Abstract
Electrospinning uses an electrical charge to draw very fine (typically on the micro or nano scale) fibers from a liquid. Electrospinning or electrostatic spinning shares characteristics of both electrospraying and conventional solution dry spinning of fibers.
The method does not need the use of coagulation chemistry or high temperatures to produce solid threads from solution. This makes the process particularly suited for the production of fibers using large and complex molecules. Because the full potential of biomaterials being used in various applications, field of nanofibers have involved considerable interest in biotechnology and medicine and there has been fast development in this area in recent years.
Introduction
Electrostatic spinning or electrospinning is a well-known established process that is able to fabricate non-woven and ultrafine nanoscale fibers with diameters tens of nanometers to microns which can be evaluated by usual non-woven fiber fabrication techniques. Electrospinning technology was coined in the 1990s by Reneker and co-workers.
The electrospinning technique possesses the unique features of simplicity, affordability, wide range of materials selection, very high surface-to-volume ratio, tunable porosity, and flexibility to adopt over a broad range of sizes and shapes.
Nanofibrous materials are being studied and developed because they embrace considerable promise for variety of applications and achieve some advantages of nanostructured materials. Nanofibrous materials can be synthesized of biocompatible and biodegradable polymers and produced by electrospinning processes. Because of the full potential of using biomaterials in different applications, field of nanofibers have attracted considerable interest in biotechnology and medicine and there has been fast development in this area in recent years.
Methods for fabrication of nanofibers
There are many ways to fabricate nanofibers, such as template synthesis (CitationLi and Xia 2004, CitationReneker and Chun 1996, CitationDoshi and Reneker 1995, CitationLiang et al. 2007), drawing (CitationZhong et al. 2011, CitationLu et al. 2005), self-assembly (CitationWilliamson and Coombes 2004, CitationNaik et al. 2003), electrospinning (CitationBadami et al. 2006, CitationBhattarai et al. 2005, CitationKweon et al. 2003, CitationYang et al. 2005, CitationZhang et al. 2005, CitationChoi et al. 2004) (random, aligned, and core-shell nanofibers), and phase separation (CitationMa and Zhang 1999, CitationWidmer et al. 1998). Because template synthesis does not able to produce continuous fibers and in drawing process only viscoelastic materials can be used which tolerate applied tensions, the three most important methods to produce nanofibers are self-assembly, electrospinning, and phase separation (CitationIto et al. 2005).
Phase separation
One of best method for production of nanoporous foams which preferentially can be used in many areas is Phase separation, but because of the long time needed to complete the entire process, this method is not the best (CitationAshammakhi et al. 2007).
The polymer solution quenched below the freezing point of solvent is freeze-dried to produce a porous structure (CitationSchugens et al. 1996). Various nanoporous foams are easily obtained through this process by modifying thermodynamic and kinetic factors. Using phase separation process, fabrication of foam scaffolds occur in five basic steps: suspension of polymer, phase separation and gelation, extraction of solvent from the gel by means of water, freezing, and then freeze-drying under vacuum (CitationMa and Zhang 1999). Influences of nanoporous morphology is determined via gelation. The creation of nanoscale fiber complex is caused by low gelation temperature, while as a consequence of crystals nucleation and their development, high gelation temperature produces the creation of platelet-like construction and is managed by increasing of cooling rate, which can produce uniform nanofibers (CitationVenugopal et al. 2008).
Self-assembly
In this method, molecules and atoms sort out and assemble themselves in the course of fragile and non-covalent forces, for example hydrophobic forces, electrostatic interactions and hydrogen bonding, and create a stable construction (CitationHartgerink et al. 2001, CitationZhang 2003). Self-assembly method can be used to make different structures, for example unilamellar and multilamellar vesicles, bilayer, nanoparticles, membranes, fibers, films, micelles, tubes and capsule (CitationVenugopal et al. 2008).
Based on the self-assembly system, an amphiphilic peptide that allows creation of thermally stable protein was designed (CitationBerndt et al. 1995).
Obtained fiber with self-assembly method can be much thinner than those produced by electrospinning, but complication of procedure with low productivityare the major problem associated with self-assembly method (CitationMa et al. 2005).
Electrospinning
Electrospinning possesses unique properties such as simplicity, affordability, high porosity (good pore size distribution), and yields continuous fibers. In this method variety of biomaterials can be used to produce nanofibers and very low amounts of initial solutions are needed.
Fibers produced using electrospinning method have the diameter in range of 3 nm to several micrometers, whereas fibers obtained using other procedures have the diameter in range of 500 nm up to a few microns (CitationZhang et al. 2005).
Because of these extremely appreciable properties, electrospinning is a most popular technique for the production of nanofibers. Nanofibers that fell on the stationary collector harvests randomly arranged nanofiber (125–600 nm) matrices, although aligned nanofiber (750–850 nm) mats are synthesized by means of rotatory or disk collector with high-pitched edge (CitationVenugopal et al. 2008).
Electrospinning machine and synthesis of nanofibers using electrospinning process
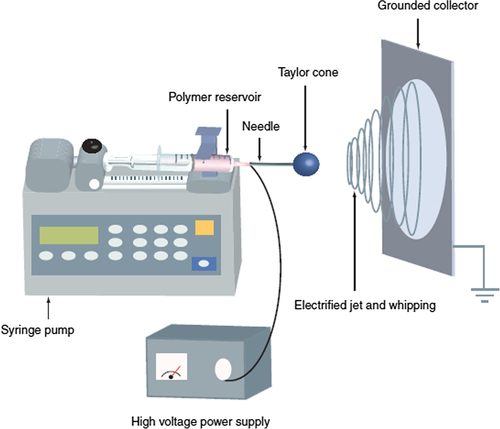
The standard electrospinning machine consists of spinneret (or needle), high-voltage power supply with a wide range of voltage, a glass syringe with a small needle, and a metal collector (). Electrospinning nanofibers can be synthesized using an electrical potential to a polymeric solution. Needle attached to and driven by a syringe pump which is accustomed to manage the flow rate and volume of the polymer is ejected. A polymer solution is loaded into the syringe that ejects the polymer solution at a constant rate (CitationMerritt et al. 2012). After loading of polymer solution, solution is charged and at the tip of the syringe an electrically charged polymer droplet is formed. Because of the repulsive force between the similar charges in electrically conductive liquid and electric field, polymer solution tends to deform the droplet into a conical-shaped structure known as Taylor cone, Taylor who has made essential studies on the jet formation (CitationTaylor 1969). Initially increase of electrical potential causes elongation of semicircular outward of solution at the tip of the needle and forms the Taylor cone. After a threshold charge density, cone becomes unstable and emits a jet of liquid (CitationHuang et al. 2003). In the presence of an electric field, the jet travels a path to the ground and as a consequence of elongation, solvent evaporation forms a continuous slim liquid fiber (CitationFrenot and Chronakis 2003, CitationReneker et al. 2000), and finally charged electro-spun fibers are collected on the Collector. The electrode plate or Collector electrode are located on a place made of acrylic acid. The electrode is usually flatted and is used for the collection of both random and aligned fibers (CitationHuang et al. 2003, CitationBaji et al. 2010, CitationPark et al. 2007).
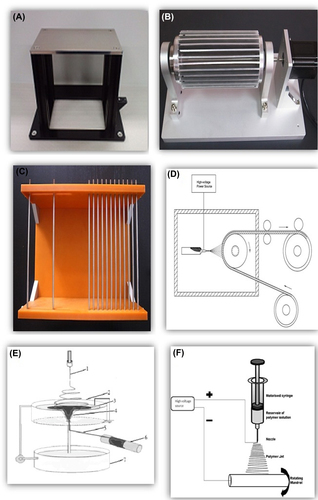
In the field of electrospinning and nanofiber production, there are two main obsession and key point; choosing polymer and collector. The alignment of the fibers () affected by the orientation of the collector and sets the morphology and the properties of synthesized nanofibers. There are different types of collectors such as plate collector (CitationTan et al. 2005), rotatory collector (CitationTeo and Ramakrishna 2006), grid-type collector (CitationTeo et al. 2011), edge-type collector (CitationChaurey et al. 2010), collector with parallel electrode (CitationKawahara et al. 2008), collector with blade auxiliary electrode (CitationJianrui et al. 2009), water bath collector (CitationPolaskova et al. 2013), continuous collector and other ().
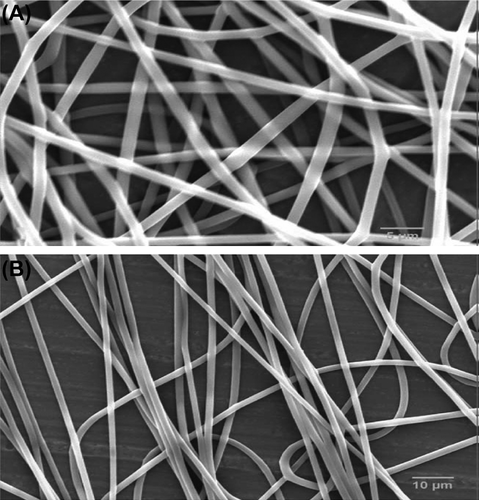
There are many biodegradable polymers can be used to produce nanofibers such as natural, synthetic, and composite of the two. Biodegradable polymers were used to develop nanofibers with different functions based on the requirement. Some can be used to deliver temporary function such as agent delivery, cell carrier, and short-time scaffolds (until new tissue become mature and independent). In this group, polymer will replaced by native tissue. Some polymers have been used for long-term purpose such as an implant in surgery. At some point in the procedure of electrospinning using synthetic polymers, the charge on the polymer solution makes it promising to govern its trajectory using an electric field (CitationHohman et al. 2001).
The most commonly used and studied synthetic polymers are PCL (CitationLuong-Van et al. 2006, CitationKhil et al. 2005, CitationVenugopal et al. 2005, CitationYoshimoto et al. 2003, CitationZeng et al. 2003, CitationBölgen et al. 2005), PLDLA (CitationCui et al. 2006, CitationZong et al. 2002), PLLA (CitationBadami et al. 2006, CitationChew et al. 2005, CitationYang et al. 2004, CitationZong et al. 2005), PLGA (CitationBadami et al. 2006, CitationChew et al. 2005, CitationYang et al. 2004, CitationZong et al. 2005, CitationLi et al. 2002, Citation2003, CitationLiang et al. 2005), and copolymers such as PCL-PEG, PCL-PLLA (CitationNikkola et al. 2005, CitationXu et al. 2004), PLGA-PEG, PLLA-PEG, and etc.
At some point in the procedure of electrospinning of synthetic polymers, the charge on the polymer solution makes it promising to govern its trajectory using an electric field (CitationJukola et al. 2008).
Studied Polymer with natural essence have been used to produce nanofibers, have drawn increasing research interests, including elastin(CitationBoland et al. 2004), collagen (CitationVenugopal et al. 2005, CitationHuang et al. 2001, CitationShields et al. 2004), silk protein (CitationJin et al. 2004, CitationKim et al. 2003, CitationMin et al. 2004), tropoelastin(CitationLi et al. 2005), elastin-mimetic peptide (CitationHuang et al. 2000), fibrin(CitationJukola et al. 2008, CitationTuzlakoglu et al. 2005), fibrinogen(CitationSindelar et al. 2006, CitationWnek et al. 2003), oxidized cellulose (CitationSon et al. 2004), and hyaluronic acid (CitationUm et al. 2004).
Furthermore, bio-corrosion of intense polymers is based on the factors such as fluctuations in pH, that is, pH-responsive polymers have also been planned (CitationPiras et al. 2006).
Blends of synthetic polymer and polymer with natural essence were also used for merging properties of both.
Studied merging polymer involved gelatin-loaded PCL (CitationMa et al. 2005), collagen-loaded PLLA-PCL (CitationHe et al. 2005), composites of PEO and silk (CitationLi et al. 2006), composites of PLLA-PCL and collagen (CitationHe et al. 2005), composites of PCL and starch (CitationUm et al. 2004), composites of hyaluronic acid and PCL (CitationYang et al. 2006),composites of PLGA with PHBV (CitationZhu et al. 2009), and composites of PLGA, elastin and collagen (CitationStitzel et al. 2000).
The method of electrospinning is influenced by two groups of factors, system factors and process factors. System factors, for example distribution and polymer molecular weight, control the proportion of degradation of nanofibers, while other system factors such as polymer solution rate, that is viscosity, outward rigidity, and conductivity, govern the nanofiber thickness and decrease the possibility for globule creation. Process factors, for example orifice thickness, flow proportion of polymer, and electric potential, impact fiber diameter, whereas other process factors, for example space between needle and collector, govern the range of solvent evaporation within nanofibers and fall on the collector, while, gesture of collector governs the form of fiber throughout fiber fall (CitationZong et al. 2002, CitationShin et al. 2001).
One of the best advantages of polymer with natural essence is similarity and is identical to some molecular substances that exist in the human body.
One disadvantage of polymer with natural essence can be their reduced mechanical properties when isolated, thus this polymer requires additional processing for handling.
Two main classes of matrix proteins in the extracellular matrix (ECM) of human body are composed of proteoglycans and fibrous proteins. In the human body fibrous proteins, depending on tissue type have fiber diameter with ranging between 50 and 150 nm (CitationElsdale and Bard 1972, CitationKadler 2004).
Polymers with natural essence that are used as biomaterials or scaffolds for tissue engineering are cellulose, gelatin, fibrin, fibrinogen, chitosan, chitin, elastin, hyaluronic acid, collagen, and silk. Fabrication of this material into scaffolds for tissue engineering may possibly convey new possessions to biomaterials. Biomaterials produced with this polymer are mechanically stronger, physically lighter and more porous, optically more tunable optical emission, chemically more reactive or less corrosive, electrically more conductive and magnetically more paramagnetic (CitationHuang et al. 2001, CitationWest and Halas 2000).
Electrospinning techniques are used for tissue engineering and mimicking of the size and morphology of natural ECM and design of collagen nanofibrous scaffolds. By this way, nanofiber of type I and III collagen were produced which can mimic properties of natural collagen (CitationMatthews et al. 2002).
Electrospinning can be used for production of nanofibrous scaffolds which mimic the natural fibrous structure in human body and regeneration of blood vessel,(CitationVenugopal et al. 2005) bones, (CitationFujihara et al. 2005) dermis, (CitationVenugopal et al. 2005, CitationVenugopal and Ramakrishna 2005), and nerve (CitationYang et al. 2004).
Composition of polycaprolactone with collagen nanofiber were produced as a goal of flexibility, elasticity and consequently promising way for the creation of smooth muscle tissues for engineering of blood vessel (CitationVenugopal et al. 2005).
Wnek et al. using fibrinogen produced a nanofiber scaffolds for wound dressing or hemostatic products (CitationWnek et al. 2003).
Gelatin promotes cell adhesion, migration and form a polyelectrolyte complex because contains Arg-Gly-Asp (RGD)-like sequence. Blends of gelatin and chitosan improve the biological and cellular activity and this composition was tested in restoring various tissues including skin, cartilage, and bone (CitationBhattarai et al. 2005, CitationHuang et al. 2005).
Coaxial electrospinning
Fabrication of core-shell nanofibers can be provided using coaxial electrospinning (also known as co-electrospinning) technique and is an adjustment or extension to the traditional electrospinning technique. As compared to traditional electrospinning, coaxial electrospinning () makes use of complex spinneret (needle) which consists of a multiple solution feed system with one or more inner channels shelled by an outer tube which is required for the injection of one material into another at the tip of the spinneret and collected into a core-sheath–structured composite fiber (CitationSun et al. 2003) to generate composite nanofibers with core-shell structures ().
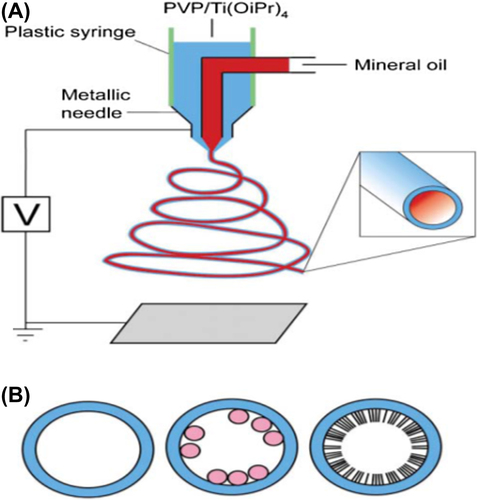
Many factors can have effect on the entrapment of components in the inner channels such as viscoelasticity of the two solutions, interfacial tension and feeding rate of the inner and outer fluids (CitationSun et al. 2003, CitationChakraborty et al. 2009).
There are variety of novel and functional polymeric nanofibers from coaxial electrospinning, such as basic bi-component nanofiber, surface-coated/-modified nanofiber, nanocomposite nanofiber, and hollow nanofibers (CitationZhang et al. 2007).
Recently, coaxial electrospinning has achieved greater than before popularity in the protein delivery field because the fabricated core-shell fibers have great potential in maintaining proteins at some point in the electrospinning procedure and it provides uniform protein distribution throughout the fibers, and proteins have potential to be delivered in an organized manner as a result of the shell blockade.
Biomedical application
Tissue engineering
One of the main areas of research in biomedical application is tissue engineering. Electrospinning is a very efficient method for production of nanofiber scaffolds. Many different types of scaffolds were produced for tissue engineering and organ regeneration, such as skin, cartilage, bone, collagen, dentin and liver (). Nanofibers have been used in making these scaffolds using both natural and synthetic polymer electrospuns. These scaffolds are used to regenerate, replace, and repair the tissue and therefore need to be well designed and must have dimensional equality.
Table I. Example of nanofiber application in tissue engineering.
As mentioned above, nanofiber scaffolds produced using electrospinning, have many good properties which require for tissue engineering such as biodegradability, large surface area, ability to maintain structural integrity with tissue, high porosity, high-quality mechanical properties, and non-toxicity to cell (CitationElsdale and Bard 1972).
Using of nanofibers composite materials, which are similar to ECM proteins such as collagen and glycosaminoglycans, can support and improve cell function and cell–cell or cell–ECM attachment. Therefore, nanofiber scaffolds produced by these materials would improve the nanofiber efficiency.
Studies have shown that thinner fibers with sizes ranging between 60 and 200 nm can increase proliferation, osteoblast adhesion, alkaline phosphatase activity, and ECM secretion on carbon nanofibers (CitationWebster et al. 1999).
Because of unique properties of core-shell nanofibers including versatility, potential for encapsulation of biological molecules, and nanocomposites as well as potential for modifying the surfaces of electrospun fibers, it can be used for tissue engineering. Another important factor that can improve nanofibers for tissue engineering is modifying the electrical and mechanical properties of the nanofibers and it is achieved by integration of nanofiber scaffolds with wall carbon nanotubes (SWNT).
The purpose and concept of drug delivery methods is to deliver a predetermined amount of drug correctly, efficiently, tissue or cell specific and for a defined period of time. Drug delivery through electrospun nanofiber has been mainly functional and useful for tissue engineering. Accordingly, dissolution rate of drug can be increased by enlargement surface area of the drug and the corresponding carrier ().
Table II. Example of nanofiber application in delivery.
Low solubility and instability make hydrophobic agents difficult to have continued release of active molecules with appropriate concentration within a satisfactory period of time.
Using of electrospun nanofiber scaffolds can be delivered both via viral and nonviral nucleic acids.
Degree of success in viral gene delivery can be determined by the parameters such as gene structure, the type of cells and viruses, and type of delivery technique.
As propose of in situ viral delivery of genes, it is necessary to develop and use of novel and more efficient carriers, for the most part polymeric carriers.
As compare to viral vectors, nonviral gene delivery and vectors have appropriate properties such as low toxicity and potential for using of large DNA with varying sizes (CitationLiao and Leong 2011).
Electrospun nanofibers have been used as scaffolds for delivery of nucleic acids (e.g., DNA and siRNA) because of owning appropriate properties such as high porosity, high surface area, interrelated pores beneficial for oxygen/nutrient transferal and unfastened bonding between fibers favorable for cell migration and infiltration (CitationYang et al. 2011) and cell adhesion/proliferation (CitationZou et al. 2012).
Improper encapsulation and transfection efficiency is one of the unsatisfactory results among the diverse techniques of blending DNA with an electrospun nanofiber scaffolds. In order to overcome this low encapsulation and transfection efficiency were tested incorporation of DNA-loaded particles into core-shell nanofibers, (CitationSaraf et al. 2010, CitationLiao et al. 2009) nanofibers, (CitationNie and Wang 2007) and surface modification (CitationKim and Yoo 2010). Another group of molecules that can be delivered using of nanofiber scaffold delivery system are growth factors or GFs which can regulate biological processes by regulating migration, proliferation, and differentiation of cells, transferring signals between cells and their ECM and by this means enhance tissue regeneration (CitationChen et al. 2010). Therefore, the incorporation of GFs with ECM-mimicking scaffolds possibly will be advantageous for tissue regeneration and other proposes (CitationTabata 2000).
Scientists achieve the controlled release of GFs but the instability of GFs hampers the thriving improvement of GF-loaded tissue-engineered scaffolds.
Various techniques were applied for GF incorporation into nanofibrous scaffolds, such as coaxial electrospinning, (CitationLiao and Leong 2011) specific or nonspecific surface modifications, (CitationZomer Volpato et al. 2012) blending, (CitationZhang et al. 2012) emulsion electrospinning (CitationTian et al. 2012, CitationYang et al. 2011) and these yielded varied levels of success.
Burst release of EGF was obtained from silk nanofibers blended with EGF, due to the hydrophobic nature of EGF (CitationSchneider et al. 2009). Different substances were used for conjugation with GFs such as polysaccharides (CitationMottaghitalab et al. 2011) and heparin (CitationZou et al. 2011).
Wound dressings
Wound dressings give a hand in shielding the wound from external microorganisms, absorbing exudates, accelerate the wound-healing process, and lastly improving surface manifestation (CitationZhang et al. 2005, CitationKhil et al. 2003).
Up till now, bioactive wound dressing materials, which typically necessitated in the initial period of wound healing incorporated with antibiotic, have been introduced such as foams, sponges, hydrogels, and films (CitationJannesari et al. 2011).
Electrospun nanofibers have great facility for wound dressing () because of owning special characteristics, for example high surface area, and as a result electrospun nanofibers can professionally suck up exudates and regulates the wound humidity (CitationKhil et al. 2003). The porosity of nanofibrous can directly impact on wound dressing because high porosity scaffold effectively contributes to air permeability and provide required oxygen for cell respiration, but small porosity contributes to preserving the wound from bacterial infections.
Table III. Example of nanofiber application in wound dressings.
Two main requirements for completely covering problematical wounds are improved hemostasis and more flexibility in dressing, which are achievable through nanofibrous dressings. Furthermore, as an esthetic point of view, nanofibers provide the better-quality advantage of scar-free regeneration (CitationTian et al. 2012, CitationBoateng et al. 2008).
Cancer therapy
Administration of anticancer drugs (both orally and intravenously) may have some disadvantages such as low efficacy, poor solubility, low instability, side effects on healthy tissues, need for several injection, and high removal rate by the reticuloendothelial system (CitationShao et al. 2011, CitationXie et al. 2010).
Scientists have been exploring many methods in order to improve a minimized unwanted side effects to healthy tissues, maximized efficiency, and extended period of function such as restricted and continued postsurgical drug delivery (CitationPradilla et al. 2006).
Blends of anticancer drugs with electrospun nanofiber scaffolds can cover up such a disadvantages and it can easily insert to the solid tumor site (). This can provide high local dosage with incorporation of small amounts of the drug but also reduces the need for frequent administrations, and therefore provide patient convenience.
Table IV. Example of nanofiber application in cancer therapy.
Conclusion
Nanofibrous materials can be synthesized of biocompatible and biodegradable polymers and produced using electrospinning processes. Continuous production of electrospun nanofibers webs with high efficiency and discontinuous production of nanofiber webs from very small amount of liquid (one droplet) for very expensive polymers usage. Production of composite materials consisting of electrospun layers with incorporated powder between nanofibers or inside nanofibers. Production of hybrid yarns—classical base yarn covered by electrospun nanofibers and is protective.
Nanofibers have applications in medicine, including artificial organ components, tissue engineering, implant material, drug delivery, wound dressing, and medical textile materials.
Authors’ contributions
AE conceived of the study and participated in its design and coordination. AA assisted in the numerical calculations. HD participated in the sequence alignment and drafted the manuscript. AA supervised the whole study. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Department of Medical Nanotechnology, and Biotechnology Faculty of Advanced Medical Science of Tabriz University for all supports provided.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ashammakhi N, Ndreu A, Yang Y, Ylikauppila H, Nikkola L, Hasirci V. 2007. Tissue engineering: a new take-off using nanofiber-based scaffolds. J Craniofac Surg. 18:3–17.
- Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. 2006. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly (lactic acid) substrates. Biomaterials. 27:596–606.
- Baji A, Mai Y-W, Wong SC, Abtahi M, Chen P. 2010. Electrospinning of polymer nanofibers: effects on oriented morphology, structures and tensile properties. Compos Sci Technol. 70:703–718.
- Berndt P, Fields GB, Tirrell M. 1995. Synthetic lipidation of peptides and amino acids: monolayer structure and properties. J Am Chem Soc. 117:9515–9522.
- Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. 2005. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 26:6176–6184.
- Boateng JS, Matthews KH, Stevens HN, Eccleston GM. 2008. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 97:2892–2923.
- Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. 2004. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 9:1422–1432.
- Bölgen N, Menceloğlu YZ, Acatay K, Vargel I, Pişkin E. 2005. In vitro and in vivo degradation of non-woven materials made of poly (ϵ-caprolactone) nanofibers prepared by electrospinning under different conditions. J Biomater Sci Polym Ed. 16:1537–1555.
- Bourget J-M, Guillemette M, Veres T, Auger FA, Germain L. 2013. Alignment of Cells and Extracellular Matrix Within Tissue-Engineered Substitutes. In: Pignatello R, ed. Advances in Biomaterials Science and Biomedical Applications. Available from: http://www.intechopen.com/books/advances-in-biomaterials-science-and-biomedical-applications/alignment-of-cells-and-extracellular-matrix-within-tissue-engineered-substitutes.
- Cao H, Jiang X, Chai C, Chew SY. 2010. RNA interference by nanofiber-based siRNA delivery system. J Control Release. 144:203–212.
- Chakraborty S, Liao IC, Adler A, Leong KW. 2009. Electrohydrodynamics: a facile technique to fabricate drug delivery systems. Adv Drug Deliv Rev. 61:1043–1054.
- Chaurey V, Chiang PC, Polanco C, Su YH, Chou CF, Swami NS. 2010. Interplay of electrical forces for alignment of sub-100 nm electrospun nanofibers on insulator gap collectors. Langmuir. 26:19022–19026.
- Chen F-M, Zhang M Wu Z-F. 2010. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 31:6279–6308.
- Chen M, Gao S, Dong M, Song J, Yang C, Howard KA, et al. 2012. Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery. ACS Nano. 6:4835–4844.
- Chen P, Wu QS, Ding YP, Chu M, Huang ZM, Hu W. 2010. A controlled release system of titanocene dichloride by electrospun fiber and its antitumor activity in vitro. Eur J Pharm Biopharm. 76:413–420.
- Chew SY, Wen J, Yim EK, Leong KW. 2005. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 6:2017–2024.
- Choi JS, Lee SW, Jeong L, Bae SH, Min BC, Youk JH, Park WH. 2004. Effect of organosoluble salts on the nanofibrous structure of electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Int J Biol Macromol. 34:249–256.
- Collector with parallel electrode. 2014. Available at: http://www.electro-spinning.com/Collectors_for_electrospun_nanofibers.html.
- Continuous collector. 2014. Available at: http://www.electro-spinning.com/Collectors_for_electrospun_nanofibers.html.
- Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. 2006. Investigation of drug release and matrix degradation of electrospun poly (DL-lactide) fibers with paracetanol inoculation. Biomacromolecules. 7:1623–1629.
- Doshi J, Reneker DH. 1995. Electrospinning process and applications of electrospun fibers. J Electrostat. 35: 151–160.
- Elsdale T, Bard J. 1972. Collagen substrata for studies on cell behavior. J Cell Biol. 54:626–637.
- Frenot A, Chronakis IS. 2003. Polymer nanofibers assembled by electrospinning. Curr Opin Coll Interface Sci. 8:64–75.
- Fujihara K, Kotaki M Ramakrishna S. 2005. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers.Biomaterials. 26:4139–4147.
- Hartgerink JD, Beniash E, Stupp SI. 2001. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 294:1684–1688.
- He W, Yong T, Teo WE, Ma Z, Ramakrishna S. 2005. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: potential vascular graft for blood vessel tissue engineering. Tissue Eng. 11:1574–1588.
- Hohman MM, Shin M, Rutledge G, Brenner MP. 2001. Electrospinning and electrically forced jets. II. Applications. Phys Fluids. (1994-present) 13:2221–2236.
- Huang L, McMillan RA, Apkarian RP, Pourdeyhimi B, Conticello VP, Chaikof EL. 2000. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules. 33: 2989–2997.
- Huang L, Nagapudi K, Apkarian RP, Chaikof EL. 2001. Engineered collagen–PEO nanofibers and fabrics. J Biomater Sci Polym Ed. 12:979–993.
- Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. 2005. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 26:7616–7627.
- Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. 2003. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 63:2223–2253.
- Im JS, Bai BC, Lee Y-S. 2010. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials. 31:1414–1419.
- Ito Y, Hasuda H, Kamitakahara M, Ohtsuki C, Tanihara M, Kang IK, Kwon OH. 2005. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J Biosci Bioeng. 100:43–49.
- Jannesari M, Varshosaz J, Morshed M, Zamani M. 2011. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomedicine. 6:993–1003.
- Jianrui Z, Yurong Y, Shicheng L. 2009. Research progress in collector and auxiliary electrode for electrospinning process. China Synthetic Fiber Industry. 5:15.
- Jin H-J, Chen J, Karageorgiou V, Altman GH, Kaplan DL. 2004. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials. 25:1039–1047.
- Jukola H, Nikkola L, Gomes ME, Reis RL, Ashammakhi N. 2008. Electrospun Starch–Polycaprolactone Nanofiber-Based Constructs for Tissue Engineering. In: Multiscale and functionally graded materials 2006: (M&FGM 2006). AIP Conference Proceedings. Volume 973, pp. 971–974.
- Kadler K. 2004. Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res C Embryo Today. 72:1–11.
- Kawahara Y, Nakayama A, Matsumura N, Yoshioka T, Tsuji M. 2008. Structure for electro–spun silk fibroin nanofibers. J Appl Polym Sci. 107:3681–3684.
- Khil MS, Bhattarai SR, Kim HY, Kim SZ, Lee KH. 2005. Novel fabricated matrix via electrospinning for tissue engineering. J Biomed Mater Res B Appl Biomater. 72:117–124.
- Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. 2003. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res B Appl Biomater. 67:675–679.
- Kim H, Yoo H. 2013. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther. 20:378–385.
- Kim HS, Yoo HS. 2010. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: in vitro and in vivo evaluation. J Control Release. 145:264–271.
- Kim SH, Nam YS, Lee TS, Park WH. 2003. Silk fibroin nanofiber. Electrospinning, properties, and structure. Polym J. 35:185–190.
- Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee HS, et al. 2003. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials. 24:801–808.
- Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. 2005. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 26:1261–1270.
- Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. 2006. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 27:3115–3124.
- Li D, Xia Y. 2004. Electrospinning of nanofibers: reinventing the wheel?Adv Mater. 16:1151–1170.
- Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. 2005. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 26:5999–6008.
- Li WJ, Danielson KG, Alexander PG, Tuan RS. 2003. Biological response of chondrocytes cultured in three–dimensional nanofibrous poly (ϵ–caprolactone) scaffolds. J Biomed Mater Res A. 67: 1105–1114.
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. 2002. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 60:613–621.
- Liang D, Hsiao BS, Chu B. 2007. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv Drug Deliv Rev. 59:1392–1412.
- Liang D, Luu YK, Kim K, Hsiao BS, Hadjiargyrou M, Chu B. 2005. In vitro non-viral gene delivery with nanofibrous scaffolds. Nucleic Acids Res. 33:e170.
- Liao I, Chen S, Liu JB, Leong KW. 2009. Sustained viral gene delivery through core-shell fibers. J Control Release. 139:48–55.
- Liao I, Leong KW. 2011. Efficacy of engineered FVIII-producing skeletal muscle enhanced by growth factor-releasing co-axial electrospun fibers. Biomaterials. 32:1669–1677.
- Liu D, Liu S, Jing X, Li X, Li W, Huang Y. 2012. Necrosis of cervical carcinoma by dichloroacetate released from electrospun polylactide mats. Biomaterials. 33:4362–4369.
- Lu H, Zhang LX, Xing WH, Wang HT, Xu NP. 2005. Preparation of TiO2 hollow fibers using poly (vinylidene fluoride) hollow fiber microfiltration membrane as a template. Mater Chem Phys. 94:322–327.
- Luong-Van E, Grøndahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. 2006. Controlled release of heparin from poly (ϵ-caprolactone) electrospun fibers. Biomaterials. 27:2042–2050.
- Luu Y, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. 2003. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J Control Release. 89:341–353.
- Ma G, Liu Y, Peng C, Fang D, He B, Nie J. 2011. Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohydr Polym. 86:505–512.
- Ma PX, Zhang R. 1999. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 46:60–72.
- Ma Z, He W, Yong T, Ramakrishna S. 2005. Grafting of gelatin on electrospun poly (caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 11:1149–1158.
- Ma Z, Kotaki M, Inai R, Ramakrishna S. 2005. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 11:101–109.
- Matthews JA, Wnek GE, Simpson DG, Bowlin GL. 2002. Electrospinning of collagen nanofibers. Biomacromolecules. 3:232–238.
- Merritt SR, Exner AA, Lee Z, von Recum HA. 2012. Electrospinning and imaging. Adv Eng Mater. 14:B266–B278.
- Min B-M, Lee G, Kim SH, Nam YS, Lee TS, Park WH. 2004. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 25:1289–1297.
- Mo X, Xu CY, Kotaki M, Ramakrishna S. 2004. Electrospun P (LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 25:1883–1890.
- Mottaghitalab F, Farokhia M, Mottaghitalab V, Ziabari M, Divsalar A, Shokrgozara MA. 2011. Enhancement of neural cell lines proliferation using nano-structured chitosan/poly (vinyl alcohol) scaffolds conjugated with nerve growth factor. Carbohydr Polym. 86:526–535.
- Naik S, Chiang AST, Thompson RW, Huang FC. 2003. Formation of silicalite-1 hollow spheres by the self-assembly of nanocrystals. Chem Mater. 15:787–792.
- Ngawhirunpat T, Opanasopit P, Rojanarata T, Akkaramongkolporn P, Ruktanonchai U, Supaphol P. 2009. Development of meloxicam-loaded electrospun polyvinyl alcohol mats as a transdermal therapeutic agent. Pharm Dev Technol. 14:73–82.
- Nie H, Wang C-H. 2007. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Control Release. 120:111–121.
- Nikkola L, Viitanen P Ashammakhi N. 2005. Multi-component implant for true controlled release of diclofenac sodium. In: Sixth International Symposium on Frontiers in Biomedical Polymers, June 16–19, 2005, Hotel Saray, Granada, Spain.
- Parallel-electrodes collector. 2014. Available at: http://www.electro-spinning.com/Collectors_for_electrospun_nanofibers.html.
- Park S, Park K Yoon H Son J Min T Kim G. 2007. Apparatus for preparing electrospun nanofibers: designing an electrospinning process for nanofiber fabrication. Polym Int. 56:1361–1366.
- Piras A, Nikkola L, Chiellini F, Ashammakhi N, Chiellini E. 2006. Development of diclofenac sodium releasing bio-erodible polymeric nanomats. J Nanosci Nanotechnol. 6:9–10.
- Plate collector. 2014. Available at: http://www.mecc.co.jp/en/html/nanon/collector/plate.html.
- Polaskova M, Cermak R, Verney V, Ponizil P, Commereuc S, Gomes MF, et al. 2013. Preparation of microfibers from wood/ionic liquid solutions. Carbohydr Polym. 92:214–217.
- Pradilla G, Wang PP, Gabikian P, Li K, Magee CA, Walter KA, Brem H. 2006. Local intracerebral administration of paclitaxel with the Paclimer® delivery system: toxicity study in a canine model. J Neurooncol. 76: 131–138.
- Reneker DH, Chun I. 1996. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 7:216.
- Reneker DH, Yarin AL, Fong H, Koombhongse S. 2000. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys. 87:4531–4547.
- Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. 2005. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 26:4606–4615.
- Rujitanaroj P-O, Wang YC, Wang J, Chew SY. 2011. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials. 32:5915–5923.
- Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. 2006. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 12:91–99.
- Said SS, Aloufy AK, El-Halfawy OM, Boraei NA, El-Khordagui LK. 2011. Antimicrobial PLGA ultrafine fibers: interaction with wound bacteria. Eur J Pharm Biopharm. 79:108–118.
- Saraf A, Baggett LS, Raphael RM, Kasper FK, Mikos AG. 2010. Regulated non-viral gene delivery from coaxial electrospun fiber mesh scaffolds. J Control Release. 143:95–103.
- Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. 2009. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 5:2570–2578.
- Schugens C, Maquet V, Grandfils C, Jerome R, Teyssie P. 1996. Polylactide macroporous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid–liquid phase separation. J Biomed Mater Res. 30:449–461.
- Shao S, Li L, Yang G, Li J, Luo C, Gong T, Zhou S. 2011. Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int J Pharm. 421:310–320.
- Shields KJ, Beckman MJ, Bowlin GL, Wayne JS. 2004. Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng. 10:1510–1517.
- Shin Y, Hohman M, Brenner MP, Rutledge GC. 2001. Experimental characterization of electrospinning: the electrically forced jet and instabilities. Polymer. 42:09955–09967.
- Sindelar T, Nikkola L, Ashammakhi N, van Griensven M, Redl H. 2006. Electrospinning of fibrinogen nanofibers. Eur Cells Mater. 11:59.
- Son WK, Youk JH Park WH. 2004. Preparation of ultrafine oxidized cellulose mats via electrospinning. Biomacromolecules. 5:197–201.
- Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, et al. 2006. Controlled fabrication of a biological vascular substitute. Biomaterials. 27:1088–1094.
- Stitzel JD, Bowlin GL, Mansfield K, Wnek GE, Simpson DG. 2000. Electrospraying and electrospinning of polymers for biomedical applications. Poly (lactic-co-glycolic acid) and poly (ethylene- co-vinylacetate). In: International SAMPE Technical Conference.
- Sun Z, Zussman E, Yarin AL, Wendorff JH, Greiner A. 2003. Compound core–shell polymer nanofibers by co–electrospinning. Adv Mater. 15:1929–1932.
- Suwantong O, Opanasopit P, Ruktanonchai U, Supaphol P. 2007. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer. 48:7546–7557.
- Tabata Y. 2000. The importance of drug delivery systems in tissue engineering. Pharm Sci Technolo Today. 3:80–89.
- Taepaiboon P, Rungsardthong U, Supaphol P. 2007. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur J Pharm Biopharm. 67:387–397.
- Tan S, Inai R, Kotaki M, Ramakrishna S. 2005. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer. 46:6128–6134.
- Taylor G. 1969. Electrically driven jets. Proc R Soc Lond.313:453–475.
- Teo W, Ramakrishna S. 2006. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 17: R89.
- Teo W-E, Inai R, Ramakrishna S. 2011. Technological advances in electrospinning of nanofibers. Sci Technol Adv Mater. 12:013002.
- Tian L, Prabhakaran MP, Ding X, Kai D, Ramakrishna S. 2012. Emulsion electrospun vascular endothelial growth factor encapsulated poly (l-lactic acid-co-ϵ-caprolactone) nanofibers for sustained release in cardiac tissue engineering. J Mater Sci. 47:3272–3281.
- Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. 2005. Nano-and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med. 16:1099–1104.
- Um IC, Fang D, Hsiao BS, Okamoto A, Chu B. 2004. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules. 5: 1428–1436.
- Venugopal J, Low S, Choon AT, Ramakrishna S. 2008. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 84:34–48.
- Venugopal J, Ma LL, Yong T, Ramakrishna S. 2005. In vitro study of smooth muscle cells on polycaprolactone and collagen nanofibrous matrices. Cell Biol Int. 29:861–867.
- Venugopal J, Ramakrishna S. 2005. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 11:847–854.
- Venugopal J, Zhang Y, Ramakrishna S. 2005. Fabrication of modified and functionalized polycaprolactone nanofibre scaffolds for vascular tissue engineering. Nanotechnology. 16:2138.
- Water bath collector. 2014. Available at: http://www.electro-spinning.com/Collectors_for_electrospun_nanofibers.html.
- Webster TJ, Siegel RW, Bizios R. 1999. Osteoblast adhesion on nanophase ceramics. Biomaterials. 20:1221–1227.
- West JL, Halas NJ. 2000. Applications of nanotechnology to biotechnology: Commentary.Curr Opin Biotechnol. 11:215–217.
- Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, et al. 1998. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 19:1945–1955.
- Williamson MR, Coombes AG. 2004. Gravity spinning of polycaprolactone fibres for applications in tissue engineering. Biomaterials. 25:459–465.
- Wnek GE, Carr ME, Simpson DG, Bowlin GL. 2003. Electrospinning of nanofiber fibrinogen structures. Nano Lett. 3:213–216.
- Xie C, Li X, Luo X, Yang Y, Cui W, Zou J, Zhou S. 2010. Release modulation and cytotoxicity of hydroxycamptothecin-loaded electrospun fibers with 2-hydroxypropyl-β-cyclodextrin inoculations. Int J Pharm. 391:55–64.
- Xu C, Inai R, Kotaki M, Ramakrishna S. 2004. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 25:877–886.
- Xu C, Inai R, Kotaki M, Ramakrishna S. 2004. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 10:1160–1168.
- Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. 2004. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 25:1891–1900.
- Yang F, Murugan R, Wang S, Ramakrishna S. 2005. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 26: 2603–2610.
- Yang F, Xu CY, Kotaki M, Wang S, Ramakrishna S. 2004. Characterization of neural stem cells on electrospun poly (L-lactic acid) nanofibrous scaffold. J Biomater Sci Polym Ed. 15:1483–1497.
- Yang Y, Ylikauppila H, Nikkola L, Ashammakhi N. 2006. Investigation of cell attachment on the scaffolds manufactured by electrospun PCL-hyaluronan blend. 04–08 September 2006, Grenoble, France.
- Yang Y, Li X, Cheng L, He S, Zou J, Chen F, Zhang Z. 2011. Core–sheath structured fibers with pDNA polyplex loadings for the optimal release profile and transfection efficiency as potential tissue engineering scaffolds. Acta Biomater. 7:2533–2543.
- Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, Li X. 2011. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 32:4243–4254.
- Yoshimoto H, Shin YM, Terai H, Vacanti JP. 2003. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 24:2077–2082.
- Yun J, Im JS, Lee Y-S, Kim H-I. 2011. Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. Eur Polym J. 47:1893–1902.
- Zeng J, Chen X, Xu X, Liang Q, Bian X, Yang L, Jing X. 2003. Ultrafine fibers electrospun from biodegradable polymers. J Appl Polym Sci. 89:1085–1092.
- Zhang S. 2003. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 21:1171–1178.
- Zhang X, Shi Z, Fu W, Liu Z, Fang Z, Lu W, Wang Y, Chen F. 2012. In vitro biocompatibility study of electrospun copolymer ethylene carbonate-ε-caprolactone and vascular endothelial growth factor blended nanofibrous scaffolds. Appl Surf Sci. 258:2301–2306.
- Zhang Y, Lim CT, Ramakrishna S, Huang ZM. 2005. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med. 16:933–946.
- Zhang Y, Su B, Venugopal J, Ramakrishna S, Lim CT. 2007. Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers. Int J Nanomedicine. 2:623.
- Zhong S, Zhang Y, Lim CT. 2011. Fabrication of large pores in electrospun nanofibrous scaffolds for cellular infiltration: a review. Tissue Eng Part B Rev. 18:77–87.
- Zhu XH, Wang CH, Tong YW. 2009. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J Biomed Mater Res A. 89:411–423.
- Zomer Volpato F, Almodóvar J, Erickson K, Popat KC, Migliaresi C, Kipper MJ. 2012. Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 8:1551–1559.
- Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, et al. 2005. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 26:5330–5338.
- Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B. 2002. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 43:4403–4412.
- Zou B, Liu Y, Luo X, Chen F, Guo X, Li X. 2012. Electrospun fibrous scaffolds with continuous gradations in mineral contents and biological cues for manipulating cellular behaviors. Acta Biomater. 8:1576–1585.
- Zou J, Yang Y, Liu Y, Chen F, Li X. 2011. Release kinetics and cellular profiles for bFGF-loaded electrospun fibers: Effect of the conjugation density and molecular weight of heparin. Polymer. 52:3357–3367.