Abstract
Forced expiratory volume in one second strongly predicts mortality from cardiovascular disease. FEV1 has been associated with aortic stiffness a strong independent predictor of cardiovascular mortality. However, the anatomical site and possible mechanisms linking aortic stiffness and lung function are unknown. We therefore examined if FEV1 and CT percent emphysema were associated with calcification of the abdominal aorta or reduced distensibility of the proximal thoracic aorta.The Multi-Ethnic Study of Atherosclerosis (MESA) measured aortic calcification on cardiac and abdominal CT scans and proximal aortic distensibility using magnetic resonance among participants aged 45–84 years without clinical cardiovascular disease. Spirometry was measured following ATS/ERS guidelines and percent emphysema was measured in the lung fields of cardiac CT scans. Multivariate analyses adjusted for age, sex, race/ethnicity and cardiovascular risk factors. Of 1,917 participants with aortic distensibility measures, 13% were current and 38% were former smokers. Eighteen percent had airflow limitation without asthma. FEV1 was associated with the extent of distal aortic calcification (0.76; 95%CI 0.60–0.97, p = 0.02) but not proximal aortic calcification or proximal aortic distensibility (−0.04 mmHg−1; 95%CI −0.16–0.09 mmHg−1, p = 0.60). Percent emphysema was associated with neither measure. FEV1 was associated with severity of distal aortic calcification where it was present independently of smoking and other cardiovascular risk factors but not with distensibility or calcification of the proximal aorta.
INTRODUCTION
Forced expiratory volume in one second strongly predicts mortality from cardiovascular disease in general population samples (Citation1). In addition, cardiovascular disease is an important cause of death in patients with chronic obstructive pulmonary disease (COPD)(Citation2).
Pulse wave velocity (PWV), which predicts cardiovascular mortality (Citation3), is measured as the rate of transmission of a pressure wave across the aorta and femoral artery (Citation4), and is thus an indirect measure of regional aortic stiffness. PWV is associated with FEV1 in men (Citation5, 6), and is higher in COPD patients than in controls matched on age, sex and smoking (Citation7, 8). However, although regional aortic stiffness has been associated with pulmonary function we are not aware of any study examining the association between pulmonary function and local arterial stiffness within the aorta.
The highly elastic proximal aorta is rich in elastin and may therefore be susceptible to elastin degradation and abnormal collagen remodelling, whereas calcification of the more distal muscular aorta may arise from systemic inflammation and oxidative stress. As such proximal aortic pathology might implicate a common susceptibility in the connective tissue of artery and lung whereas distal aortic pathology might implicate systemic inflammation which is associated with airflow limitation (Citation9, 10). Abdominal aortic calcification is itself a strong predictor of cardiovascular mortality (Citation11, 12), yet the relationship of FEV1 to aortic calcification has not been established.
Therefore we tested the hypothesis that decrements in the FEV1 and increments in percent emphysema on computed tomography (CT) scans are associated with reduced distensibility of the ascending aorta on magnetic resonance (MR) and calcification of the vasculature from the ascending aorta to the iliac arteries(Citation13).
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study designed to investigate sub-clinical cardiovascular disease in individuals without clinical cardiovascular disease (Citation14). In 2000–2002, MESA recruited 6,814 men and women ages 45–84 years old from six U.S. communities: Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles, CA. MESA participants were European-American white, African-American, Hispanic, or Asian-American (mostly of Chinese origin). Exclusion criteria were clinical cardiovascular disease, weight > 300 lbs, pregnancy or impediment to long-term participation(Citation14). MESA protocols and all studies described herein have been approved by the Institutional Review Boards of all collaborating institutions.
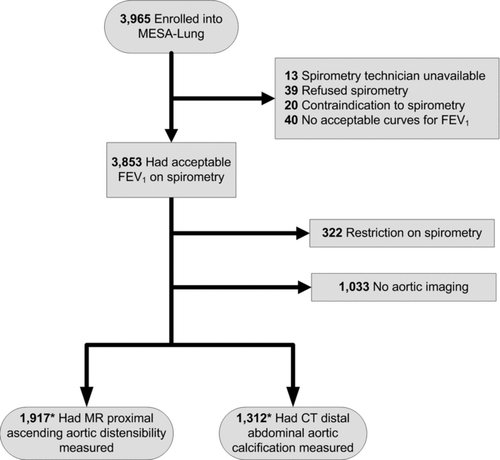
The MESA Lung Study enrolled 3,965 MESA participants of 4,483 sampled randomly from among those who consented to genetic analyses, underwent baseline measures of endothelial function, and attended an examination during the MESA-Lung recruitment period in 2004–2006 (99%, 89%, and 91% of the MESA cohort, respectively). Chinese-Americans were over sampled to improve the precision of estimates for this group. For the current cross-sectional analysis related to obstructive lung disease, we excluded participants with restrictive spirometry, defined as a forced vital capacity (FVC) less than the lower limit of normal (LLN)(Citation15) with a forced expiratory volume in one second (FEV1)/FVC ratio above 0.70.
Aortic calcification
CT scans of the abdomen were acquired on multidetector (MD) and electron beam (EBT) scans in 2002 and 2005 in a randomly selected subset of the cohort. CT images were analyzed centrally using a standard protocol by the MESA CT Reading Center. Calcification in the wall of the distal abdominal aorta in an 8 cm in length segment proximal to the aortic bifurcation was measured. For consistency with previous MESA studies, calcification was identified as a plaque of ≥1mm2 with a density of ≥130 Hounsfield units (Hu) and quantified using the Agatston scoring method(Citation13). Calcification in the proximal abdominal aorta and each iliac artery was scored similarly.
Similar methods were used on cardiac CT scans in 2000–02(Citation16) to measure ascending aortic calcification and descending thoracic aortic calcification. Calcification was identified as plaque of ≥4.6 mm3 and ≥5.5 mm3 on EBT and MDCT respectively as previously described(Citation17).
Proximal aortic distensibility
Consenting participants underwent a cardiac MR scan in 2000–2002. The protocol, its reliability and characteristics of MESA participants with and without MR measures have been previously described (Citation18). All imaging was performed on 1.5 T magnets with a 4-element phased-array surface coil positioned anteriorly and posteriorly, electrocardiographic gating, and brachial artery blood pressure monitoring.
Aortic cross sectional area at the level of the right pulmonary artery during systole and diastole were calculated in a subset of the cohort using an automated contour routine using the software FLOW (Medis, Netherlands). Brachial blood pressure was measured with the participant supine in the MRI scanner before and after the scan. Proximal aortic distensibility, a well-validated measure of proximal aortic stiffness (Citation18, Citation4), was calculated as 1000 × (maximum area –minimum area) / (minimum area × brachial pulse pressure) (Citation18). Left ventricular stroke volume was calculated as previously described (Citation19).
Spirometry
Spirometry was conducted in 2004–2006 in accordance with the American Thoracic Society/European Respiratory Society guidelines (Citation20). All participants performed at least three acceptable manoeuvres. Tests were conducted using a dry-rolling-sealed spirometer and software that performed automated quality checks as manoeuvres were performed (Occupational Marketing, Inc., Houston, TX). All spirometry exams were reviewed by one investigator and each test was graded for quality (Citation21). Participants with no acceptable curves were excluded from spirometry analyses.
CT percent emphysema
Quantitative measures of emphysema on CT scan were performed on the lung fields of full-inspiration cardiac CT scans acquired in 2000–2002, which imaged approximately 70% of the lung volume from the carina to the lung bases (Citation22). Two scans were performed on each participant; the scan with the higher air volume was used for analyses except in cases of discordant scan quality, in which case the higher quality scan was used.
Image attenuation was assessed using modified Pulmonary Analysis Software Suite (Citation23) at a single reading centre by trained readers without knowledge of other participant information. CT percent emphysema low attenuation area was defined as the percentage of the total voxels in the lung which fell below −910 HU. The ICC on blinded re-reads was 0.94. Attenuation of inside and outside air was measured, and CT percent emphysema measures corrected for each were obtained for use in sensitivity analyses.
Percent emphysema measures from the carina to lung base are highly correlated (r = 0.99) with full-lung measures on the same full-lung scans in smokers. Emphysema measures from MESA cardiac scans correlated with full-lung scans (e.g., r = 0.93 on MD-CT scanners) (Citation22).
Potential confounders
Age, gender, race/ethnicity, educational attainment, and medical history were self-reported. Current smoking was defined as self-report of a cigarette in the last 30 days or urinary cotinine level at the time of CT exam of greater than 100 ng/ml (Immulite 2000 Nicotine Metabolite Assay; Diagnostic Products Corp., Los Angeles, CA).
Asthma was defined as self-report of physician-diagnosed asthma before age 45 years in order to avoid including participants with COPD in the definition of asthma, as misdiagnosis is common and differential by gender(Citation24).
Height, weight and resting blood pressure were measured using standard techniques, the latter with the Dinamap Monitor PRO 100 (Critikon, Tampa, FL) automated oscillometric device. Hypertension was defined as a systolic blood pressure >140 mm Hg, a diastolic blood pressure > 90 mm Hg, or currently taking medications for BP control (Citation25).
Glucose and lipids were measured after a 12-h fast. The presence of diabetes mellitus was based on self-reported physician diagnosis, use of insulin and/or oral hypoglycaemic agent, or a fasting glucose value >126 mg/dL measured by rate reflectance spectrophotometry (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY). Total cholesterol was measured using a cholesterol oxidase method (Roche Diagnostics), as was HDL after precipitation of non-HDL cholesterol with magnesium/dextran, triglycerides using Triglyceride GB reagent (Roche Diagnostics). LDL-cholesterol was calculated in plasma specimens having a triglyceride value <400 mg/dl using the formula of Friedewald et al.
Statistical analysis
Demographics and medical characteristics were tabulated by respiratory condition/spirometric criteria. Among individuals without self-reported asthma before age 45, participants with normal lung function and with various degrees of severity of severity of airflow obstruction were compared. Multiple linear regression of proximal aortic distensibility on lung density and lung function was performed. Analyses adjusted for study site, age, race/ethnicity, gender, height, body mass index, cigarette smoking status, pack-years, diabetes, hypertension, educational attainment (as a measure of socioeconomic status), serum low density lipoprotein, high density lipoprotein, and glucose concentration, antihypertensive therapy, and lipid lowering therapy.
We employed relative risk regression with robust standard errors (log-link and Poisson error distribution) to estimate associations between relevant variables and the presence of detectable calcification. Among participants with detectable calcification we modelled the log-transformed magnitude of distal abdominal aortic calcification using linear regression. The exponentiated coefficients are presented, and may be interpreted as multiplicative (i.e., ratio) change in average distal abdominal aortic calcification (Citation26). Formal adjustments for multiple comparisons were not performed (Citation27), but rather the results of all analyses are presented.
Sensitivity analyses were performed using emphysema measures corrected for both outside and inside air. Interactions were tested with multiplicative terms in regression models. Analyses were performed with SPSS version 14 (Chicago, Illinois, USA) and R 2.8.1 (R Foundation, Vienna, Austria). P-values were two-tailed with p < 0.05 considered statistically significant.
RESULTS
Of the participants in the MESA Lung Study with valid spirometry measures and without restrictive lung disease, 1,917 had proximal aortic distensibility MR measures and 1,312 had distal abdominal aortic CT measures (). Those with proximal aortic distensibility MR measures were younger and less likely to have hypertension and diabetes but were otherwise similar to those without such measures. Participants with distal abdominal aortic calcification measures were very similar to those without (Online Supplement).
Participants with proximal aortic distensibility measures were on average 60 years old and 47.1% were male. Nine percent reported a diagnosis of asthma before age 45 years, and 148 (7.7%) of the remaining participants had airflow obstruction on spirometry. Those with airflow obstruction were older, more likely to be male and to have hypertension, diabetes, chronic bronchitis and a history of smoking (). Similar associations were found for the sub-group with distal abdominal aortic calcification measures ().
Table 1 Characteristics of participants with proximal aortic distensibility measures, stratified by respiratory status/condition
Table 2 Characteristics of participants with distal abdominal aortic calcification measures, stratified by respiratory status/condition
Pulmonary function in relation to aortic calcification
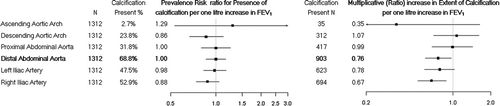
Of the 1,312 participants who had distal abdominal aortic calcification measured, 903 (68.8%) had detectable calcification. Although the presence of distal abdominal aortic calcification was not associated with FEV1 (RR 0.92; 95% CI 0.80 to 1.07; p = 0.28) lower FEV1 was associated with a greater multiplicative (ratio) increment in the extent of distal abdominal aortic calcification after adjusting for study centre, age, sex, race/ethnicity, height, and BMI (FEV1, 0.57, 95% CI, 0.45 to 0.72, p < 0.001). The strength of the association was attenuated but still evident after additional adjustment for cigarette smoking status, pack-years, diabetes, hypertension, educational attainment, serum low density lipoprotein, high density lipoprotein, and glucose concentration, antihypertensive therapy, and lipid lowering therapy (0.76, 95% CI 0.60 to 0.97, p = 0.02).
The FEV1/FVC ratio was also associated with the extent of distal abdominal aortic calcification after minimal adjustment, and similar trends were evident in the full model, although the associations were no longer statistically significant ().
Table 3 Multivariate analysis –distal abdominal aortic calcification and proximal aortic distensibility in relation to FEV1, FVC, the FEV1/FVC ratio, and CT percent emphysema
Similar associations between FEV1 and vascular calcification were found in the more distal iliac arteries (). In contrast, there was no evidence for an association between FEV1 and ascending aortic calcification or descending thoracic aortic calcification (). A similar pattern was found for the FEV1/FVC ratio, but not for the FVC (data not shown).
Pulmonary function in relation to proximal aortic distensibility
Similar to findings for thoracic aortic calcification, proximal aortic distensibility was not associated with pulmonary function after adjusting for study center, age, sex, race/ethnicity, height, and BMI (−0.02 mmHg−1, 95% CI −0.15 to 0.11 mmHg−1, p = 0.75) or after adjusting for the additional covariates as described above (−0.04, 95% CI −0.16 to 0.09, p = 0.60). Results for the FEV1/FVC ratio were similarly null ().
CT percent emphysema in relation to proximal aortic distensibility, and distal abdominal aortic calcification
There was no evidence to suggest that CT percent emphysema was associated with abdominal aortic calcification (1.05, 95% CI 0.72 to 2.06, p = 0.47) or aortic distensibility (0.03 mmHg−1, 95% CI -0.02 to 0.09 mmHg−1, p = 0.19) in the full multivariate models adjusted for study site, age, race/ethnicity, gender, height, body mass index, cigarette smoking status, pack-years, diabetes, hypertension, educational attainment, serum low density lipoprotein, high density lipoprotein, and glucose concentration, antihypertensive therapy, lipid lowering therapy and scanner type and protocol.
Airflow obstruction on spirometry in relation to proximal aortic distensibility, and distal abdominal aortic calcification
Similar results for distal abdominal aortic calcification were obtained when participants were compared by respiratory condition/spirometric criteria. Compared to participants with normal lung function without self-reported asthma, distal abdominal aortic calcification was higher for participants with moderate airflow obstruction (1.24, 95% CI 0.87 to 1.79), and higher still in those with severe airflow obstruction (1.31, 95% CI 0.55 to 3.13).
Reduced proximal aortic distensibility was not evident in participants with moderate or severe airflow obstruction, indeed compared to participants with normal lung function, proximal aortic distensibility was higher for those with moderate airflow obstruction (0.24 mmHg−1, 95% CI 0.02 to 0.46 mmHg−1) and severe airflow obstruction (0.09 mmHg−1, 95% CI -0.44 to 0.62 mmHg−1).
Sensitivity analyses
Sensitivity analyses limited to participants who had smoked greater than 10, 20 and 50 pack-years produced similar results. Exclusion of participants with asthma diagnosed before the age of 45 did not affect the results; neither did exclusion of participants with any diagnosis of asthma, nor use of CT percent emphysema measures corrected for outside air or airways air, or use of the 15th percentile point. Additional adjustment for self-reported use of bronchodilators, steroids, or methylxanthines and measures of inflammation (serum interleukin-6 and C-reactive protein levels) had little impact on the results.
There was no evidence for effect modification on an additive scale by gender, race/ethnicity, smoking status, study site, CT scanner type or severity of airflow obstruction on spirometry, nor any evidence of statistically significant departures from linearity for any of the main relationships (data not shown).
DISCUSSION
In this unique and large study, an obstructive pattern of spirometry was associated with greater extent of calcification in the distal aorta. In contrast, obstructive spirometry was associated neither with proximal aortic calcification nor with proximal aortic distensibility on MRI. Furthermore, there was a graded increase in the magnitude of the association between pulmonary function and the extent of calcification from the proximal thoracic to distal abdominal aorta.
Previous population-based studies that have examined associations between pulmonary function and PWV are limited to two European studies of 194 and 827 middle aged men (Citation6), in which associations were found, and one negative study in 678 healthy Japanese-Americans (Citation28). Aortic PWV has also previously been found to be higher in patients with COPD than controls matched on age, sex and smoking (Citation7, 8).
Abdominal aortic calcification is associated with PWV (Citation29, 30), and, like PWV, is a cardiovascular risk factor (Citation11, 12). Therefore, it is of some interest that calcification is related to pulmonary function. Our study adds to the literature by demonstrating that pathological changes in the distal aorta are more likely to explain the association between pulmonary function and aortic stiffness than changes in the proximal aorta.
These findings may generalise to patients with COPD as, despite loss of statistical power as a consequence of categorisation, similar results for abdominal aortic calcification and proximal aortic distensibility were obtained when participants were compared by respiratory condition, as defined spirometrically.
The mechanisms underlying the distal aortic calcification are unknown, but inflammatory mediators such as TNF-alpha, high sensitivity C-reactive protein and oxidised lipids, which are increased in COPD (Citation9, Citation31), could mediate an association between impaired lung function and vascular calcification which occurs both in the intima in atheromatous plaques, and in the medial elastic laminae. C-reactive protein has been implicated in atheroma formation (Citation32). TNF-alpha causes smooth muscle cells to undergo osteoblastic differentiation and mineralisation thereby promoting calcification (Citation33). Oxidised lipids also promote smooth muscle cell osteoblastic differentiation, but inhibit this process in bone-derived preosteoblasts (Citation34), which is of particular interest since osteoporosis is inversely associated with arterial stiffness in COPD (Citation7).
We did not find any attenuation in the association between FEV1 and abdominal aortic calcification after adjusting for C-reactive protein and interleukin-6. However, both measures show considerable variability and were only measured at a single time point, while measures of TNF-alpha and oxidised lipids were not available for the majority of participants.
Comprehensive assessments meant that we were able to adjust for a wide range of potential confounders including cigarette smoke exposure and cardiovascular risk factors, although we did not measure arterial oxygen tension. Nevertheless, acute changes in oxygenation appear unlikely to affect vascular calcification.
Spirometry was performed without administering a bronchodilator, and as such a proportion of participants with airflow obstruction may have had asthma. However, excluding participants who had ever had a diagnosis of asthma did not affect our findings, nor did confining the analysis to those participants with a 50 or greater pack-year smoking history. Moreover, participants with asthma had higher aortic distensibity and lower calcification.
MESA CT measures of emphysema were obtained from the lung fields of cardiac CT scans and therefore visualization of the lung apices was limited. Nonetheless, the CT percent emphysema measures were highly reproducible and validated (Citation22).
Lung function was assessed approximately 4 years after the other measures. However, the expected mean change in FEV1 over a period of 4 years in a population-based study such as MESA is small (Citation35), and in a previous population-based study aortic PWV was found to be associated with FEV1 measured approximately 10 years prior as well as FEV1 measured contemporaneously, suggesting that relationships between pulmonary function and aortic stiffness are reasonably consistent over time (Citation6).
MESA excluded participants with known cardiovascular disease at baseline. However, this is likely to lead to an underestimation of the strength of the association between aortic calcification and FEV1.
Power was limited for participants with severe airflow obstruction, and caution is required in extrapolating these findings to this group. Nevertheless, there was no evidence for any departure from linearity across the range of lung function, and results comparing participants with moderate and severe airflow obstruction on spirometry were consistent with those obtained measuring pulmonary function in the full cohort.
CONCLUSION
In conclusion, FEV1 was associated with distal aortic calcification where it was present independently of smoking and other cardiovascular risk factors but not with distensibility or calcification of the proximal aorta. Therefore pathological changes in the distal aorta are more likely to explain the association between pulmonary function and aortic stiffness than changes in the proximal aorta.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The MESA and MESA-Lung Studies are conducted and supported by the NHBLI (contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 and grants R01 HL-077612 and R01 HL-075476) in collaboration with the MESA and MESA-Lung Investigators. This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications and significant comments have been incorporated prior to submission for publication. David McAllister is supported by a Fellowship Grant from Chest, Heart and Stroke Scotland. The authors thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. The authors wish to thank Dr Firas Ahmed for his help with coding. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
REFERENCES
- Hole DJ, Watt GCM, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. Br Med J 1996 Sep 21; 313(7059):711–715.
- McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: Operations of the TORCH Clinical Endpoint Committee. Thorax 2007 May 1; 62(5):411–415.
- Willum Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006 Feb 7; 113(5):664–670.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, on behalf of the European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 2006 Nov 1; 27(21):2588–2605.
- Zureik M, Benetos A, Neukirch C, Courbon D, Bean K, Thomas F, Ducimetiere P. Reduced pulmonary function is associated with central arterial stiffness in men. Am J Respir Crit Care Med 2001 Dec 15; 164(12):2181–2185.
- Bolton CE, Cockcroft JR, Sabit R, Munnery M, McEniery CM, Wilkinson IB, Ebrahim S, Gallacher JE, Shale DJ, Ben-Shlomo Y. Lung function in mid-life compared with later life is a stronger predictor of arterial stiffness in men: The Caerphilly Prospective Study. Intl J Epidemiol 2009; 38(3):867–876.
- Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007 Jun 15; 175(12):1259–1265.
- Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, MacNee W. Vascular Dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009 Sep 15; 180(6):513–520.
- Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004 Jul 1; 59(7):574–580.
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005 May 1; 25(5):932–943.
- Wilson PWF, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001 Mar 20; 103(11):1529–1534.
- Rodondi N, Taylor BC, Bauer DC, Lui L, Vogt MT, Fink HA, Browner WS, Cummings SR, Ensrud KE. Association between aortic calcification and total and cardiovascular mortality in older women. J Intern Med 2007; 261(3):238–244.
- Michos ED, Vaidya D, Gapstur SM, Schreiner PJ, Golden SH, Wong ND, Criqui MH, Ouyang P. Sex hormones, sex hormone binding globulin, and abdominal aortic calcification in women and men in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2008 Oct; 200(2):432–438.
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs Jr. DR, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002 Nov 1; 156(9):871–881.
- Hankinson J, Odencrantz J, Fedan K. Spirometric Reference values from a sample of the general U.S. Population. Am J Respir Crit Care Med 1999 Jan 1; 159(1):179–187.
- Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology 2005 Jan 1; 234(1):35–43.
- Takasu J, Budoff MJ, O'Brien KD, Shavelle DM, Probstfield JL, Carr JJ, Katz R. Relationship between coronary artery and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2009 Jun; 204(2):440–446.
- Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, Bluemke DA. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the multi-ethnic study of atherosclerosis [MESA]). Am J Cardiol 2008 Aug 15; 102(4):491–496.
- Heckbert SR, Post W, Pearson GDN, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: The Multiethnic Study of Atherosclerosis. J Am Coll Cardiol 2006 Dec 5; 48(11):2285–2292.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Van Der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005 Aug 1; 26(2):319–338.
- Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults. Chest 2010 Jan 1; 137(1):138–145.
- Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, Barr RG. Reproducibility and validity of lung density measures from cardiac CT Scans—The multi-ethnic study of atherosclerosis (MESA) lung study. Acad Radiol 2009 Jun; 16(6):689–699.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003 May 22; 348(21):2059–2073.
- Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest 2001 Jun 1; 119(6):1691–1695.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003 Dec 1; 42(6):1206–1252.
- Erbel R, Delaney JA, Lehmann N, McClelland RL, Möhlenkamp S, Kronmal RA, Schmermund A, Moebus S, Dragano N, Stang A, Jöckel K, Budoff MJ, on behalf of the Multi-Ethnic Study of Atherosclerosis and the Investigator Group of the Heinz Nixdorf Recall Study. Signs of subclinical coronary atherosclerosis in relation to risk factor distribution in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR). Eur Heart J 2008 Nov 2; 29(22):2782–2791.
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990 Jan; 1(1):43–46.
- Taneda K, Namekata T, Hughes D, Suzuki K, Knopp R, Ozasa K. Association of lung function with atherosclerotic risk factors among Japanese Americans: Seattle Nikkei Health Study. Clin Exp Pharmacol Physiol 2004; 31(s2):S31–S34.
- McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki-Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB, on behalf of the Anglo-Cardiff Collaboration Trial Investigators. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension 2009 Mar 1; 53(3):524–531.
- Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 2000 Jul 1; 15(7):1014–1021.
- Santus P, Sola A, Carlucci P, Fumagalli F, Di Gennaro A, Mondoni M, Carnini C, Centanni S, Sala A. Lipid peroxidation and 5-lipoxygenase activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005 Apr 15; 171(8):838–843.
- Libby P. Inflammation in atherosclerosis. Nature 2002 Dec 19;420(6917):868–874.
- Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-{alpha} promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000; Nov 21;102(21):2636–2642.
- Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 1997; Apr 1;17(4):680–687.
- Jiang R, Burke GL, Enright PL, Newman AB, Margolis HG, Cushman M, Tracy RP, Wang Y, Kronmal RA, Barr RG. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol 2008; Sep 15; 168(6):602–610.