Abstract
Recent studies have shown strong associations between chronic exercise and improved spirometric values. Building on these findings, we investigated whether habitual lifetime exercise influences six-minute walk test performance (6MWT) in subjects with at least 10 pack-years smoking history. The 6MWT was chosen for its correlation with performance on activities of daily living and predictive value for inactivity, morbidity and mortality in individuals with chronic obstructive pulmonary disease (COPD) versus spirometric values, which are less adept at predicting functional status. Because COPD is a global cause of disability, therapeutic measures that delay symptom-induced immobility are more cost-effective versus late-stage interventional therapies. Among 49 subjects, we compared lifetime exercise assessed with a validated physical activity questionnaire with six-minute walk distances (6MWD). The American College of Sports Medicine's (ACSM) recommended exercise levels (30 minutes/day, 1000 kcal/week) were used as a benchmark. Among subjects with spirometry-determined lung obstruction (n = 21), those who have not met at least half of ACSM guidelines (500 kcal/week) were classified as “Inactive,” while others were “Active.” A significant difference in 6MWD was found between Inactive and Active subjects: (1123.86ft vs. 1468.25ft, STDev = 210.07 vs. 240.25, p = 0.0045). This difference was not found in subjects without lung obstruction. Pack-years was a significant covariate: subjects who smoked less walked farther distances. No relation was found between exercise and predicted FEV1%. In summary, our case-control study suggests that meeting even half of ACSM exercise guidelines could improve functional status in smokers if habitual exercise is adopted early in life.
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is a debilitating airway disorder currently ranked as the fourth-leading cause of death in the United States, and is predicted by the World Health Organization to become the fourth-leading cause of death worldwide by 2030 (Citation1, 2). Between 5–10% of the U.S. population are affected by COPD, with increasing prevalence among women (Citation3).
Although the manifestations of COPD vary, the most common are reduced airflow from lung obstruction, parenchymal destruction (emphysema), chronic lung and/or systemic inflammation, reduced exercise capacity and a sense of breathlessness (dyspnea) (Citation4). Lung airflow is limited by obstructive fibrosis, while emphysema reduces airway patency and elastic recoil, culminating in air trapping, progressive airflow limitation and dyspnea upon exertion (Citation5). Several co-morbidities may also accompany COPD, such as cardiovascular disease (Citation6), muscle wasting (Citation7), osteoporosis (Citation8), anxiety/depression (Citation9) and anemia (Citation10), and can be present during the disease's early stages (Global Initiative for Chronic Obstructive Lung Disease Stage I or more) (Citation11).
Collectively, the clinical features and co-morbidities of COPD contribute toward gradual deconditioning that results in severe disability. As patients suffer increasing dyspnea, physical exertion is discouraged and inactivity results in muscle atrophy and loss of lean body mass (Citation12), which cyclically worsens dyspnea and further discourages exertion. In late stages, this cycle severely limits the ability to perform activities of daily living (ADL), which reduces overall functional status, psychosocial well-being, and Health Related Quality of Life (HQRL) (Citation13). Because COPD is projected to become the fifth global cause of disability by 2020, efforts to counteract COPD symptom-induced deconditioning are crucial for public health.
An interventional program for COPD-associated deconditioning is pulmonary rehabilitation (PR), which is multidisciplinary and incorporates multiple modalities. Among these are individualized physical training, dietary intervention, self-management education and psychological support (Citation14). Although PR programs vary, most target the cyclical dyspnea-muscle wasting of COPD through exercise, with reported improvements in patient exercise tolerance, perceived dyspnea and functional status (Citation15–18).
Despite its success, PR remains primarily palliative in nature and is unable to reverse the relentless progression of COPD. In addition, the multicausal nature of COPD and its diverse systemic influences render pharmacological attempts at reversing disease progress implausible, particularly when most drugs improve spirometric values that correlate poorly with functional status. Therefore, greater attention has been devoted to prophylactic measures through the health benefits of recreational exercise. In light of PR's success and of the demonstrated benefits of chronic physical activity (Citation19–22), researchers have proposed exercise as potential measure of affecting COPD development. The high costs of palliative care and exacerbation-induced hospitalizations in later COPD stages (Citation23) underscore the value of early prevention, since symptom-induced inactivity begins early in the course of the disease (Citation24).
Recreational exercise is proposed to slow COPD deconditioning through several mechanisms, primarily via an anti-inflammatory effect. Because the disease involves a high state of lung inflammation (Citation25, 26), which often “spills over” (Citation27) into systemic circulation, the reported anti-inflammatory effects (Citation28) of moderate exercise could provide a counteractive force (Citation29). New research highlights the role of skeletal muscle as an endocrine organ producing a mixture of cytokines that cumulatively exert anti-inflammatory effects (Citation30, 31).
Combined with a reduction in adipose tissue, which has been to shown to secrete pro-inflammatory mediators (Citation32), an increase in skeletal muscle mass and accompanying chronic anti-inflammatory cytokine release may provide benefits when performed over many years. Improvements in pulmonary vasculature, respiratory muscle mass (and reduced breathing effort), breathing efficiency (Citation33) and psychosocial well-being (Citation34) are also possible contributors.
As a starting point for comparing performed physical exercise versus disease severity, researchers relied on the forced expiratory volume in 1 second (FEV1). The FEV1 has been in use for several years as a marker for COPD progression, and has proven reliability as a predictor for morbidity and mortality (Citation35). Moreover, a reduction in spirometry-determined lung function is a risk factor for impaired health status (Citation36), cardiovascular disease, stroke and lung cancer (Citation37, 38). The FEV1 and Forced Vital Capacity (FVC) provide a simplified staging of disease progression that is reproducible and standardized (Citation39).
In 2002, Jakes et al examined a population of 12,283 men and women aged 45–74 years for an association between physical activity and changes in FEV1 (Citation40). Exercise histories were procured through questionnaires, with three levels of physical activity considered: [1] vigorous physical recreational activity, [2] stair climbing and [3] television viewing. Overall, the study found a negative association between television viewing and FEV1 in males and females, and a positive association of stair climbing and vigorous recreational activity with FEV1 in both sexes, after adjusting for confounders such as age, height and smoking.
Another study by Garcia-Aymerich and associates using a population-based sample (n = 6,790) compared FEV1 and FVC decline rates between physically active and sedentary smokers for 11 years, and found that smokers with moderate to high physical activity had reduced lung function loss versus those who were sedentary after adjusting for confounders (Citation41). The study also found a dose-based effect in active smokers, with greater activity associated with less lung function loss or COPD incidence. Together, these findings demonstrate a strong association between exercise and improved lung function, including resilience against COPD onset.
To supplement these findings, we performed an analysis of exercise history versus a validated complement to spirometry: the 6-minute walk test (6MWT) (Citation42). As a submaximal field test (versus laboratory-based), the 6MWT resembles physical exertion in real life and is validated with respect to several physiologic and quality of life measurements (Citation43). A recent study by Pitta et al. using triaxial accelerometers found a strong correlation between 6MWD and several physiologic surrogates of physical activity, including quadriceps force, movement intensity and handgrip force (Citation13). In contrast, the FEV1 does not correlate well with functional status (Citation44, 45).
Once FEV1 values fall below 35% predicted, large variability exists for exercise capacity (Citation46, 47); some subjects are able to live an active lifestyle while others cannot (Citation24). Clinical outcomes such as dyspnea (Citation48) and health status (Citation49) also do not correlate well with spirometry, likely due to the exam's artificial nature (Citation50). Furthermore, there is substantial variability in 6MWD among spirometry-classified severe COPD subjects, underscoring the dissociation between lung function and actual health status.
This is not surprising, since emerging evidence suggests dyspnea to depend largely on inspiratory airflow limitation versus expiratory (Citation51, Citation52). For an assessment with greater relevance to clinical outcomes, the 6-minute walk is a logical step. Therefore, we investigated the relationship between chronic lifetime exercise and 6MWD in subjects with at least 10 pack-years smoking history using a reproducible historical physical activity questionnaire (HPAQ) (Citation53).
METHODS
Subject recruitment
Institutional Review Board approval from the University of California, San Diego (UCSD) was obtained for work with human study subjects. We performed a convenience sample of a study called COPDGene® at the UCSD Hillcrest Clinical Trials Center. Inclusion criteria for the parent study included race of African-American or Caucasian, age 45 years or older and evidence of at least 10 pack-years of smoking. A diagnosis of COPD was not required. After recruitment, subjects were anonymized with code numbers for privacy protection. Please refer to Supplement 1 for full Inclusion/Exclusion criteria.
Subject classification
Post-albuterol sulfate (Horsham, PA, USA) spirometry was performed to categorize subjects into two groups: With-lung-obstruction (GOLD) and Normal (NM). GOLD subjects had Global Initiative for Chronic Obstructive Lung Disease (GOLD)-classified COPD Stage I (“Mild COPD,” FEV1 ≥ 80% of predicted, FEV1/FVC < 0.70) or worse, while NM subjects were GOLD-classified as normal or Stage 0. Please refer to Supplement 2 for spirometry protocols.
Historical Physical Activity Questionnaire
The exercise questionnaire asked subjects to report how many average hours per week that were spent for diverse physical activities. The metabolic equivalent of task values (Citation54) (MET) for these activities were assessed and multiplied by the reported time expenditures and current body weight to yield average kilocalorie expenditures per week, per life segment, starting from the age range of [a] 14–21, [b] 22–34, [c] 35–50, and [d] 50+. The values were then plotted for each subject (Age Range x Kcal), and the Area Under Curve was calculated to yield a lifetime activity score (LAS). This score was later compared with 6MWD.
Six-minute walk test
The test was performed in a 30 m hallway at least 30 minutes after bronchodilator administration for spirometry. No encouragement was given for any subject, and all performed (to our knowledge) the test either for the first time, or after a few months have lapsed from a prior 6MWT (lessening the likelihood of a “learning effect”). Oxygen saturation with a pulse-oximeter was determined every 120 m, as well as a subject self-assessment (e.g., dyspnea, back or joint pain).
Analysis
The main outcome for this study was 6MWD and the effect of the LAS. For comparison, a score of 3,000 equates to an average weekly kcal expenditure of 1,000 kcal, which is the minimum amount of exercise recommended by the American College of Sports Medicine (Citation55) (ACSM) for health maintenance (30 min of moderate exercise per day). Covariates of gender, BMI and pack-years were examined. Descriptive data was calculated as mean and standard deviations for continuous data and percent for categorical data. Student t-tests were analyzed by evidence of lung obstruction or no lung obstruction. Correlations were run by lung disease status and lifetime activity. Analysis of variance with covariates (smoking, gender, BMI) were calculated using variables with correlations < 0.20. Statistical analyses were performed using Systat software on a Windows®-based operating system.
RESULTS
Forty-nine subjects between December 2009 and May 2010 agreed to participate. Subjects had an average age of 56 ± 8 years, were predominately males (n = 30, 61%), and had histories of 41 ± 16 pack-years, with 39 (80%) still smoking actively. Initially, all 49 participants were divided into two groups according to LAS: (A) LAS < 3,000; (B) LAS ≥ 3,000. Although average values for FEV1 and 6MWD were higher for B than for A, no statistical significance was found due to high standard deviations (1314.5ftA vs 1397.18ftB; FEV1 = 73.75%A vs 81.55%B; nA = 20, nB = 29).
Next, all subjects were divided into GOLD and NM groups, according to spirometry performance. Within these groups, the subgroups A and B were formed according LAS, therefore determining four groups:
NM –classified by spirometry as without lung obstruction
NM-A: LAS < 3,000
NM-B: LAS ≥ 3,000
GOLD –GOLD Stage 1 or worse
GOLD-A: LAS < 3,000
GOLD-B: LAS ≥ 3,000
A comparison of FEV1, 6MWD, BMI and pack-years was performed among NM subjects between subgroups A and B (NM-A vs NM-B), and found no significant differences. However, the same comparison was performed among GOLD subjects between A and B (GOLD-A vs GOLD-B), and a notable (but not quite statistically significant) difference was found between average walk-distances: 1253.90 ftA vs 1462.95 ftB; StDev = 305.82 ftA vs 211.98 ftB; nA = 11, nB = 10; p = 0.0875. No relation was determined for FEV1 due to high standard deviations, although the average for A was lower than B (55.73% vs 63.8% predicted; p = 0.3964). Pack-years was a significant covariate among all GOLD subjects: those with lower pack-years performed better (p < 0.03).
We then observed a natural split at the LAS of 1,500: subjects above 1,500 performed significantly more exercise while those below this level had much lower scores (1,834.5 or higher; 1,197.13 or lower; median = 1,515.82). Splitting GOLD subjects at 1,500, we reformed GOLD-A and GOLD-B and reanalyzed 6MWD, finding a significant difference: 1123.85 ftA vs 1468.25 ftB; StDev = 210.07 ftA vs 240.25 ftB; nA = 7, nB = 14; p = 0.0045. Again, no relation was determined for FEV1 due to high standard deviations, although the average of A was lower than B (53.85% vs 62.43% predicted; p = 0.3953).
Figure 1 Comparison of 6-minute walk distances between “Active” (top) and “Inactive” (bottom) groups. Inactives walked an average of 1123.85 ft, versus 1468.25 ft for Actives.
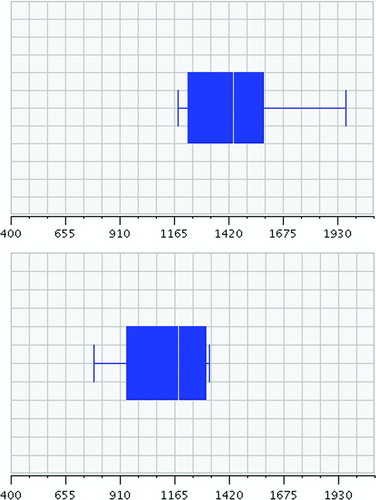
Finally, a comparison of 6MWD, FEV1, LAS, pack-years and BMI was performed between NM and GOLD subjects, finding no statistical difference. There was no relation between FEV1 and LAS across all categories. However, we did find a correlation between lower LAS and lower pack-years among NM subjects. NM subjects who exercised less than 1,000 kcal/wk (A) smoked less versus their more active peers (B) (29.29 pkyrsA vs 45.79 pkyrsB; StDev = 15.58 pkyrsA vs 19.11 pkyrsB; nA = 9, nB = 19; p = 0.0328). This was not observed among GOLD subjects. The association was even more pronounced among smokers who exceeded ACSM-recommendations by 3 times (B), due to organized sports or personal training habits (33.44 pkyrsA vs 53.15 pkyrsB; StDev = 16.221 pkyrsA vs 18.89 pkyrsB; nA = 18, nB = 10; p = 0.0074).
DISCUSSION
Our findings show a significant link between inactivity and lower 6MWD in subjects who are GOLD Stage 1 or worse. This link first becomes noticeable when average weekly exercise falls below the ACSM-recommended expenditure of 1,000 kcals, and becomes substantial when weekly exercise falls below half this amount. Indeed, the 7 subjects who exercised less than 500 kcals a week actually expended an average of 260 kcal per week. Four had walk-distances less than 350 m, which places them at greater risk for acute-exacerbation induced hospitalization and a worse prognostic index (Citation56). The remaining three averaged a walk-distance of less than 400 m, which is an indicator of a daily walking time of less than 30 min and general inactivity (Citation13).
Although this link between inactivity and lower 6MWD was not as statistically significant among subjects who exercised 1,000 kcal/wk or more, there was still a clear difference: those who exercised less than 1,000 kcal/wk averaged a walk-distance of 1253.90 ft vs 1462.95 ft (p = 0.0875). This suggests a dose-based effect of exercise on functional status among subjects with spirometry-diagnosed lung obstruction, i.e., those who have met ACSM physical activity guidelines throughout their lives, despite chronic smoking, are less likely to suffer COPD-symptom induced deconditioning versus peers who have not met guidelines, regardless of lung function. Recall that there was no relation between FEV1 and LAS nor 6MWD across all categories of subjects. Even in our comparison of 6MWD between GOLD Stage 3 subjects (A) and all remaining participants (B), we found no statistically significant difference (1178.80 ftA vs 1384.41 ftB; StDev = 366.93 ftA vs 253.72 ftB; nA = 5, nB = 44; p = 0.1071).
Figure 2 Pack-years versus 6MWD. Those with lower pack-years had higher 6 minute walk distances (p < 0.03).
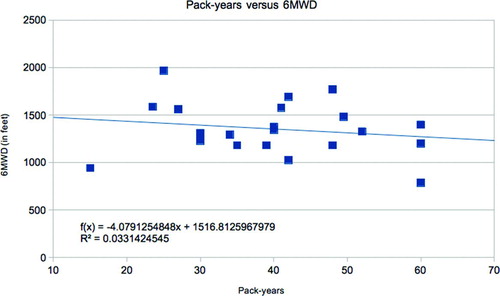
We did not find decreased walk-distances in NM subjects with low lifetime activity scores, no matter how inactive they had been throughout their lives. There was no correlation between LAS and 6MWD, FEV1 nor BMI. Curiously, we did find a correlation between higher LAS and higher pack-years in NM subjects only. Those who were vigorously active (B) by exercising an average of 3,000 kcal/wk smoked significantly more than those who were not (A); (33.44 pkyrsA vs 53.15 pkyrsB; StDev = 16.221 pkyrsA vs 18.89 pkyrsB; nA = 18, nB = 10; p = 0.0074). This finding poses many questions. So far, we can only account for this observation by suggesting that rigorous physical activity prompts stress relief through smoking. However, this does not account for why this association was not found among GOLD subjects.
We are also unable to explain by there was no association between LAS and FEV1. We cannot account for why some subjects who led highly active lifestyles succumbed to lung function loss while others did not. In addition, the link between chronic exercise and better 6MWD was only found among subjects who were GOLD-Stage 1 or worse, and not among NM subjects. This finding underscores the complex pathogenesis of COPD and the need for further research of its diverse etiologies. However, the possibility for exercise to have provided an anti-inflammatory effect against systemic inflammation, and therefore affected COPD onset, should be considered.
Several limitations of the study should be noted. First, our results do not support the findings of Jake and Garcia-Aymerich; namely, that prolonged, chronic exercise improves lung function as determined by spirometry. Many factors may account for this discrepancy, particularly our limited sample size. Because the study was an unfunded convenience sample, study subjects were less easy to recruit and could only contribute a limited amount of time towards our study. In contrast, the research of Jakes and Garcia-Aymerich involved large and extensive analyses of thousands of study subjects; therefore, while our study does not support the idea of exercise affecting lung function, it in no way negates their findings. We feel that our best conclusion regarding exercise and lung function is that more research is needed via a greater sample size.
Table 1 Demographics and outcome variables for those with COPD N = 21
Second, many limitations are intrinsic to the nature of questionnaires. One is the problem of memory bias, especially when recalling events from several decades ago. Studies on questionnaire-related memory bias show conflicting evidence, with some indicating an overall lack of reliability and validity while other long-term studies show otherwise (Citation57). Blair et al. tested the Minnesota Leisure Time Physical Activity survey in 500 subjects by comparing baseline answers of activity performed the prior year with those procured 1–10 years later (asking of same year prior to the study), and found modest correlation coefficients (0.20 to 0.50) regardless of elapsed time between assessments (Citation58). In addition, percent agreement between baseline reported physical activity and later reassessed activity ranged between 60 to 75%. However, participants of the Blair study recalled historical physical activity from a maximum of 10 years prior, not decades ago. Literature evidence suggests memory bias to be a notable problem when recalling events as early as 3 months ago (Citation59), while others highlight the integrity of long-term memory in advanced age (Citation60).
Moreover, our study used an abridged version of the interview-administered HLAQ that was changed in two main areas. First, the original HLAQ recommended a training session and “take-home” work to refresh memories of past exercise events, which could cumulatively require 2–3 hours of involvement. Because our study was unfunded, we deemed it unlikely to retain volunteer subjects’ attention spans for more than a half hour; therefore, our abridged version was made keeping the core mechanics of the HLAQ (i.e., MET and kcal calculations) while forgoing lifetime exercise questions in specific detail. Second, because literature evidence suggests memory recall of past “vigorous” exercise events to be the most reproducible (Citation61), we replaced the original “checklist” of activities (that subjects checked off to obtain MET values) with a blank sheet, asking subjects to recall only events that were most meaningful to them.
This was performed to reduce the risk of exaggeration by subjects checking off frivolous activities. Chasan-Taber et al. has tested the reproducibility of Kriska's HLAQ among 134 women with a one-year reassessment interval, and found intraclass correlation coefficients of 0.82 for total lifetime activity, and values between 0.78 to 0.87 for differing intensity lifetime activities. For age-interval correlations, the study reported 0.73 for total activities in the age of menarche to 21, 0.70 for 22–34, 0.78 for 35–50, and 0.83 for ages 51–65 (Citation53). Overall, the study suggests the HLAQ to be reliable; therefore, we were comfortable abridging and using the HLAQ based on literature evidence for the higher reliability of simpler questionnaires versus complex versions (Citation62, 63). However, we advise follow-up study testing for the reproducibility of our abridged HLAQ, in addition to further investigation of our results considering lifetime activity and 6MWD.
Other intrinsic limitations concern standard MET values and cultural factors influencing self-reported physical activity. Most compendia of MET values include energy expenditures of activities for young adults in standardized environments, and do not account for physiological differences (age, fitness level) between individuals (Citation64). Cultural influences also skew reported results, such as the desirability of reporting recreational sports versus sedentary activities (Citation59, Citation65). Another problem is overreporting/exaggerating, particularly of sporting events where subjects experience interspersed “downtime” (e.g., socializing, sitting out) (Citation66). All of these are inherent problems to memory recall studies, and their hindrance of accurate historical activity assessments should be noted. Although our study attempted to procure lifetime activity levels, our aim was not for great accuracy but rather for generalized caloric expenditures to rank respondents for comparative purposes.
Table 2 Comparison of 6MWD between Active and Inactive subjects with lung obstruction
Overall, we believe the present study supports our hypothesis that lifetime chronic exercise improves functional status in subjects with smoking-induced lung obstruction. Furthermore, daily exercise need not be substantial but only 30 min/day, as recommended by the ACSM. A useful instrument for procuring lifetime exercise histories is the HLAQ that was modified for use in our study. In contrast to the original questionnaire, which required a training period of a few hours and “take-home” work for recalling lifetime exercise, ours only required an interview lasting 15–30 min, yet yielded useful results. In addition to the original HPAQ, this abridged version can be useful for other applications such as heart disease and diabetes, is easily administered, and can be calculated with spreadsheet software. We support our abridged version in future research for validation purposes, as well as the original HLAQ.
In conclusion, our study does show a notable link between chronic exercise and improved 6MWD, which correlates well with HRQL. A significant implication is that pulmonary rehabilitation should not only intervene for late-stage COPD, but as early GOLD Stage 0, i.e., the “at-risk” stage. We feel that a PR program for boosting daily physical activity should begin as soon as possible once COPD is suspected, for the cost of a simple, daily health maintenance program should pale in comparison to an acute-exacerbation hospitalization. In addition, the many benefits to cardiovascular health and mental wellness of exercise will save in the long term over the considerable costs of late-stage medical intervention. Overall, we recommend that more research be devoted to prophylactic exercise therapy for COPD patients as early as GOLD Stage-0.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Mannino D, Homa D, Akinbami L, Ford E, Redd S. Chronic obstructive pulmonary disease surveillance — United States, 1971–2000, Morb Mortal Wkly Rep 51 (SS06) 2002; 1–16.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3: e442. doi:10.1371/journal.pmed.0030442.
- Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest 2003; 123:1684–1692.
- Kim HC, Mofarrahi M, Hussain SNA. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008 December; 3(4):637–658.
- Barnes PJ. Chronic obstructive pulmonary disease: a growing but neglected global epidemic. PLoS Med 2007; 4(5): e112. doi:10.1371/journal.pmed.0040112.
- Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med 2000; 160: 2653–2658.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153:976–980.
- Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med 2003; 114:10–14.
- Wagena EJ, Arrindell WA, Wouters EF, van Schayck CP. Are patients with COPD psychologically distressed? Eur Respir J 2005; 26:242–248.
- Similowski T, Agusti A, MacNee W, Schšnhofer B. The potential impact of anaemia of chronic disease in COPD. Eur Respir J 2006; 27:390–396.
- Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences –Clinical impact, mechanisms, and potential for early intervention. COPD: J Chron Obstruct Pulmon Dis 2008; 5(4):235–256.
- Jounieaux V, Mayeux I. Oxygen cost of breathing in patients with emphysema or chronic bronchitis in acute respiratory failure. Am J Respir Crit Care Med 1995; 152(6 Pt 1):2181– 2184.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171:972−−977.
- Reardon J, Casaburi R, Morgan M, Nici L, Rochester C. Pulmonary rehabilitation for COPD. Respir Med 2005; Dec;99 Suppl B:S19–S27.
- Kirsten DK, Taube C, Lehnigk B, Jorres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respir Med 1998; 92:1191–1198.
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Amer J Med 2000; 109:207–212.
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003; 163(5):585–591.
- Coventry PA, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: Systematic review and meta-analysis. J Psychosom Res 2007; 63(5):551–565.
- American Thoracic Society. Pulmonary rehabilitation–1999. Am J Respir Crit Care Med 1999; 159:1666–1682.
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16:3–63.
- Thune I, Furberg A-S. Physical activity and cancer risk: dose-response and cancer, all sites and site specific. Med Sci Sports Exer 2001; 33:S530–S550.
- Lamonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol 2005; 99(3):1205–1213.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422.
- Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, Wouters E, Sethi S, Cooper CB. COPD as a lung disease with systemic consequences—clinical impact, mechanisms, and potential for early intervention. COPD 2008 Aug;5(4):235–256. Review. PubMed PMID: 18671149.
- Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease: Inflammation of small airways and lung parenchyma. AM J Respir Crit Care Med 1999; 160:S21–S25.
- Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4:512–521.
- Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm 2010; 2010:585989. Epub 2010 Apr 20.
- Wilund KR. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin Sci 2007; 112(11–12):543–555.
- Dentener MA, Creutzberg EC, Schols AM, Systematic anti-inflammatory mediators in COPD: increase in soluble interleukin 1 receptor II during treatment of exacerbations. Thorax 2001; 56:721–726.
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exer Sport Sci Rev 2005; 33(3):114–119.
- Pedersen BK, Steensberg A, Fischer C, Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 2003; 24(2–3):113–119.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 2001; 60(3): 349–356.
- Casaburi R, Porszasz J, Burns MR, Carithers ER, Chang RS, Cooper CB. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 155:1541– 1551.
- Kayahan B, Karapolat H, Atýntoprak E, Atasever A, Oztürk O. Psychological outcomes of an outpatient pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease. Respir Med 2006; 100:1050–1057.
- Sabia S, Shipley M, Elbaz A, Marmot M, Kivimaki M, Kauffmann F, Singh-Manoux A. Why does lung function predict mortality? Results from the Whitehall II Cohort Study. Am J Epidemiol 2010; Dec 15;172(12):1415–1423.
- Roche N, Dalmay F, Perez T, Kuntz C, Vergnengre A, Neukirch F, Giordanella J-P, Huchon G. Impact of chronic airflow obstruction in a working population. Eur Respir J. 2008; 31:1227–1233.
- Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005; 127:1952–1959.
- Cook NR, Hebert PR, Satterfield S, Height, lung function, and mortality from cardiovascular disease among the elderly. Am J Epidemiol 1994; 139:1066–1076.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005 Aug; 26(2):319–338.
- Jakes RW, Day NE, Patel B, Khaw KT, Oakes S, Luben R, Welch A, Bingham S, Wareham NJ. Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation into Cancer-Norfolk Prospective Population Study. Am J Epidemiol 2002; 156;139–147.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Ant JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med 2007 Mar 1; 175(5):458–463.
- American Thoracic Society Statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Brown CD, Wise RA. Field tests of exercise in COPD: the six-minute walk test and the shuttle walk test. COPD. 2007 Sep; 4(3):217–223.
- Carrasco Garrido P, de Miguel Diez J, Rejas Gutierrez J, Centeno AM, Gobartt Vazquez E, Gil de Miguel A, Garcia Carballo M, Jiminez Garcia R. Negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes 2006 May 23; 4:31.
- McGavin CR, Artvinli M, Naoe H, McHardy GJR. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. BMJ 1978; 2:241–243.
- Cooper CB. Airflow obstruction and exercise. Respir Med 2009; 103(3):325–334.
- Foglio K, Carone M, Pagani M, Bianchi L, Jones PW, Ambrosino N. Physiological and symptom determinants of exercise performance in patients with chronic airway obstruction, Respir Med 2000; 94(3):256–263.
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea: contents, inter-observer agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85:751–758.
- Reardon JZ, Lareau SC, ZuWallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Amer J Med 2006; 119(10): Supplement 1, The role of patient-centered outcomes in defining the clinical course of chronic obstructive pulmonary disease, October 2006, Pages 32–37, ISSN 0002-9343, DOI: 10.1016/j.amjmed.2006.08.005.
- Wise RA. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med 2006; 119:4–11.
- Taube C, Burghart L, Paasch K, Factor analysis of changes in dyspnea and lung function parameters after bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 162:216–220.
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001 Nov; 56(11):880–887.
- Chasan-Taber L, Bianca Erickson J, Philip NC, Validity and reproducibility of a physical activity questionnaire in women. Med Sci Sports Exer 2002 Jun; 34(6):987–992.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, Jacobs DR Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000 Sep; 32(9 Suppl):S498–504.
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995 Feb 1; 273(5):402–407.
- Celli R, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata R, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012.
- Shephard RJ. Limits to the measurement of habitual physical activities by questionnaires. Br J Sports Med 2003; 37:197–206.
- Blair S, Dowda M, Pate RR, Reliability of long-term recall of participation in physical activity by middle-aged men and women. Am J Epidemiol 1991; 133:266–275.
- Shephard RJ. Fitness of a nation: lessons from the Canada Fitness Survey. Basel, Switzerland: Karger; 1986.
- Cumming RG, Klineberg RJ. A study of the reproducibility of long-term recall in the elderly. Epidemiology 1994; 5:116–119.
- van Poppel M, Chinapaw M, Mokkink L. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med 2010 Jul 1; 40(7):565–600.
- Shephard RJ. Assessment of occupational fitness in the context of human rights. Can J Sport Sci 1990; 15:89–95.
- Sallis JF, Buono MJ, Roby JJ, Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc 1993; 25:99–108.
- Rikli RE. Reliability, validity and methodological issues in assessing physical activity in older adults. Res 2000; 71:89–96.
- Klesges RC, Eck LH, Mellon MW, The accuracy of self-reports of physical activity. Med Sci Sports Exerc 1990; 22:690–697.
- Shephard RJ. Normal levels of activity in Canadian city dwellers. Can Med Assoc J 1967; 96:912–914.
- COPDGene. Protocol, Revision 16 Jun 2008; 18–24.
- COPDGene. Manual of Procedures. https://biosweb.njc.org/sec/copdgene/mainpage.scm (Authorized IP Address Required for Login, Accessed February 2011).
