Abstract
The development of specialized nanoparticles (NPs) for use in the detection and treatment of cancer is increasing. In the last few years, gold nanoparticles (AuNPs) have been greatly studied in biological and photothermal therapeutic status. AuNPs can bind to a wide range of organic molecules, and their synthesis is easy. In this review, we summarize the characteristics of AuNPs, their contributions to tumor destruction, their toxicity, and their potential in the treatment of breast and ovarian cancer.
Keywords::
Introduction
The use of nanoparticles (NPs) in the diagnosis and treatment of cancer is developing (CitationZwicke et al. 2012, CitationYan et al. 2003). Angiogenesis, the formation of new blood vessels, is essential for the growth and progression of tumors (CitationMukherjee et al. 2005, CitationCarmeliet and Jain 2000). It has been argued that gold nanoparticles (AuNPs) could be used in almost all medical applications—diag nostics, therapy, prevention, and hygiene (CitationKhlebtsov and Dykman 2011). AuNPs have recently been demonstrated in cell imaging (CitationBriñas et al. 2008), targeted drug delivery (CitationChithrani et al. 2006), cancer diagnostics, and therapeutic applications (CitationHoek and Stillman 2003, CitationHainfeld et al. 2008).
A wealth of information on how to obtain and use colloidal gold in biology and medicine, as well as how it functions, can be found in books and reviews (CitationDykman and Khlebtsov 2011). Treatment with high specificity and low side effects is the goal of modern therapy. Clinical applications of recombinant protein drugs have been limited due to their low bioavailability, rapid clearance in vivo, systemic cytotoxicity, and high production cost (11) (CitationLedley 1996). In the last few years, AuNPs have been greatly studied for their biological and photothermal therapeutic status (CitationHuang et al. 2008) (). AuNPs can bind to a wide range of organic molecules (CitationChen et al. 2008), and their synthesis is easy (CitationPaciotti et al. 2004). AuNPs have also been used as a platform for novel experimental cancer therapy (CitationPaciotti et al. 2004, CitationBaptista 2012). It was estimated that in 2013, 91,730 women would be diagnosed with a gynecologic cancer and some 28,080 would die from the disease. The American Cancer Society estimated that about 232,670 new cases of invasive breast cancer would be diagnosed in women in 2014. With respect to ovarian cancer, 22,240 new cases and 14,030 deaths had been estimated.
Figure 1. Improved and sustained therapeutic effect of chemotherapeutic agents using nanotechnology. (A) Oral or intravenous route for delivery of conventional formulations and nanocarriers. (B) Pictorial structures of various drug delivery devices such as polymer–drug conjugates, dendrimers, polymer micelles, polymer NPs, and lipid NPs/capsules. (C) Immunoconjugate nanosystem route for improved therapeutic efficacy. Adapted from (CitationYallapu et al. 2010).
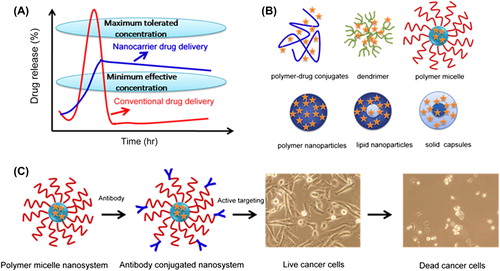
Breast cancer is the most common cancer in women. Early diagnosis and appropriate treatment are essential. Noninvasive diagnostic methods are especially needed for the screening (CitationRajadhyaksha et al. 1995). In this review, we summarize the characteristics of AuNPs, their contributions to tumor destruction, their toxicity, and their potential in the treatment of breast and ovarian cancer.
Biological properties of gold nanoparticles
Gold is one of the first metals to have been discovered (CitationDykman and Khlebtsov 2011). Gold compounds were used in Chinese culture and in India since early 2500–2600 BC (17)(CitationKumar et al. 2011). They utilized it for medicinal purposes (CitationMahdihassan 1985).
Inorganic NPs such as gold, silver, and copper have resplendent colors due to the presence of the surface plasmon resonance (SPR) band (CitationBrongersma 2003). The SPR induces a strong absorption of the incident light, and thus can be measured using a UV-Vis absorption spectrometer (20) (CitationLink et al. 1999). AuNPs might also reduce angiogenesis. The vascular endothelial growth factor/vascular permeability factor (VEGF/ VPF; refs. (CitationSenger et al. 1986, CitationLeung et al. 1989)) and the basic fibroblast growth factor (bFGF; ref. (CitationRisau 1997)) are two critical cytokines for the stimulation of angiogenesis.
AuNPs can also be tuned by surface modifications, such as coating the core with a layer of silica (CitationJain et al. 2006). AuNPs show the SPR band at around 520 nm in the visible region. The SPR band is affected by the particle size (CitationJain et al. 2006, CitationLink and El-Sayed 1999).
AuNPs have strong binding affinity towards thiols, disulfides, phosphine, and amines (CitationSastry et al. 2002).
The strong affinity of gold towards thiol and amine functionalities (according to the SHAB principle) allows many different biological ligands such as DNA, peptides, proteins, antibodies, viruses, and receptors to coat the gold surface (CitationLove et al. 2005). AuNPs have been in active use in the identification of chemical and biological agents (CitationMaunsbach and Afzelius 1998).
AuNPs are used as a probe for electron microscopy or as a vehicle to deliver biomolecules into cells (). Recently, Bhattacharya et al. showed that AuNPs can be used as active agents to interfere directly with the cellular processes, and that they possess antiangiogenic and antitumor properties (CitationMukherjee et al. 2005). For example, in 2001, there was an investigation of AuNPs that had been functionalized with cationic quaternary ammonium groups and then electrostatically bound to plasmid DNA. This composite particle could protect the DNA from enzymatic degradation and could regulate DNA transcription of T7 RNA polymerase (CitationMcIntosh et al. 2001, CitationHan et al. 2006). Sokolov et al. used AuNPs conjugated to epidermal growth factor receptor (EGFR) antibody for identification of precancerous lesions. This receptor is overexpressed in cervical cancer cells. Therefore, treatment with these gold conjugates enhances the finding of cervical cancer cells due to the raised SPR scattering of the AuNPs by laser scanning confocal reflectance (CitationSokolov et al. 2003).
AuNPs were used for therapeutic purposes in 1997, when the successful application of colloidal gold in a patient with rheumatoid arthritis was first reported (CitationRichards et al. 2002). AuNPs were used to enhance cancer apoptosis by radiotherapy (CitationHainfeld et al. 2004).
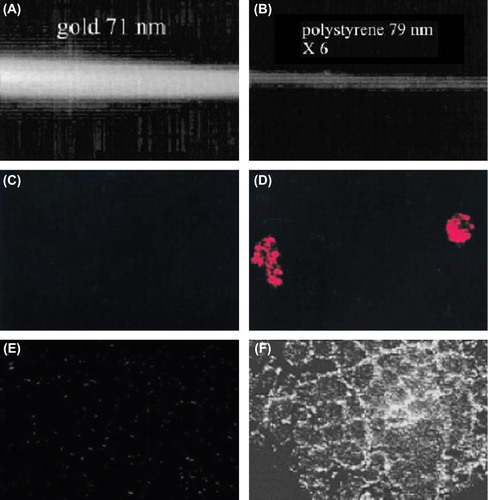
Figure 2. (A and B) Comparison of scattering properties of AuNPs (A) and polystyrene NPs (B) of the same size (B). (C and D) Comparison of reflectance images of SiHa cells labeled with BSA/Au conjugates (C) and anti-EGFR gold conjugates (D). (E and F) Comparison of reflectance images of cervical biopsies labeled with anti-EGFR antibodies/AuNP conjugates for normal tissue (E) and abnormal tissue (F). Due to strong scattering from targeted AuNPs, cancer cells and tissues can be differentiated from normal ones. All images were taken by a laser scanning confocal microscope in reflectance mode. Adapted from (CitationHuang and El-Sayed 2010) (68).
NPs can be used for site-specific delivery of cytotoxic drugs, with the potential to reduce toxicity and reduce harmful side effects (CitationPeer et al. 2007).
The options of using AuNPs conjugated with the following antitumor agents were proposed: prospidin (CitationDykman and Khlebtsov 2011), tamoxifen (CitationDreaden et al. 2012), and Herceptin (CitationEghtedari et al. 2008).
Many cancerous cells in the breast, ovary, brain, and kidney have overexpression of folate receptors (FR) (CitationToffoli et al. 1997). Recently, gold nanoconjugates (AuNCs) were synthesized, to use the FR on different cancer cells. Polyethylene glycol (PEG) was used as a target. It is a linker between folic acid and adapted drug molecules. PEGylated molecules minimize the toxic effects. They also aid in stabilization during blood circulation, with minimal degradation and nonimmunogenicity (CitationDixit et al. 2006).
AuNPs in breast cancer research
Rapid selective detection of cancer cells is an important challenge for the diagnosis and treatment of tumors (CitationRevenko et al. 2010). Wentong Lu has shown that when multifunctional oval-shaped AuNPs are mixed with the breast cancer SK-BR-3 cell line, a distinct color change occurs and two-photon scattering intensity increases by about 13-fold. Our experimental data of the HaCaT noncancerous cell line and of the MDA-MB-231 breast cancer cell line clearly demonstrate that our colorimetric and TPS assays are highly sensitive to SK-BR-3 and can distinguish from other breast cancer cell lines which express low levels of HER-2 (CitationLu et al. 2010).
Dreaden EC has shown enhanced potency and selective intracellular delivery of tamoxifen using AuNPs targeted to ER(+) breast cancer cells in vitro (CitationDreaden et al. 2009).
It was found that AuNC–Her could escape the endolysosomal pathway and enter the nucleus of cancer cells to enhance the therapeutic efficacy of Herceptin. The nuclear localization effect of AuNC–Her enhances the anticancer therapeutic efficacy of Herceptin, as evidenced by the induction of DNA damage (CitationWang et al. 2011).
AuNPs conjugate with Herceptin. It increases the uptake of AuNPs and the drug to overcome Herceptin resistance. It is essential for treating breast cancer cells (CitationNahta and Esteva 2006).
Application of 300 kVp (peak cells led to 5.1 times more DNA double-strand breaks in Herceptin–PEG–AuNPs than in PEG–AuNPs (CitationChattopadhyay et al. 2010). Herceptin–AuNR conjugates have also been shown to be taken up into tumor cell endosomes and lysosomes rather than attaching to the cellular membrane (CitationChen and Irudayaraj 2009).
Although Herceptin has been shown to be effective against HER-2 overexpression in breast cancer, some patients who receive it develop resistance to the agent (CitationNahta and Esteva 2006).
Au3Cu1 nanoshells have also been found to be effective contrast agents for MRI (CitationSu et al. 2007). The use of AuNPs is also being explored in ‘‘theranostics,’’ applications in which diagnosis and treatment are combined. Multifunctional NPs made of magnetic NPs, AuNS, and upconversion NPs could accumulate in tumors, enabling detection by upconversion luminescence and MRI, to increase survival from photothermal treatment combined with a magnetic field (CitationCheng et al. 2012).
AuNPs have been used to detect breast cancer in several diverse applications, ranging from the evaluation of pathology specimens to noninvasive in vivo imaging. Park et al. found that two-photon-induced photoluminescence (TPIP) could be used to visualize tumor cells embedded with gold, in vivo (CitationPark et al. 2008).
The transferrin receptor participates in cellular proliferation, and overexpression of the transferrin receptor has been observed in breast tumors. Thus, the tumoral intake of AuNPs would be enhanced by conjugating them with transferrin (CitationLi et al. 2009). Breast cancer cells also have a high expression level of receptors for luteinizing hormone-releasing hormone (LHRH) (CitationJin et al. 2008). Dumbbell-shaped AuFe3O4 nanoparticles conjugated with a trastuzumab and platinum complex showed target-specific delivery of platinum compounds to HER-2-positive SK-BR-3 cells. That cisplatin was released at lower pH levels led the investigators to suggest that the intracellular release of cisplatin (and hence its cytotoxicity) was stimulated by the lower pH in endosomes after uptake of the conjugates (CitationXu et al. 2009). The lower pH levels in cellular endosomes could also facilitate the release of drugs from chloroquine-conjugated AuNPs, which also have antitumor activity in breast cancer, and lead to increased cytotoxicity in MCF-7 cells (CitationJoshi et al. 2012).
AuNPs and ovarian cancer
Ovarian cancer is the fifth most prevalent cancer among women. There are no early symptoms for ovarian cancer, which hinders detection until it reaches advanced stages (CitationChauhan et al. 2009). In fact, of the top ten cancer types afflicting American women in 2008, ovarian cancer had the highest death incidence ratio, even exceeding that of lung cancer (CitationGreenlee et al. 2000).
Some of the greatest challenges in the diagnosis and treatment of ovarian cancer stem from its heterogeneous nature. The term “ovarian cancer” refers not to a single disease, but to a various group of malignancies disturbing the ovary. On the other hand, ovarian tumors may expand from one of three cell types: epithelial cells, sex cord-stromal cells (including granulosa, theca, and hilus cells), or germ cells (oocytes) (CitationChen et al. 2003).
Importantly, ovarian cancer patients are often initially responsive to these therapeutic modalities but eventually become resistant to therapy. Therefore, drug resistance remains the major obstacle in the treatment of ovarian cancer. One way to improve the efficacy and specificity of chemotherapeutic agents is through nanotechnology-based formulations (encapsulated, conjugated, or entrapped/loaded forms in nanocarriers or drug delivery vehicles/vectors). Nanotechnology-mediated therapies promote a controlled delivery of chemotherapeutic drug (s) in a targeted way, which directly acts on the cancer site for prolonged periods of time, with minimal or no organ toxicity normally seen (CitationYallapu et al. 2010).
In cancer treatments, NPs can rely more on the enhanced permeability and retention effect caused by leaky tumor vasculatures for better drug accumulation at the tumor sites (CitationAgarwal and Kaye 2003).
Ovarian cancer is the fifth most prevalent of the gynecologic malignancies among women in the Western world, with a lifetime risk of 1.4 to 1.8%. There are no early symptoms for ovarian cancer, which hinders detection until it reaches advanced stages. Primary management consists of an extensive surgery, in combination with chemotherapy post-surgery (CitationAgarwal and Kaye 2003, CitationHerzog and Pothuri 2006).
Over the past decade, nanotechnology has received considerable attention for cancer therapy (CitationArvizo et al. 2010). At the molecular level, treatment with AuNPs has been seen to alter the profiles of a series of secretory cytokines (CitationQiao et al. 2011).
Cisplatin and carboplatin (CBP) have been the most effective chemotherapeutic regimens for more than two decades (CitationMcGuire and Ozols 1998).
Cisplatin retains its cytotoxicity in the nanoconjugated form. Fascinatingly, nanoconjugation in the form of Au–PSH–CP–FA protects the cytotoxic effect of CP to normal cells while enhancing cytotoxic effects on tumor cells (CitationPatra et al. 2010).
Cisplatin treatment has been found to upregulate the expression of several stem cell markers such as ALDH1, CD24, CD44, CD133, EpCAM, Nanog, Oct-4, and Sox2, and increase the side population (SP) simultaneously. In addition, pretreatment with AuNPs also prevented cisplatin-induced acquired ‘stemness’ and enrichment of SP cells.
Progress in nanomedicine has led to the development of molecular targeted NP therapeutic carriers. These NP drug carriers can be targeted against cancer cells using cancer cell surface markers to directly deliver therapeutics to cancer cells (CitationWang et al. 2008) . Another exciting aspect of the behavior of AuNPs is their unequal cytotoxicity toward cancer cells. Metallic materials may arrest cells at the G2/M phase, the most radiosensitive phase of the cell cycle, and thus unreasonably increase the sensitivity of cancer cells toward radiation (CitationTurner et al. 2005). On the other hand, Glu–AuNPs trigger activation of the CDK kinases, leading cancer cells to gather in the G2/M phase. Consequently, after treatment with Glu–AuNPs, cancer cells were found to be more sensitive to radiotherapy (CitationOjeda et al. 1994).
It is well recognized that the FRs are overexpressed in a number of malignant cells such as ovarian, kidney, lung, breast, endometrial, colorectal, brain, etc., compared to their normal complement (CitationPatra et al. 2010, CitationTorchilin 2007).
AuNPs were found to inhibit the expression of stem cell markers and reduce the pool of SP cells. Exploring the signaling that regulates AuNP-mediated inhibition of stemness will help to identify key players involved in the cisplatin resistance and develop AuNP-based combination therapy (CitationXiong et al. 2014).
Inhibition of Akt/NF-κB signaling by AuNPs provides an inorganic nanomaterial-based therapeutic approach for sensitizing cells to cisplatin by decreasing EMT and stemness, and thus may play an important role in therapeutic management of ovarian cancer (CitationXiong et al. 2014).
Conclusions
NPs possess great potential for future clinical applications in imaging, diagnosis, and therapeutics. Further clinical investigations will determine the translation of AuNPs toward clinical applications. AuNPs show promise for both the imaging and treatment of breast and ovarian cancers. They possess characteristics which could improve ovarian cancer therapy.
Authors’ contributions
LK and TK conceived of the study and participated in its design and coordination. AA participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University, for all support provided. This work is funded by a 2014 grant by the Drug Applied Research Center, Tabriz University of Medical Sciences.
Declaration of interest
The authors have no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agarwal R, Kaye SB. 2003. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 3:502–516.
- Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, et al. 2010. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 10:2543–2548.
- Baptista PV. 2012.Could gold nanoprobes be an important tool in cancer diagnostics? Expert Rev Mol Diagn. 12:541–543.
- Briñas RP, Hu M, Qian L, Lymar ES, Hainfeld JF. 2008. Gold nanoparticle size controlled by polymeric Au (I) thiolate precursor size. J American Chem Soc. 130:975–982.
- Brongersma ML. 2003. Nanoscale photonics: nanoshells: gifts in a gold wrapper. Nat Mat. 2:296–297.
- Carmeliet P, Jain RK. 2000. Angiogenesis in cancer and other diseases . Nature. 407:249–257.
- Chattopadhyay N, Cai Z, Pignol J-P, Keller B, Lechtman E, Bendayan R, Reilly RM. 2010. Design and characterization of HER-2-targeted gold nanoparticles for enhanced X-radiation treatment of locally advanced breast cancer. Mol Pharm. 7:2194–2206.
- Chauhan SC, Kumar D, Jaggi M. 2009. Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res. 2:21.
- Chen J, Irudayaraj J. 2009. Quantitative investigation of compartmentalized dynamics of ErbB2 targeting gold nanorods in live cells by single molecule spectroscopy. ACS Nano. 3:4071–4079.
- Chen PC, Mwakwari SC, Oyelere AK. 2008. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnol Sci Appl. 1:45–65.
- Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. 2003. Pathology and classification of ovarian tumors. Cancer. 97:2631–2642.
- Cheng L, Yang K, Li Y, Zeng X, Shao M, Lee ST, Liu Z. 2012. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 33:2215–2222.
- Chithrani BD, Ghazani AA, Chan WC. 2006. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6:662–668.
- Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. 2006. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem. 17:603–609.
- Dreaden EC, Gryder BE, Austin LA, Tene Defo BA, Hayden SC, Pi M, et al. 2012. Antiandrogen gold nanoparticles dual-target and overcome treatment resistance in hormone-insensitive prostate cancer cells. Bioconjug Chem. 23:1507–1512.
- Dreaden EC, Mwakwari SC, Sodji QH, Oyelere AK, El-Sayed MA. 2009. Tamoxifen− poly (ethylene glycol)− thiol gold nanoparticle conjugates: enhanced potency and selective delivery for breast cancer treatment. Bioconj Chem. 20:2247–2253.
- Dykman L, Khlebtsov N. 2011. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 3:34.
- Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M. 2008. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 9:287–291.
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics.2000. CA Cancer J Clin. 50:7–33.
- Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. 2008. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 60:977–985.
- Hainfeld JF, Slatkin DN, Smilowitz HM. 2004. The use of gold nanoparticles to enhance radiotherapy in mice. Physics in medicine and biology. 49:N309.
- Han G, Martin CT, Rotello VM. 2006. Stability of gold nanoparticle‐bound DNA toward biological, physical, and chemical agents. Chem Biol Drug Des. 67:78–82.
- Herzog TJ, Pothuri B. 2006. Ovarian cancer: a focus on management of recurrent disease. Nat Clin Pract Oncol. 3:604–611.
- Hoek M, Stillman B. 2003. Chromatin assembly factor 1 is essential and couples chromatin assembly to DNA replication in vivo. Proc Natl Acad Sci U S A. 100:12183–12188.
- Huang X, El-Sayed MA. 2010. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 1:13–28.
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. 2008. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 23:217–228.
- Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. 2006. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 110:7238–7248.
- Jin H, Hong B, Kakar SS, Kang KA. 2008. Tumor-specific nano-entities for optical detection and hyperthermic treatment of breast cancer. Adv Exp Med Biol. 614:275–284.
- Joshi P, Chakraborti S, Ramirez-Vick JE, Ansari Z, Shanker V, Chakrabarti P, Singh SP. 2012. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Collids Surf B Biointerfaces. 95:195–200.
- Khlebtsov N, Dykman L. 2011. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 40:1647–1671.
- Kumar A, Boruah BM, Liang XJ. 2011. Gold nanoparticles: promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J Nanomater. 2011:22.
- Ledley FD. 1996. Pharmaceutical approach to somatic gene therapy. Pharm Res. 13:1595–1614.
- Leung DW, Cachianes G, Kuang W-J, Goeddel DV, Ferrara N. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 246:1306–1309.
- Li JL, Wang L, Liu XY, Zhang ZP, Guo HC, Liu WM, Tang SH.2009. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 274:319–326.
- Link S, El-Sayed MA. 1999. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B. 103:4212–4217.
- Link S, Wang ZL, El-Sayed MA. 1999. Alloy formation of gold-silver nanoparticles and the dependence of the plasmon absorption on their composition. J Phys Chem B. 103:3529–3533.
- Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. 2005. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev. 105:1103–1170.
- Lu W, Arumugam SR, Senapati D, Singh AK, Arbneshi T, Khan SA, et al. 2010. Multifunctional oval-shaped gold-nanoparticle-based selective detection of breast cancer cells using simple colorimetric and highly sensitive two-photon scattering assay. ACS Nano. 4:1739–1749.
- Mahdihassan S. 1985. Cinnabar-gold as the best alchemical drug of longevity, called Makaradhwaja in India. Am J Chin Med. 13:93–108.
- Maunsbach AB, Afzelius BA. 1998. Biomedical Electron Microscopy: Illustrated Methods and Interpretations. San Diego, London: Academic Press.
- McGuire WP, Ozols RF (Eds.). 1998. Chemotherapy of advanced ovarian cancer. Semin Oncol. 25:340–348.
- McIntosh CM, Esposito EA, Boal AK, Simard JM, Martin CT, Rotello VM. 2001. Inhibition of DNA transcription using cationic mixed monolayer protected gold clusters. J Am Chem Soc. 123:7626–7629.
- Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, et al. 2005. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 11:3530–3534.
- Nahta R, Esteva FJ. 2006. Molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 8:667–674.
- Ojeda F, Diehl HA, Folch H. 1994.Radiation induced membrane changes and programmed cell death: Possible interrelationships. Scanning Microsc. (3):645–651.
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. 2004. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 11:169–183.
- Park J, Estrada A, Sharp K, Sang K, Schwartz JA, Smith DK, et al. 2008. Two-photon-induced photoluminescence imaging of tumors using near-infrared excited gold nanoshells. Opt Express. 16:1590–1599.
- Patra CR, Bhattacharya R, Mukherjee P. 2010. Fabrication and functional characterization of goldnanoconjugates for potential application in ovarian cancer. J Mater Chem. 20:547–554.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. 2007. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnol. 2:751–760.
- Qiao Y, Huang X, Nimmagadda S, Bai R, Staedtke V, Foss CA, et al. 2011. A robust approach to enhance tumor-selective accumulation of nanoparticles. Oncotarget. 2:59.
- Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. 1995. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 104:946–952.
- Revenko AS, Kalashnikova EV, Gemo AT, Zou JX, Chen HW. 2010. Chromatin loading of E2F-MLL complex by cancer-associated coregulator ANCCA via reading a specific histone mark. Mol Cell Biol. 30:5260–5272.
- Richards DG, McMillin DL, Mein EA, Nelson CD. 2002. Gold and its relationship to neurological/glandular conditions. Int J Neurosci. 112:31–53.
- Risau W. 1997. Mechanisms of angiogenesis. Nature. 386:671–674.
- Sastry M, Rao M, Ganesh KN. 2002. Electrostatic assembly of nanoparticles and biomacromolecules. Acc Chem Res. 35:847–855.
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. 1986. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 46:5629–5632.
- Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. 2003. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 63:1999–2004.
- Su CH, Sheu HS, Lin CY, Huang CC, Lo YW, Pu YC, et al. 2007. Nanoshell magnetic resonance imaging contrast agents. J Am Chem Soc. 129:2139–2146.
- Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. 1997. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 74:193–198.
- Torchilin VP. 2007. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 9:E128–E47.
- Turner J, Koumenis C, Kute TE, Planalp RP, Brechbiel MW, Beardsley D, et al. 2005. Tachpyridine, a metal chelator, induces G2 cell-cycle arrest, activates checkpoint kinases, and sensitizes cells to ionizing radiation. Blood. 106:3191–3199.
- Wang AZ, Gu F, Zhang L, Chan JM, Radovic-Moreno A, Shaikh MR, Farokhzad OC. 2008. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin Biol Ther. 8:1063–1070.
- Wang Y, Chen J, Irudayaraj J. 2011. Nuclear targeting dynamics of gold nanoclusters for enhanced therapy of HER2 + breast cancer. Acs Nano. 5:9718–9725.
- Xiong X, Arvizo RR, Saha S, Robertson DJ, McMeekin S, Bhattacharya R, Mukherjee P. 2014. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget. 5:6453–6465.
- Xu C, Wang B, Sun S. 2009. Dumbbell-like Au− Fe3O4 nanoparticles for target-specific platin delivery. J Am Chem Soc. 131:4216–4217.
- Yallapu MM, Jaggi M, Chauhan SC. 2010. Scope of nanotechnology in ovarian cancer. J Ovarian Res. 3:19.
- Yan H, LaBean TH, Feng L, Reif JH. 2003. Directed nucleation assembly of DNA tile complexes for barcode-patterned lattices. Proc Natl Acad Sci U S A. 100:8103–8108.
- Zwicke GL, Mansoori GA, Jeffery CJ. 2012.Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 3.

