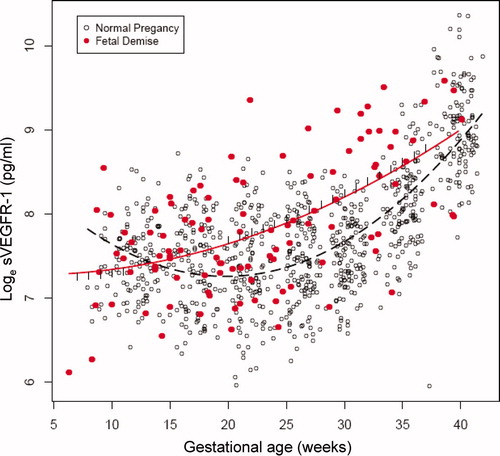
Figure 2. Maternal plasma concentrations (actual data on a loge scale) of soluble endoglin (sEng) in women with a normal pregnancy (ˆ) and patients destined to have a fetal death (•). Each curve represents a quadratic model fit of the concentrations as a function of gestational age in normal pregnant women (dashed line) and those with a fetal death (solid line) without adjusting for covariates. The short vertical lines on the solid curve denote statistical significance between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates.
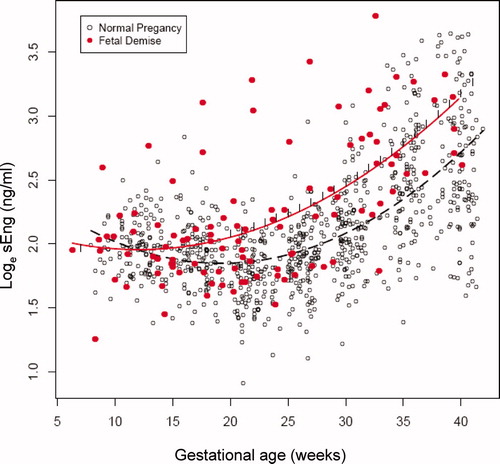
Figure 3. Maternal plasma concentrations (actual data on a loge scale) of placental growth factor (PlGF) in women with a normal pregnancy (ˆ) and patients destined to have a fetal death (•). Each curve represents a quadratic model fit of the concentrations as a function of gestational age in normal pregnant women (dashed line) and those with a fetal death (solid line) without adjusting for covariates. The short vertical lines on the solid curve denote statistical significance between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates.
Longitudinal analysis of plasma sVEGFR-1, sEng and PlGF concentrations
A linear mixed-effects model was used to assess the relationship between fetal death and angiogenic/anti-angiogenic factor plasma concentrations while adjusting for gestational age at venipuncture, maternal age (years), BMI (Kg/m2), smoking, nulliparity and duration of sample storage (years). Overall, the average profiles of analyte concentrations as a function of gestational age for sVEGFR-1, sEng and PlGF were different between the two groups as determined by the p-values (p < 0.05) for the ‘Fetal death × GA’ and ‘Fetal death × GA2’ coefficients in (see also ). The inclusion of the interaction terms ‘Fetal death × GA’ and ‘Fetal death × GA2’ in the mixed effects model allowed evaluation of the significance and magnitude of the differences in plasma angiogenic/anti-angiogenic factor concentrations between groups at the origin of the GA scale (i.e. GA = 0). Since a gestational age of 0 does not have a meaningful interpretation, the origin was shifted to 7 weeks. demonstrates that the difference in concentrations of each analyte at 7 weeks was significant (sVEGFR-1: p = 0.0002; sEng: p = 0.0464 and PlGF: p < 0.0001; see supplementary Table I).
Table III. Longitudinal analysis of the association between anti-angiogenic (sVEGFR-1 and sEng) or angiogenic factor (PIGF) and fetal death after adjusting for confounding factors.
By preserving the same model structure, the differences in plasma angiogenic/anti-angiogenic factor concentrations between the two groups while adjusting for all covariates were evaluated from 7 to 40 weeks of gestation ( and supplementary Table I). The magnitude of differences in plasma concentrations of sVEGFR-1, sEng and PlGF between the two groups was a function of gestational age. The maternal plasma sVEGFR-1 concentrations were significantly lower (26–48%) among patients destined to have a fetal death than those of the control group from 7 to 11 weeks of gestation (each p < 0.05; ). Subsequently, maternal plasma sVEGFR-1 concentrations in the cases increased until term, while those of normal pregnancy trended down in the second trimester and rose again in the third trimester (see ). Maternal plasma sVEGFR-1 concentrations in women destined to have a fetal death were significantly higher (26–51%) than in those with a normal pregnancy from 20 to 37 weeks of gestation (p < 0.05; ).
Table IV. The statistical differences (p) and percentage changes [(fetal death − control)/control × 100] in the maternal plasma concentrations of angiogenic and anti-angiogenic factors between patients with a fetal death and normal pregnant women according to gestational age.
Similarly, plasma sEng concentrations of women destined to have a fetal death were lower (21%) at 7 weeks of gestation (p = 0.04) and became significantly higher (18–62%) between 20 and 40 weeks of gestation than those of women destined to have a normal pregnancy (p < 0.05; ). In contrast to the anti-angiogenic analytes, the maternal plasma PlGF concentrations were higher (46–262%) among patients destined to have a fetal death than in women who had a normal pregnancy outcome between 7 and 14 weeks of gestation (p < 0.05; ). After the first trimester, plasma concentrations of PlGF in patients with fetal death increased at a slower rate than those with a normal pregnancy until the early third trimester (see ). Between 22 and 39 weeks of gestation, the maternal plasma PlGF concentrations became significantly lower (26–46%) among patients destined to have a fetal death than in those in the control group (p < 0.05; ). Note that the assessment of the magnitude and significance of the between group differences shown in and supplementary Table I included an adjustment for all covariates explored in this study.
Collectively, patients destined to have a fetal death compared to those who had a normal pregnancy were characterised by higher plasma concentration of PlGF in the first trimester, and lower plasma concentrations of sVEGFR-1 and sEng. This profile changed to favour higher concentrations of sVEGFR-1 and sEng, but lower PlGF in the second and third trimesters.
Longitudinal analysis of the ratio between angiogenic factor (PlGF) and anti-angiogenic factor (sVEGFR-1 and/or sEng) concentrations
A similar longitudinal analysis was conducted on the ratios of analytes [PlGF/sVEGFR-1, PlGF/sEng, and PlGF/(sVEGFR-1 × sEng)] instead of individual concentrations (see and supplementary Table II). The ratio of PlGF/sVEGFR-1 × sEng was significantly higher in women destined to have a fetal death between 7 and 13 weeks of gestation (94–781%) and significantly lower (44–75%) between 20 and 40 weeks of gestation than those in normal pregnant women (each p < 0.05; and ). The ratio of PlGF/(sVEGFR-1 × sEng), PlGF/sEng or PlGF/sVEGFR-1 differed significantly (p < 0.05) between patients with fetal death and those with normal pregnancy in 28 out of 34 (82%) gestational weeks evaluated ( and supplementary Table II). However, among the three ratios evaluated, the ratio PlGF/(sVEGFR-1 × sEng) provided the best discrimination between women destined to have a fetal death and those destined to have a normal pregnancy outcome (as determined by the number of weeks from 7 to 40 when the differences were statistically significant and also based on the magnitude of the differences expressed in percentages, and supplementary Table II). When the patients with placental abruption and those who had an SGA fetus were excluded, the ratios of PlGF/(sVEGFR-1 × sEng) were significantly higher in women destined to have a fetal death between 7 and 12 weeks of gestation (103–585%) and significantly lower (47–61%) between 21 and 34 weeks of gestation than those in normal pregnant women (each p < 0.05).
Table V. The statistical differences (p) and percentage changes [(fetal death − control)/control × 100] in the ratio of maternal plasma concentrations of angiogenic and anti-angiogenic factors between patients with a fetal death and normal pregnant women according to gestational age.
Figure 5. The ratio (loge scale) of maternal plasma concentrations of PlGF/(sEng × sVEGFR-1) in women with a normal pregnancy (ˆ) and patients destined to have a fetal death (•). Each curve represents a quadratic model fit of the concentrations as a function of gestational age in normal pregnant women (dashed line) and those with a fetal death (solid line) without adjusting for covariates. The short vertical lines on the solid curve denote statistical significance between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates.
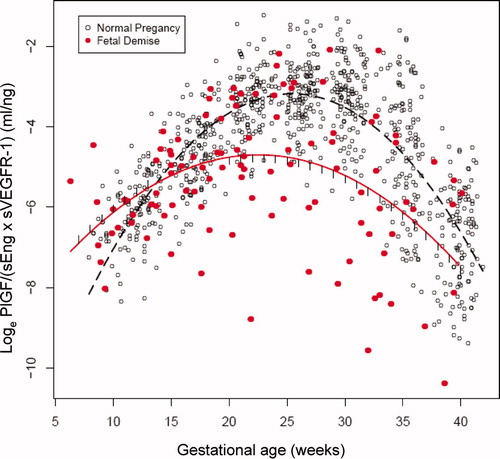
Longitudinal analysis of the ratio of PlGF/(sVEGFR-1 × sEng) in fetal death in preterm and term gestations
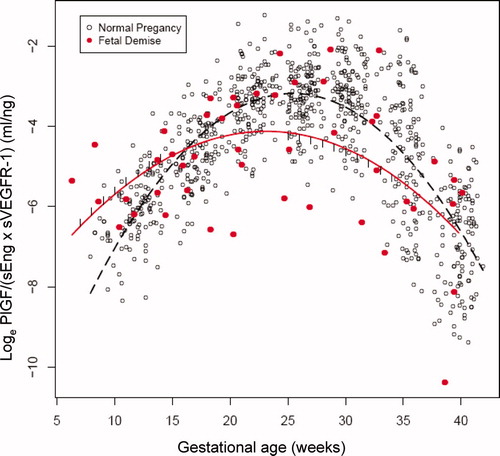
We subdivided patients with fetal death into those who were diagnosed before or after 37 weeks and compared the results with those of normal pregnancy ( and supplementary Table III). The ratio of PlGF/(sVEGFR-1 × sEng) in patients with preterm fetal death was significantly higher between 7 and 11 weeks of gestation (111–328%) and significantly lower (49–94%) between 20 and 37 weeks of gestation than those in normal pregnant women (each p < 0.05; and ). In patients with term fetal death, the ratio of PlGF/(sVEGFR-1 × sEng) was significantly higher between 7 and 14 weeks of gestation (119–1146%) and significantly lower (48–54%) between 25 and 34 weeks of gestation than those in normal pregnant women (each p < 0.05; and ). The ratio of PlGF/(sVEGFR-1 × sEng) in patients with fetal death in preterm and term gestation was significantly different from that of normal pregnant women in 74% (23/31) and in 53% (18/34) of the number of weeks evaluated (supplementary Table III).
Table VI. The statistical differences (p) and percentage changes [(fetal death − control)/control ×100] in the ratio of maternal plasma concentrations of angiogenic and anti-angiogenic factors [PlGF/ (sVEGFR-1 × sEng)] between patients with a fetal death and normal pregnant women stratified by gestational age at which fetal death was diagnosed.
Figure 6. The ratio (loge scale) of maternal plasma concentrations of PlGF/(sEng × sVEGFR-1) in women with a normal pregnancy (ˆ) and patients destined to have a fetal death before 37 weeks of gestation (•). Each curve represents a quadratic model fit of the concentrations as a function of gestational age in normal pregnant women (dashed line) and those with a fetal death (solid line) without adjusting for covariates. The short vertical lines on the solid curve denote statistical significance between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates.
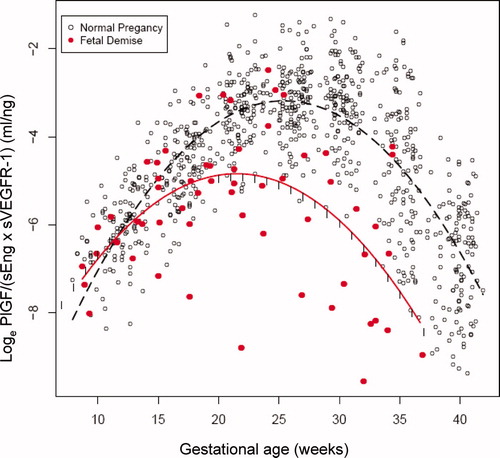
Figure 7. The ratio (loge scale) of maternal plasma concentrations of PlGF/(sEng × sVEGFR-1) in women with a normal pregnancy (ˆ) and patients destined to have a fetal death after 37 weeks of gestation (•). Each curve represents a quadratic model fit of the concentrations as a function of gestational age in normal pregnant women (dashed line) and those with a fetal death (solid line) without adjusting for covariates. The short vertical lines on the solid curve denote statistical significance between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates.
Umbilical and uterine artery Doppler velocimetry in patients with a fetal death
Seventeen patients (89%) in the fetal death group had results of Doppler interrogation on umbilical and uterine arteries. The median gestational age at Doppler examination was 24 weeks (range 15.7–32.4) and the median duration from the examination to the diagnosis of fetal death was 57 days (range 15–151 days). The median umbilical artery PI was 1.5 (range 0.9–2.2). There were two patients who had Doppler examination at <20 weeks and there was no standard curve for umbilical artery PI published to interpret the results. One-fifth (3/15) of patients destined to develop fetal death had an abnormal umbilical artery PI. Similarly, the median mean uterine artery (average from the left and the right) PI was 0.9 (range 0.54–2.01). Four patients (23.5%) had an abnormal mean uterine artery PI. Three of them also had an abnormal umbilical artery PI.
Histological examination of the placenta
Ten (53%) placentas of patients with a fetal death were available for histological examination. Forty percent (4/10) had lesions consistent with maternal under perfusion (villous infarction, increased syncytial knots, intervillous fibrin, mural hypertrophy of the decidual arterioles and acute atherosis of the basal plate arterioles) according to the criteria proposed by Redline et al. [Citation88]. Other lesions were chronic villitis (n = 3), avascular villi (n = 1), and villous stromal-vascular karyorrhexis (n = 1).
Comments
Principal findings of the study
(1) This is the first longitudinal study, that we are aware of, reporting a change in biological markers before fetal death. The analytes measured included angiogenic and anti-angiogenic factors implicated in the genesis of pregnancy complications; (2) Patients destined to have a fetal death had a higher maternal plasma concentrations of PlGF and lower plasma concentrations of sVEGFR-1 and sEng in the first trimester than women destined to have a normal pregnancy; (3) In contrast, during the second and third trimesters, patients destined to have a fetal death had higher plasma concentrations of sVEGFR-1 and sEng, but lower plasma PlGF concentrations than women destined to have a normal pregnancy; and (4) The ratio of PlGF/(sVEGFR-1 × sEng) in patients destined to have a fetal death is higher in the first trimester, and lower in the second and third trimesters than that in normal pregnant women. The association between fetal death and the profile of the ratio of PlGF/(sEng × sVEGFR-1) is present in both preterm and term fetal deaths.
Fetal death has a unique anti-angiogenic profile
We have previously proposed that fetal death is one of the ‘great obstetrical syndromes’ [Citation1]. The observations herein demonstrate that mothers destined to have a fetal death have a different angiogenic and anti-angiogenic profile than the profiles previously described in other obstetrical syndromes in the context of longitudinal studies (e.g. preeclampsia and SGA) [Citation84]. The most obvious difference among fetal death, preeclampsia and SGA is that a subset of patients destined to have a fetal death had higher concentrations of PlGF in the first trimester of pregnancy and lower concentrations of sVEGFR-1 compared to those who had a normal pregnancy. The opposite is the case for preeclampsia, in which PlGF concentrations are lower in the first trimester while there is no difference in the maternal plasma concentration of sVEGFR-1 [Citation37,Citation84,Citation89]. In SGA, the maternal plasma concentrations of sEng are higher in the first trimester, while PlGF concentrations are lower than in normal pregnancy [Citation84]. Of interest is that in a subset of patients destined to develop preterm labour with intact membranes, changes in PlGF, sVEGFR-1 and sEng in maternal plasma were not detected in the first trimester in a longitudinal study reported by our group [Citation90]. Collectively, these observations suggest that the behaviour of maternal concentrations of angiogenic and anti-angiogenic factors among different complications of pregnancy differ according to the specific phenotype. Thus, it is not simply a matter of whether there is an angiogenic or anti-angiogenic profile, but also when such a profile exists and what specific growth factors are involved.
Changes in maternal plasma sVEGFR-1
Patients destined to have a fetal death, compared to those destined to have a normal pregnancy, had lower maternal plasma sVEGFR-1 concentrations in the first trimester (between 7 and 11 weeks), which then became higher between 20 and 37 weeks of gestation. This observation is consistent with a previous cross-sectional study which demonstrated that high maternal serum concentrations of sVEGFR-1 between 10 and 14 weeks were associated with a reduced risk of stillbirth [Citation57]. Previous studies indicate that patients destined to develop early-onset preeclampsia and late-onset preeclampsia have a significantly higher plasma sVEGFR-1 concentration than those destined to have a normal pregnancy only after 26 (range 24–28) and 30 (range 28–32) weeks of gestation, respectively [Citation37,Citation41,Citation84]. Moreover, the magnitude of the changes is much higher in patients destined to develop preeclampsia than those destined to have a fetal death.
The changes in sVEGFR-1 concentrations in patients who delivered an SGA neonate were less dramatic than those observed in patients who developed preeclampsia [Citation84]. Indeed, significantly higher maternal plasma concentrations of sVEGFR-1 were reported among patients with an SGA neonate at the time of diagnosis, in particular, among those with Doppler abnormalities in the uterine and umbilical arteries [Citation70]. However, in a previous longitudinal study reported by our group, there was no significant difference in the changes of maternal plasma sVEGFR-1 concentrations between patients who were destined to deliver an SGA neonate and those with a normal pregnancy [Citation84]. This suggests that sVEGFR-1 may be more important in determining the phenotype of preeclampsia and fetal death than that of SGA without Doppler abnormalities.
Changes in maternal plasma sEng
The pattern of maternal plasma sEng concentrations among patients destined to have a fetal death throughout pregnancy is unique. During the first trimester, the maternal plasma concentration of sEng is lower than that of women destined to have a normal pregnancy only at 7 weeks of gestation, and the change over time (advancing gestational age) is subtle in comparison to the magnitude of the change of sVEGFR-1. After the first trimester, the sEng concentrations increased in women destined to have a fetal death in comparison to those who went on to have a normal pregnancy; these became statistically significant between 20 and 40 weeks of gestation.
Our group reported that sEng was elevated from the 10th week of gestation among patients who were destined to deliver an SGA neonate [Citation84]. In contrast, patients who developed preterm preeclampsia had a significant elevation of the maternal plasma concentrations of this analyte starting at 23 weeks of gestation, while those who subsequently developed term preeclampsia had a significant increase in maternal plasma sEng concentration only after 30 weeks of gestation [Citation84]. Similarly, in the study conducted by Levine et al. [Citation46], sEng was increased among patients who developed preterm preeclampsia or delivered an SGA neonate between 17 and 20 weeks of gestation, and between 25 and 28 weeks among those destined to develop term preeclampsia.
Changes in maternal plasma PlGF
The changes in the maternal plasma PlGF concentrations among patients destined to have a fetal death demonstrated a different pattern than those previously reported in patients destined to develop preeclampsia or deliver an SGA neonate [Citation84]. While our previous longitudinal study demonstrated that the plasma PlGF concentrations in patients destined to develop preeclampsia or deliver an SGA neonate were significantly lower than those with a normal pregnancy in the first trimester from 10 to 11 weeks [Citation84], the plasma PlGF concentration in patients with a fetal death reported herein was higher than that in normal pregnant women between 7 and 14 weeks. Moreover, the gestational age at which maternal plasma PlGF concentration peaked among patients destined to have a fetal death was slightly earlier (approximately 26 weeks) than that of normal pregnant women (approximately 28–30 weeks), and that of patients with an SGA neonate or term preeclampsia (approximately 27 weeks) as reported in a previous longitudinal study [Citation84]. In contrast, patients with preterm preeclampsia had an earlier peak in their maternal plasma PlGF concentrations (before 25 weeks of gestation) [Citation84] and substantially lower PIGF concentrations during the third trimester than normal pregnant women [Citation37,Citation84].
Possible mechanisms to explain the changes in angiogenic and anti-angiogenic factors in fetal death
Recent studies using hysteroscopy [Citation91], hysterectomy specimens [Citation91–93], Doppler velocimetry [Citation94–97] and an advanced oxygen sensing probe [Citation98–100] suggest that the circulation in the intervillous space is not established until 10–12 weeks [Citation101]. Before this time, extravillous trophoblast plugs the tips of the spiral arteries; therefore, the conceptus is in a state of hypoxia. Upon dislodgement of the trophoblast plugs between 10 and 14 weeks, maternal blood enters the intervillous space and the oxygen tension increases. These developmental stages are designed to limit exposure of the trophoblast to oxygen [Citation98,Citation102–104]. The latter can induce the production of reactive oxygen radicals and oxidative stress and damage the placenta [Citation105–109]. Untimely and premature opening of the spiral arteries leads to a state of hyperoxia in the intervillous space, and this can damage the placenta and lead to a spontaneous abortion [Citation110–112].
A recent study of pregnancies undergoing voluntary termination between 6 and 12 weeks of gestation demonstrated an inverse relationship between the partial pressure of oxygen and the concentrations of sVEGFR-1 in blood from the placental bed, suggesting that the changes in oxygen tension can modulate the expression of specific placental proteins including anti-angiogenic factors in early pregnancy [Citation113]. Moreover, high oxygen tension (40%) has been shown to up-regulate PlGF and down-regulate sVEGFR-1 protein expression in term villous explants [Citation114,Citation115]. Thus, it is possible that the observed lower plasma concentrations of sVEGFR-1 and higher plasma concentrations of PlGF in patients destined to have a fetal death in the early first trimester compared to those with a normal pregnancy result from abnormal high oxygen tension in the intervillous space.
Although the profile of angiogenic and anti-angiogenic factors in the second and third trimesters of patients destined to have a fetal death is somewhat similar to that of those destined to develop preeclampsia, the magnitude of the changes is much higher in those with preeclampsia, especially for early-onset disease. It is noteworthy that in contrast to patients destined to have a fetal death, those who subsequently developed preeclampsia have lower plasma concentrations of PlGF than normal pregnant women, while there is no difference in sVEGFR-1 or sEng concentrations in the first trimester. Future studies should be performed to determine the biological activity of maternal blood (functional assay) and confirm that there is an anti-angiogenic state in patients with a fetal death.
Strengths and limitations of this study
The strengths of this study are its longitudinal nature and that several analytes have been measured. This represents the first longitudinal study of angiogenic and anti-angiogenic factors in women destined to have a fetal death. Another strength of this study is the analytical approach. Many longitudinal studies have been analysed using a cross-sectional approach. We have previously discussed the limitations of such an approach [Citation84].
It is now accepted that testing hypotheses about the association between the maternal plasma concentration of anti-angiogenic/angiogenic factors or other analytes and important covariates [such as pregnancy outcome (normal pregnancy or fetal death), gestational age, BMI, etc.] requires a different set of analytical tools. The classical use of generalised linear models or repeated measure analysis of variance has limitations. Generalised linear models could overestimate the significance of the covariates, while the use of repeated measure ANOVA has limitations when there is missing data. These problems are overcome by using the linear mixed-effects model analysis. Other statistical approaches may also be appropriate. It is important to stress that whatever analytical approach is employed, it must take into account the correlated nature of the observations and missing values. A mixed-effects model addresses this issue by allowing each individual to have its own ‘random effect’ on the baseline analyte concentration. Therefore, these models have the capability to fit the observed data much better than generalised linear models.
We have incorporated all the available data in the figures to enable the reader to visualise the main trends in the raw data on a logarithmic scale in each study group, while the individual profiles are presented in . The curves in the figures presented in the manuscript represent a quadratic fit of the analyte concentration based on the gestational age alone. The purpose of these overall curves is to provide the reader with a description of the behaviour of the group's average concentration for a particular analyte at a given gestational age.
Limitations of this study are that the change of plasma angiogenic/anti-angiogenic factor concentrations in fetal demise was based on the assumption that the early or late fetal demise group had similar profiles of angiogenic/anti-angiogenic factor concentrations. We tried to address this potential problem by comparing plasma angiogenic/anti-angiogenic concentrations between cases of fetal death that occurred before 24 or 28 weeks of gestation and controls and could not demonstrate a statistically significant difference between the two groups. However, we acknowledge that we have a small sample size (n = 3 and 4, respectively), and the small number of repeat measurements for each patient were obtained before 24 or 28 weeks of gestation. It is possible that we do not have the statistical power to detect a difference. However, we have demonstrated that patients destined to have a fetal death (preterm and term) had a different profile of maternal angiogenic/anti-angiogenic factors from that of women with normal pregnancies. Since the mechanisms leading to a fetal death in the second and third trimester may be different [Citation8,Citation116], one would expect that the gestational age at which the fetal death was diagnosed might influence the maternal profile of angiogenic and anti-angiogenic factors. A larger study that is specifically designed to address this question is necessary; yet the low prevalence of fetal death in the general obstetric population makes this a challenge. It could also be argued that the observed higher PlGF concentrations in women destined to have a fetal death than those in the control group could result from undetectable concentrations of PlGF in the controls in the first trimester. We have replaced these values with 99% of the smallest non-zero concentration observed in the entire dataset. This was done to reduce the chance of type I error for this observation. The development of more sensitive assays for PlGF should address this problem, and we will be in a position to employ such assays in the near future.
Conclusions
(1) Patients destined to have a fetal death have higher maternal plasma concentrations of PlGF and lower plasma concentrations of sVEGFR-1 and sEng in the first trimester than women destined to have a normal pregnancy; (2) In contrast, during the second and third trimesters, patients destined to have a fetal death have higher plasma concentrations of sVEGFR-1 and sEng, but lower plasma PlGF concentrations than that of women destined to have a normal pregnancy; (3) Changes in maternal plasma concentrations of these angiogenic/anti-angiogenic factors, especially in the first trimester, are different from those we have previously reported in patients destined to develop preeclampsia or SGA. The mechanisms responsible for the occurrence of a fetal death may be operational in the first trimester of pregnancy. This has important implications because it requires a major emphasis on the organisation of prenatal care so that we can focus on the study of biological markers of disease and interventions in early pregnancy.
Acknowledgement
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference
- Romero R. The child is the father of the man. Prenat Neonat Med 1996;1:8–11.
- Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern.Fetal Neonatal Med 2009;22:636–639.
- Di Renzo GC. The great obstetrical syndromes. J Matern. Fetal Neonatal Med 2009;22:633–635.
- Sarhanis P Pugh DH. Resolution of pre-eclampsia following intrauterine death of one twin. Br J Obstet Gynaecol 1992;99:159–160.
- Heyborne KD, Porreco RP. Selective fetocide reverses preeclampsia in discordant twins. Am J Obstet Gynecol 2004;191:477–480.
- Bschierl F, Beinder E. Temporary resolution of preeclamptic symptoms after intrauterine death of one twin. Hypertens Pregnancy 2005;24:313–317.
- Audibert F, Saloman LJ, Frydman R. Selective fetocide reverses preeclampsia in discordant twins. Am J Obstet Gynecol 2005;193:894–895.
- Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol 2005;193:1923–1935.
- Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ 2005;331:1113–1117.
- Korteweg, FJ, Gordijn, SJ, Timmer, A, Erwich, JJ, Bergman, KA, Bouman, K, Ravise, JM, Heringa, MP, Holm, JP. The Tulip classification of perinatal mortality: introduction and multidisciplinary inter-rater agreement. BJOG. 2006;113:393–401.
- Silver, RM. Fetal death. Obstet Gynecol 2007;109:153–167.
- Reddy UM, Goldenberg R, Silver R, Smith GC, Pauli RM, Wapner RJ, Gardosi J, Pinar H, Grafe M, Kupferminc M, et al Stillbirth classification – developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol 2009;114:901–914.
- Vergani P, Cozzolino S, Pozzi E, Cuttin MS, Greco M, Ornaghi S, Lucchini V. Identifying the causes of stillbirth: a comparison of four classification systems. Am J Obstet Gynecol 2008;199:319–314.
- Flenady V, Froen JF, Pinar H, Torabi R, Saastad E, Guyon G, Russell L, Charles A, Harrison C, Chauke L, et al An evaluation of classification systems for stillbirth. BMC Pregnancy Childbirth 2009;9:24.
- Lu JR, McCowan L. A comparison of the Perinatal Society of Australia and New Zealand-Perinatal Death Classification system and relevant condition at death stillbirth classification systems. Aust N Z J Obstet Gynaecol 2009;49:467–471.
- Froen JF, Pinar H, Flenady V, Bahrin S, Charles A, Chauke L, Day K, Duke CW, Facchinetti F, Fretts RC, et al Causes of death and associated conditions (Codac): a utilitarian approach to the classification of perinatal deaths. BMC Pregnancy Childbirth 2009;9:22.
- Varli IH, Petersson K, Bottinga R, Bremme K, Hofsjo A, Holm M, Holste C, Kublickas M, Norman M, Pilo C, et al The Stockholm classification of stillbirth. Acta Obstet Gynecol Scand 2008;87:1202–1212.
- Korteweg FJ, Gordijn SJ, Timmer A, Holm JP, Ravise JM, Erwich JJ. A placental cause of intra-uterine fetal death depends on the perinatal mortality classification system used. Placenta 2008;29:71–80.
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol 2003;189:861–873.
- Blackwell S, Romero R, Chaiworapongsa T, Kim YM, Bujold E, Espinoza J, Camacho N, Hassan S, Yoon BH, Refuerzo JS. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern.Fetal Neonatal Med 2003;14:151–157.
- McClure EM, Goldenberg RL. Infection and stillbirth. Semin. Fetal Neonatal Med 2009;14:182–189.
- Measey MA, Charles A, d'Espaignet ET, Harrison C, Deklerk N, Douglass C. Aetiology of stillbirth: unexplored is not unexplained. Aust N Z J Public Health 2007;31:444–449.
- Froen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 2004;83:801–807.
- Pedersen NG, Figueras F, Wojdemann KR, Tabor A, Gardosi J. Early fetal size and growth as predictors of adverse outcome. Obstet Gynecol 2008;112:765–771.
- Gardosi J, Clausson B, Francis A. The value of customised centiles in assessing perinatal mortality risk associated with parity and maternal size. BJOG 2009;116:1356–1363.
- Smith SK, He Y, Clark DE, Charnock-Jones DS. Angiogenic growth factor expression in placenta. Semin Perinatol 2000;24:82–86.
- Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003;110 (Suppl 1):S10–S18.
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–439.
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439–442.
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995;376:66–70.
- Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol 1999;106:1019–1022.
- Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 2001;184:1267–1272.
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003;188:177–182.
- Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 2003;88:2348–2351.
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 2003;88:5555–5563.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658.
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683.
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004;190:1541–1547.
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ, Preeclampisa is associated with reduced serum levels of placental growth factor. Am J Obstet Gynecol 1998;179:1539–1544.
- McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol 2004;191:1240–1246.
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern.Fetal Neonatal Med 2005;17:3–18.
- Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Goncalves LF, Edwin S, Mazor, M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med 2005;18:9–16.
- Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol 2005;193:984–989.
- Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2005;122:33–39.
- Nadar SK, Karalis I, Al Yemeni E, Blann AD, Lip GY. Plasma markers of angiogenesis in pregnancy included induced hypertension. Thromb Haemost 2005;94:1071–1076.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005.
- Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol 2006;194:551–556.
- Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;195:255–259.
- Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab 2006;91:180–184.
- Schlembach D, Wallner W, Sengenberger R, Stiegler E, Mortl M, Beckmann MW, Lang U. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol 2007;29:407–413.
- Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol 2007;196:239–246.
- Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, Medina L, Edwin S, Hassan S, Carstens M, et al Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol 2007;196:326.e1–326.e13.
- Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res 2007;30:151–159.
- Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol 2007;197:211–214.
- Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol 2007;109:1368–1374.
- Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension 2007;50:137–142.
- Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, Connor JM, Dobbie R. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol 2007;109:1316–1324.
- Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol 2007;197:28–36.
- Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, et al The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–287.
- Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, Bartz J, de Barros SC, Cecatti JG, Costa R, et al Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol 2008;199:268–269.
- Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, et al A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–1038.
- Shokry M, Bedaiwy MA, Fathalla MM, Alsemary A, Elwakil S, Murphy A. Maternal serum placental growth factor and soluble fms-like tyrosine kinase 1 as early predictors of preeclampsia. Acta Obstet Gynecol Scand 2010;89:143–146.
- Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol 2007;197:176.
- Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol 2007;197:174–175.
- Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol 2008;198:175–176.
- Savvidou MD, Yu CK, Harland LC, Hingorani AD, Nicolaides KH. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am J Obstet Gynecol 2006;195:1668–1673.
- Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol 2006;195:201–207.
- Stepan H, Kramer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab 2007;92:2831–2834.
- Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–57.
- Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, et al The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med 2008;21:25–40.
- Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol 2006;108:338–344.
- Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne's syndrome). J Matern Fetal Neonatal Med 2006;19:607–613.
- Stepan H, Faber R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med 2006;354:1857–1858.
- Stepan H, Faber R. Cytomegalovirus-induced mirror syndrome associated with elevated levels of angiogenic factors. Obstet Gynecol 2007;109:1205–1206.
- Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, Erez O, Gotsch F, Mazaki-Tovi S, et al Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol 2008;198:382–388.
- Smulian JC, Ananth CV, Vintzileos AM, Scorza WE, Knuppel RA. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol 2002;100:1183–1189.
- Gruenberger W, Gerstner GJ. The causes of antepartum fetal death: a clinico-pathological study. Clin Exp Obstet Gynecol 1980;7:210–214.
- Page EW, Christianson R. The impact of mean arterial pressure in the middle trimester upon the outcome of pregnancy. Am J Obstet Gynecol 1976;125:740–746.
- Ngoc NT, Merialdi M, bdel-Aleem H, Carroli G, Purwar M, Zavaleta N, Campodonico L, Ali MM, Hofmeyr GJ, Mathai M, et al Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bull World Health Organ 2006;84:699–705.
- de Courcy-Wheeler RH, Wolfe CD, Warburton F, Goodman J, Reynolds F, Gamsu H. The association between small size for gestational age and perinatal and neonatal death in a UK Regional Health Authority. Paediatr Perinat Epidemiol 1995;9:431–440.
- Cnattingius S, Haglund B, Kramer MS. Differences in late fetal death rates in association with determinants of small for gestational age fetuses: population based cohort study. BMJ 1998;316:1483–1487.
- Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, Kusanovic JP, Erez O, Bujold E, Goncalves LF, et al Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med 2007;20:495–507.
- Hladunewich MA, Steinberg G, Karumanchi SA, Levine RJ, Keating S, Kingdom J, Keunen J. Angiogenic factor abnormalities and fetal demise in a twin pregnancy. Nat Rev Nephrol 2009;5:658–662.
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, et al A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23.
- Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. A national birth weight distribution curve according to gestational age in chile from 1993 to 2000. Rev Med Chil 2004;132:1155–1165.
- Gomez O, Figueras F, Fernandez S, Bennasar M, Martinez JM, Puerto B, Gratacos E. Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet Gynecol 2008;32:128–132.
- Arduini D, Rizzo G. Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat.Med 1990;18:165–172.
- Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta 2005;26 (Suppl A):S114–S117.
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 2004;89:770–775.
- Chaiworapongsa T, Romero R, Tarca A, Pedro KJ, Mittal P, Kwon KS, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med 2009;22:1122–1139.
- Hustin J, Schaaps JP. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987;157:162–168.
- Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat 1960;94:297–328.
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999;181:718–724.
- Jauniaux E, Jurkovic D, Campbell S. In vivo investigations of the anatomy and the physiology of early human placental circulations. Ultrasound Obstet Gynecol 1991;1:435–445.
- Jaffe R, Woods JR, Jr. Color Doppler imaging and in vivo assessment of the anatomy and physiology of the early uteroplacental circulation. Fertil Steril 1993;60:293–297.
- Coppens M, Loquet P, Kollen M, De NF, Buytaert P. Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol 1996;7:114–121.
- Carbillon L, Challier JC, Alouini S, Uzan M, Uzan S. Uteroplacental circulation development: Doppler assessment and clinical importance. Placenta 2001;22:795–799.
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 1992;80:283–285.
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 2000;157:2111–2122.
- Jauniaux E, Watson A, Ozturk O, Quick D, Burton G. In-vivo measurement of intrauterine gases and acid-base values early in human pregnancy. Hum Reprod 1999;14:2901–2904.
- Kliman HJ. Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion. Am J Pathol 2000;157:1759–1768.
- Graham CH, Postovit LM, Park H, Canning MT, Fitzpatrick TE. Adriana and Luisa Castellucci award lecture 1999: role of oxygen in the regulation of trophoblast gene expression and invasion. Placenta 2000;21:443–450.
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science 1997;277:1669–1672.
- Jauniaux E, Gulbis B, Burton GJ. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the fetus – a review. Placenta 2003;24 (Suppl A):S86–S93.
- Watson AL, Palmer ME, Jauniaux E, Burton GJ. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997;18:295–299.
- Watson AL, Skepper JN, Jauniaux E, Burton GJ. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J Clin Endocrinol Metab 1998;83:1697–1705.
- Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol 2003;162:115–125.
- Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol 2003;34:1265–1275.
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update 2006;12:747–755.
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 2004;11:342–352.
- Jauniaux E, Burton GJ. Pathophysiology of histological changes in early pregnancy loss. Placenta 2005;26:114–123.
- Carter AM. Placental oxygen consumption. Part I: in vivo studies – a review. Placenta 2000;21 (Suppl A):S31–S37.
- Muttukrishna S, Suri S, Groome N, Jauniaux E. Relationships between TGFbeta proteins and oxygen concentrations inside the first trimester human gestational sac. PLoS One 2008;3:e2302.
- Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen – a review. Placenta 2000;21 (Suppl A):S16–S24.
- Khaliq A, Dunk C, Jiang J, Shams M, Li XF, Acevedo C, Weich H, Whittle M, Ahmed A. Hypoxia down-regulates placenta growth factor, whereas fetal growth restriction up-regulates placenta growth factor expression: molecular evidence for ‘placental hyperoxia’ in intrauterine growth restriction. Lab Invest 1999;79:151–170.
- Korteweg FJ, Erwich JJ, Holm JP, Ravise JM, van der MJ, Veeger NJ, Timmer A. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol 2009;114:809–817.