Abstract
Objective: To investigate audiological and quality of life outcomes for a new active transcutaneous device, called the bone conduction implant (BCI), where the transducer is implanted under intact skin. Design: A clinical study with sound field audiometry and questionnaires at six-month follow-up was conducted with a bone-anchored hearing aid on a softband as reference device. Study sample: Six patients (age 18–67 years) with mild-to-moderate conductive or mixed hearing loss. Results: The surgical procedure was found uneventful with no adverse events. The first hypothesis that BCI had a statistically significant improvement over the unaided condition was proven by a pure-tone-average improvement of 31.0 dB, a speech recognition threshold improvement in quiet (27.0 dB), and a speech recognition score improvement in noise (51.2 %). At speech levels, the signal-to-noise ratio threshold for BCI was − 5.5 dB. All BCI results were better than, or similar to the reference device results, and the APHAB and GBI questionnaires scores showed statistically significant improvements versus the unaided situation, supporting the second and third hypotheses. Conclusions: The BCI provides significant hearing rehabilitation for patients with mild-to-moderate conductive or mixed hearing impairments, and can be easily and safely implanted under intact skin.

| Abbreviations | ||
| ABG | = | Air-bone-gap |
| AC | = | Air conduction |
| AP | = | Audio processor |
| APHAB | = | Abbreviated profile of hearing aid benefit |
| AV | = | Aversiveness of sound |
| BAHA | = | Bone-anchored hearing aid |
| BBC | = | Bridging bone conductor |
| BC | = | Bone conduction |
| BCD | = | Bone conduction device |
| BCI | = | Bone conduction implant |
| BEST | = | Balanced electromagnetic separation transducer |
| Bi | = | Bilateral |
| BN | = | Listening against background noise |
| CT | = | Computed tomography |
| EC | = | Ease of communication |
| F | = | Female |
| GBI | = | Glasgow benefit inventory |
| L | = | Left |
| M | = | Male |
| MRI | = | Magnetic resonance imaging |
| NSP | = | Nasal sound pressure |
| PTA | = | Pure tone average |
| R | = | Right |
| RV | = | Listening under reverberant conditions |
| SNR-threshold | = | Signal-to-noise ratio threshold |
| SRS | = | Speech recognition score |
| SRT | = | Speech recognition threshold |
| Uni | = | Unilateral |
There is a rapidly growing interest in different bone conduction devices (BCD) available for hearing-impaired patients. Since the end of the 1970s, the bone-anchored hearing aid (BAHA) is a common choice of hearing rehabilitation for patients with mild-to-moderate conductive and mixed hearing loss. Today more than 150 000 patients have been treated with this percutaneous solution (CitationCochlear, 2013; CitationOticon Medical, 2014). However, due to some complications related to the skin penetration of the traditional BAHA titanium abutment (CitationDun et al, 2012; CitationKiringoda & Lustig, 2013; CitationSnik et al, 2005), a trend is seen towards transcutaneous devices (both passive and active), characterized by the most important feature of keeping the skin intact. A passive transcutaneous device, which is a skin drive device, uses implanted magnet(s) for the attachment of an external audio processor (AP) on the head over the implant. The AP incorporates the transducer, which transmits vibrations through the skin before vibrating the bone. Available passive transcutaneous devices on the market are Sophono Alpha (Sophono™, Boulder, USA) and Baha® Attract (Cochlear® BAS, Mölnlycke, Sweden). An active transcutaneous device, which is a direct drive device, has an implanted transducer in direct contact to the bone, and here only the AP (without transducer) is attached on the intact skin via retention magnets. Currently, there is one active transcutaneous bone conduction device available on the market, Bonebridge™ (MED-EL, Innsbruck, Austria), while the present bone conduction implant (BCI), developed in cooperation between research groups at Chalmers University of Technology and Sahlgrenska University Hospital in Gothenburg, Sweden, is in the clinical trial phase which is reported in this paper.
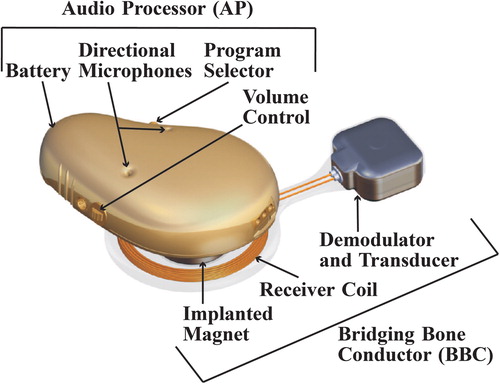
The basic design of the BCI is described in . The AP incorporates microphones, battery and digital signal processor. For the electromagnetic transmission of the sound signal to the implant, there is an inductive link over the skin. This inductive link includes a modulator circuitry and a transmitter coil in the AP, and a receiver coil and a demodulator circuitry in the implant. The implant, called bridging bone conductor (BBC), also includes the transducer, which is attached in the temporal bone. The BCI transducer, with size 12.0 × 14.0 × 7.4 mm, fits normal-sized temporal bones (CitationReinfeldt et al, 2015a). It is positioned in a 4 to 5 mm deep recess, which is situated approximately 15 mm behind the bony ear-canal opening far from the inner ear structures and the facial nerve. The dura and the sigmoid sinus are in the majority of cases not encountered (CitationReinfeldt et al, 2015a). The BCI transducer is attached to the bone via a flat surface contact, and the transmission properties of this type of contact have been investigated in preclinical studies (H kansson et al, 2010; CitationTaghavi et al, 2013; CitationEeg-Olofsson et al, 2014a), showing equally good transmission as a screw attachment of the transducer. Moreover, the BCI surgery is considered safe and easy (CitationEeg-Olofsson et al, 2014b). No special surgical tools are needed except for implant dummies to achieve the correct position and size of the recess, and furthermore, a functionality test of the implant is done before closing the surgical incision.
Figure 1. 3D model of the audio processor (including digital signal processor, modulator, transmitter coil and retention magnet (not shown), directional microphones, battery, program selector, and volume control), and the bridging bone conductor (including receiver coil, internal retention magnet, demodulator, and transducer).
The BCI stimulates the cochleae in the same way as a percutaneous BAHA, and also provides similar hearing sensitivity. The inductive link attenuates the power output about 10 to 15 dB (CitationTaghavi et al, 2012a, Citation2012b). This loss can be regained by positioning the transducer closer to the cochlea. In previous studies, it was found that the sensitivity of the cochlea depends on the transducer position (CitationEeg-Olofsson et al, 2008, Citation2013; CitationHåkansson et al, 2008, 2010; CitationReinfeldt et al, 2014; CitationStenfelt & Goode, 2005). More specifically, the sensitivity, measured in hearing thresholds, is increased 3 to 14 dB at 125 to 8000 Hz by changing the stimulation from the BAHA position to the BCI position (CitationReinfeldt et al, 2014). Furthermore, the transducer in the BCI is of balanced electromagnetic separation transducer (BEST) type (CitationHåkansson, 2003) with a high frequency boost, providing higher output force around 4500 Hz. Depending on the BAHA model used, the hearing sensitivity from a BAHA and a BCI should be about the same, since the hearing sensitivity loss caused by the inductive link, the increased sensitivity from changes in stimulation position, and the high frequency boost essentially cancel out each other (CitationHåkansson et al, 2010).
Feedback is a major issue in today's hearing aids; however, it is less of a problem for the BCI compared to the BAHA. In a study by CitationTaghavi et al (2012c), it was shown that the margin to feedback and device instability is larger for the BCI compared to the Baha Classic 300 (Cochlear® BAS, Mölnlycke, Sweden). The main reasons are that the microphone and the transducer are mechanically separated by a substantial distance in the BCI, where the transducer is capsuled, situated under the skin, and not placed in the same housing as the microphone. Another advantage of the BCI is that the AP has a low profile, which is causing less wind turbulence. The BCI AP only protrudes in total about 9 mm from the skin and has a more smooth integration with the skull shape. The improved feedback margin and low profile of the AP increases the possibility and flexibility to use different kinds of headwear without disturbing feedback noise.
Magnetic resonance imaging (MRI) compatibility is one of the biggest challenges for transcutaneous devices with magnets implanted. In a study by CitationFredén Jansson et al (2014), the demagnetization and torque was studied for different types of retention magnets that could be used for the BCI. Based on these results, the BCI implant might be considered to be MR-conditional under the condition that the maximum static field of the MRI scanner is 1.5 Tesla, and if a compression band around the skull fixates the implant; however, this is not yet approved and requires further verification.
After extensive preclinical trials of the BCI, the first clinical trial is ongoing after approval from the Swedish Medical Agency and the Regional Ethical Review Board in the late 2012 to operate up to 20 patients. Follow-up visits are taking place at 1, 3, 6, and 12 months after fitting. The 1-month results from the first patient were published in CitationEeg-Olofsson et al (2014b), showing significant improvement in warble tone thresholds, speech recognition thresholds, and speech in noise test. To date, in total six patients have been treated with the BCI, and all of them have passed the 6-month follow-up visit.
The aim of this study is to present audiometric results and patient related outcome measures from two validated questionnaires, at the 6-month follow-up visits for the first six patients implanted with the BCI. The following three hypotheses are formulated:
The BCI improves the sensitivity to sound and the intelligibility of speech at normal conversation levels relative to the unaided condition.
The BCI has the same or better sensitivity to sound and intelligibility of speech at normal conversation levels relative to a conventional bone conduction reference device.
Comparing with the unaided situation the scores of the questionnaires using the BCI will be the same or better than scores for the reference device.
Materials and Methods
Subjects
Six patients have been included in the clinical study after baseline tone and speech audiometry. Audiograms for each of these patients are shown in . The etiology of hearing loss, implant side, gender, and age at implantation are shown in . The inclusion criteria for the clinical study are as follows:
Unilateral or bilateral conductive hearing loss with air-bone gap of at least 20 dB (average over 500, 1000, 2000, and 4000 Hz),
Normal or near normal sensorineural hearing: pure-tone-average bone conduction (PTABC) of 30 dB HL (average over 500, 1000, 2000, and 4000 Hz) or better,
To either reject or be unable to use conventional air conduction (AC) hearing aids.
To be accessible for multiple follow-up visits according to the protocol and be motivated to be one of the first patients using the BCI.
Figure 2. Patient audiograms with air conduction (AC) and masked bone conduction (BC) thresholds. Blue symbols = left; red symbols = right; x = left AC; o = right AC;] = left masked BC; and [= right masked BC. In patients 2 and 4, some BC thresholds were not reached due to over-masking (]] or [[). Pat = patient; R = right; and L = left.
![Figure 2. Patient audiograms with air conduction (AC) and masked bone conduction (BC) thresholds. Blue symbols = left; red symbols = right; x = left AC; o = right AC;] = left masked BC; and [= right masked BC. In patients 2 and 4, some BC thresholds were not reached due to over-masking (]] or [[). Pat = patient; R = right; and L = left.](/cms/asset/c8a11275-ce42-44a8-9edc-2acdf2e28679/iija_a_996826_f0002_oc.jpg)
Table 1. Type of hearing loss, implant side, gender, and age at implantation described for each patient (Uni = unilateral, Bi = bilateral, R = right, L = left, F = female, M = male).
Reference device
Before the BCI surgery, the patients were fitted with a reference device, the Ponto Pro Power (Oticon Medical, Askim, Sweden) on a softband. The reference device was fitted using in-situ thresholds, including skin compensation (up to 10 dB extra gain at high frequencies), and disabling automatic functions. The feature of attenuating the vibrations around the generic resonance frequency at approximately 750 Hz in this device was not possible to turn off. This resonance needs extra damping in direct drive applications (provided by a hard-coded notch filter in the Ponto Pro Power), which is not needed for skin drive applications where the skin provides this dampening. After the patients have used the reference device for about one month (still prior to the BCI surgery), full audiometric assessments and two questionnaires (the Swedish abbreviated profile of hearing aid benefit (APHAB) and Glasgow benefit inventory (GBI)) were used to evaluate the subjective rehabilitation effect for each patient. The audiometric tests were the same as for the BCI, as described in the section Audiometric testing.
Surgical procedure
The BCI surgical procedure was done under general anesthesia. A straight line indicating the level of the AP was marked on the patient so that the AP microphones were positioned just above the superior level of the pinna. A postauricular C-shaped incision was made down to the bone and an anterior flap was raised so that the posterior border of the ear-canal opening was visualized. A posterior flap was also separated from the bone to allow for the coil and retention magnet of the BBC () to be inserted. Then, a recess for the transducer was drilled with the anterior border 15 mm from the ear-canal opening. A small channel and a shallow recess for the BBC were thereafter drilled posteriorly. After the drilling procedure, the BBC was inserted under the posterior flap and the transducer was secured in the drilled recess in one of two ways. For the first patient, an approximately 40 mm long titanium plate was placed above the transducer casing and attached with two 3 mm titanium screws on both sides of the transducer. However, this method was abandoned, since it required more space on each side of the transducer, and since it proved to be time consuming to bend the titanium plate in an exact way. For the remaining patients, small holes were drilled 1–2 mm from the edge, one on each side of the recess, and through these holes a 0.4 mm titanium wire was inserted. This wire was stretched and tightened over the transducer casing, which has a compliant layer of silicone on top, and finally the ends were twisted three turns, and then cut and placed in the bone bed on the side of the transducer. Before closing the incision, the implant functionality was verified by electrically stimulating the implant by a specially designed external audio processor and measuring the resulting nasal sound pressure. The nasal sound pressure was also measured at all follow-up visits, and showed good coherence between the visits. The surgical procedure of patient 1 is also described in CitationEeg-Olofsson et al (2014b). For patients with known defects of the mastoid, such as congenital malformations or cavities after mastoid surgery, preoperative planning is recommended for the optimal position of the BBC, which enables the bottom surface of the transducer to safely rest in direct contact with the bone.
Fitting of the audio processor
The fitting of the external AP took place about one month after surgery. Fitting was generally done in a linear fashion using computer-based software. No automatic features were activated, but modest compression was used in some patients, decided in the interaction between the patient and the operator. No specific fitting algorithms taking the patients’ hearing thresholds into account have been developed so far. A description of the AP's digital signal processor can be found in CitationTaghavi et al (2014). All patients were provided with up to four programs with different frequency characteristics to be tested in various listening situations. For the audiometric tests in this clinical study, the program (and volume control setting) that was preferred during normal listening situations was used.
Audiometric testing
Four audiometric test methods were used to evaluate the hearing with and without the device (aided and unaided condition), both directly after the fitting procedure and also 1, 3, 6, and 12 months after this procedure. The audiometric tests performed are listed below.
Sound field warble tone thresholds were tested at 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz with sound coming from a loudspeaker in front of the patient according to standard (CitationISO 8253-1, 2010).
Sound field speech recognition thresholds (SRT) in quiet were measured from frontal direction, using lists of Swedish spondees and following standard CitationISO 8253-3, 2012.
Sound field speech recognition score (SRS) in noise with speech at 4 dB higher level than the noise was measured at 63 dB SPL for both aided and unaided condition. Lists with 50 Swedish phonemically balanced words and pre-recorded noise were played from frontal direction, and the test followed the procedure in standard CitationISO 8253-3, 2012.
Sound field signal-to-noise ratio thresholds (SNR-threshold) that yields 50% intelligibility with five-word sentences (Swedish Hagerman sentences (CitationHagerman, 1982)) was done for the aided condition with both speech and noise from the frontal direction following the procedure in CitationHagerman (1993). The speech level was kept constant at 63 dB SPL, while the noise level was adjusted in order to achieve 50% speech intelligibility.
The speech material for SRS was played from the CD ‘Tal i brus’, and the speech material for SRT and SNR-thresholds from the CD ‘Svensk talaudiometri’. The speech and noise levels were controlled by an AC40 (Interacoustics AS, Assens, Denmark) audiometer. All audiometric testing was performed in a sound insulated room of 16 m3. Prior to the clinical study, all equipment was calibrated according to standard procedures.
Blocking of the non-test ear was applied during all measurements for patients with AC hearing better at the non-implanted ear than at the implanted ear, if anatomically feasible. These preconditions applied to patients 1, 3, 5, and 6. The purpose of blocking was only to remove the AC sound in the contralateral ear, which was done effectively by inserting a foam ear-plug (E-A-R Classic Soft) as deep as possible to minimize the occlusion effect (CitationStenfelt & Reinfeldt, 2007), and also covering the ear with circum-aural earmuffs (Peltor™ 3M™ Svenska AB, Sollentuna, Sweden). The non-implanted ear should not be masked, since that would reduce the sensitivity of the non-implanted ear to vibrations transmitted by the implant, which is not the case in real life.
To minimize the order effects, the measurement order was varied between the follow-up visits for each patient. However, for practical reasons, the measurements had to be performed in two blocks — unaided and aided condition. All unaided measurements were performed in one sequence, either before or after all aided measurements. The order of the two blocks was randomized.
Patient related outcome measures
In addition to the audiometric testing, patient related outcome measures were completed in form of Swedish APHAB and GBI questionnaires, six months after fitting of the BCI audio processor. In APHAB, four subscales are covered: ease of communication (EC), listening against background noise (BN), listening under reverberant conditions (RV), and aversiveness of sound (AV) (CitationCox & Alexander, 1995). A difference between the unaided and the aided condition of at least 22 points was needed for individual subscales in EC, BN, and RV to be judged as a real difference (CitationCox & Alexander, 1995). The GBI is measuring the patient benefit in the general, the social support, and the physical health subscale scores (CitationRobinson et al, 1996). The scores are given on a scale of −100 to +100, where positive scores imply benefit in quality of life.
In the APHAB questionnaire, unaided and aided condition is evaluated every time that the questionnaire is completed. Therefore, in the present study, the unaided condition was evaluated both when comparing with the reference device (before the BCI surgery), and when comparing with the BCI after six months of use. The improvement for each device was calculated by comparing the aided with the unaided condition as reported at each specific time.
Calculations
For each patient, the hearing improvement was calculated as the difference between the unaided and the aided condition. The results for the BCI and the reference device were separated in order to assess statistical significance in improvement for each device, and to be able to compare the devices. The comparisons between the devices were made both at an individual level and at a group level. For the SNR-threshold test, however, comparing aided and unaided condition does not add to the discussion since they were measured at different speech levels. Instead, a useful result was obtained by comparing the aided BCI and reference device results directly.
Wilcoxon signed rank test was used to test significant differences between unaided and aided condition (with null hypothesis of same thresholds unaided and aided), as well as between the devices (with null hypothesis of same improvement from the BCI and from the reference device).
Results
Audiometric outcomes
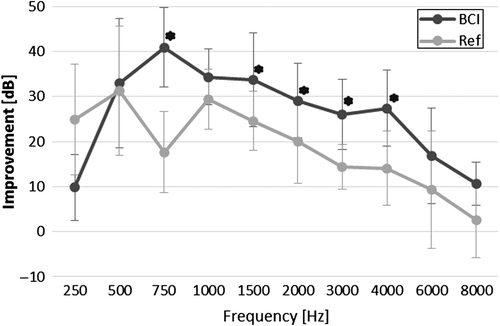
shows a graphic representation of the improvement of the warble tone thresholds at each tested frequency for the BCI, and for the reference device, in terms of mean improvement together with standard deviation. The BCI provided these patients with an improvement of 10–41 dB in the frequency range 250 to 8000 Hz. The corresponding improvement for the reference device was 3–31 dB. Wilcoxon signed rank test showed that the improvements were statistically significant over the unaided condition for both devices in all frequencies except at 8000 Hz. When comparing the improvements of the devices, the null hypothesis (same improvement from the BCI and the reference device) could be rejected for frequencies of 750, 1500, 2000, 3000, and 4000 Hz, showing better improvement from the BCI than from the reference device. The PTA4 improvement (average over 500, 1000, 2000, and 4000 Hz) for the BCI was in average 31.0 ± 8.0 dB, while the PTA4 improvement for the reference device was 23.7 ± 6.8 dB. The PTA3 improvement (average over 500, 1000, and 2000 Hz) was 32.2 ± 8.0 dB for the BCI, and 26.9 ± 7.4 dB for the reference device.
Figure 3. Tone threshold improvements for BCI and reference device, including mean improvement and standard deviation. BCI PTA4 = 31.0 ± 8.0 dB, and Ref PTA4 = 23.7 ± 6.8 dB. Stars are included at frequencies where the BCI has statistically higher improvement than the reference device (α = 0.05).
The SRT improvement using the BCI and the reference device is described in . Once again, the BCI was better than the unaided condition in all patients (27.0 ± 7.6 dB). As can be seen in the table, four of the patients also had a better SRT with the BCI compared to the reference device, which had a lower mean (23.1 ± 7.5 dB). The average improvement over the unaided condition was statistically significant for both devices (α = 0.05) and slightly better (not statistically significantly better) for the BCI as compared to the reference device.
Table 2. Speech recognition threshold (SRT) and speech recognition score (SRS) improvements for BCI and reference device over the unaided condition for all patients, mean improvement and standard deviation (std).
also shows the statistically significant improvement in SRS, both for the BCI and for the reference device (α = 0.05) over the unaided condition. Five of the patients performed better with the BCI than with the reference device. Furthermore, the average SRS improvement was higher using the BCI compared to using the reference device (BCI: 51.2 ± 8.9 %-units, reference device: 43.5 ± 15.5 %-units); however, the difference between the devices was not statistically different.
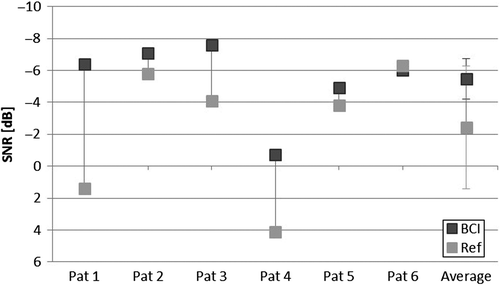
shows the SNR-thresholds using the BCI and the reference device. Increasing negative SNR-thresholds means that the patient reaches the same speech intelligibility with a higher noise level. As can be seen in the figure, the BCI shows better or similar results compared to the reference device for all patients; however, it was not statistically different according to the Wilcoxon signed rank test. On average the BCI gave a SNR-threshold of −5.5 ±2.3 dB, while the reference device gave an average SNR-threshold of −2.4±3.8 dB. It should be noted that in the unaided condition, the speech level of 63 dB SPL was too low and no patient reached 50% speech intelligibility even without noise.
Patient-related outcome measures
The APHAB and GBI questionnaires were completed by the patients for the reference device before the surgery, and for the BCI after six months. Patient-related outcome measure results for the reference device and for the six-month follow-up BCI are reported for all six patients in .
Figure 5. APHAB improvements in the four categories (ease of communication (EC), listening against background noise (BN), listening under reverberant conditions (RV), and aversiveness of sound (AV)) for the BCI and for the reference device (Ref). Mean improvements (bars) and standard deviations (error bars) are included.
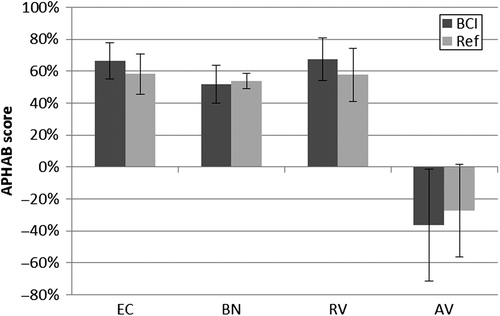
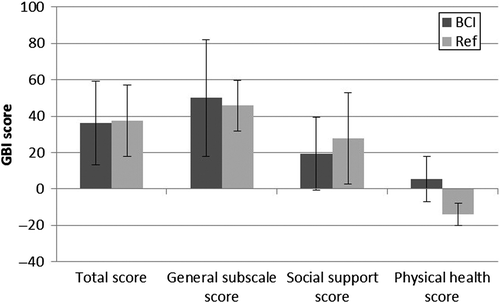
Figure 6. GBI results in the categories total score, general subscale score, social support score, and physical health score. Results for both the BCI and the reference device (Ref) are presented. Mean improvements (bars) and standard deviations (error bars) are included.
The APHAB results (), divided in the four subscales EC, BN, RV and AV, are presented as improvements from unaided to aided condition. For the subscales EC, BN, and RV, all patients experienced an improvement above the critical 22-point level with both devices over the unaided condition. All improvements, seen in , for the first three categories, EC, BN, and RV, are statistically significant, and no significant differences between the devices were found, in support of the third hypothesis of this study.
An improvement in the GBI was experienced by all patients for the total score and for the general subscale score, and by almost all patients for the social support score. The GBI result showed statistically significant improvement in the average total and general subscale scores for both devices (α = 0.05) (). The social support score and the physical health score were not significantly changed for either of the devices, and furthermore, no statistically significant differences were found between the devices.
Discussion
The results obtained for the first six patients in this clinical study of the BCI show statistically significant hearing improvement over the unaided condition. Based on the surgical experience gained from the six patients, the surgical procedure is concluded to be safe and uncomplicated.
Audiometric results
The audiometric testing showed statistically significant hearing improvements for the BCI over the unaided condition, which supports the first hypothesis of this study. In general, the hearing improvements were similar or better for the BCI as compared to the reference device, which also support the second hypothesis. The BCI gave statistically better results than the reference device for the tone thresholds at 750 and 1500–4000 Hz. It should be noted that the 750-Hz tone threshold difference is underestimating the performance of the Ponto Pro Power, because of that it is tailor made for the percutaneous application. A plausible explanation for the better SNR-threshold of the BCI could be that the high frequencies in the speech signal, which are present in normal speech, are more attenuated when vibrations pass through the skin, as in the reference device.
The audiometric and questionnaire results in this study are similar to results in studies of the other active transcutaneous BCD, Bonebridge (MED-EL, Innsbruck, Austria). CitationManrique et al (2014) presented the surgical technique and audiological outcomes of five patients using the Bonebridge. They concluded a 35.62 ± 12.09 dB improvement by comparing the preoperative unaided PTA4-AC (with head-phones) with the aided PTA4 in sound field. For the same calculation in this study, the improvement was 34.1 ± 9.6 dB. CitationIhler et al (2014) concluded from six Bonebridge patients that the average improvement (called ‘functional gain’ and calculated in the same way as in CitationManrique et al (2014)) was 33.6 ± 7.2 dB, and that the mean GBI improvement was + 36.1 points. These results are also similar to the results of this study. Speech tests in CitationManrique et al (2014) and in CitationIhler et al (2014) were performed in quiet, and are therefore not comparable to the ones in this study, where a speech-to-noise ratio of 4 dB was used. By observing the sound field warble tone threshold data that CitationSprinzl et al (2013) obtained from 12 patients after three months with Bonebridge, their average improvement in aided sound field PTA4, calculated as in this study, was approximately 25 dB. Their improvement in SRT between unaided and aided condition was on the average 25.3 dB. CitationBarbara et al (2013) presented an average sound field PTA4 improvement of 36.5 dB and an average SRT improvement of 36 dB for four patients with Bonebridge. Hence, there are some variations in the improvement data for the Bonebridge, and the BCI results in this study are at least equal to the Bonebridge results.
The standard deviations are high in some of the measurements, especially for the SRS data of the reference device, as seen in . Variations may depend on many different factors, such as cognitive factors, level of fatigue, mood and concentration at that specific measurement time, and variation in fitting accuracy.
It should be emphasized that comparing two different BC devices, besides the psychoacoustic variance, always means uncertainties due to stimulation position, tissues for sound transmission, transducer characteristics, and fitting procedure. For SRT in quiet, patient 2 performs worse compared to the reference device. A possible explanation could be that the BCI transducer is positioned further back due to a radical mastoid cavity. On the other hand, patient 2 performs better in SRS in noise, while patient 3 performs worse with the BCI than with the reference device in both SRT in quiet and SRS in noise. Both devices perform significantly better than the unaided for all these patients so the differences between the devices might be considered as minor as compared with the total rehabilitation effect relative to unaided condition. More patients are needed to increase the accuracy of the results and give more detailed inclusion criteria for the BCI.
An interesting comparison can be made to the first patients who were treated by BAHA. In a study by CitationTjellström and Håkansson (1995), approximately 120 patients (110–127 patients depending on which test) who had received the HC 200 processor was tested with practically the same protocol as was used in this study. It was found that the improvement with the HC 200 over the unaided condition was (present BCI results within parentheses): PTA4 = 29.4 (31.0) dB; SRT = 26.5 (27.0) dB; SRS = 41.6 (51.2) %. Obviously, the first generation BCI performs a bit better than the first generation BAHA, but one should keep in mind that the patient selection criteria was a bit different. For more comparison details, see CitationReinfeldt et al (2015b) and CitationCarlsson et al (1986).
As an efficiency parameter for BCDs, the closure (or non-closure) of the air-bone gap (ABG) is often used. Although the ABG is important as an indication for BCD in general (a substantial ABG implies better speech perception with a BCD as compared to conventional air-conduction hearing aids (Citationde Wolf, 2011)), it can be quite misleading. If the contralateral BC function is much better than the ipsilateral BC function at some frequencies, the functional (aided) ABG is determined by the contralateral BC and ipsilateral AC thresholds. For these reasons, the ABG is not addressed in this paper. See CitationReinfeldt et al (2015b) for more details.
Learning effects from the speech audiometry tests are considered negligible for the patients in this study. CitationHagerman and Kinnefors (1995) investigated learning effects for patients with and without hearing impairments, and with hearing impairment divided into patients with SRT-threshold better than and worse than 0 dB. They concluded that the learning effects are negligible for patients with SRT-thresholds better than 0 dB, which applies to all patients in the current study.
The fitting of the BCI device was mainly done manually in an interaction between the patient and the investigator, considering the patient's preoperative AC and BC hearing thresholds. Moreover, all subsequent adjustments were based on feedback from the patient. Although the devices are fitted using the same methodology in order to achieve reliable data, the outcome of the audiometric measurements are naturally affected by the fitting of the device.
Patient-related outcome measures
In general, there were significant improvements for the BCI over the unaided condition. No significant differences were found between the BCI and the reference device, in support of the third hypothesis of this study. However, the AV category in APHAB gave somewhat worse results for the devices compared to the unaided condition. Furthermore, the physical health score in GBI was worse for the reference device compared to the unaided condition. In the EC, BN, and RV categories, all patients improved with both the BCI and the reference device above the critical 22-point level (CitationCox & Alexander, 1995), which is unusual in studies of bone conduction devices, where previous investigations have shown improvements of 16 to 34% of the patients (CitationDesmet et al, 2014; Citationde Wolf et al, 2010).
In the APHAB questionnaire, the patient answers questions about the unaided condition and the aided condition. Therefore, in the present study, the unaided and the reference device was evaluated before surgery, and the unaided (once again) and the BCI was evaluated after six months of use. The improvement for each device was calculated by comparing the aided with the unaided condition as reported at each specific time. However, a general finding, also reported by CitationPfiffner et al (2011), is that the unaided condition is considered slightly worse in later sessions. This might be a reflectance of patients being more aware of their difficult unaided hearing situation, as an outcome of participating in the study (CitationPfiffner et al, 2011).
Surgical procedure
The experience from six BCI implantations confirms that the surgical procedure is straightforward, safe, and uncomplicated. Two explanations for this conclusion are that the engagement in the mastoid bone is shallow and that the implant is far from delicate structures, such as the inner ear and the facial nerve. Moreover, the surgery required less space and was less time consuming by changing the method of securing the transducer casing from a titanium plate to the use of a titanium wire. By using the titanium wire, it is also easier to remove and replace the implant if necessary. The titanium wire has shown to provide sufficient retention force to ensure an efficient transmission in the transducer-to-bone interface.
The BCI transducer is attached to the bone via a flat surface contact, whereas in all other BCDs, a screw attachment is used. A reason not to use a screw attachment in the bottom of the recess is that the screw would have deepened the engagement into the bone. To use screws on the lateral sides of the transducer case was not considered, as the highest transmission sensitivity was found when stimulating as close as possible to the cochlea, and also that screws may be more difficult to handle at installation and in a possible future explantation. The transmission properties of this flat surface contact have been investigated in preclinical studies (CitationHåkansson et al, 2010; CitationTaghavi et al, 2013), showing equally good transmission properties as a screw attachment of the transducer. The transducer-to-bone contact improved over time, and indicated stiffer and osseointegrated bone close to the implant, in a long-term study on sheep (CitationTaghavi et al, 2013; CitationEeg-Olofsson et al, 2014a).
The BCI design enables a straightforward surgical procedure in patients with normal temporal bones and no history of previous surgeries. In those cases, pre-operative CT-scanning is not necessary since the BCI fits normal temporal bone sizes, as evaluated by CitationReinfeldt et al (2015a). In all six patients, the BCI protrudes approximately 2 mm above the bone surface facilitating the application of sufficient retention force towards the bone. In patients with known defects of the mastoid or cavities after mastoid surgery on the BCI side, preoperative CT is recommended. A preoperative CT scan was done in patients number 2 and 3, and showed to be helpful in determining the location of the BCI transducer. Also in patient number 1, a preoperative CT was taken, but that was done mainly as a precaution as this was the first patient treated with the BCI.
An exchange of the implant in patient 5 was decided after eight months, because the retention magnet came loose inside its titanium capsule, which created a clicking sound when attaching and removing the AP from its position on the head. It was easy to remove the implant after cutting the titanium wire, since there was no adherence between the implant and the bone on the bottom of the recess. This event shows that in situations when explantation of the BCI implant is needed, e.g. for a necessary MRI of the brain where distortions of the MRI image must be avoided, the procedure is quick and safe.
Conclusions
The BCI is a new direct drive active transcutaneous bone conduction device that does not need a percutaneous abutment, like the BAHA. This paper reports the results of the first six patients in an ongoing clinical study using the BCI, approved by the Swedish Medical Agency and the Regional Ethical Review Board. At the six-month follow-up of this clinical study, it was found that:
The BCI offers the patients significantly improved audiometric results and subjective outcome compared to the unaided condition, in support of the first hypothesis of the study,
The BCI audiometric results and the subjective outcome are similar or better compared to a conventional bone conduction device (BAHA on softband), in support of the second and third hypothesis of the study,
The BCI leaves the skin intact and no skin complications were seen, and
The BCI surgery was found to be straightforward, safe, and uncomplicated.
Longer follow-up time in more patients is needed to further ascertain clinical outcomes and establish more definite audiological parameters. In summary, it was found that the BCI can provide hearing rehabilitation for patients with mild-to-moderate conductive or mixed hearing impairments, and is a realistic alternative to bone conduction devices on the market.
Acknowledgements
The study was financed in various stages by Vinnova, Swedish Research Council, Hearing Research Foundation, Promobilia, and by grants from the Swedish state, under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALF-agreement).
Declaration of interest: All authors report no declaration of interest, except the co-author Bo Håkansson, who holds several patents related to the BCI device.
References
- Barbara M., Perotti M., Gioia B., Volpini L. & Monini S. 2013. Transcutaneous bone-conduction hearing device: Audiological and surgical aspects in a first series of patients with mixed hearing loss. Acta Otolaryngol, 133, 1058–64.
- Carlsson P., Håkansson B., Rosenhall U. & Tjellström A. 1986. A speech-to-noise ratio test with the bone-anchored hearing aid: a comparative study. Otolaryngol Head Neck Surg, 94, 421–426.
- Cochlear Bone Anchored Solutions celebrated 100 000 patients at the Osseo 2013 meeting, Newcastle, UK.
- Cox R.M. & Alexander G.C. 1995. The abbreviated profile of hearing aid benefit. Ear Hear, 16, 176–186.
- de Wolf M.J.F., Shival M.L.C., Hol M.K.S., Mylanus E.A.M., Cremers C.W.R.J. et al. 2010. Benefit and quality of life in older bone-anchored hearing aid users. Otol Neurotol, 31, 766–772.
- de Wolf M.J.F. 2011. Bone anchored hearing aid: Clinical outcomes of the linear incision technique and benefit assissment. Thesis, Radboud University Nijmegen Medical Centre, ISBN 978-90-9026047-1.
- Desmet J., Wouters K., De Bodt M. & Van de Heyning P. 2014. Long-term subjective benefit with a bone-conduction implant sound processor in 44 patients with single-sided deafness. Otol Neurotol, 35, 1017–1025.
- Dun C.A.J., Faber H.T.F., de Wolf M.J.F., Mylanus E.A.M., Cremers C.W.R.J. et al. 2012. Assessment of more than 1000 implanted percutaneous bone conduction devices: Skin reactions and implant survival. Otol Neurotol, 33, 192–98.
- Eeg-Olofsson M., Stenfelt S., Tjellström A. & Granström G. 2008. Transmission of bone-conducted sound in the human skull measured by cochlear vibrations. Int J Audiol, 47, 761–9.
- Eeg-Olofsson M., Stenfelt S., Taghavi H., Reinfeldt S., Håkansson B. et al. 2013. Transmission of bone conducted sound: Correlation between hearing perception and cochlear vibration. Hear Res, 306, 11–20.
- Eeg-Olofsson M., Lith A., Håkansson B., Reinfeldt S., Taghavi H., Fredén Jansson KJ., Johansson C. 2014a. Evaluation of Bone Tissue Formation in a Flat Surface Attachment of a Bone Conduction Implant: A Pilot Study in a Sheep Model. Audiol Neurotol Extra, 4, 62–76.
- Eeg-Olofsson M., Håkansson B., Reinfeldt S., Taghavi H., Lund H. et al. 2014b. The bone conduction implant: First implantation, surgical and audiologic aspects. Otol Neurotol, 35, 679–685.
- Fredén Jansson K.J., Håkansson B., Taghavi H., Reinfeldt S. & Eeg-Olofsson M. 2014. MRI induced torque and demagnetization in retention magnets for bone conduction implants. IEEE Trans Biomed Eng, 61, 1887–1893.
- Hagerman B. 1982. Sentences for testing speech intelligibility in noise. Scand Audiol, 11, 79–87.
- Hagerman B. 1993. Efficiency of speech audiometry and other tests. Br J Audiol, 27, 423–425.
- Hagerman B. & Kinnefors C. 1995. Efficient adaptive methods for measuring speech reception threshold in quiet and in noise. Scand Audiol, 24, 71–77.
- Håkansson B. 2003. The balanced electromagnetic separation transducer: A new bone conduction transducer. J Acoust Soc Am, 113, 818–25.
- Håkansson B., Eeg-Olofsson M., Reinfeldt S., Stenfelt S., Granström G., 2008. Percutaneous vs. transcutaneous bone condution implant system: A feasibility study on a cadaver head. Otol Neurotol, 29, 1132–1139.
- Håkansson B., Reinfeldt S., Eeg-Olofsson M., Östli P., Taghavi H. et al. 2010. A novel bone conduction implant (BCI): Engineering aspects and pre-clinical studies. Int J Audiol, 49, 203–215.
- Ihler F., Volbersw L., Blum J., Matthias C. & Canis M. 2014. Preliminary functional results and quality of life after implantation of a new bone conduction hearing device in patients with conductive and mixed hearing loss. Otol Neurotol, 35, 211–215.
- ISO 8253-1, 2010. Acoustics - Audiometric test methods - Part 1: Pure-tone air and bone conduction audiometry. ISO.
- ISO 8253-3, 2012. Acoustics - Audiometric test methods - Part 3: Speech audiometry. ISO.
- Kiringoda R. & Lustig L.R. 2013. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol, 34, 790–794.
- Manrique M., Sanhueza J., Manrique R. & de Abajo J. 2014. A new bone conduction implant: Surgical technique and results. Otol Neurotol, 35, 216–220.
- Oticon Medical. 2014. Oticon Medical: Taking ideas further. http://www.oticonmedical.com/∼asset/cache.ashx?id = 7229&type = 14&format = web
- Pfiffner F., Caversaccio M.D. & Kompis M. 2011. Comparisons of sound processors based on osseointegrated implants in patients with conductive or mixed hearing loss. Otol Neurotol, 32, 728–735.
- Reinfeldt S., Håkansson B., Taghavi H. & Eeg-Olofsson M. 2014. Bone conduction hearing sensitivity in normal-hearing subjects: Transcutaneous stimulation at BAHA vs. BCI position. Int J Audiol, 53, 360–369.
- Reinfeldt S., Östli P., Håkansson B., Taghavi H., Eeg-Olofsson M. & Stalfors J. 2015a. Study of the feasible size of a bone-conduction implant (BCI) transducer in the temporal bone. Otol Neurotol, Published Ahead-of-Print.
- Reinfeldt S., Håkansson B., Taghavi H. & Eeg-Olofsson M. 2015b. New developments in bone-conduction hearing implants: A review. Medical Devices: Evidence and Research, 8, 79–93.
- Robinson K., Gatehouse S. & Browning G.G. 1996. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol, 105, 415–422.
- Snik A., Mylanus E., Proops D., Wolfaardt J., Hodgetts W. et al. 2005. Consensus statements on the BAHA system: Where do we stand at present? Ann Otol Rhinol Laryngol, Suppl 195, 2–12.
- Sprinzl G., Lenarz T., Ernst A., Hagen R., Wolf-Magele A. et al. 2013. First European multicenter results with a new transcutaneous bone conduction hearing implant system: Short-term safety and efficacy. Otol Neurotol, 34(6), 1076–1083.
- Stenfelt S. & Goode R. 2005. Transmission properties of bone- conducted sound: measurements in cadaver heads. J Acoust Soc Am, 118, 2373–2391.
- Stenfelt S. & Reinfeldt S. 2007. A model of the occlusion effect with bone-conducted stimulation. Int J Audiol, 46, 595–608.
- Taghavi H., Håkansson B. & Reinfeldt S. 2012a. A novel bone conduction implant system: Analog radio frequency data and power link design. In: Proceedings of the 9th IASTED international conference on biomedical engineering, Innsbruck, Austria. 327–335.
- Taghavi H., Håkansson B. & Reinfeldt S. 2012b. Analysis and design of RF power and data link using amplitude modulation of Class-E for a novel bone conduction implant. IEEE Transactions on Biomedical Engineering, 59, 3050–3059.
- Taghavi H., Håkansson B., Reinfeldt S., Eeg-Olofsson M. & Akhshijan S. 2012c. Feedback analysis in percutanous bone-conduction device and bone-conduction implant on a dry cranium. Otol Neurotol, 33, 413–420.
- Taghavi H., Håkansson B., Eeg-Olofsson M., Johansson C., Tjellström A. et al. 2013. A vibration investigation of a flat surface contact to skull bone for direct bone conduction transmission in sheep skulls in vivo. Otol Neurotol, 34, 690–698.
- Taghavi H., Håkansson B., Reinfeldt S., Eeg-Olofsson M., Fredén Jansson K.J. et al. 2014. Technical design of a new bone conduction implant (BCI) system. Int J Audiol – Accepted after revision.
- Tjellström A. & Håkansson B. 1995. The bone anchored hearing aid: Design principles, indications, and long-term clinical results. Otolaryngologic clinics of north America, 28(1), 53–72.