Abstract
Background:
More than 100,000 patients each year in Denmark experience nosocomial infections, erroneous medication, or pressure ulcers while hospitalized. The Danish Safer Hospital Program includes 12 bundles for improving patient safety through the introduction and maintenance of evidence-based routine treatment or standard procedures.
Objective:
To determine cost-effectiveness of implementing the Ventilator bundle (VB), thereby reducing ventilator-associated pneumonia (VAP), when treating a ventilated patient, compared to standard procedure.
Setting and patients:
A hypothetical population of intensive care patients in a Danish ICU, ventilated for >48 h.
Methods:
Cost-effectiveness analysis of the implementation of VB. The outcomes were prevention of VAP and prevention of death. Model inputs were evidence based from literature along with data from Kolding Hospital. A hypothetical population of intensive care patients in a Danish ICU, ventilated for >48 h was used.
Results:
The cost per VAP episode prevented was ∼€4451, and cost per death prevented was ∼€31,792. The incremental cost-effectiveness scatter plot showed that VB was more effective in 99.9%, and 42.6% have lower cost and better outcome for prevention of VAP. The incremental cost-effectiveness scatter plot showed that VB was more effective in 85.9%, and 31.6% have lower cost and better outcome for death prevented.
Limitations:
The study was a retrospective cost analysis where incidence rates were based on best evidence, even if it did not cover all elements in the VB. The perspective of this study was seen from a third-party payer, e.g., the hospital, thus societal costs and direct medical costs post-hospitalization for patients with VAP were not considered.
Conclusion:
We found that implementation of VB is potentially cost-effective when considering prevention of one case of VAP or death, based on a Danish ICU as a case study.
Introduction
Despite increased focus on patient safety, ∼100,000 patients each year in Denmark experience nosocomial infections, erroneous medication, or pressure ulcers while hospitalizedCitation1. Inadvertent conditions are costly for hospitals and the society, additionally influence the patient’s quality-of-life, and may in severe cases be fatal. In order to prevent these inadvertent conditions, five Danish hospitals are participating in the Danish Safer Hospital Program, which includes implementation and evaluation of 12 treatment guidelines, known as bundles, focusing on improving patient safety through the introduction and maintenance of evidence-based routine treatment or standard procedures, e.g. the AMI-, Septic-, or Ventilator bundle. This article will focus on the cost-effectiveness of the Ventilator bundle (VB), a guideline developed to decrease nosocomial infections and time spent in a mechanical ventilator for patients requiring mechanical ventilation admitted to an ICUCitation1.
Patients suffering from a disease making them dependent on mechanical ventilation are at risk of developing Ventilator-Associated Pneumonia (VAP), as a consequence of treatment and not the primary reason for their hospitalization. VAP is defined as a newly-developed infection of the lungs after >48 h of mechanical ventilation and with a Clinical Pulmonary Infection Score >6Citation2. VAP has been shown to prolong the duration of mechanical ventilation, length of stay (LOS) in intensive care unit (ICU), and increased risk of mortalityCitation3,Citation4.
The Danish VB contains five elements, which are not included in standard procedure of VAP:
Elevation of the head of the bed between 30–45° has been shown to significantly reduce the risk of developing VAP by decreasing the aspiration of secretionsCitation5,Citation6.
Sedation protocol, designed to minimize the time a patient is mechanically ventilated and to reduce complications, which limits the risk of developing VAP by daily interrupting the sedation of the ventilated patientCitation7,Citation8.
Extubation protocol, with the intent to wean the patient from mechanical ventilation as early as possible by extubating the patient, which in studies has been shown to shorten the time spent receiving mechanical ventilation and thereby the total LOS in the ICUCitation9,Citation10.
Oral decontamination using antiseptics has been reported in several studies to reduce the risk of developing VAP by limiting hostile bacteria in the upper airwaysCitation11–15.
Deep venous thrombosis (DVT) prophylaxis seeks to prevent peripheral thrombosis in ventilated patientsCitation6.
The studies mentioned above have demonstrated how the risk of developing VAP can be reduced with implementation of one of the different elements of VB in the ICUCitation5–15. However, there are only limited investigations of the health economic potential of these five elements. No previous studies have calculated the cost-effectiveness of implementing all five elements in the VB in an ICU. Estimating the cost-effectiveness of implementing the Danish VB will allow a comparison to the existing standard procedure relevant for treating ventilated patients. This can further be used in a decision model to evaluate whether it is recommendable to implement VB at the ICU, given a fixed set of variables. We hypothesize that, despite a possible higher direct cost, it might be cost-effective to implement the VB when treating a ventilated patient, compared to standard procedure, as there may be cost-savings associated with the prevention of VAP. To investigate this, we have constructed a decision model and a cost-effectiveness analysis, to compare the costs and potential effects of implementing the VB compared to the standard procedure. The analysis will be based on best international evidence combined with direct costs from Kolding Hospital.
Methods
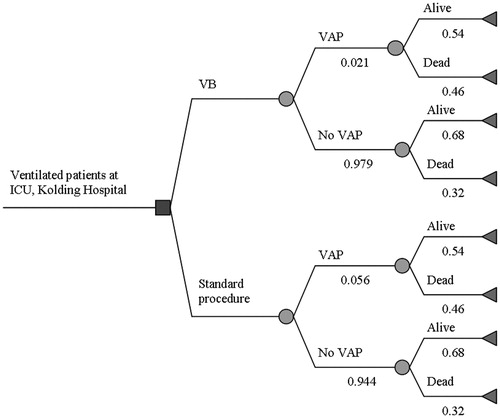
A decision analytic model, a cohort model, was constructed to assess the potential effects and costs of implementing VB compared to the standard procedure, see . An incremental cost-effectiveness ratio per VAP prevented and death prevented was estimated using TreeAge (Pro 2010; TreeAge Software, Williamstown, MA). Model inputs were taken from the literature as well as local data from Kolding HospitalCitation16–18.
Figure 1. Illustration of the decision model for the Ventilator Bundle (VB). Starting from the left, the blue decision node represents the choice of whether to implement the VB in the ICU, Kolding Hospital. The green chance nodes represent the probabilities of developing VAP both for VB and Standard procedure and probability of death. The red terminal end-points illustrate the end of each possible path in the decision tree and are associated with individual costs and effects.
Systematic literature search
We performed a systematic literature search in PubMed (including MEDLINE) and Cochrane Library, to determine the best available evidence for evaluation of the potential of introducing VB. The following keywords: ventilator associated pneumonia, oral decontamination, oral care, ventilator weaning, supine position, extubation, mortality, incidence rate, risk factors, length of stay, were used, along with various combinations of these keywords. The end date of search was May 25, 2011 and it was conducted by all authors. We chose to only include studies from the last 10 years, as treatment protocol and intensive care has developed rapidly during the last two decades. To identify incidence rates for VAP before and after implementation of intervention, average length of stay in ICU, prolonged length of stay in the ICU due to VAP, and mortality rates before and after implementation of intervention were identified and evaluated according to criteria decided by the authors. For studies that did not report an incidence rate, but did include number of patients in the study and number of patients that developed VAP, incidence rates were calculated.
In order for the studies to be included, they had to fulfill the following criteria:
The study must be performed at an ICU;
Data included in the study must be of recent date (2001 or more recent);
The study must report incidence rate (%) before and after implementation;
The study must include >100 subjects;
The study population must be >18 year of age;
The study must include one or more elements from the VB;
VAP must be diagnosed after 48 h;
Only full-text studies are included; and
The article must be published in English.
The studies included in all fulfilled the inclusion criteria, and, on the basis of expert opinions from physicians at Kolding Hospital, only Lansford et al.Citation19 and Mori et al.Citation14 were considered for the evaluation of incidence rate. Only one study found significant reduction in the mortality between patients developing VAPCitation4. Only one study revealed a significant difference for increased LOS for a patient with VAPCitation20.
Table 1. Presentation of the systematic literature search.
Data input
When performing the cost-effectiveness analysis, the following data were required for input: The time spent on implementation of the different elements in VB were measured by physicians and nurses at ICU on Kolding Hospital during their daily routines, listed in minutes (). These data were later validated by Hillerød Hospital, which also participates in the Danish Safer Hospital Program. Only costs relevant for the hospital were included in the study, thus no outpatient costs were included. Labor cost per effective hour for nurses specialized in intensive care and chief physicians was calculated from wage statistics for 2010 and covers gross salary including pension and pay supplements. Average cost of stay per day includes ventilator equipment, medication, staff, materials-and activity cost, service and maintenance of department, and property management. Cost of treatment for one episode of VAP includes diagnostics and antibiotic treatment. The cost of death for a moderately complicated patient is included as the incremental cost of mortality for inpatients. All the above-mentioned costs were retrieved in Danish Kroner (DKK) and converted into Euros (€) (). The average prolongation of hospitalization due to VAP was included and referred to as the increased length of stay (LOS) with VAP. Antibiotics cost was included in cost of VAP (), and used in one-way sensitivity analysis. The effect measures chosen for this analysis were number of cases of VAP prevented and number of deaths prevented. The effects were immediate for this study’s perspective and therefore not discounted, whereas capital costs were discounted (e.g. the mechanical ventilator, monitor, and moisturizers were discounted over 8 years at 3%). To avoid double counting when considering staff salary, implementation of VB and the time nurses spent on VB were calculated and subtracted from the cost of a bed day. Based on previous studies, the implementation of the elements in the VB is not associated with adverse events, thus will not be included. All costs were critically evaluated and conservatively approximated to limit the risk of over-estimating the ICER for VAP or death prevented. All unit-costs were 2010-prices.
Table 2. Time and cost (€) estimated for each element in the VB. Time illustrates the amount of minutes per days the health personnel uses to conduct the element for each patient, where cost for these have been calculated as time*hourly set salary for the health personnel. Cost for DVT prophylaxis and oral decontamination have been calculated by Pharmacy, Kolding Hospital. All data were price level 2010.
Table 3. Evidence-based model inputs and estimates by Kolding Hospital, along with distributions for each variable. Cost of VB includes capital costs, and these were discounted at 3% for 8 years.
Table 4. The expected costs and effects are displayed, which have been calculated from a cohort of 140 patients for each treatment.
Sensitivity analysis
The sensitivity analyses included a one-way analysis to identify the variables of the model, which cause important changes of the ICER as well as a probabilistic sensitivity analysis (PSA) of the total parameter uncertaintyCitation20,Citation21.
We have chosen to perform a one-way sensitivity analysis, including best- and worst case for each variable. Expert opinions from physicians at Kolding Hospital have been used to identify worst- and best case for cost of treatment and cost of stay in an ICU bed per day. Furthermore, the cost of death in the ICU for worst- and best case was changed according to the Danish DRG case mix system. All other inputs in the model were changed ±25% in best- and worst case.
We also performed a 2nd order Monte Carlo simulation with 1000 expected values for VB and standard procedure. For the PSA calculations, relevant distributions for the input were used. The distributions for costs are right-tailed; therefore, γ-distributions were applied, calculated by mean and standard deviations derived from literature or local data. For incidence rates, β-distributions were applied, as these can only assume the value from 0 to 1. Increased LOS due to VAP was normally distributed with mean and standard deviations from Kolding Hospital. Data from Kolding Hospital on LOS without VAP were analyzed with SPSS Software, illustrating a distribution with a right tail, implying a γ-distribution, calculated from mean and standard deviations of the data set from Kolding Hospital. All other standard deviations were calculated from ±25% of means from the input. The results of the 2nd order Monte Carlo simulation were interpreted by use of the cost-effectiveness acceptability curve (CEAC).
Furthermore, we calculated the expected costs and health outcomes from a cohort simulation of 140 patients corresponding to the annual number of patients at the intensive care unit at Kolding Hospital.
Results
The result of the cohort simulation is shown in , from which it is seen that VB has a potentially positive effect on the incidence of VAP compared to standard procedure, in the ICU, Kolding Hospital, with a catchment population of 140 patients per year. The cost of implementing VB is ∼€21,810 more per year than standard procedure. As five cases of VAP are being prevented, the ICER (base-case) was €4451 per case of VAP prevented. Furthermore, the ICER was DKK €31,792 per death prevented.
The one-way sensitivity analysis () revealed ICER intervals ranging from €910–11,333 per case of VAP prevented and €9032–80,949 per death prevented.
Table 5. One-way sensitivity analysis for each variable, demonstrating the individual influence for each variable on ICER.
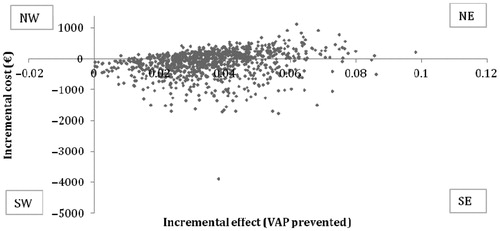
The results of the two PSA are illustrated in the incremental cost-effectiveness (ICE) scatter plot. The first illustrates the ICE scatter plot for 1000 hypothetical expected values (EVs) from the analysis of VAP prevented (), in which 42.6% have lower cost and better outcome (Quadrant SE) for VB compared to standard procedure. Higher cost and better effect are given in 57.3% (Quadrant NE). Lower effect and higher cost are given at 0.1% (Quadrant NW).
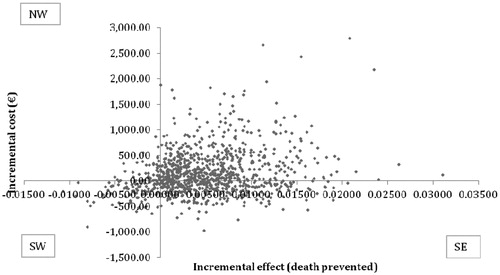
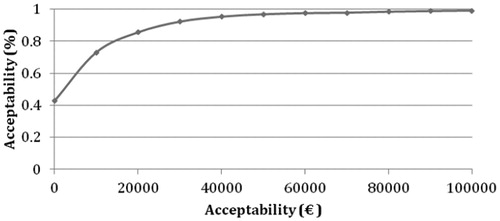
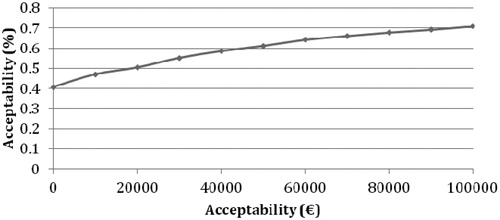
When the ICE scatter plot for 1000 hypothetical EVs from the PSA is plotted for deaths prevented (), 31.6% had lower cost and a better effect in the VB compared to standard procedure (Quadrant SE). A better effect at a higher cost was found in 54.3% (NE). Both lower cost and lower effect will result in 9% according to the PSA (Quadrant SW), and lastly 5.1% were both more expensive and had a lower effect (Quadrant NW). The outcome from the CEAC for patients in VB illustrates the cost-effectiveness of a hypothetical cohort of 1000 patients for implementation of VB for both VAP and death prevented, e.g., showing a cost-effectiveness of >80% per VAP prevented and >50% per death prevented, at an acceptability threshold of €20,000 ( and ).
From the one-way sensitivity analysis it was observed that the variability related to the incidence rate for old treatment and treatment length were the two parameters having the largest influence on the model, which also contributes to collected variability influencing ICER, as seen in the PSA for both VAP and death prevented. Both PSAs for VAP and death prevented show collective variability of the ICER, from which it is seen that the VB is predominantly cost-effective and in the majority of cases also cost-saving.
Discussion
In this study we have investigated the potential effects of implementing VB at the ICU, Kolding Hospital compared to the standard procedure of treating a patient in mechanical ventilation. This was displayed in a decision model and elaborated further in a cost-effectiveness analysis providing a mean ICER of €4451/VAP prevented or €31,792/death prevented. Additionally the sensitivity analysis was used to strengthen our model and provided an ICER ranging from €910–11,333 per VAP prevented or €9032–80,949 per death prevented. Based on the ICE scatter plot, our model would be effective in 99.9% (NE and SE quadrants in the ICE scatter plot); and in 42.6% of cases it would furthermore be cost-saving (Quadrant SE) in prevention of VAP. The ICE scatter plot for death prevented showed the model would be cost-saving in at least 31.6% (Quadrant SE) and more effective in 85.9% (Quadrant NE + SE), where the cost-saving result (31.6%) represents VB dominating the standard treatment and the more cost-effective result (85.9%) is further dependent on the willingness to pay by the decision-makers for new interventions.
The robustness of the study
The structural composition of the models used in this study is based on the international guidelinesCitation16 to limit bias, which potentially could affect the results. Furthermore, data used as effect measurements were only included from relevant articles of high levels of evidence. Similarly, costs provided by Kolding Hospital were critically evaluated.
The systemic literature search indicates, except for oral decontamination, that only a few studies reported effects of the elements included in VB. Several studies have reported incidence rates much higher than those chosen for this study, but only incidence rates below 10% as base case were considered for our cost-effectiveness analysis, as physicians at Kolding Hospital estimated this to be somewhat similar to incidence in Danish ICUs. In addition, a base case below 10% was also chosen as this is a conservative approximation and thereby limits the risk of over-estimating the ICER for VAP or death prevented. Our cost-effectiveness analysis is based on a calculated average of incidence rates from Mori et al.Citation14 and Lansford et al.Citation19 (5.6 vs 2.1%), but investigations reporting incidence rates based on implementation of VB in a Danish ICU would have strengthened our results. According to Hanberger et al.Citation22, Scandinavian incidence rates and antibiotic resistance rates are considerably lower compared to Southern European countries and relatively lower than North America. Thus, it is expected that a Danish ICU will have a lower incidence rate of VAP compared to the one reported in the American study by Lansford et al.Citation19, and therefore introducing the ventilator bundle at a Danish ICU would be less cost-effective.
The actual effect of implementing VB or at least one element is dependent on high compliance, particularly by the nurses. If the nurses do not follow the guidelines from the Danish Society for Patient Safety, or the patient’s condition prevents them (if they cannot endure having their head elevated), the compliance decreases, and the risk of developing VAP with VB remains unchanged, thus under-estimating the actual effect of implementing VB. In addition, it could be a problem that the effect measurements used in our models came from studies which did not investigate-the effect of implementing all five elements. The actual effect might therefore be higher if the effect of all five elements in VB were investigated, and the one used in our models may, therefore, be an under-estimate. Lastly, one should acknowledge the time spent by nurses and physicians, listed in , are based on a Danish ICU as a case study, and these may be different in other countries.
The ICE scatter plot of prevented death, showed 85.9% of the cases, had a higher effect for VB compared to standard procedure. There is an ongoing discussion whether mortality can be used as an outcome when considering implementing VBCitation23,Citation24. As studies report on different populations, different ways to determine if cause of death was directly caused by VAP, different patient groups, different pathologic conditions and treatments in the case of VAP vary between ICUs, it is difficult to match studies. One should acknowledge that ventilated patients often suffer from serious conditions, are heavily medicated, and are, even without VAP, at great risk of dyingCitation24. Several studies have demonstrated increased risk of mortality in the case of VAPCitation23, whereas others have found no significant difference in mortality, despite patients suffering from VAPCitation3,Citation23. Therefore, it can be argued whether the reduction from 46% to 32% in risk of mortality, if VAP is preventedCitation4 in this study, is representative for a Danish ICU. No conclusions should solely be based on our potential ICER of €31,792/death prevented if VB was implemented.
In addition, the total cost of an average bed day is not specific to patient groups, and the exact cost of one bed day for a ventilated patient might be higher, as this patient group often suffers from serious conditions and are heavily medicated. If the average bed day cost actually is higher for a patient receiving mechanical ventilation, the ICER would favor the implementation of VB, as seen in the one-way sensitivity analysis.
Only prevention of VAP or death were considered as relevant outcomes for this study; however, for future studies, one might consider including additional outcomes, e.g., peripheral thrombosis and other complications as a consequence of mechanical ventilation or VAP, relevant for the patient’s safety and cost of treatment.
Conclusion
In conclusion we found that implementation of VB is potentially cost-effective, with an ICER of €4451/VAP prevented or €31,792/death prevented, based on a Danish ICU as case study.
Transparency
Declaration of funding
This study had no funding or financial relationships with an external sponsor.
Declaration of financial/other interests
The authors of this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgments
The authors would like to thank Jane Stab Nielsen, Torben Gilsaa (Chief physicians, Kolding Hospital), and Jacob Brix Petersen (Planning and Finance Department, Kolding Hospital) for their inputs, discussion, and data contributions to this article. Authors Møller, Hansen, Ehlers and Jensen made equal contributions to the writing and editing of this manuscript.
References
- Dansk Selskab for Patientsikkerhed. Patientsikkert sygehus - respirator pakken. 2010;1-10
- Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis 2010;51(1 Suppl):131-5
- Heyland DK, Cook DJ, Griffith L, et al. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian critical trials group. Am J Respir Crit Care Med 1999;159:1249-56
- Ibrahim EH, Tracy L, Hill C, et al. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest 2001;120:555-61
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 1999;354:1851-8
- Wip C, Napolitano L. Bundles to prevent ventilator-associated pneumonia: how valuable are they? Curr Opin Infect Dis 2009;22:159-66
- Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med 2004;32:1272-6
- Kress JP, Gehlbach B, Lacy M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med 2003;168:1457-61
- Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish lung failure collaborative group. N Engl J Med 1995;332:345-50
- Frutos-Vivar F, Ferguson ND, Esteban A, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 2006;130:1664-71
- Chan EY, Ruest A, Meade MO, et al. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 2007;334:889
- Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med 2006;173:1348-55
- Fourrier F, Dubois D, Pronnier P, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo controlled multicenter study. Crit Care Med 2005;33:1728-35
- Mori H, Hirasawa H, Oda S, et al. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med 2006;32:230-6
- Tantipong H, Morkchareonpong C, Jaiyindee S, et al. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect Control Hosp Epidemiol 2008;29:131-6
- Kristensen FB, Sigmund H, Andersen SE, et al. Metodehåndbog for medicinsk teknologi vurdering. Copenhagen, Denmark: Sundhedstyrelsen, 2007
- Buxton MJ, Drummond MF, Van Hout BA, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ 1997;6:217-27
- Drummond MF, O’Brien BJ, Stoddart GL, et al. The methods of economic evaluation of health care programmes. Oxford University, England: Oxford University Press, 2007
- Lansford T, Moncure M, Carlton E, et al. Efficacy of a pneumonia prevention protocol in the reduction of ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt) 2007;8:505-10
- Tan-Torres Edejer T, Baltussen R, Adam T, et al. WHO guide to cost-effectiveness analysis - making choices in health. Geneva: World Health Organization, 2003
- Tonnelier JM, Prat G, Le Gal G, et al. Impact of a nurses’ protocol-directed weaning procedure on outcomes in patients undergoing mechanical ventilation for longer than 48 hours: a prospective cohort study with a matched historical control group. Crit Care 2005;9:83-9
- Hanberger H, Diekema D, Fluit A, et al. Surveillance of antibiotic resistance in European ICUs. J Hosp Infect 2001;48:161
- Safdar N, Dezfulian C, Collard HR, et al. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 2005;33:2184-93
- Solomkin JS. Cost-effectiveness issues in ventilator-associated pneumonia. Respir Care 2005;50:956-64
- Rose L, Baldwin I, Crawford T, et al. Semirecumbent positioning in ventilator dependent patients: a multicenter, observational study. Am J Crit Care 2010;19:100-8
- Quenot JP, Ladoire S, Devoucoux F, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med 2007;35:2031-6
- van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med 2006;34:396-402
- Bergmans DC, Bonten MJ, Gaillard CA, et al. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo controlled study. Am J Respir Crit Care Med 2001;164:382-8
- Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115-21