Abstract
This retrospective cohort study aimed to analyze the prescribing practices of general practitioners treating patients with newly diagnosed chronic obstructive pulmonary disease (COPD), and to assess characteristics associated with initial pharmacotherapy. Patients were identified in the General Practice Research Database, a population-based UK electronic medical record (EMR) with data from January 1, 2008 to December 31, 2009. Patient characteristics, prescribed COPD pharmacotherapies (≤12 months before diagnosis and within 3 months following diagnosis), co-morbidities, hospitalizations, and events indicative of a possible COPD exacerbation (≤12 months before diagnosis) were analyzed in 7881 patients with newly diagnosed COPD. Most patients (64.4%) were prescribed COPD pharmacotherapy in the 12 months before diagnosis. Following diagnosis, COPD pharmacotherapy was prescribed within 3 months in 85.0% of patients. Short-acting bronchodilators alone (22.9%) or inhaled corticosteroids + long-acting beta-2 agonists (ICS+LABA, 22.1%) were prescribed most frequently. Compared with other pharmacotherapies, the prevalence of severe airflow limitation was highest in patients prescribed ICS+LABA+long-acting muscarinic antagonists (LAMA). Moderate-to-severe dyspnea was identified most frequently in patients prescribed a LAMA-containing regimen. Patients prescribed an ICS-containing regimen had a higher prevalence of asthma or possible exacerbations recorded in the EMR than those not prescribed ICS. In conclusion, pharmacotherapy prescribed at initial COPD diagnosis varied by disease severity indicators as assessed by airflow limitation, dyspnea, history of asthma, and possible exacerbations. Frequent prescription of COPD pharmacotherapies before the first-recorded COPD diagnosis indicates a delay between obstructive lung disease presentation in primary care practice and assignment of a medical diagnosis.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and treatable condition that is characterized by persistent airflow limitation. It is usually progressive and associated with an enhanced, chronic inflammatory response in the respiratory system to noxious particles or gases (Citation1).
A variety of pharmacotherapies are available for the treatment of COPD. The most commonly prescribed inhaled pharmacotherapies include: short- and long-acting beta-2 agonists (SABAs and LABAs, respectively), short- and long-acting muscarinic antagonists (SAMAs and LAMAs), inhaled corticosteroids (ICSs), and the combination of a LABA and an ICS, either used in one inhaler or separately, or in combination with a LAMA (Citation2).
Guidelines that are available to aid pharmacotherapy decisions include the well-established Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy (Citation1); those from the American College of Physicians, American College of Chest Physicians, American ≠Thoracic Society, and European Respiratory Society (Citation3); and national guidelines, such as those from the National Institute for Health and Clinical Excellence (NICE) in the UK (Citation4).
When treating COPD in primary care, recent studies have shown that physician adherence to prescribing guidelines may be suboptimal (5–9). In the UK, a national audit has shown that patients are not benefitting from many of the interventions recommended in NICE guidance (Citation4, Citation10); for example, 11% of patients treated for acute COPD exacerbations had not been receiving COPD pharmacotherapy. In the USA, studies have shown that 23–52% of patients did not receive any COPD-related pharmacotherapy following diagnosis (Citation5, 6, Citation11). Furthermore, outside North America and Europe, global COPD guidelines appear to have limited reach and application (Citation12). Low adherence to guidelines has been attributed to factors including a lack of familiarity with guidelines, physician doubt in the ability to change patient behavior, and time constraints (Citation8, 9).
There is limited literature describing patient and disease factors that relate to physician prescribing. Large electronic medical record (EMR) databases based on routinely delivered patient care, such as the UK General Practice Research Database (GPRD), can be useful for studying prescribing practices. The GPRD, to which general practices have contributed medical history data since 1987, has been used extensively in studying patients with COPD. The GPRD algorithm used to identify COPD diagnosis has been validated (Citation13, 14). Since April 2004, the introduction of the Quality Outcomes Framework in the UK has mandated practices to document care for COPD using predefined clinical standards that include COPD diagnosis confirmed with spirometry and the monitoring of breathlessness using the Medical Research Council (MRC) dyspnea scale.
The aims of this analysis were to describe prescribing practices of general practitioners (GPs) for patients newly diagnosed with COPD, and to assess patient characteristics associated with initial COPD pharmacotherapy, in order to assess unmet needs in COPD and to target interventions to educate and support physicians who treat patients with COPD.
Methods
Study design
A retrospective cohort of patients newly diagnosed with COPD (incident COPD cases) was identified in the GPRD from January 1, 2008 to December 31, 2009. Patient data were included for the 12 months preceding the first diagnosis of COPD (COPD index date) and for 3 months of follow-up. Prescribed pharmacotherapies and corresponding patient characteristics were analyzed for all newly diagnosed patients. Anonymized patient data were analyzed. Read codes, used to identify medical conditions or pharmacotherapies, were reviewed by a clinician or pharmacist. Previous validation studies have confirmed the completeness and high quality of the computer-recorded general practice data and the accuracy of COPD diagnosis in the GPRD (Citation13, 14). The study protocol was reviewed and approved by the Independent Scientific Advisory Committee of the UK Medicines and Healthcare Products Regulatory Agency.
Patients
Patients were identified as incident COPD cases according to the following criteria: at least 40 years of age at the time of incident diagnosis; at least one COPD-related medical code first recorded between January 1, 2008 and December 31, 2009; a diagnosis of COPD confirmed by spirometry (forced expiratory volume in one second/forced vital capacity [FEV1/FVC] ≤0.7); at least 12 months of medical history before the COPD index date; and 24 months of follow-up, unless death occurred (Citation15). Patients with a record of a prior diagnosis of COPD or COPD-related medical code any time in the past history were excluded. The first 3 months of follow-up are reported here.
Study variables
Patient characteristics were assessed at COPD index date. Patient data were grouped according to age (40 to ≤65 years and ≥65 years), body mass index (BMI; using the World Health Organization classifications of underweight if ≤18.5 kg/m2, normal if 18.5–24.9 kg/m2, overweight if 25.0–29.9 kg/m2, obese if ≥30.0 kg/m2), and smoking status (ex-smoker, current smoker, never smoker, other smoker [data not available or passive tobacco exposure]). Severity of airflow limitation at the index date was defined based on the 2006 GOLD lung obstruction classification, using the first post-bronchodilator FEV1 measurement following or occurring on incident diagnosis and classified as mild (FEV1 ≥ 80% predicted), moderate (50% ≤ FEV1 ≤ 80% predicted), severe (30% ≤ FEV1 ≤50% predicted) or very severe (FEV1 ≤ 30% ≠predicted) impairment (Citation16).
Co-morbid conditions were evaluated using adjusted Charlson's co-morbidity index scores (with acquired immunodeficiency syndrome removed due to a low prevalence in the general population) (Citation17). The prevalence of selected diseases listed in Charlson's co-morbidity index (acute myocardial infarction, congestive heart disease, cancer, peripheral vascular disease, renal disease), with the addition of depression, anxiety, and asthma, were recorded if a diagnostic medical code (Read code) was present at any time in a patient's record. A medical code for a diagnosis of asthma ever in the patient history was considered to be a possible indicator of asthma. It was not considered necessarily a ‘true’ diagnosis of asthma, as the presence of a code may represent COPD that was initially misdiagnosed as asthma.
Healthcare utilization, including exacerbations
Healthcare utilization, as an indicator of disease burden, was assessed in the 12 months before the new COPD diagnosis. All-cause hospitalizations (excluding COPD hospitalizations) were identified by GPRD medical codes. Events indicative of possible moderate COPD exacerbations included a medical diagnosis of acute bronchitis, or concurrent treatment with oral corticosteroids and antibiotics. The number of GP visits (≠in-office visits only, maximum of one visit counted per day) during the 12 months before the COPD index date was standardized per 365.25 days. The MRC score for dyspnea severity was defined based on recordings between the index date and 3 months of follow-up, where available (Citation18, 19).
COPD pharmacotherapy
Prescribed COPD pharmacotherapies were identified within 3 months (including day of diagnosis) after a new diagnosis. The numbers and proportions of patients in each of the following categories were determined: no prescription for COPD pharmacotherapy; prescription for a short-acting bronchodilator (SABD) alone (a SABA alone, a SAMA alone, or SABA+SAMA); a LABA alone; an ICS alone; a LAMA alone; LABA+LAMA; ICS+LABA; ICS+LAMA; and ICS+LABA+LAMA (triple therapy). The duration of each prescription was assumed to be 30 days. Combination pharmacotherapies were defined as two or more unique medication prescriptions on the same day or within a 30-day period of each other, and could include individual pharmacotherapies or dual combination products. The prescribed pharmacotherapy categories were mutually exclusive, with the exception of SABD use in patients who were prescribed maintenance pharmacotherapy. The proportion of patients prescribed various COPD pharmacotherapies was determined for the total COPD population, and stratified by selected patient and ≠disease characteristics.
Statistical methods
Logistic regression was used to examine the factors determining the choice of initial maintenance pharmacotherapy (prescribed within the 3-month follow-up period) using three backwards selection models for: ICS-containing combinations (ICS, ICS+LABA, ICS+LAMA, ICS+LABA [single inhaler]) versus long-acting bronchodilators only (LABDs [LAMA, LABA, LABA+LAMA]); triple therapy versus ICS-combination pharmacotherapy; and triple therapy versus LABDs. Variables examined included age, sex, BMI, smoking status, airflow limitation, Charlson's co-morbidity index score, individual co-morbidities, and healthcare utilization events that occurred in the 12 months before the COPD index date, including events indicative of possible exacerbations, all-cause hospitalizations, and number of GP office visits. MRC dyspnea scores in the 3-month follow-up period could not be included as this information was missing for more than 20% of patients. Statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, North Carolina, USA).
Results
A study population of 7881 patients newly diagnosed with COPD was identified from the GPRD (). A slight majority of patients were male (55.0%) and ≥65 years old (59.1%), and 38.6% of patients were classified as current smokers (). A total of 70.1% of patients had mild or moderate airflow limitation ().
Figure 1. Cohort selection. COPD = chronic obstructive pulmonary disorder; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; GOLD = Global Initiative for Obstructive Lung Disease; GPRD = General Practice Research Database.
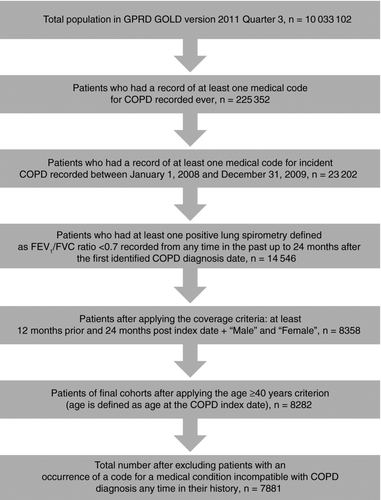
Table 1. Selected demographic and disease characteristics by pharmacotherapy prescribed at and up to 3 months following initial COPD diagnosis
COPD prescription frequencies at incident COPD diagnosis
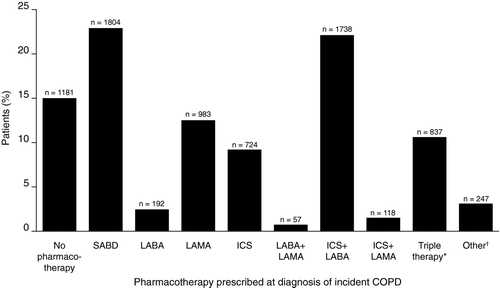
The most frequently prescribed COPD pharmacotherapies were an SABD alone (22.9%) and ICS+LABA (22.1%), followed by LABD(s) alone (15.6%), triple therapy (10.6%), and an ICS alone (9.2%) (). A small proportion of patients were prescribed other pharmacotherapies, including ICS+LAMA (1.5%) and LABA+LAMA (0.7%) (). For 15.0% of patients, no COPD pharmacotherapy was prescribed within 3 months following a new diagnosis of COPD.
Figure 2. Prevalence of COPD prescription (mutually exclusive groups) in the 3 months following initial COPD diagnosis *Triple therapy refers to ICS+LABA+LAMA. ÜOther refers to home-based oxygen therapy, oral corticosteroids, and theophylline and its derivatives. COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroid; LABA = long-acting beta-2 agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting beta-2 agonist; SABD = short-acting bronchodilator.
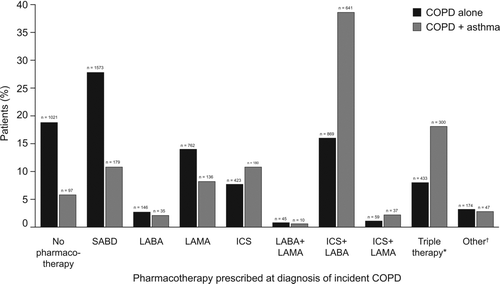
Age, smoking status, and BMI did not vary appreciably among pharmacotherapies prescribed (). A prior diagnosis code of asthma was identified in 32.9% of patients overall (). The proportion of patients with a prior asthma diagnosis varied considerably among prescription categories: no COPD pharmacotherapy: 14.7%; SABD alone: 18.3%; LABA alone: 26.0%; LAMA alone: 23.9%; ICS alone: 45.0%; ICS+LABA: 52.1%; and triple therapy: 50.2%. The distribution of pharmacotherapies among patients with at least one asthma code and COPD compared with COPD alone was notably different (). An ICS containing pharmacotherapy was prescribed for 68% of patients with COPD and at least one code for asthma compared with 43% for patients with COPD without an asthma code.
Figure 3. Distribution of COPD pharmacotherapy prescriptions in the 3 months following initial COPD diagnosis in patients with COPD and a prior diagnosis of asthma and patients with COPD alone. *Triple therapy refers to ICS+LABA+LAMA. ÜOther refers to home-based oxygen therapy, oral corticosteroids, and theophylline and its derivatives. COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroid; LABA = long-acting beta-2 agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting beta-2 agonist; SABD = short-acting bronchodilator.
The frequency of prescription category was stratified by GOLD stage of airflow limitation and MRC score at COPD index date (). GOLD stage of airflow limitation was unknown for 240 (3.1%) patients. The proportion of patients with GOLD stages III or IV airflow limitation (severe or very severe COPD) ranged from 22.5% for patients prescribed SABD alone to 43.5% for patients prescribed triple therapy, whereas 14.5% who were prescribed no pharmacotherapy had GOLD stages III or IV of airflow limitation. In total, 45.2% of patients with GOLD stages I or II airflow limitation, and 61.9% with GOLD stages III or IV airflow limitation, were prescribed an LABD, either alone or in combination. An ICS, either alone or in combination, was prescribed to 40.1% of patients with GOLD stages I or II airflow limitation, and 52.1% with GOLD stages III or IV airflow limitation. MRC score at COPD index date was unknown for 36.0% of patients. Of those patients assigned MRC scores, the proportion of patients with moderate-to-severe dyspnea (MRC score ≥3) ranged from 14.1% of patients prescribed no pharmacotherapy to 30.5% of patients prescribed triple therapy () (Citation19).
Prescription frequencies and exacerbations before incident COPD diagnosis
A total of 64.4% of patients were prescribed COPD pharmacotherapy before diagnosis; 59.3% were prescribed an SABD. In 37.3% of patients prescribed no pharmacotherapy, 58.5% of patients prescribed a LAMA, 73.1% of patients prescribed ICS+LABA, and 75.8% of patients prescribed triple therapy, a prescription of an SABD in the 12 months before the COPD index date was identified. In 25.6% of patients prescribed ICS+LABA, and 21.9% of patients prescribed triple therapy on or within 12 months of incident COPD diagnosis, a previous prescription for an ICS was identified. In 16.7% of patients prescribed a LAMA and in 55.7% of patients prescribed triple therapy on or within 12 months of incident COPD diagnosis, a previous prescription for ICS+LABA was recorded ().
Table 2. Selected disease characteristics of patients in the 12 months before initial COPD diagnosis by pharmacotherapy prescribed at or up to 3 months following diagnosis
Evidence of a possible exacerbation in the 12 months before a new diagnosis of COPD was recorded in 37.9% of the total cohort (). One or more events suggestive of possible exacerbation occurred in 25.7% of patients prescribed no pharmacotherapy, 30.4% of patients prescribed an SABD, 37.3% of patients prescribed a LAMA, 45.1% of patients prescribed ICS+LABA, and 51.1% of patients prescribed triple therapy ().
Factors associated with prescription of initial COPD pharmacotherapy
GPs were more likely to prescribe an initial treatment of triple therapy than another ICS-containing pharmacotherapy in patients with: [1] GOLD stages III or IV airflow limitation, or [2] at least one possible exacerbation in the 12 months before COPD index date. GPs were more likely to prescribe an initial treatment of triple therapy than to prescribe a LABD in patients with: [1] GOLD stages III or IV airflow limitation; [2] at least one possible exacerbation; [3] at least one all-cause hospitalization in the 12 months before COPD index date; or [4] a prior diagnosis of depression or asthma. GPs were more likely to prescribe an initial treatment of an ICS-containing pharmacotherapy than a LABD in patients with: [1] at least one possible exacerbation in the 12 months before COPD index date; or [2] prior diagnosis of asthma ().
Table 3. Factors associated with physicians’ choice of initial COPD pharmacotherapy (output from the final statistical model)
Discussion
Previous studies have indicated that a high proportion of patients with COPD in the UK are diagnosed at advanced stages of the disease (Citation20). Results from this study, based on longitudinal, EMR data from a UK GPRD newly diagnosed COPD cohort, confirm these findings. In this study, most patients (55.8%) were prescribed a maintenance pharmacotherapy (LAMA, ICS, ICS+LABA, ICS+LAMA or triple therapy) within 3 months of diagnosis.
Many of these patients had also been prescribed a maintenance pharmacotherapy before diagnosis. The finding that most patients prescribed triple therapy at COPD diagnosis had received a prescription for ≠maintenance pharmacotherapy in the previous 12 months suggests that GPs prescribed LABD and ICS pharmacotherapies as first-line treatment without recording a COPD diagnosis. Although it is not possible to ascertain the rationale for not recording a COPD diagnosis, the possibility of prescribing based on a historical or concurrent asthma diagnosis was explored. Approximately half of the patients prescribed ICS+LABA had an ICS+LABA prescription in the previous 12 months, which may partly be attributed to a prior diagnosis of asthma (52.1% of all patients prescribed ICS+LABA had a prior asthma diagnosis). These data are in line with findings from an Italian general practice study, which showed that 32.5% of patients with COPD in 2008 received an ICS+LABA combination (Citation21).
In our study, the percentage of patients prescribed ICS+LABA following an initial COPD diagnosis was considerably lower in patients without compared with those with a prior asthma diagnosis (22.1% vs 52.1%, respectively). Patients with COPD and a prior diagnosis of asthma were less likely to be prescribed SABD only or LAMA only as initial therapy than patients in the overall COPD population. The prescription of ICS monotherapy in our study (10.8% of patients with COPD and asthma and 7.7% of patients with COPD alone) is also in line with the Italian study, which showed that 15.3% of patients with COPD in 2008 received ICS monotherapy (Citation21), although ICS monotherapy is not indicated for the treatment of COPD.
The 15.0% of patients with COPD in this study who were not prescribed pharmacotherapy in the 3 months following diagnosis may not be accurately classified as unexposed to COPD pharmacotherapy, as 41.1% of them had been prescribed a COPD pharmacotherapy in the 12 months before diagnosis. Thus, only 8.8% of the cohort was consistently never prescribed COPD pharmacotherapy in the 12 months preceding, or 3 months following, incident COPD diagnosis. Nevertheless, both estimates of 15.0% and 8.8% are lower than the reported percentages of patients with COPD recorded without any prescribed COPD pharmacotherapy in published US studies (23–52%) (Citation5, 6, Citation11).
In this study, the number of events indicative of a possible prior exacerbation and the percentage of patients with severe airflow limitation were also higher than would be expected for a population with newly diagnosed COPD. Approximately 27% of the newly diagnosed cohort had severe or very severe airflow limitation, similar to the airflow limitation reported in a Greek, primary care prevalent COPD cohort (20%) (Citation22). Compared with patients with COPD who were included in the Greek primary care study, and in a recent, European, health-related quality of life study, patients enrolled in this study were more likely to be female (45.0% vs 12.4% and 30.7%) and less likely to be current smokers (38.0% vs 45.9% and 45.1%), respectively (Citation22, 23).
The proportion of patients with COPD who were underweight was less than in a recent UK study of ≠pulmonary rehabilitation outpatients (4.7% vs 16.1%), although the proportions of patients who were overweight or obese were similar (33.0% vs 27.6% and 25.5% vs 25.5%, respectively) (Citation24). The percentage of patients who experienced one or more events indicative of a possible exacerbation in the 12 months before COPD diagnosis (37.9%) is higher than previously reported exacerbation percentages in other primary care populations (21.0%) (Citation25).
The frequency of prescriptions for COPD pharmacotherapies in the prior 12 months, the number of events indicative of a possible exacerbation, and the percentage of patients with severe airflow limitation, suggest that most of the newly diagnosed COPD cohort had a prior history of COPD, asthma, or other symptomatic respiratory disease that was undiagnosed or unrecorded in the primary care EMR. The findings from this study indicate that a record of an incident COPD diagnosis may not accurately reflect the timing of initial care for COPD. For future studies, use of the first diagnosis code in the record plus the recording of COPD pharmacotherapy and/or an event indicative of a possible exacerbation may provide more accurate timing of COPD occurrence.
Results from this study also demonstrate that there are identifiable COPD clinical factors and co-morbid conditions associated with a GP's choice of prescribed pharmacotherapy at diagnosis. At initial COPD diagnosis, prescriptions varied by severity of airflow limitation, MRC dyspnea score, history of asthma, and events indicative of possible exacerbations in the previous year. Compared with patients prescribed other pharmacotherapies, patients prescribed a SABD only had lower levels of airflow limitation and dyspnea. Patients prescribed a LAMA or triple therapy had more dyspnea; thus, the LABD component was ≠prescribed to provide bronchodilation and treat symptoms. Patients prescribed LABA+ICS or triple therapy more often had a history of asthma and events indicative of possible exacerbations. Prescription of ICS-containing pharmacotherapy may represent treatment of concurrent/historical asthma and the prevention of exacerbations in patients with previous exacerbations. Multivariate analyses confirmed these findings.
GPs’ choice of prescribed pharmacotherapy may not be consistent with the GOLD strategy. The 2006 GOLD strategy, relevant to this study period, suggests the use of additive pharmacotherapy for COPD with increasing GOLD stage (Citation16). The 2006 GOLD recommendations suggested prescription of the following pharmacotherapies: as-needed SABD pharmacotherapy for GOLD stage I; SABD plus an LABD when needed for GOLD stage II; an additional ICS if there are repeated exacerbations for GOLD stage III; and additional oxygen therapy if there is chronic respiratory failure for GOLD stage IV. In this study, about 45% of patients with GOLD stage I or II of airflow limitation, and 62% with GOLD stage III or IV of airflow limitation, were prescribed an LABD, either alone or in combination. The 2006 GOLD strategy suggests that all patients with GOLD stages III and IV are prescribed at least one LABD when needed, implying a 37% non-adherence rate to the strategy in this study.
In addition, about 40% of patients with GOLD stage I or II airflow limitation were prescribed an ICS, whereas the 2006 GOLD strategy suggests that an ICS is ≠introduced at GOLD stage III if patients are experiencing repeated exacerbations. It has been recognized for many years that many physicians do not adhere to prescribing guidelines for patients with COPD (Citation26). Consistent with other published studies on guideline non-adherence, these results indicate that many GPs may not follow the 2006 GOLD strategy (5, 6, 21, 27–30). Patients with late-stage COPD are likely to have more symptoms and exacerbations, and GPs may consider these factors when choosing pharmacotherapy.
The reasons for non-adherence to guidelines may be manifold and include lack of familiarity, lack of awareness, lack of time, and disagreement with the guidelines (Citation31). A healthcare utilization study of patients diagnosed with COPD between 2000 and 2005 in the US showed that treatment practices were not in line with the GOLD strategy but correlated with the number of previous respiratory healthcare visits (Citation32). Guidelines are not mandatory and although GPs may use them for information, situations in clinical practice may require departure from guidelines, allowing treatment decisions as part of a holistic approach to patient care, alongside other clinical factors and preferences.
In the 2006 GOLD strategy, guidance on pharmacotherapy is based mainly on spirometric classification (Citation16). The 2010 NICE guideline 101 (Citation4), which has been adopted by the British Thoracic Society, and the 2011 (Citation33) and 2013 (Citation1, Citation4) GOLD strategies use the same ≠spirometric classification of airflow obstruction as GOLD 2006 (Citation16), but take into account previous exacerbations and assessment of symptoms or health status, including breathlessness, to reduce the risk of future exacerbations when considering pharmacotherapy (Citation16). A comparison of how the different guidelines define the patient groups recommended for the prescription of each pharmacotherapy category is shown in . Although this study was based on data recorded before the latest updates of the GOLD strategy, prescribing recommendations were not based solely on degree of airflow limitation, but also accounted for MRC dyspnea score, history of exacerbations, and asthma history, as do the 2010 NICE guidelines and the 2011 and 2013 GOLD strategies.
Table 4. First choice COPD pharmacotherapy recommendations from the 2010 NICE clinical guideline 101 (4) and the 2006 (4,16), 2011 (33) and 2013 (1,4) GOLD strategies
A limitation of the GPRD data is that the data are recorded electronically by healthcare providers in the primary care setting only. Thus, pharmacotherapies prescribed and outcomes recorded in hospital may be under-reported (Citation34). Future studies could use linked Hospital Episode Statistics data to improve data capture for hospitalized events, such as severe COPD exacerbations. GPs often receive communications from specialists, discharge summaries from hospitals, and test results from pathology laboratories in hard copy, and the GP must manually enter these into the electronic database. Therefore, it is likely that only test results requiring additional follow-up or clearly refuting a differential diagnosis are entered into the GPRD (Citation34). Lastly, the data analyzed are based on linked prescriptions written for pharmacotherapies of interest, rather than pharmacotherapy dispensed or administered, which could lead to exposure misclassification.
Conclusions
Most patients with a new record of a COPD diagnosis between January 2008 and December 2009 in the GPRD were prescribed a maintenance pharmacotherapy within 3 months of diagnosis. Many patients received previous prescriptions for COPD pharmacotherapy and were treated to reduce symptoms before the first COPD diagnosis recorded, or were treated based on a prior asthma diagnosis. Thus, there may be a delay in COPD diagnosis recorded in the EMR until the later stages of the disease. GPs prescribed COPD pharmacotherapy according to lung function, but also with consideration of other factors, such as MRC dyspnea score, history of events indicative of possible COPD exacerbations, and asthma history. Although these prescribing patterns were not entirely concordant with COPD guidelines in place at the time of the study, they are consistent with the 2010 NICE guideline and 2011 GOLD strategy, which include consideration of dyspnea and exacerbations as a more holistic patient assessment (Citation4, Citation33). These findings indicate that there is a need for earlier diagnosis of COPD in patients in the UK to ensure that they are prescribed the most appropriate treatment. Future studies should assess ≠factors associated with the delay in COPD diagnosis and barriers to adherence to updated clinical guidelines to inform interventions aimed at improving care and outcomes in patients with COPD.
Declaration of Interest Statement
All authors are employees of GlaxoSmithKline R&D and own stocks and shares of GlaxoSmithKline Plc.
This study was funded by GlaxoSmithKline Research and Development (R&D) (Protocol WEUSKOP5904).
Editorial support was provided by Jane Davies, ≠Stephen Moore, and Carol Cooper of Caudex Medical (supported by GlaxoSmithKline Plc).
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (updated). 2013. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
- Antus B. Pharmacotherapy of chronic obstructive pulmonary disease: a clinical review. ISRN Pulmonology 2013; 2013.
- Qaseem A, Wilt TJ, Weinberger SE, Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3):179–191.
- NICE. Management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update). 2010. Available from: http://www.nice.org.uk/nicemedia/live/13029/49397/49397.pdf. Accessed: 2012 December 3.
- Diette GB, Orr P, McCormack MC, Is pharmacologic care of chronic obstructive pulmonary disease consistent with the guidelines? Popul Health Manag 2010; 13(1):21–26.
- Fitch K, Iwasaki K, Pyenson B, Variation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysis. Curr Med Res Opin 2011; 27(7):1425–1429.
- Jochmann A, Neubauer F, Miedinger D, General practitioner's adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly 2010; 21 Apr.
- Perez X, Wisnivesky JP, Lurslurchachai L, Barriers to adherence to COPD guidelines among primary care providers. Respir Med 2012; 106(3):374–381.
- Salinas GD, Williamson JC, Kalhan R, Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis 2011; 6:171–179.
- The Royal College of Physicians of London, British Thoracic Society, British Lung Foundation. Report 5 of The National Chronic Obstructive Pulmonary Disease Audit 2008: survey of COPD care within UK General Practices. London, UK: Royal College of Physicians; 2008. Report No. 5.
- Heins-Nesvold J, Carlson A, King-Schultz L, Patient identified needs for chronic obstructive pulmonary disease versus billed services for care received. Int J Chron Obstruct Pulmon Dis 2008; 3(3):415–421.
- Aisanov Z, Bai C, Bauerle O, Primary care physician perceptions on the diagnosis and management of chronic obstructive pulmonary disease in diverse regions of the world. Int J Chron Obstruct Pulmon Dis 2012; 7:271–282.
- Soriano JB, Maier WC, Visick G, Validation of general practitioner-diagnosed COPD in the UK General Practice Research Database. Eur J Epidemiol 2001; 17(12):1075–1080.
- Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 1991; 302(6779):766–768.
- NHS Employers. Quality and outcomes framework guidance for GMS contract 2009/10. March 2009. Available from: http://www.nhsemployers.org/aboutus/publications/documents/qof_guidance_2009_final.pdf. Accessed: 2012 December 3.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2006. Available from: http://www.who.int/respiratory/copd/GOLD_WR_06.pdf. Accessed: 2013 June 17.
- Khan NF, Perera R, Harper S, Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010; 11:1.
- Bestall JC, Paul EA, Garrod R, Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54(7):581–586.
- NICE. MRC dyspnoea scale. 2007. Available from: http://www.nice.org.uk/usingguidance/commissioningguides/pulmonaryrehabilitationserviceforpatientswithcopd/mrc_dyspnoea_scale.jsp. Accessed: 2013 January 10.
- Bastin AJ, Starling L, Ahmed R, High prevalence of undiagnosed and severe chronic obstructive pulmonary disease at first hospital admission with acute exacerbation. Chron Respir Dis 2010; 7(2):91–97.
- Cazzola M, Segreti A, Bettoncelli G, Change in asthma and COPD prescribing by Italian general practitioners between 2006 and 2008. Prim Care Respir J 2011; 20(3):291–298.
- Minas M, Hatzoglou C, Karetsi E, COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim Care Respir J 2010; 19(4):363–370.
- Jones PW, Brusselle G, Dal Negro RW, Patient-centred assessment of COPD in primary care: experience from a cross-sectional study of health-related quality of life in Europe. Prim Care Respir J 2012; 21(3):329–336.
- Greening NJ, Evans RA, Williams JE, Does body mass index influence the outcomes of a Waking-based pulmonary rehabilitation programme in COPD? Chron Respir Dis 2012; 9(2):99–106.
- Bischoff EW, Schermer TR, Bor H, Trends in COPD prevalence and exacerbation rates in Dutch primary care. Br J Gen Pract 2009; 59(569):927–933.
- Roche N, Lepage T, Bourcereau J, Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary disease. Eur Respir J 2001;18(6):903–908.
- Asche CV, Leader S, Plauschinat C, Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis 2012; 7:201–209.
- Bourbeau J, Sebaldt RJ, Day A, Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J 2008; 15(1):13–19.
- Chavez PC, Shokar NK. Diagnosis and management of chronic obstructive pulmonary disease (COPD) in a primary care clinic. COPD 2009;6(6):446-451.
- Cazzola M, Bettoncelli G, Sessa E, Primary care of the patient with chronic obstructive pulmonary disease in Italy. Respir Med 2009; 103(4):582–588.
- Desalu OO, Onyedum CC, Adeoti AO, Guideline-based COPD management in a resource-limited setting - physicians’ understanding, adherence and barriers: a cross-sectional survey of internal and family medicine hospital-based physicians in Nigeria. Prim Care Respir J 2013; 22(1):79–85.
- Seaman J, Leonard AC, Panos RJ. Health care utilization history, GOLD guidelines, and respiratory medication prescriptions in patients with COPD. Int J Chron Obstruct Pulmon Dis 2010; 5:89–97.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease (revised). December 2011. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed: 2012 December 3.
- GPRD database. August 2010. Available from: http://www.itmat.upenn.edu/ctsa/acard/docs/all_Description_GPRD_FINAL.pdf. Accessed: 2013 January 10.

