Abstract
Earlier diagnosis of COPD is a major public health challenge as symptoms may be attributed to the normal consequences of aging. The optimum strategy for identifying patients with COPD remains to be determined. People aged 35 and over (n = 1896) on a GP practice register were randomised to either invitation or an opportunistic lung health check which included spirometry, quadriceps strength and MRC dyspnoea score. Then, 101 participants subsequently completed the General Practice Physical Activity Questionnaire. A total of 335 attended over a 15-week period; 156 were in the invitation group and 179 from the opportunist group. In 25 persons, spirometry was unsatisfactory or contraindicated. Spirometry was normal in 204(65.8%) and restrictive in 36(11.6%). 70(22.6%) had airflow obstruction, corresponding to Global Initiative for Chronic Lung Disease (GOLD) stages I-IV in 18(5.8%), 35(11.3%), 14(4.5%) and 3(1.0%), respectively. The opportunist group were significantly more likely to have airflow obstruction 30.1% vs 14.3% (p = 0.001). Breathlessness was reported commonly (40.5%) and quadriceps strength correlated significantly with MRC dyspnoea score independent of age, sex, pack-years smoked, fat-free mass and FEV1 percent predicted. This relationship was also present in the subgroup of healthy participants (n = 143). 51.5% of participants screened were classified as “inactive” and this group were weaker and more breathless than those who were more active. Airflow obstruction was more common in those screened opportunistically. Breathlessness and inactivity are common in patients taking part in spirometry screening. Breathlessness is significantly associated with leg strength independent of spirometry and should be amenable to interventions to increase physical activity.
Keywords :
Introduction
The diagnosed prevalence of chronic obstructive pulmonary disease is well below expected levels (Citation1) and a significant proportion of patients admitted to hospital with an acute exacerbation have not previously been diagnosed (Citation2). The undiagnosed “missing millions” (Citation3) are thus missing out on optimal therapy and management. Guidelines for the management of chronic obstructive pulmonary disease (COPD) stress the importance of early diagnosis (Citation4) and the incremental cost-effectiveness ratio of opportunistic case-finding has been estimated as a cost per quality adjusted life year of only £814, which is much lower than many funded healthcare interventions (Citation5). Admission rates could be reduced by better identification and management and a diagnosis of COPD can promote smoking cessation (Citation6–8) even in the absence of symptoms.
There is a growing literature describing the importance of skeletal muscle weakness in apparently healthy populations (Citation9) as well as in COPD (Citation10,11) and other chronic conditions (Citation12). The promotion of physical activity is a public health priority, because inactivity is associated with the development of many major public health problems, including cardiovascular disease, diabetes and COPD (Citation13).
Weakness has recently been recognised as a feature of early COPD (Citation14,15) but this and other studies in the literature (Citation11,Citation16) have been limited by the use of hospital clinic-based cohorts rather than the more general population. It is not clear to what extent the breathlessness and weakness occurring in early COPD can be differentiated from that due to sedentarism and a consequent lack of cardiovascular fitness. This is further complicated by the consistent observation that physical inactivity is associated with more rapid decline in FEV1 (Citation17).
In addition, few data exist to inform optimum screening strategies for COPD. Spirometry screening, although primarily intended to narrow the significant gap between observed and predicted COPD prevalence (Citation18,19), may be a useful opportunity to intervene and encourage lifestyle changes. We therefore addressed two questions. First, what are the determinants of breathlessness in smokers or ex-smokers participating in spirometry screening in primary care? Second, whether two different screening methods, invitation or opportunistic, would have similar pick-up rates for COPD.
Methods
This prospective, cross-sectional study was carried out between September and December 2008. It was approved by The Riverside Research Ethics Committee and all participants provided written informed consent and the trial was registered on the NIHR UKCRN Network: UKCRN ID 6438.
Subject recruitment
All patients on the practice list over the age of 35 were randomised either to an “invitation” or an “opportunistic” screening arm. Patients in the invitation arm received a letter asking them to attend for a “lung health check” if they were a current or former smoker, or to disregard the letter if not (Appendix 1). Subjects in the “opportunistic” arm had their records flagged, and if they attended the practice were invited by the reception or practice nursing staff to participate if they were eligible. Those with a diagnosis of lung cancer or other terminal conditions, active tuberculosis, or another co-morbidity that made them unable to perform spirometry, or who had significant musculoskeletal disease affecting their ability to make a maximum muscle contraction in their legs were excluded as well as never-smokers. Patients were not excluded from screening if they had a diagnosis of COPD, so that this prior diagnosis could be confirmed.
Measurements
All assessments were made by a specialist respiratory physiotherapist (JLK) with experience in performing the measurements described. The lung health screening consisted of a short symptom questionnaire (Appendix 2) detailing smoking history, cough and breathlessness, questions about prior diagnoses and The Medical Research Council (MRC) Dyspnoea Scale. This is a self-reported score which rates breathlessness on a scale from 1 (normal) to 5 (breathless while washing and dressing or too breathless to leave the house) (Citation20). Diagnostic information on diagnoses and medications and other treatments received was also available from the clinic's computer records.
Body mass index (BMI) and fat-free mass (FFM) measured using bioelectrical impedance analysis (Bodystat 1500, Bodystat Ltd, Douglas, Isle of Man) was recorded. Smoking status was confirmed by measurement of exhaled carbon monoxide level (Pico Smokerlyzer, Bedfont, UK). Spirometry was performed with the subjects seated and wearing a nose clip. The best of three measurements confirmed by the device's internal algorithm to be consistent with ATS/ERS guidelines was taken (Citation21).
Up to 8 attempts at technique correction were allowed. If the spirometry result was within the normal range (for FEV1, FVC and FEV1/FVC ratio) this was accepted as normal even if variability guidelines were not met. Where a restrictive or obstructive pattern was observed, but individual tests showed excessive variability these subjects were excluded from analysis. An obstructive pattern of spirometry was defined as an FEV1/FVC ratio of less than the lower limit of normal (LLN) and a restrictive one as an FEV1 <80% predicted with an FEV1/FVC ratio >LLN. Airflow obstruction was categorised according to GOLD grade as I FEV1>80%, II FEV1 80%-50%, III FEV1 50%-30% and IV FEV1 <30% predicted.
Quadriceps strength was tested using a hand held dynamometer (Lafayette Manual Muscle Test System) (Citation22). The subject was assessed in an upright sitting position at the edge of a plinth, with the whole thigh supported and hips and knees positioned to 90 degrees flexion. They were asked to keep their hands in their lap to disallow contribution of the upper limbs to stabilisation. The dynamometer, held in the dominant hand of the investigator, was placed on the right shin of the subject just above the ankle. On the investigator's instructions, the subjects were asked to push as hard as they could against the pad, trying to straighten their leg from the knee. Vigorous encouragement was given during contractions. A “make” test, where the subject pushes as hard as they can against the tester was used, with the peak force achieved over a 5 second effort recorded in Kg. The device's testing range was adjusted to either high (0–136.1 kg) or low (0–22.6 kg) to give the most accurate result. At least 3 tests were performed, more if the subject was improving with practice, or the investigator judged that maximum effort had not been achieved. Because of mechanical advantage, no subject was able to generate more force than that opposed by the investigator.
After the end of the initial data collection we decided to further characterise the cohort by obtaining data on physical activity levels. Following ethical approval for a protocol amendment, participants were telephoned and the General Practice Physical Activity Questionnaire (GPPAQ) administered (Citation23). This is a validated screening tool for assessing physical activity levels in patients aged 16–74 in primary care. It provides a quick, simple 4-level physical activity index consisting of: Active, Moderately Active, Moderately Inactive, and Inactive.
Analysis
Two analyses were undertaken. The first was to compare the diagnostic rates between the two arms of the screening study. The second was to investigate the relationship between quadriceps strength and breathlessness. This was analysed using stepwise linear regression analysis, to include the effect of potential confounding factors, including age, gender, smoking status and pack-years exposure, as well as fat-free mass and reported physical activity level where that had been obtained. A sub-analysis was performed in healthy subjects—defined as people with normal spirometry (FEV1 > 80% predicted and FEV1/FVC ratio greater than the LLN) with neither self-reported, nor documented cardiorespiratory disease nor other co-morbidities likely to influence strength or exercise capacity. Values are given as mean(SD) unless otherwise specified. StatView 5 was used to perform statistical analysis and a p-value of <0.05 was taken as significant.
Results
Comparison of invitation and opportunistic screening
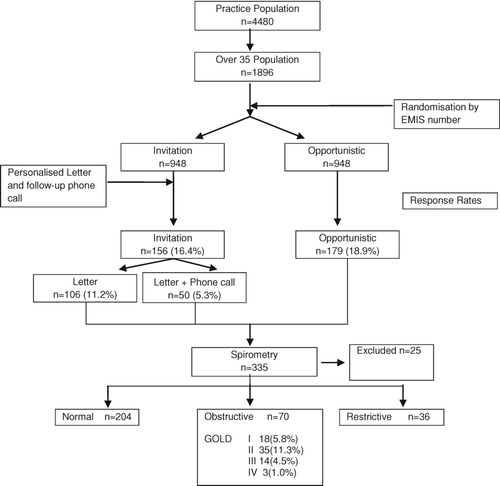
From a practice population of 4480, the 1896 individuals over the age of 35 were randomised to 2 groups of 948. Numbers are outlined in the CONSORT diagram (). In total, 335 people took part in the lung health screening. 156 attended in response to invitation and 179 were screened opportunistically, representing 16.4% and 18.9% of the two groups respectively. In 21 (6.3%) spirometry was technically unsatisfactory and 3 were excluded; 1 recent eye haemorrhage; 1 previous lobectomy; 1 failed to fill in the questionnaires. In the remaining 310 subjects spirometry was normal in 204 (65.8%) and restrictive in 36 (11.6%). Of these, 70 (22.6%) had airflow obstruction, corresponding to Global Initiative for Chronic Lung Disease (GOLD) stages I-IV in 18 (5.8%), 35 (11.3%), 14 (4.5%) and 3 (1.0%), respectively.
The opportunist group were significantly more likely to have airflow obstruction 30.1% vs 14.3% (p = 0.001) and also were more symptomatic than those attending in response to invitation (). Of those identified as having airflow obstrction by screening, 21 had a prior diagnosis of COPD. Prior to screening there were a total of 42 patients on the practice COPD register.
Table 1. Participant characteristics by screening groups
Of the opportunistic screening group 32.4% were attending the practice to see a doctor GP, 42.6% a practice nurse and 2.3% had an appointment with a counselor, with 22.7% declining to answer this question.
Compared to the screened population (both groups combined) those who were not screened were younger 53.6(13.7) years vs 48.7(12.3) years (p < 0.0001); less likely to be male 60.9% vs 51.4% (p = 0.002). There was no significant difference in documented smoking status or co-morbidities between those screened and not screened.
Quadriceps strength, breathlessness and spirometry in the whole population
Some patients declined to perform the quadriceps strength tests so data are reported for 301 subjects (59.8% male) (). Of these, 201 (66.8%) had normal spirometry, 60 (19.9%) had an obstructive pattern (GOLD stages I-IV 17 (28.3%), 27 (45.0%), 12 (20.0%) and 4 (6.7%), respectively) and 40 (13.3%) had a restrictive one. MRC dyspnoea score results ranging from 1–5 were 179 (59.5%), 86 (28.6%), 25 (8.3%), 8 (2.7%) and 3 (1.0%) respectively meaning that 122 (40.5%) had an MRC score ≥2 and were thus defined as “breathless.” There was a significant negative correlation between quadriceps strength and MRC dyspnoea score (ANOVA p < 0.0001) in the whole group and the different spirometry classes ().
Figure 2. Group mean values (± SD) for voluntary quadriceps strength stratified by self-reported MRC dyspnoea scores. There was significant reduction in strength with increasing dyspnoea score in all subjects (* p < 0.0001, † p = 0.0002, ‡ p = 0.0067, § p = 0.156), ‘healthy’ subjects (ll p = <0.0001, ** p = 0.0031), those with obstructive spirometry (†† p = 0.0427) and the ‘inactive’ subjects (‡‡ p = 0.047). There was no significant trend in the restrictive group. Error bars represent standard error of the mean.
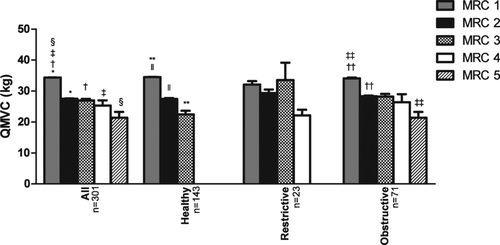
Table 2. Comparison of breathless and not-breathless subjects
Univariate analysis revealed significant correlations between MRC dyspnoea score and QMVC, BMI, age, pack-years smoking exposure, and spirometric values (). Using stepwise regression QMVC, percent predicted FEV1, BMI and pack-years smoking exposure were retained in a model that explained 50% of the variation in the MRC score; MRC score = 2.005 + 0.043(BMI) - 0.023(QMVC) - 0.014 (FEV1%predicted) + 0.012 (pack-years of smoke exposure).
Table 3. Correlates of MRC dyspnoea score
Subjects reporting breathlessness i.e., with an MRC dyspnoea score ≥2, (n = 122 (40.5%)) were weaker - QMVC 27.1(9.1) kg vs 34.3(9.1) kg (p < 0.0001) and also older, with a greater cigarette exposure, lower spirometry values and a higher BMI (). In the breathless group 59 (48.4%) had normal spirometry, 41 (33.6%) were obstructive; 9 (7.4%) GOLD I, 18 (14.8%) GOLD II, 10 (8.2%) GOLD III, 4 (3.3%) Gold IV, respectively, and 22 (18.0%) had a restrictive pattern. For not-breathless patients, the corresponding numbers were normal 142 (79.4%), and GOLD I 8 (4.5%), II 9(5.0%), III 2 (1.1%), IV 0 (0%), and 18 (10.0%) restrictive.
Subgroup analysis of apparently ‘healthy’ participants
Out of all people screened, 143 met the study definition of ‘healthy’ with normal spirometry and no diagnosed illnesses. Of these 111 (77.6%) had a normal MRC score, 28(19.6%) had a score of 2 and 4 (2.8%) had a score of 3. People in this category who were breathless were again significantly weaker than those with a normal MRC dyspnoea score; QMVC 26.8 (7.5) kg vs 34.5 (8.0) kg (p < 0.0001). Stepwise regression retained the same variables as in the whole population with the following model explaining 59% of the variance in MRC dyspnoea score; MRC score = 1.38 + 0.048(BMI) - 0.009(FEV1%predicted) –0.023(QMVC) + 0.014(pack-years)
Physical activity
At the end of the study period we attempted to contact 217 of the participants to complete the GPPAQ (this number was determined by the duration of funding available). They were telephoned twice from their GP's practice and a message left to call back. This led to the questionnaire being completed for 101 (46.5%). The sample group's characteristics did not differ significantly from those of the whole study population ().
Non-completion of the form was mostly due to unanswered phone calls 28 (12.9%) or non-response to messages left 19 (11.1%). Nineteen patients (8.8%) had left the practice and 8(3.7%) had died. There were incorrect contact numbers recorded for 5 (2.3%) and the absence of an interpreter excluded a further 5 (2.3%). Three patients were reported to be too unwell by family members, 2 people declined. Homelessness, holiday, prison and drug rehabilitation also prevented individual patients from answering. Nine (4.1%) interviewees were in the group previously excluded from analysis. Of the 101 responders, less than half were currently employed, 36 (35.7%). There were 27 (26.7%) retirees, 7 (6.9%) people who had retired due to their health and 31 (30.7%) people who reported being unemployed.
People who were classified as inactive by GPPAQ (n = 52 (52%) were older, more likely to have airway obstruction, less likely to be healthy and less likely to have be in current employment (). Inactive subjects were weaker; QMVC 28.6 (9.1) kg v 34.1 (8.9) kg (p = 0.0024) and more breathless; MRC Dyspnoea Score 1.8 (1.0) v 1.1 (0.4) (p < 0.0001), than more active individuals ( and and ).
Figure 3. Activity level was defined using the GP physical activity questionnaire (GPPAQ) 86% of the active group reported a normal MRC dyspnoea score of 1 with the remaining active subjects scoring 2, “shortness of breath when hurrying or walking up a slight hill”. The inactive group were significantly more breathless (p < 0.0001).
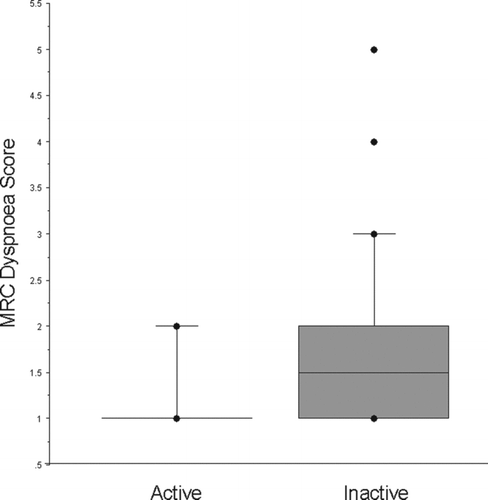
Figure 4. Quadriceps strength was significantly lower in those who were inactive; QMVC 28.6 (9.1)kg v 34.1 (8.9)kg (p = 0.0024). Activity level was defined using the GP physical activity questionnaire (GPPAQ).
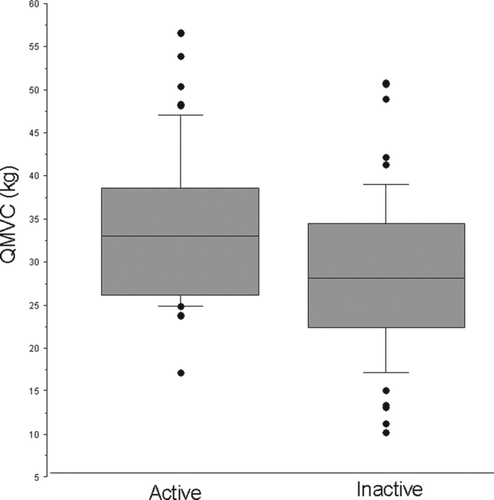
Table 4. Comparison of participants in physical activity questionnaire subgroup to whole population
Table 5. Values are Mean (SD) unless otherwise specified. *p < 0.05 comparing active to inactive subjects
The determinants of breathlessness in the subgroup completing the GPPAQ were the same as those observed in the whole group, with percent predicted FEV1, BMI, QMVC and pack-years smoking exposure retained in stepwise regression analysis as well as inactivity giving the model; MRC score = 1.519 + 0.044(BMI) - 0.020(QMVC) - 0.012(FEV1%predicted) + 0.011(pack-years of smoke exposure) + 0.384(inactive = 1 GPPAQ score) (r2 = 0.54).
Discussion
The main findings of this study were, first, that there was a higher rate of airflow obstruction detected in patients screened opportunistically in primary care than in those responding to an invitation to undergo screening, which is likely to be due to a healthy responder effect. Second, amongst respondents, breathlessness in this population was significantly associated with quadriceps weakness, an association that was independent of spirometry and other demographic and anthropometric characteristics. Importantly, the association between breathlessness and weakness was present both in the whole group screened and in a subset defined as ‘healthy’ on the basis of their medical history and normal spirometry. Both breathlessness and quadriceps weakness were more pronounced in inactive subjects.
Significance of findings
We found that opportunistic screening was more likely to identify patients with COPD than an invitation based approach, but in both approaches a similar proportion of the targeted population were screened. The identified prevalence of COPD was similar to other studies conducted in general practice (Citation24) but to our knowledge no prior studies have compared screening strategies or evaluated quadriceps strength. Both strategies identified significant numbers of new COPD diagnoses allowing for the possibility of anticipatory care such as inhaled medications for symptoms and physical activity promotion either through general advice or pulmonary rehabilitation programs.
Skeletal muscle strength is an important factor in determining peak exercise capacity (Citation25,26). The current data extend this by demonstrating that muscle weakness is also relevant to self-reported breathlessness during usual daily activities in a primary care population both with and without abnormal spirometry. It follows therefore, that if breathlessness is related to muscle weakness, then intervention to improve muscle function through increased physical activity and/or exercise training is likely to be of benefit in relieving it.
It has already been established that quadriceps weakness is associated with increased mortality both in the presence (Citation11,Citation27) and absence (Citation9,Citation22,Citation28) of chronic disease, which would support the promotion of exercise programs to improve skeletal muscle function. Physical inactivity, measured in the present study using the GPPAQ, provides a likely mechanism linking breathlessness and weakness. Weaker, deconditioned muscles will produce more lactate and thus a greater CO2 burden and ventilatory demand for any given activity, driving a vicious cycle of reduced activity and breathlessness (Citation29,30).
The finding that leg weakness is associated with breathlessness in this population, likely due to physical inactivity, should encourage primary care physicians towards making a positive diagnosis of deconditioning as a cause of breathlessness in this context and incentivise patients as well as to increase their physical activity level with a range of attendant health benefits (Citation31). Informing patients that smoking is itself associated with muscle weakness (Citation15) could provide additional motivation to some individuals. Future research programs should, using qualitative techniques, focus on identifying and overcoming the barriers to exercise in smokers; typically such patients may be younger than the clinic population of patients with COPD and their responsibilities (e.g., paid employment, younger children) may preclude a traditional pulmonary rehabilitation program.
Skeletal muscle weakness and COPD
The major purpose of spirometry screening has been the early identification of respiratory disease, principally COPD and our findings have specific relevance for these patients beyond the general observation linking leg strength and breathlessness. In COPD, skeletal muscle depletion is associated with reduced exercise capacity (Citation25,Citation32,Citation33) and quality of life (Citation10) and quadriceps strength has been shown to predict survival (Citation34). This underpins the effectiveness of pulmonary rehabilitation which is recommended in COPD and other chronic respiratory diseases (Citation35,36). The strong correlation between quadriceps strength and breathlessness in the subgroup who did have obstructive spirometry underlines the importance of skeletal muscle impairment as a cause of limitation. This is particularly important as it is potentially reversible whereas to date, apart from smoking cessation, therapies aimed at altering the natural history of COPD have been of limited benefit.
Few data exist describing the time course and progression of skeletal muscle impairment in COPD, although it is increasingly recognised that weakness can occur early in the course of the disease (Citation14,15) and that fat free mass is lower and declines more rapidly, in patients with frequent exacerbations (Citation37). An important limitation of previous reports has been that the patients were derived from a hospital clinic population but the current data confirms that weakness is present in patients with mild disease in a primary care population. It remains to be established to what extent, if at all, the muscle dysfunction observed in early COPD differs from that occurring in ostensibly healthy but sedentary individuals, particularly those with a significant smoking history.
Certainly, a low level of physical activity is associated with increased mortality (Citation38) and increasing the level of physical activity in mid-life can significantly reduce mortality rates (Citation31). Inactivity along with poor diet is estimated to cause 16.6% of all deaths in the United States, a proportion that is rising while the proportion attributable to smoking is falling (Citation13). In The Copenhagen City Heart Study, people with low levels of self-reported physical activity were more likely to develop COPD than those who were physically active (Citation39,40) and a number of other studies have also linked low levels of physical activity to an accelerated decline in FEV1 (Citation17,Citation41–46).
Many of the co-morbidities associated with COPD, including coronary artery disease, peripheral vascular disease, osteoporosis and glucose intolerance have also been associated with inactivity (Citation47) and for a given lung function impairment, inactive COPD patients have more co-morbidity (Citation48). These data suggest that physical inactivity drives both the progression of COPD and common co-morbidities. The GPPAQ gives a quick, valid but subjective measure of activity level. The possibility that strength measurements could provide a useful surrogate for measurement of physical activity deserves further study.
Methodological issues
Subject selection
As spirometry screening becomes more widespread it is important to quantify and address the health needs of the specific population who are participating in these programmes. Because the study targeted people with a smoking history, we cannot comment on whether the relationship between strength and breathlessness is present in never smokers. There is however, a growing body of evidence to suggest that smoking has a deleterious effect on skeletal muscle (Citation9,Citation15,Citation49–53). Moreover, pilot data from our group suggests that in patients with COPD, smoking cessation is associated with an increase in muscle mass (Citation37).
Physical activity subgroup
The decision to use the GPPAQ was taken after the initial data collection had been completed so this data was retrospective and incomplete. Despite this, the characteristics of those in whom it was measured did not differ significantly from those of the whole study population. The measure of activity levels by the GPPAQ is validated for use in general practice and in this sample gave meaningful representation of the actual activity performed by the patient as well as showing relationship to strength and breathlessness, both factors of physical fitness. Ideally we would have measured physical activity directly but that would have added to the cost of the study and time involved and would be less relevant for routine clinical practice.
Conclusion
Quadriceps weakness is associated with breathlessness in patients with a smoking history undergoing spirometry screening, whether or not they have obstructive spirometry. Breathlessness was a common symptom and our findings would support the evaluation of early exercise interventions to reduce dyspnoea. In early cardiorespiratory disease it may be difficult to separate the occurrence of weakness and breathlessness associated with reduced physical activity from any specific disease-related effect. The comparison of screening strategies suggests that opportunistic screening rather than screening by invitation is likely to identify more patients with COPD.
Declaration of Interests
All authors declare that they have no conflicts of interest. The study was conceived by NSH, SLE, MAS and JF. JLK performed the measurements and with MAS and NSH performed the analysis. JLK and NSH prepared the first draft to which all authors contributed and approved the final version. NSH is the guarantor of the paper.
Acknowledgments
Source of Support: The study was funded by a Trevor Clay grant from The British Lung Foundation and supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, who part fund MIP's salary.
References
- Nacul L, Soljak M, Samarasundera E, COPD in England: a comparison of expected, model-based prevalence and observed prevalence from general practice data. J Publ Health (Oxf) 2011; 33:108–116.
- Bastin A, Starling L, Ahmed R, High prevalence of undiagnosed and severe chronic obstructive pulmonary disease at first hospital admission with acute exacerbation. Chron Respir Dis 2010; 7:91–97.
- British Lung Foundation. Invisible Lives. Chronic Obstructive Pulmonary Disease (COPD)—Finding the missing millions, November 2007
- Rabe KF, Hurd S, Anzueto A, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am J Respir Crit Care Med 2007; 176:532–555.
- National Institute for Clinical Excellence. National Clinical Guideline on Management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; 59:i1–232.
- Bednarek M, Gorecka D, Wielgomas J, Smokers with airway obstruction are more likely to quit smoking. Thorax 2006; 61:869–873.
- Gorecka D, Bednarek M, Nowinski A, Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest 2003; 123:1916–1923.
- Parkes G, Greenhalgh T, Griffin M, Effect on smoking quit rate of telling patients their lung age: The Step2quit randomised controlled trial. BMJ 2008; 336:598–600.
- Ruiz JR, Sui X, Lobelo F, Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008; 337:a439. doi: 10.1136/bmj.a439.
- Shrikrishna D, Hopkinson NS. Chronic obstructive pulmonary disease: consequences beyond the lung. Clin Med 2012; 12:71–74.
- Swallow EB, Reyes D, Hopkinson NS, Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62:115–120.
- Gosker HR, Lencer NHMK, Franssen FME, Striking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPD. Chest 2003; 123:1416–1424.
- Mokdad AH, Marks JS, Stroup DF, Actual causes of death in the United States, 2000. JAMA 2004; 291:1238–1245.
- Shrikrishna D, Patel M, Tanner RJ, Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 2012, in press.
- Seymour JM, Spruit MA, Hopkinson NS, The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J 2010; 36:81–88.
- Man WD, Soliman MG, Nikoletou D, Non-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax 2003; 58:665–669.
- Hopkinson NS, Polkey MI. Does physical inactivity cause chronic obstructive pulmonary disease? Clin Sci (Lond) 2010; 118:565–572.
- Nacul L, Soljak M, Samarasundera E, COPD in England: a comparison of expected, model-based prevalence and observed prevalence from general practice data. J Publ Health 2010: 10.1093/pubmed/fdq1031.
- Falzon C, Elkin SL, Kelly JL, Can financial incentives for improvements in healthcare quality enhance identification of COPD in primary care? Thorax 2011; 66:630.
- Bestall JC Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–586.
- Miller MR, Hankinson J, Brusasco V, Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Buchman AS, Boyle PA, Wilson RS, Pulmonary function, muscle strength and mortality in old age. Mechan Ageing Develop 2008; 129:625–631.
- Department of Health (UK). The General Practice Physical Activity Questionnaire (GPPAQ) 2006. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicy AndGuidance/DH_063812
- Ulrik CS, Lokke A, Dahl R, Early detection of COPD in general practice. Int J Chron Obstruct Pulmon Dis 2011; 6:123–127.
- Hamilton AL, Killian KJ, Summers E, Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995; 152:2021–2031.
- Jones NL, Killian KJ. Exercise limitation in health and disease. N Engl J Med 2000; 343:632–641.
- Hülsmann M, Quittan M, Berger R, Muscle strength as a predictor of long-term survival in severe congestive heart failure. European Journal of Heart Failure 2004; 6:101–107.
- Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Med Sci Sports Exer 2002; 34:740–744.
- Casaburi R, Patessio A, Ioli F, Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis 1991; 143:9–18.
- Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease. Clin Med 2006; 6:190–196.
- Byberg L, Melhus H, Gedeborg R, Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. BMJ 2009; 338:b688. doi: 10.1136/bmj.b688.
- Bernard S, LeBlanc P, Whittom F, Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158:629–634.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153:976–980.
- Swallow EB, Reyes D, Hopkinson NS, Quadriceps strength predicts mortality in patients with moderate to severe Chronic Obstructive Pulmonary Disease. Thorax 2006: 62:115–120.
- Dodd JW, Hogg L, Nolan J, The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011; 66:425–429.
- Nici L, Donner C, Wouters E, American Thoracic Society/European Respiratory Society Statement on Pulmonary Rehabilitation. Am J Respir Crit Care Med 2006; 173:1390–1413.
- Hopkinson NS, Tennant RC, Dayer MJ, A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res 2007; 8:25.
- Jonker JT, De Laet C, Franco OH, Physical activity and life expectancy with and without diabetes: Life table analysis of the Framingham Heart Study. Diabetes Care 2006; 29:38–43.
- Garcia-Aymerich J, Lange P, Benet M, Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: A population-based cohort study. Am J Respir Crit Care Med 2007; 175:458–463.
- Garcia-Aymerich J, Lange P, Serra I, Time-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: An application of the marginal structural model. Ann Epidemiol 2008; 18:775–783.
- Jakes RW, Day NE, Patel B, Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation into Cancer-Norfolk Prospective Population Study. Am J Epidemiol 2002; 156:139–147.
- Pelkonen M, Notkola I-L, Lakka T, Delaying Decline in Pulmonary Function with Physical Activity: A 25-Year Follow-up. Am J Respir Crit Care Med 2003; 168:494–499.
- Twisk J, Staal B, Brinkman M, Tracking of lung function parameters and the longitudinal relationship with lifestyle. Eur Respir J 1998; 12:627–634.
- Canoy D, Pekkanen J, Elliott P, Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax 2007; 62:396–402.
- Cheng YJ, Macera CA, Addy CL, Effects of physical activity on exercise tests and respiratory function. Br J Sports Med 2003; 37:521–528.
- Sandvik L, Erikssen G, Thaulow E. Long term effects of smoking on physical fitness and lung function: a longitudinal study of 1393 middle aged Norwegian men for seven years. BMJ 1995; 311:715–718.
- Decramer M, Rennard S, Troosters T, COPD as a lung disease with systemic consequences—Clinical impact, mechanisms, and potential for early intervention. COPD: J Chron Obstruct Pulmon Dis 2008; 5:235–256.
- Watz H, Waschki B, Boehme C, Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: A cross-sectional study. Am J Respir Crit Care Med 2008; 177:743–751.
- Wust RC, Morse CI, de Haan A, Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol 2008; 104:103–110
- Morse CI, Wust RCI, Jones DA, Muscle fatigue resistance during stimulated contractions is reduced in young male smokers. Acta Physiol 2007; 191:123–129
- Petersen AMW, Magkos F, Atherton P, Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab 2007; 293:E843–848
- Rapuri PB, Gallagher JC, Smith LM. Smoking is a risk factor for decreased physical performance in elderly women. J Gerontol A Biol Sci Med Sci 2007; 62:93–99
- Lee JS, Auyeung TW, Kwok T, Associated factors and health impact of sarcopenia in older Chinese men and women: A cross-sectional study. Gerontology 2007; 53:404–410