Abstract
Mesenchymal stem cells (MSCs) are non-hematopoietic, multipotent progenitor cells which reside in bone marrow (BM), support homing of hematopoietic stem cells (HSCs) and self-renewal in the BM. These cells have the potential to differentiate into tissues of mesenchymal origin, such as fibroblasts, adipocytes, cardiomyocytes, and stromal cells. MSCs can express surface molecules like CD13, CD29, CD44, CD73, CD90, CD166, CXCL12 and toll-like receptors (TLRs). Different factors, such as TGF-β, IL-10, IDO, PGE-2, sHLA-G5, HO, and Galectin-3, secreted by MSCs, induce interaction in cell to cell immunomodulatory effects on innate and adaptive cells of the immune system. Furthermore, these cells can stimulate and increase the TH2 and regulatory T-cells through inhibitory effects on the immune system. MSCs originate from the BM and other tissues including the brain, adipose tissue, peripheral blood, cornea, thymus, spleen, fallopian tube, placenta, Wharton's jelly and umbilical cord blood. Many studies have focused on two significant features of MSC therapy: (I) MSCs can modulate T-cell-mediated immunological responses, and (II) systemically administered MSCs home in to sites of ischemia or injury. In this review, we describe the known mechanisms of immunomodulation and homing of MSCs. As a result, this review emphasizes the functional role of MSCs in modulating immune responses, their capability in homing to injured tissue, and their clinical therapeutic potential.
Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic, multipotent progenitor cells, which exist in the bone marrow (BM)(CitationBen-Ami et al. 2011). They are responsible for the homing of hematopoietic stem cells (HSCs) and their self-renewal in the BM(CitationMaitra et al. 2004). These cells are capable of differentiating in vitro and in vivo into more cells of mesenchymal lineage, as well as adipocytes, chondrocytes, osteocytes, tenocytes, fibroblasts, cartilage, bone, cardiomyocytes, skeletal myocytes, visceral cells, mesoderm, ectodermal cells (e.g. neurons), endodermal cells (e.g. hepatocytes), and stromal cells(CitationKrampera et al. 2006a, CitationGebler et al. 2012, CitationWang et al. 2009).
In addition, MSCs have been found to supply cytokine and growth factor support for expansion of hematopoietic and embryonic stem cells(CitationAggarwal and Pittenger 2005). These cells, which are also well known as multipotent stromal or mesenchymal cells, were discovered by Friedenstein and his colleagues in 1970. MSCs are capable of dividing up to 50 times in about 10 weeks, in vitro(CitationLotfinegad 2014). The presence of non-hematopoietic stem cells in the bone marrow was first revealed by the observation of the German pathologist Cohnheim, 130 years ago(CitationChamberlain et al. 2007). This class of the multipotent progenitors were spindle-shaped, plastic-adherent, and non-phagocytic, with fibroblast-like morphology(CitationMohammadian and Shamsasenjan 2013).
MSCs are well-known for the expression of surface markers such as:
CD105 (SH2), CD73 (SH3, SH4), stromal antigen-1, CD90, CD44, CD166 (VCAM), CD54, CD102 (ICAM-2), and CD49 (VLA) (CitationVolarevic et al. 2011). Conversely, MSCs are distinguished from HSCs in that they lack the cell surface markers, CD11b, CD11c, CD14, CD19, CD31, CD34, CD45, CD79a, and the HLA-DR, lymphocyte function-associated antigen1(LFA1), erythrocytes (glycophorin A), platelet and endothelial cell markers. The commonly known phenotypes and markers are listed in (CitationVolarevic et al. 2011, CitationGhannam et al. 2010a, CitationShi et al. 2011). ().
Table I. Phenotypes, lineage-specific and functional markers of MSCs (Krampera et al. 2006, CitationPountos et al. 2007, CitationBühring et al. 2007, CitationDeans and Moseley 2000, CitationDevine et al. 2002, CitationFickert et al. 2004, CitationJorgensen et al. 2004, CitationOtto and Rao 2004, CitationPittenger and Martin 2004, CitationSimmons and Torok-Storb 1991, CitationBarry et al. 2001, CitationTse et al. 2003, CitationLe Blanc et al. 2003, CitationXu et al. 2004, CitationVogel et al. 2003, CitationMajumdar et al. 2003, CitationBruder et al. 1998, CitationGronthos et al. 1994, CitationPotian et al. 2003).
It is believed that MSCs are remnants of embryonic stem cells which remain in the adult human body, and express embryonic stem cell markers including: HOX, SSEA-1, Nanong, Oct-4, Rex-1 and GATA-4(CitationKrampera et al. 2006b, CitationLotfinegad 2014). In addition to the BM, MSCs originate from other sources, including the liver, lung, brain, adipose tissue, peripheral blood, cornea, synovium, thymus, dental pulp, periosteum, tendon, spleen, fallopian tube, placenta, amniotic fluid, Wharton's jelly and umbilical cord blood(CitationLotfinegad 2014). In vitro and in vivo, MSCs release IL-6, IL-7, IL-8, IL-10, IL-11, IL-12, IL-14, IL-15, sHLA-G5, PGE2, M-CSF, IDO,TGF-β, hepatocyte growth factor (HGF), inducible nitric oxide synthase (iNOS), Galectin-3, and hemooxygenase (HO). The potential for self-renewal and multipotency are the hallmarks of MSCs (CitationMohammadian and Shamsasenjan 2013, CitationShi et al. 2011, CitationMoriscot et al. 2005). Pevsner-Fischer et al. displayed that cultured MSCs express toll-like receptor (TLR) molecules 1 to 9. Activation of MSCs by TLR ligands provoked IL-6 secretion and NF-kB nuclear translocation(CitationYagi et al. 2010, CitationPevsner-Fischer et al. 2007).
It has been identified that TLRs mediate responses of bone marrow-derived progenitor cells. A new study has described the significance of TLRs in migration and immune regulation of MSCs(CitationYagi et al. 2010, CitationPevsner-Fischer et al. 2007, CitationNagai et al. 2006, CitationRyan et al. 2007). It is predicted that MSCs constitute about 0.001% of mononucleotide cells in the BM, while their proportion declines with age(CitationMohammadian and Shamsasenjan 2013, CitationKitoh et al. 2004, CitationMueller and Glowacki 2001).
This review presents the immunomodulatory mechanism of MSCs on immune cells. In addition, homing of MSCs and the current uses of these cells in medicine are discussed briefly.
Immunomodulatory effects of MSCs on immune cells
It has been agreed that the potential of MSCs to modulate immune responses is due to both cell-cell interactions and paracrine effects(CitationLotfinegad 2014). MSCs can down-regulate the strength of an immune response by influencing both natural and adaptive immunity(CitationBen-Ami et al. 2011). MSCs can inhibit innate immune system cells (DCs, NK, monocyte and neutrophil) and adaptive immune system cells (B, TH1 and T-CTL). MSCs also induce stimulation of the TH2 and regulatory T-cells by the inflammatory microenvironment.
Innate immune system cells
Natural killer cells
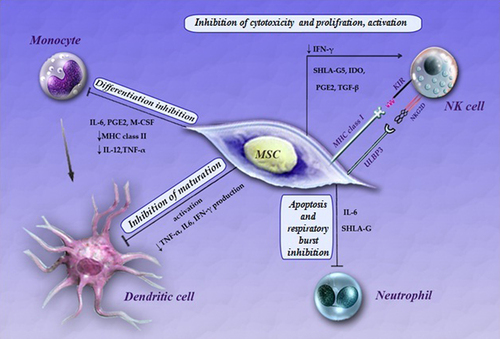
Natural killer cells (NK cells) are the main effector cells in inherent immunity, and are commonly thought to play a basic role in antiviral responses(CitationYagi et al. 2010). MSCs hinder the proliferation of IL-2-induced NK cells, which is mostly mediated by the soluble immunosuppressive factors, transforming growth factor-ß (TGF-ß), soluble human leukocyte antigen-G (sHLA-G), prostaglandin E2 (PGE2) and indoleamine2,3-dioxygenase, in addition to cell-cell contact(CitationBen-Ami et al. 2011, CitationGebler et al. 2012, CitationLotfinegad 2014, CitationYagi et al. 2010, CitationSpaggiari et al. 2008, CitationGonen-Gross et al. 2010, CitationAbdi et al. 2008). MSCs can exert more influence on innate immunity during their inhibition of the cytotoxicity of NK cells by down-regulating the expression of NKp30, NKp44, NKG2D and DNAM-1-activating receptors on these cells, and also by inhibiting proliferation and inducing suppression of IFN-γ(CitationBen-Ami et al. 2011, CitationSpaggiari et al. 2008, CitationSpaggiari et al. 2006). Several studies have demonstrated that MSCs suppress NK cell proliferation and IFN-ɤ production driven by IL-2 or IL-15, but only partially inhibit the proliferation of activated NK cells(CitationAggarwal and Pittenger 2005, CitationShi et al. 2011, CitationRyan et al. 2007, CitationSotiropoulou et al. 2006, CitationRasmusson et al. 2003, CitationMaccario et al. 2005). In contrast, Krampera et al. described that NK cells cultured for 4–5 days with IL-2 in the presence of MSCs showed reduced cytolytic potential against K562 cells, and this suppressive effect might be attributed to the IFN-γ produced by NK cells(CitationShi et al. 2011). Recently, Prigione et al. discovered that the inhibitory effect of MSCs on the proliferation of invariant NK T (iNKT, Vα24 + Vß11+) and γδT(Vδ2+) cells in the peripheral blood is mediated by secreting prostaglandin E2 (PGE2), before IDO and TGF-β1. On the other hand, cytokine production and cytotoxic activity of the cells were only moderately affected by MSCs. Vδ2 + cells also function as expert antigen-presenting cells for naive CD4+ T cell response, and MSCs do not restrain antigen processing/presentation of activated Vδ2 + Tcells to CD4+ T-cells. ()(CitationShi et al. 2011, CitationPrigione et al. 2009).
Figure 1. Effects of Mesenchymal stem cells (MSCs) on the innate immune system. MSCs exert an influence on a variety of cells of the immune system. Mechanisms governing these interactions include secretion of soluble paracrine factors by MSCs and direct cell–cell contact between MSCs and innate immune cells. Abbreviations: IFN gamma (interferon γ), IDO (indoleamine 2,3-dioxygenase), IL-2 (interleukin 2), IL-6 (interleukin 6), prostaglandin E2 (PGE2), transforming growth factor beta (TGFβ) TNF-α (tumor necrosis factor α), soluble human leukocyte antigen-G5 (sHLA-G5) M-CSF (monocyte-colony stimulating factor), MHC (major histocompatibility complex), KIR (killer inhibitory receptor), NK(natural killer cell), PD-1(programmed death 1), PD-L1(programmed death ligand1).
Dendritic cells
Dendritic Cells (DCs) participate with a key function in the beginning of primary immune responses, which depend on the maturation and activation steps of DCs. Immature DCs function as guards in peripheral tissues, by increased antigen uptake and processing, with low capability to stimulate T-cells(CitationYagi et al. 2010, CitationBanchereau and Steinman 1998, CitationMellman and Steinman 2001). In the past, MSCs hampered the in vitro maturation of monocytes and hematopoietic progenitor cells into DCs, in addition to down-regulating the cell surface expression of MHC class II, CD11c, CD83 and co-stimulatory molecules on mature DCs(CitationBen-Ami et al. 2011, CitationJiang et al. 2005).
MSCs may also regulate immune reaction during interaction with DCs. MSCs could inhibit differentiation of monocytes into DCs; however, they could also inhibit the maturation of DCs, giving rise to immature DCs that could consequently render T-cells anergic. MSCs have also been shown to modify the cytokine secretion profile of DCs to up-regulate regulatory cytokines, for example IL-10, and down-regulate inflammatory cytokines like IFN-ɤ, IL-12, and TNF-α, and induce further anti-inflammatory effect or tolerant DC-phenotypes(CitationAbdi et al. 2008, CitationRyan et al. 2005, CitationNauta et al. 2006). Spaggiari et al. confirmed that MSCs powerfully inhibit the maturation and functioning of immunomodulators of mesenchymal stem cell monocyte-derived DCs, by interfering selectively on the generation of immature DCs by means of inhibitory mediator MSC-derived PGE2, but not IL-6. On the other hand, the fundamental mechanism in the up-regulation of PGE2 in monocyte–MSC co-cultures remains unclear. Ramasamy et al. showed that the cell cycle in DCs was arrested in the G0/G1 phase upon contact with MSCs. A new study has reported that MSCs isolated from human adipose tissue are more potent immunomodulators for the differentiation of human DCs than MSCs derived from the BM (CitationShi et al. 2011, CitationSpaggiari et al. 2009, CitationRamasamy et al. 2007, CitationIvanova-Todorova et al. 2009). One more MSC-secreted factor, IL-6, has been reported to be involved in the inhibition of the differentiation of monocytes to DCs, diminishing their stimulatory capacity on T-cells(CitationGhannam et al. 2010b, CitationJiang et al. 2005, CitationDjouad et al. 2007).
Myeloid DCs are the main potent antigen-presenting cells, important in the induction of immunity and tolerance. Through maturation, immature DCs acquire the expression of co-stimulatory molecules and up-regulate the expression of MHC class I and class II molecules collectively, with further cell surface markers such as CD11c, CD80, CD83 and CD86. In vitro, MSCs inhibit the maturation of monocytes and the development of CD34+ hematopoietic progenitor cells into DCs, as shown by a decline in cell surface expression of MHC class II and co-stimulatory molecules, in addition to a reduced production of IL-12 and TNFα. This outcome is at least partly mediated through the production of IL-6 by activated MSCs or PGE-2, which are directly responsible for blocking DC maturation. These results propose that MSCs might regulate DC maturation to an anti-inflammatory or regulatory phenotype responsible for a satisfactory T-cell response(CitationAggarwal and Pittenger 2005, CitationGhannam et al. 2010a, CitationJiang et al. 2005, CitationSpaggiari et al. 2009, CitationDjouad et al. 2007). The effect of MSCs is controlled toward primary phases of DC maturation, as verified by alterations in the expression of the DC surface markers CD80, CD86, CD83, and the secretion of the polarizing cytokine IL-12. DCs which are produced in the presence of MSCs secrete low levels of IL-12 and TNF-α, but elevated levels of IL-1β, IL-10; in addition, they express low levels of MHC class II surface antigens. Most recent studies propose that antigen processing and presentation by MHC class II surface antigens are impaired(CitationGebler et al. 2012, CitationNauta et al. 2006, CitationZhang et al. 2004). For the first time, Di Nicola et al. showed the repression of cell-mediated immune connections by co-culturing DCs, irradiated allogenic lymphocytes or phytohemaglutinin (PHA)-stimulated T-cells with irradiated MSCs, in a mixed lymphocyte reaction (MLR)(CitationLotfinegad 2014). They found that MSCs delayed the up-regulation of CD1A, CD40, CD80 (B7-1), CD86 (B7-2) and HLA-DR through DC maturation, even as CD83 increased. Significantly, DCs isolated from cultures that were co-cultured with MSCs showed a decreased potential to activate CD4+ cells in the presence of MLCs(CitationAggarwal and Pittenger 2005, CitationMaccario et al. 2005, CitationJiang et al. 2005, CitationZhang et al. 2004, CitationLe Blanc and Ringden 2007, CitationBeyth et al. 2005). In the presence of MSCs, IL-10-secreting plasmacytoid DCs, characterized by the expression of the BDCA4 antigen, increased after stimulation by lipopolysaccharide(CitationAggarwal and Pittenger 2005, CitationLe Blanc and Ringden 2007). CD14+ monocytes activate MSCs to secrete soluble factors as well as IL-1β that inhibit alloreactive T-cells. () (CitationLe Blanc and Ringden 2007, CitationGroh et al. 2005).
Neutrophils
Neutrophils are the first cells that arrive at inflammatory tissue, and these cells secrete cytokines. One more MSC-produced factor, IL-6, has been shown to be engaged in the inhibition of monocyte differentiation to DCs, diminishing their stimulation capacity on T-cells. Similarly, the production of IL-6 by MSCs has also been reported toward stoppage of apoptosis of lymphocytes and neutrophils(CitationGhannam et al. 2010b, CitationJiang et al. 2005, CitationDjouad et al. 2007, CitationRaffaghello et al. 2008, CitationXu et al. 2007). MSCs greatly inhibit the in vitro secretion of hydrogen peroxide in activated neutrophils, therefore these stem cells can potentially control the intensity of a respiratory burst upon inflammatory stimulation(CitationBen-Ami et al. 2011, CitationRaffaghello et al. 2008). With respect to cells of the inherent immune system, MSCs can significantly decrease the power of the respiratory burst and apoptosis, which is a vital factor of the phagocytic role of neutrophils. This can be a serious process whereby MSCs can control the intensity of tissue injury following ischemic and ischemia/reperfusion damage(CitationMazaheri et al. 2012, CitationHirata et al. 1993). Hyperactivated T-lymphocyte helper 1 (Th1) produces proinflammatory cytokines such as IL-2, IL-6, IL-8, IL-17, TNF-α and IFN-γ. These cytokines stimulate neutrophils and activate monocytes. Activated monocytes stimulate Th1 differentiation by secreting IL-12, and the hyperfunction of neutrophils causes a tissue wound. Altogether, the connection between APCs, hypersensitivity of T-lymphocytes, and hyperactivity of neutrophils, might be the major cause for immune responses in Behcet's disease (BD). () (CitationMazaheri et al. 2012, CitationTürsen 2012, CitationKapsimali et al. 2010, CitationTursen 2009, CitationHirohata and Kikuchi 2003).
Adaptive immune system cells
B-cells
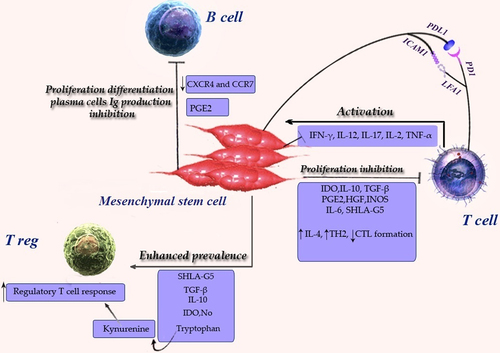
MSCs are capable of modulating the immune response of B-cells. It has been demonstrated that in a co-culture method of stimulated B-cells and MSCs, the proliferation of B-cells as well as the secretion of antibodies (IgA, IgG, and IgM) were inhibited in plasma cells(CitationLotfinegad 2014, CitationCorcione et al. 2006). In murine studies, MSCs have been stated to inhibit the proliferation of B-cells, stimulated through anti-CD40L and IL-4, or by pokeweed mitogen and protein A, as in Staphylococcus aureus(CitationNauta and Fibbe 2007, Schwartz et al. 2007, CitationGlennie et al. 2005, CitationZhang et al. 2005, CitationTögel et al. 2005, CitationLe Blanc et al. 2004a, CitationBreitbach et al. 2007). Allogeneic MSCs have been revealed to restrain the proliferation, activation and IgG secretion of B-cells, as shown in BXSB mice that were utilized as an investigational model for human systemic lupus erythematous(CitationNauta and Fibbe 2007, CitationAugello et al. 2005, CitationDeng et al. 2005). Krampera et al. demonstrated that MSCs only decreased the proliferation of B cells in the presence of IFN-γ. The suppressive effect of IFN-ɤ was probably attributed to its capacity to stimulate the secretion of IDO by MSCs, which in turn suppresses the proliferative response of effector cells during the tryptophan pathway(CitationNauta and Fibbe 2007, CitationKrampera et al. 2006a). Due to the fact that B-cell activation is mostly T-cell dependent, the influence of MSCs on the activity of T-cells might also not directly suppress B-cell functions. Additionally, MSCs have been shown to apply a direct influence on B-cells through cell to cell contact and during secretion of paracrine molecules(CitationCorcione et al. 2006, CitationAugello et al. 2005, CitationWeil et al. 2011, CitationGerdoni et al. 2007). MSCs arrest B-cells in the G0/G1 phase of the cell cycle, without apoptosis(CitationMohammadian and Shamsasenjan 2013, CitationCampagnoli et al. 2001). MSCs down-regulate the expression of the chemokine receptors CXCR4 and CXCR5, in addition to CCR7B, as well as lead to chemotaxis of CXCL12, the CXCR4 ligand, CXCL13 and CXCR5 ligand, suggesting that elevated numbers of MSCs influence the chemotactic properties of B-cells(CitationChamberlain et al. 2007, CitationVolarevic et al. 2011, CitationShi et al. 2011, CitationAbdi et al. 2008, CitationLe Blanc and Ringden 2007, CitationCorcione et al. 2006, CitationDeng et al. 2005). These findings cannot support the potential therapeutic utilization of MSCs in autoimmune diseases, where the B-cells play a major role ()(CitationLe Blanc and Ringden 2007). Also, MSCs were observed to increase the CD40 expression and the ectopic hyperexpression of the CD40 ligand on the B-cells of BXSB mice(CitationShi et al. 2011, CitationDeng et al. 2005).
Figure 2. Effects of Mesenchymal stem cells (MSCs) on the adaptive immune system. These effects promote an overall anti-inflammatory and immunosuppressive state. Abbreviations: IFN gamma (interferon γ), IDO (indoleamine 2,3-dioxygenase), IL-2(interleukin 2), IL-4 (interleukin 4) IL-10 (interleukin 10), IL-6 (interleukin 6), IL-12 (interleukin 12), IL-17 (interleukin17),prostaglandin E2(PGE2), transforming growth factor beta (TGFβ), hepatocyte growth factor (HGF), induced nitric oxide synthases (iNOS), soluble human leukocyte antigen-G5 (sHLA-G5), ICAM 1(Intercellular adhesion molecule 1), LFA 1(Lymphocyte function-associated antigen-1), TH 2 (T helper 1), Treg (T regulatory).
T-cells
Mesenchymal stem cells are immunosuppressive by inhibiting the response of naive and memory T-cells in MLC, which are made by mitogens. Repression is MHC-free and mainly manifests if MSCs are added on the earliest day of the 6-day culture. The amount of restraint is dosage- dependent(CitationPevsner-Fischer et al. 2007, CitationTse et al. 2003, CitationLe Blanc et al. 2003, CitationPotian et al. 2003, CitationLe Blanc and Ringden 2007). Manifest reserve is detected when more numbers of MSCs are present (MSC/lymphocyte ratio > 1/10). In distinction, the adding of MSCs at a low ratio (1/100–1/10 000) frequently increases proliferation(CitationPotian et al. 2003, CitationLe Blanc and Ringden 2007, CitationLe Blanc et al. 2003, CitationLiu et al. 2004). Tse et al. demonstrated that nearness to MSCs was significant in suppressing T-cell responsiveness and recommended that direct interaction between lymphocytes and MSCs was more significant than soluble mediators in the immunosuppressive function of MSCs(CitationYagi et al. 2010, CitationTse et al. 2003). Krampera et al. stated that inhibition needs the presence of MSCs and MSC-T-cell interaction in culture(CitationYagi et al. 2010, CitationKrampera et al. 2003).
Regulatory T cells
Although MSCs powerfully hamper T-cell proliferation, they can protect the role of CD4+ CD25+ CD127–, forkhead box P3 (FoxP3)+ regulatory T cells (Treg)(CitationLe Blanc and Ringden 2007). MSCs raised the amount of CD4+ CD25high, CD4+ CTLA4+ and CD4 + CD25+ CTLA4+ cells in IL-2-motivated lymphocytes and MLC(CitationAggarwal and Pittenger 2005, CitationMaccario et al. 2005, CitationLe Blanc and Ringden 2007). In contrast, the amount of CD25+ and CD38+ cells diminished in the presence of MSCs in mitogen-stimulated lymphocyte cultures ()(CitationLe Blanc and Ringden 2007, CitationGroh et al. 2005). MSCs also generate bone morphogenic protein-2 (BMP-2), which mediates immunosuppression through the production of CD8+ regulatory T cells(CitationLe Blanc and Ringden 2007, CitationDjouad et al. 2003).
T-helper and cytotoxic T-cells
The presence of signals that support the development of the Th1, such as CD3, CD28, IL-4, IL-2 and IL-12 stimulation, cause naive T-cells mature into IFN-γ-secreting cells. If MSCs are present in the culture, IFN-γ secretion is decreased. Hence, MSCs provoke a bias towards Th2 differentiation(CitationAggarwal and Pittenger 2005, CitationLe Blanc and Ringden 2007). Mesenchymal stem cells suppress CD8 + T-cell-mediated lysis if added at the beginning of the MLC (CitationRasmusson et al. 2003, CitationLe Blanc and Ringden 2007). Cytotoxicity was not affected if MSCs were added in the cytotoxic stage(CitationPotian et al. 2003, CitationRasmusson et al. 2003, CitationMaccario et al. 2005, CitationLe Blanc and Ringden 2007, CitationAngoulvant et al. 2004). Lysis was partly abrogated by the addition of IL-2. MSCs might hinder the afferent stage of alloreactivity and stop the growth of cytotoxic T-cells. When cytotoxic T-cells are activated, MSCs are not effective. In vivo studies are essential to clarify this point(CitationLe Blanc and Ringden 2007, CitationAngoulvant et al. 2004). Human MSCs limit the structure of CD4+ and CD8+ T cells by soluble factors(CitationTse et al. 2003, CitationPotian et al. 2003, CitationLe Blanc and Ringden 2007, CitationCorcione et al. 2006, CitationDi Nicola et al. 2002). The suppressive factor is not constitutively produced by MSCs, since cell culture supernatants do not suppress T-cell proliferation(CitationMaitra et al. 2004, CitationPotian et al. 2003, CitationLe Blanc and Ringden 2007, CitationAugello et al. 2005, CitationLe Blanc et al. 2004b). This result may be characteristic of the inhibition of cell division, which is supported through the gathering of cells in the G0/G1 phase of the cell cycle. At the molecular level, cyclin D2 expression is down-regulated, whereas p27 expression is up-regulated; this might clarify why T-cell proliferation, before activation, and IFN-γ secretion, are affected with MSC(CitationShi et al. 2011, CitationGlennie et al. 2005). Liu et al. clarified that the addition of antibodies specific to FasL and TGF-β1 satisfied suppression by MSCs in concanavalin A-stimulated MLC in a dose-dependent style, other than anti-IL-10, had no effect(CitationLe Blanc and Ringden 2007, CitationLiu et al. 2004). Mesenchymal stem cells may inhibit T-cell proliferation through the secretion of indoleamine 2, 3-dioxygenase (IDO). IDO is induced via IFN-γ, catalyzes the alteration of tryptophan to kynurenine, and inhibits T-cell responses through tryptophan diminution(CitationLe Blanc and Ringden 2007, CitationMunn et al. 1998).
Meisel et al., using the Western blotting technique, revealed that human MSCs do not constitutively express IDO, but the expression is provoked by IFN-γ. IFN-γ also aroused IDO enzyme activity in dose-dependent behavior. Important IDO activity was detected in T-cells stimulated with mitomycin C-treated PBMC, in the presence of MSCs(CitationLe Blanc and Ringden 2007, CitationMeisel et al. 2004). PGE2, which is produced by cyclooxygenase (COX) enzymes, induces regulatory T-cells. (15-750). MSCs constitutively express COX-1 and COX-2 (CitationAggarwal and Pittenger 2005, CitationLe Blanc and Ringden 2007, CitationArikawa et al. 2004) together. While purified T-cells were co-cultured by MSCs, both COX-2 and PGE2 production were boosted(CitationAggarwal and Pittenger 2005, CitationTse et al. 2003, CitationLe Blanc and Ringden 2007). Inhibitors of PGE2 synthesis restored the majority of the proliferation of phytohemaglutinin-activated (PHA) lymphocytes co-cultured with MSCs. Tse et al. studied alloreactive lymphocytes in contrast to mitogen-stimulated cultures. They set up that neither MSC production of IL-10, TGFb1, and PGE2, nor tryptophan reduction, was responsible for the suppression in MLC(CitationAggarwal and Pittenger 2005, CitationTse et al. 2003). Di Nicola et al. recommended that HGF worked synergistically through TGF-β1, to challenge T-cell detection by simultaneous neutralization of HGF and TGF-β1 in the later study restoring T-cell proliferation(CitationYagi et al. 2010, CitationDi Nicola et al. 2002). One more statement exhibited that quantitative real-time PCR confirmed important HGF mRNA up-regulated by IFN-γ and TNFα(CitationYagi et al. 2010, CitationEnglish et al. 2007). NO (nitric oxide) stops the proliferation of T-cells by suppressing the phosphorylation of signal transducer and activator of transcription-5 (STAT5), a transcription factor vital for T-cell activation and proliferation(CitationShi et al. 2011, CitationBingisser et al. 1998). Ding et al. reported that matrix metalloproteinases (MMPs), in particular MMP-2 and MMP-9, produced by MSCs, mediate the suppressive activity of MSCs through diminution of CD25 expression on responding T-cells within a model of allogeneic islet transplant(CitationDing et al. 2009). In an experimental model of arthritis, MSCs reduced antigen-specific Th1/Th17 cell expansion and reduced the production of cytokines released via Th1/Th17 cells, for example IFN-γ and IL-17, and caused the Th2 cells to raise production of IL-4 and IL-10 in lymph node joints(CitationAggarwal and Pittenger 2005, CitationShi et al. 2011, CitationKrampera et al. 2003, CitationZappia et al. 2005). Conversely, a new study reported that MSCs might provoke apoptosis in activated T-cells[CD3+ and bromodeoxyuridine BrdU+], but not in the resting T-cells[CD3+ and BrdU–]; this leads to clear reduction of delayed-type hypersensitivity (DTH) response in vivo with inducing NO production(CitationLim et al. 2010). A recent study demonstrated that the negative co-stimulatory molecule B7-H4 was involved in the immunosuppressive effect of MSCs on T-cell activation and proliferation by the generation of cell cycle arrest and the inhibition of nuclear translocation of the nuclear factor (NF)-kappa B(CitationSensebe et al. 2010). MSCs inhibit Th17 differentiation from naive T-cells. MSCs can also decrease the expression of major histocompatibility complex class E (MHC class E)(CitationMazaheri et al. 2012, CitationGhannam et al. 2010b). Conversely, in one study, it was found that CD25 and CTLA-4 (cytotoxic T lymphocyte-associated antigen-4) surface expression, and Foxp3 mRNA levels, were not dependent on whether CD4+ T-cells were cultured in the presence of MSCs(CitationKrampera et al. 2006b). Furthermore, MSCs have also been reported to influence the cytokine secretion profile of the different T-cell subsets, since their addition to an in vitro activated T-cell culture leads to reduced production of the pro-inflammatory cytokines: IFN-γ, TNF-α, IL-6, IL-17, and enhanced levels of anti-inflammatory cytokines, for example IL-4 and IL-10. On the whole, these outcomes could show a probable MSC-mediated alteration in Th1/Th2 balance(CitationZappia et al. 2005, CitationKong et al. 2009). MSCs can hamper T-cell proliferation by engaging the inhibitory molecule programmed death 1(PD-1) to its ligands PD-L1 and PD-L2, thus producing soluble factors that suppress T-cell proliferation (such as TGF-β or IL-10) and during interaction through DCs(CitationVolarevic et al. 2011, CitationNauta and Fibbe 2007, CitationVolarevic et al. 2009). MSCs increase Th2 and IL-4 production, regulatory T-cell response and decrease activation by foreign antigen, cytotoxic T-cells and IFN-γ production(CitationWeil et al. 2011). The generation of HLA-G5 by MSCs has more lately been revealed to suppress T-cell proliferation, in addition to cytotoxicity of NK cells T-cells, and to increase the generation of regulatory T (Treg) cells. Cell contact between MSCs and activated T-cells stimulated IL-10 production, which was necessary to induce the release of soluble HLA-G5. () (CitationGhannam et al. 2010a, CitationSelmani et al. 2008, CitationNasef et al. 2009).
Homing of MSCs
Homing is the procedure by which cells migrate to, and engraft within, the tissue in which they are able to apply local, efficient effects. While the homing of leukocytes to places of inflammation is well studied, the methods of progenitor cell homing to places of ischemia or damage are weakly recognized(CitationImhof and Aurrand-Lions 2004, CitationLuster et al. 2005). Homing engages a cascade of incidents begun with shear-resistant adhesive interactions between flowing cells and the vascular endothelium at the target tissue (Stage I). This procedure is mediated via ‘homing receptors’ expressed on circulating cells that involve related endothelial co-receptors, causing in cell-tethering and rolling contacts on the endothelial surface. This is characteristically pursued via chemokine-generated activation of integrin adhesiveness (Stage II), hard adhesion (Stage III) and extravasation (Stage IV)(CitationYagi et al. 2010, CitationSackstein 2005). MSCs expressed chemokine receptors for homing of immune cells such as: CCR1, CCR2, CCR3, CCR4, CCR7, CCR8, CCR10, CCL2, CCL3, CCL4, CCL5, CCL7, CCL20, CCL26, CX3CL1, CXCL1, CXCL2, CXCL3, CXCL5, CXCL8, CXCL10, CXCL11 and CXCL12, but not CXCR4, suggesting that CXCR4 can simply be significant for the trafficking of mature stem cell populations and receptor tyrosine kinase growth factor receptors such as platelet-derived growth factor (PDGF) and insulin-like growth factor1(IGF-1)(CitationLotfinegad 2014, CitationYagi et al. 2010, CitationMcTaggart and Atkinson 2007, CitationHoogduijn et al. 2010). Integrins have been identified to play a significant role in cell adhesion, migration, and chemotaxis(CitationRidger et al. 2001, CitationWerr et al. 1998). Integrin α4/β1-VCAM contact has been known to regulate T-cell and NK trafficking(CitationWoodside et al. 2006). Integrin β1 engages cell to-cell adhesion, which can be essential for the anchorage of the engrafted cells. As expected, blockade of integrin β1 reduces neutrophil migration to the lung through inflammation(CitationYagi et al. 2010, CitationRidger et al. 2001). Ruster et al. explained that MSCs react in an organized style through endothelial cells, not only through integrin α4/β1-VCAM-1 interaction or integrin β1, but also via the endothelial phenotype, P-selectin, MMP-2 production, and cytokines(CitationRüster et al. 2006).
Fibronectin attaches extracellular matrix constituents like collagen, fibrin and heparan sulfate proteoglycans. It plays a significant role in cell adhesion, growth, migration, as well as differentiation, and it is significant for the injury healing processes. Records of previous studies show that contact between integrin α4 and β1-fibronectin plays a significant role in transmigration of MSCs into the extracellular matrix(CitationRuoslahti 1984, CitationValenick et al. 2005). Stromal cell-derived factor 1 (SDF-1), which is formally identified as chemokine (C-X-C motif) ligand 12 (CXCL12), is a minute chemotactic cytokine that activates leukocytes and is frequently stimulated through proinflammatory stimuli like TNF-α or IL-1(CitationFedyk et al. 2001). The receptor for this chemokine is CXCR4 and the SDF-1-CXCR4 communication is regarded to be private (CitationMa et al. 1998).
Interaction between SDF-1 and its ligand CXCR4 co-operates a significant function in homing, bone marrow retention and mobilization, as shown in studies on engraftment of hematopoietic stem/progenitor cells(CitationChamberlain et al. 2007, CitationPeled et al. 1999). MSCs migrated considerably in response to SDF-1 and CX3CL, consistent with their corresponding expression of chemokine receptors CXCR4 or CX3CR1. Unexpectedly, Basic Fibroblast Growth Factor (bFGF) might have contrasting effects on MSC migration, depending on the concentration(CitationYagi et al. 2010). MSCs have an important role in co-transplantation by hematopoietic stem cells, by producing SDF-1, Flt-3 ligand and stem cell factor, together with expressing extra-cellular matrix proteins including fibronectin, laminin and vimentin, which have a critical function in HSC homing in the bone marrow niche(CitationMohammadian and Shamsasenjan 2013, CitationHorwitz et al. 2011, CitationDelalat et al. 2009, CitationAkbari et al. 2007).
Medical applications of MSCs
Stem cells in general and MSCs in particular, by their adaptable increase and differentiation potential, are considered perfect candidates for utilization in regenerative medical procedures(CitationMcTaggart and Atkinson 2007). One of the most significant properties that make MSCs a special device for cell-based therapeutic approaches, is their capability to escape from immune refusal; therefore, HLA-matching is not of much significance for their implant and HLA-mismatched donors can also be selected (CitationSiegel et al. 2009, CitationDazzi and Marelli Berg 2008). Major roles of MSCs are correlated to their diverse therapeutic properties, like their anti-inflammatory and immunomodulatory effects, the secretion of mediators that initiate or support tissue renovation and tissue substitution with the potential of multipotent differentiation (CitationCaplan and Dennis 2006, CitationWaszak et al. 2012, CitationDu et al. 2013). The major significant therapeutic areas comprise ischemic cardiac disease, graft-versus-host disease (GVHD), chronic obstructive pulmonary disease, Crohn's and Behcet's disease(CitationMazaheri et al. 2012, CitationDu et al. 2013). MSCs infusion can also be very useful in cord blood transplantation where the restricted amount of stem cells delays engraftment and favors graft rejection. The cell therapy approach has also been utilized as prophylaxis in GVHD in HSC transplantation. The therapeutic efficiency was related to reduced antigen-specific Th1/Th17 cell expansion, increased production of IL-10 and generation of CD4+,CD25+,FoxP3 +Treg cells via the ability to suppress self-reactive T-effector responses(CitationGhannam et al. 2010a, CitationGonzález et al. 2009). Growth of autoimmune diabetes results from immune cell dysfunction to maintain peripheral and central tolerance. MSCs can be useful in regulating Treg/auto reactive T-cell balance. The earliest proposed function of MSCs was the stimulation of the regeneration of endogenous insulin-secreting cells, and next, inhibition of the T-cell-mediated immune responses against newly produced beta cells(CitationUrban et al. 2008). MSCs have been brought into clinical therapy for numerous reasons: to differentiate and repair injured tissues, to increase hematopoietic engraftment following transplant through the production of growth factors, and for immunosuppressant function in GVHD. Since the immunomodulatory methods vary between murine and human MSCs, animal forms cannot mimic the medical position(CitationLazarus et al. 1995, CitationKoç et al. 2000). Recently studies in pathological models have also revealed that MSC can home in to damaged kidneys and make simple renovations(CitationMcTaggart and Atkinson 2007). The proof-of-principle essential to utilize of MSCs in vivo has been shown in a series of trials:(I) MSCs might engraft into mouse tissues after infusion and use a site-specific differentiation, which is due to their exclusive immunological properties that permit engraftment with no rejection; (II) in humans, autologous enlarged MSCs in vitro could be infused intravenously with no toxicity; (III) transplantation of autologous MSCs in arrangement by HSCs lead to improved HSC engraftment; and(IV) allogeneic transplantation of MSCs decreased the frequency and intensity of acute and chronic GVHD (CitationGebler et al. 2012, CitationSato et al. 2010, CitationTolar et al. 2010). Additionally, MSCs have been used for the conduct of different autoimmune diseases leading to the stimulation of T-cell tolerance and damaged pathogenic T and B cell responses. BM-derived MSCs can also suppress the proliferation of PBMCs, independent of their supply (autologous or allogeneic), subtype of autoimmune disease and form of conduct(CitationMacDonald et al. 2011). In the case of tissue renovation, the anti-inflammatory activity of MSCs resulted in the production of anti-inflammatory macrophages, which were important for increasing tissue repair(CitationKim and Hematti 2009). Moreover, MSCs also have therapeutic potential in treating pulmonary fibrosis, acute renal nephropathy, and in inhibiting the progress of diabetes. MSC transplantation promotes the extension and growth of B-cells and renal glomeruli as well as decreasing collagen expression and inflammation in fibrosis(CitationLee et al. 2009, CitationVija et al. 2009). MSCs express high levels of arylsulfatase A and α-l-iduronidase. The absence of these enzymes cause breakdown to hydrolyze a different substrate, leading to its accumulation and the dysfunction of several organs, the most severe being mental retardation. The lack of arylsulfatase A is the cause of metachromatic leukodystrophy, and the deficit of α-l-iduronidase is the cause of Hurler's disease, disorders that can possibly be prevented via allogeneic hematopoietic stem cell transplantation (HSCT), which is just potential therapy(CitationGroth and Ringdén 1984, CitationKrivit et al. 1999). MSCs can be exploited to treat bone disorders (e.g., osteogenesis imperfecta). Five patients with osteogenesis imperfecta, treated with bone marrow transplantation, had donor osteoblast engraftment, novel dense bone shape, an augmentation in complete bone mineral content, increase in development rate and decreased frequencies of bone cracks. This proposes that HSCT leads to engraftment of practical MSCs. Gene-marked MSCs, to recognize the cells after infusion, were given to six children who had undergone HSCT for severe osteogenesis imperfecta(CitationSillence et al. 1978, CitationHorwitz et al. 1999, CitationHorwitz et al. 2001, CitationHorwitz et al. 2002). A bone marrow biopsy demonstrated 0.3%–7.4% Y-chromosome-positive cells by fluorescent in situ hybridization (FISH),signifying engraftment of the donor MSCs. Lee et al. stated the case of a patient with acute leukemia, who accepted a peripheral blood stem cell graft collectively via MSCs since her HLA-haploidentical father was treated by regular immunosuppression(CitationLe Blanc and Ringden 2007, CitationLee et al. 2002).
Conclusion
Mesenchymal Stem Cells (MSCs) have a capacity to home in and integrate into damaged tissues. MSCs provide immunomodulatory effects by paracrine and/or cell-cell contact that inhibit innate immune system cells (DCs, NK cells, monocytes and neutrophils) and adaptive immune system cells (B, TH1 and T CTL). Also, MSCs stimulate Th2 and regulatory T-cells by the inflammatory microenvironment. Therefore, the use of MSCs could lead to various therapeutic possibilities such as supporting tissue regeneration and correcting inherited disorders. A rational understanding of the mechanisms of action of MSCs allows the translation of our basic knowledge of MSC biology into the design of new clinical therapies .The potential antiproliferative and immunomodulatory function of MSCs is being intensely studied by various groups, with the hope that MSCs may be developed as a therapeutic strategy for autoimmune disease, HSCT, BMT(Bone marrow Transplantation) and as a useful tool for cell-based therapy. Autologous transplantation of MSCs has a high ability to produce the desired results in clinical therapies, but it could induce tumors, because MSCs can undergo spontaneous transformation exhibiting a tumorigenic potential with immunosuppression effects. Also, allogeneic MSCs might have a potential risk of infections obtained from donors. The opportunity exists to utilize genetic engineering of MSCs to state particular factors for homing and therapy. Finally, clinical trials with MSCs will afford a rich resource of information that can be studied widely in the laboratory and will play an important role in clinical therapy. In the present review, the comprehensive definition, sources, markers, and receptors of MSCs, as well as the immunomodulatory effect of these cells on innate and adaptive immune system cells, homing to the damaged tissues and therapeutic aspects in a variety diseases, have been reviewed. We hope that using MSCs in the treatment of autoimmune diseases, BMT, HSCT and cell-based therapy will be investigated more in the near future.
Authors’ contributions
AA, AR, and FSTM conceived of the study and participated in its design and coordination. AM, K S, SK, Mt, AS, VZ, and MGG participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Department of Hematology, Faculty of Medicine, Tabriz University of Medical Sciences for all supports provided.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. 2008. Immunomodulation by mesenchymal stem cells a potential therapeutic strategy for type 1 diabetes. Diabetes. 57:1759–1767.
- Aggarwal S, Pittenger MF. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105:1815–1822.
- Akbari A, Mozdarani H, Akhlaghpoor S, Pourfatollah A, Soleimani M. 2007. Evaluation of the homing of human CD34 + cells in mouse bone marrow using clinical MR imaging. Pak J Biol Sci. 10: 833–842.
- Angoulvant D, Clerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y, Eljaafari A 2004. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 41:469–476.
- Arikawa T, Omura K, Morita I 2004. Regulation of bone morphogenetic protein 2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 200:400–406.
- Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G 2005. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 35:1482–1490.
- Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252.
- Barry F, Boynton R, Murphy M, Zaia J. 2001. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 289: 519–524.
- Ben-Ami E, Berrih-Aknin S, Miller A. 2011. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 10:410–415.
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al 2005. Human mesenchymal stem cells alter antigen-presenting cell maturation, induce T-cell unresponsiveness. Blood. 105: 2214–2219.
- Bingisser RM, Tilbrook PA, Holt PG, Kees UR 1998. Macrophage- derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 160: 5729–5734.
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. 2007. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 110:1362–1369.
- Bruder SP, Ricalton NS, Boynton RE, Connolly TJ, Jaiswal N, Zaia J, Barry FP. 1998. Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J Bone Miner Res. 13:655–663.
- Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. 2007. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 1106:262–271.
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM 2001. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, bone marrow. Blood. 98:2396–2402.
- Caplan AI, Dennis JE 2006. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 98:1076–1084.
- Chamberlain G, Fox J, Ashton B, Middleton J. 2007. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25:2739–2749.
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. 2006. Human mesenchymal stem cells modulate B-cell functions. Blood. 107:367–372.
- Dazzi F, Marelli Berg FM 2008. Mesenchymal stem cells for graft versus host disease: Close encounters with T cells. Eur J Immunol. 38:1479–1482.
- Deans RJ, Moseley AB. 2000. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 28:875–884.
- Delalat B, Pourfathollah AA, Soleimani M, Mozdarani H, Ghaemi SR, Movassaghpour AA, Kaviani S 2009. Isolation, ex vivo expansion of human umbilical cord blood-derived CD34 + stem cells, their cotransplantation with or without mesenchymal stem cells. Hematology. 14:125–132.
- Deng W, Han Q, Liao L, You S, Deng H, Zhao RC 2005. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T, B lymphocytes from BXSB mice. DNA Cell Biol. 24:458–463.
- Devine MJ, Mierisch CM, Jang E, Anderson PC, Balian G. 2002. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 20:1232–1239.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. 2002. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 99:3838–3843.
- Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ 2009. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9. Diabetes. 58:1797–1806.
- Djouad F, Charbonnier LM, Bouffi C, Louis Plence P, Bony C, Apparailly F, et al 2007. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin 6 dependent mechanism. Stem cells. 25:2025–2032.
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. 2003. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 102:3837–3844.
- Du Z, Wei C, Cheng K, Han B, Yan J, Zhang M, et al. 2013. Mesenchymal stem cell–conditioned medium reduces liver injury, enhances regeneration in reduced-size rat liver transplantation. J Surg Res. 183:907–915.
- English K, Barry FP, Field-Corbett CP, Mahon BP 2007. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 110:91–100.
- Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA 2001. Expression of stromal-derived factor-1 is decreased by IL-1, TNF, in dermal wound healing. J Immunol. 166:5749–5754.
- Fickert S, Fiedler J, Brenner RE. 2004. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 6:R422–R432.
- Gebler A, Zabel O, Seliger B. 2012. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med.18:128–134.
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. 2007. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 61:219–227.
- Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. 2010a. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 1:2.
- Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H 2010b. Mesenchymal stem cells inhibit human Th17 cell differentiation, function, induce a T regulatory cell phenotype. J Immunol. 185: 302–312.
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F 2005. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 105:2821–2827.
- Gonen-Gross T, Goldman-Wohl D, Huppertz B, Lankry D, Greenfield C, Natanson-Yaron S, et al. 2010. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLoS One. 5:e8941.
- González MA, Gonzalez Rey E, Rico L, Büscher D, Delgado M 2009. Treatment of experimental arthritis by inducing immune tolerance with human adipose derived mesenchymal stem cells. Arthritis Rheum. 60:1006–1019.
- Groh ME, Maitra B, Szekely E, Koç ON 2005. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 33:928–934.
- Gronthos S, Graves S, Ohta S, Simmons P. 1994. The STRO-1 + fraction of adult human bone marrow contains the osteogenic precursors. Blood84:4164–4173.
- Groth CG, Ringdén O. 1984. Transplantation in relation to the treatment of inherited disease. Transplantation. 38:319–326.
- Hirata Y, Sugita T, Gyo K, Yanagihara N 1993. Experimental vestibular neuritis induced by herpes simplex virus. Acta Otolaryngol Suppl. 113:79–81.
- Hirohata S, Kikuchi H 2003. Behcet's disease. Arthritis Res Ther. 5: 139–146.
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke M-H 2010. The immunomodulatory properties of mesenchymal stem cells, their use for immunotherapy. Int Immunopharmacol. 10:1496–1500.
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. 2002. Isolated allogeneic bone marrow-derived mesenchymal cells engraft, stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 99:8932–8937.
- Horwitz EM, Maziarz RT, Kebriaei P 2011. MSCs in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 17:S21–S29.
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. 1999. Transplantability, therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 5:309–313.
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. 2001. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 97:1227–1231.
- Imhof BA, Aurrand-Lions M 2004. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 4:432–444.
- Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, et al. 2009. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 126:37–42.
- Jiang X-X, Zhang Y, Liu B, Zhang S-X, Wu Y, Yu X-D, Mao N. 2005. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 105: 4120–4126.
- Jorgensen C, Gordeladze J, Noel D. 2004. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 15:406–410.
- Kapsimali VD, Kanakis MA, Vaiopoulos GA, Kaklamanis PG 2010. Etiopathogenesis of Behçet's disease with emphasison the role of immunological aberrations. Clin Rheumatol. 29:1211–1216.
- Kim J, Hematti P. 2009. Mesenchymal stem cell–educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 37:1445–1453.
- Kitoh H, Kitakoji T, Tsuchiya H, Mitsuyama H, Nakamura H, Katoh M, Ishiguro N. 2004. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis—a preliminary result of three cases. Bone. 35:892–898.
- Kong Q-F, Sun B, Bai S-S, Zhai D-X, Wang G-Y, Liu Y-M, et al. 2009. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF. J Neuroimmunol. 207:83–91.
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM 2000. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 18:307–307.
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. 2006a. Role for interferon in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 24: 386–398.
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F 2003. Bone marrow mesenchymal stem cells inhibit the response of naive, memory antigen-specific T cells to their cognate peptide. Blood. 101:3722–3729.
- Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. 2006b. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 6:435–441.
- Krivit W, Peters C, Shapiro EG. 1999. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol. 12:167–176.
- Lazarus H, Haynesworth S, Gerson S, Rosenthal N, Caplan A 1995. Ex vivo expansion, subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 16: 557–564.
- Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, et al. 2004a. Mesenchymal stem cells inhibit the expression of CD25 (interleukin 2 receptor), CD38 on phytohaemagglutinin activated lymphocytes. Scand J Immunol. 60:307–315.
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O 2004b. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 363:439–1441.
- Le Blanc K, Ringden O 2007. Immunomodulation by mesenchymal stem cells, clinical experience. J Intern Med. 262:509–525.
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. 2003. HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 31: 890–896.
- Le Blanc K, Tammik L, Sundberg B, Haynesworth S, Ringden O 2003. Mesenchymal stem cells inhibit, stimulate mixed lymphocyte cultures, mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 57:11–20.
- Lee JW, Gupta N, Serikov V, Matthay MA. 2009. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 9:1259–1270.
- Lee ST, Jang JH, Cheong JW, Kim JS, Maemg HY, Hahn JS, et al. 2002. Treatment of high risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co infusion of T cell depleted haematopoietic stem cells and culture expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol. 118:1128–1131.
- Lim J-H, Kim J-S, Yoon I-H, Shin J-S, Nam H-Y, Yang S-H, et al. 2010. Immunomodulation of delayed-type hypersensitivity responses by mesenchymal stem cells is associated with bystander T cell apoptosis in the draining lymph node. J Immunol. 185: 4022–4029.
- Liu J, Lu X, Wan L, Li Y, Li S, Zeng L, et al. 2004. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 36:3272–3275.
- Lotfinegad P. 2014. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull. 4:5.
- Luster AD, Alon R, von Andrian UH 2005. Immune cell migration in inflammation: present, future therapeutic targets. Nat Immunol. 6:1182–1190.
- MacDonald GI, Augello A, De Bari C. 2011. Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 63:2547–2557.
- Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, et al. 2005. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4 + T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 90:516–525.
- Maitra B, Szekely E, Gjini K, Laughlin M, Dennis J, Haynesworth S, Koc O. 2004. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 33:597–604.
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. 2003. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 10:228–241.
- Mazaheri T, Esmaeilzadeh A, Mirzaei MH 2012. Introducing the immunomodulatory effects of mesenchymal stem cells in an experimental model of Behçet's disease. J Med Hypotheses Ideas. 6:23–27.
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. 1998. Impaired B-lymphopoiesis, myelopoiesis, derailed cerebellar neuron migration in CXCR4-and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 95:9448–9453.
- McTaggart SJ, Atkinson K 2007. Mesenchymal stem cells: Immunobiology, therapeutic potential in kidney disease (Review Article). Nephrology (Carlton). 12:44–52.
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D 2004. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase–mediated tryptophan degradation. Blood. 103:4619–4621.
- Mellman I, Steinman RM. 2001. Dendritic cells-specialized and regulated antigen processing machines. Cell. 106:255–258.
- Mohammadian M, Shamsasenjan K. 2013. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 3:433.
- Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. 2005. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 23:594–603.
- Mueller SM, Glowacki J. 2001. Age related decline in the osteogenic potential of human bone marrow cells cultured in three dimensional collagen sponges. J Cell Biochem. 82:583–590.
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 281:191–1193.
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. 2006. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 24:801–812.
- Nasef A, Zhang Y, Mazurier C, Bouchet S, Bensidhoum M, Francois S, et al. 2009. Selected Stro 1 enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int J Lab Hematol. 31:9–19.
- Nauta AJ, Fibbe WE 2007. Immunomodulatory properties of mesenchymal stromal cells. Blood. 110:3499–3506.
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. 2006. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 177:2080–2087.
- Otto W, Rao J. 2004. Tomorrow’s skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 37:97–110.
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. 1999. Dependence of human stem cell engraftment, repopulation of NOD/SCID mice on CXCR4. Science. 283:845–848.
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, et al. 2007. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 109:1422–1432.
- Pittenger MF, Martin BJ. 2004. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 95:9–20.
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. 2003. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 171:3426–3434.
- Pountos I, Corscadden D, Emery P, Giannoudis PV. 2007. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 38:S23–S33.
- Prigione I, Benvenuto F, Bocca P, Battistini L, Uccelli A, Pistoia V. 2009. Reciprocal interactions between human mesenchymal stem cells and T cells or invariant natural killer T cells. Stem Cells. 27:693–702.
- Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, et al. 2008. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells26:151–162.
- Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. 2007. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 83:71–76.
- Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. 2003. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 76:1208–1213.
- Ridger VC, Wagner BE, Wallace WA, Hellewell PG 2001. Differential effects of CD18, CD29, CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol. 166:3484–3490.
- Ruoslahti E 1984. Fibronectin in cell adhesion, invasion. Cancer Metastasis Rev. 3:43–51.
- Ryan JM, Barry FP, Murphy JM, Mahon BP. 2005. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2:8.
- Ryan JM, Barry F, Murphy JM, Mahon BP. 2007. Interferon does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 149: 353–363.
- Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, et al. 2006. Mesenchymal stem cells display coordinated rolling, adhesion behavior on endothelial cells. Blood. 108:3938–3944.
- Sackstein R 2005. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 12:444–450.
- Sato K, Ozaki K, Mori M, Muroi K, Ozawa K 2010. Mesenchymal stromal cells for graft-versus-host disease: basic aspects and clinical outcomes. J Clin Exp Hematop. 50:79–89.
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, et al. 2002. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells.J Clin Invest. 109:1291–1302.
- Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. 2008. Human leukocyte antigen G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte, natural killer function, to induce CD4 + CD25highFOXP3 + regulatory T cells. Stem Cells. 26:212–222.
- Sensebe L, Krampera M, Schrezenmeier H, Bourin P, Giordano R 2010. Mesenchymal stem cells for clinical application. Vox Sang. 98:93–107.
- Shi M, Liu ZW, Wang FS. 2011. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 164:1–8.
- Siegel G, Schäfer R, Dazzi F 2009. The immunosuppressive properties of mesenchymal stem cells. Transplantation87:S45–S49.
- Sillence DO, Rimoin DL, Danks DM. 1978. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig Artic Ser. 15:113–129.
- Simmons PJ, Torok-Storb B. 1991. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 78:55–62.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. 2006. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 24:74–85.
- Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. 2009. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 113:6576–6583.
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. 2008. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood. 111:1327–1333.
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. 2006. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 107:1484–1490.
- Tolar J, Le Blanc K, Keating A, Blazar BR. 2010. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 28: 1446–1455.
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. 2003. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 75:389–397.
- Tursen U 2009. Activation markers in Behcet disease. Turkderm- Archives of the Turkish Dermatology, Venerology. 43:74–86.
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C 2005. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 289:F31–FF42.
- Türsen ÜPathophysiology of the Behçet’s Disease. Pathol Res Int. 2012: 2011.
- Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori V, Uher F 2008. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 26:244–253.
- Valenick LV, Hsia HC, Schwarzbauer JE 2005. Fibronectin fragmentation promotes 4 1 integrin-mediated contraction of a fibrin– fibronectin provisional matrix. Exp Cell Res. 309:48–55.
- Vija L, Farge D, Gautier J-F, Vexiau P, Dumitrache C, Bourgarit A, et al. 2009. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 35:85–93.
- Vogel W, Grunebach F, Messam CA, Kanz L, Brugger W, Buhring HJ. 2003. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica88:126–133.
- Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML 2009. Interleukin-1 receptor antagonist (IL-1Ra), IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 43:255–263.
- Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. 2011. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 29:5–10.
- Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. 2009. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 126:220–232.
- Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thébaud B 2012. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 21:2789–2797.
- Weil BR, Manukyan MC, Herrmann JL, Abarbanell AM, Poynter JA, Wang Y, Meldrum DR 2011. The immunomodulatory properties of mesenchymal stem cells: implications for surgical disease. J Surg Res. 167:78–86.
- Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L 1998. 1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med. 187:2091–2096.
- Woodside DG, Kram RM, Mitchell JS, Belsom T, Billard MJ, McIntyre BW, Vanderslice P 2006. Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion, soluble VCAM-1 binding to integrin 4 1. J Immunol. 176:5041–5049.
- Xu G, Zhang Y, Zhang L, Ren G, Shi Y 2007. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 361:745–750.
- Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, et al. 2004. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood). 229:623–631.
- Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. 2010. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 19:667.
- Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al 2004. Effects of mesenchymal stem cells on differentiation, maturation, function of human monocyte-derived dendritic cells. Stem Cells Dev. 13:263–271.
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, et al. 2005. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 195:16–26.
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. 2005. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 106:1755–1761.

