Abstract
Objectives:
Knee cartilage damage is a common cause of referral for orthopedic surgery. Treatment aims to reduce pain and symptoms by repairing cartilage. Microfracture, the current standard of care, yields good short-term clinical outcomes; however, treatment might fail after 2–3 years. A Chitosan-Beta glycerolphosphate-based medical device (BST-CarGel) is used as an adjunct to microfracture and demonstrates improvements in quantity and quality of repaired tissue, potentially reducing the risk of treatment failure. This study aimed to establish the economic value of BST-CarGel vs microfracture alone in knee cartilage repair from the societal perspective, using Germany as the reference market.
Methods:
A decision tree with a 20-year time-horizon was constructed, in which undesirable clinical events were inferred following initial surgery. These events consisted of pain management, surgery, and total knee replacement. Clinical outcomes were taken from the pivotal clinical trial, supplemented by other literature. Data and assumptions were validated by a Delphi panel. All relevant resource use and costs for procedures and events were considered.
Results:
In a group of patients with all lesion sizes, the model inferred that BST-CarGel yields a positive return on investment at year 4 (with 20-year cumulative cost savings of €6448). Reducing the incremental risk of treatment failure gap between the device and microfracture by 25–50% does not alter this conclusion. Cost savings are greatest for patients with large lesions; results for patients with small lesions are more modest.
Limitations:
Clinical evidence for microfracture and other interventions varies in quality. Comparative long-term data are lacking. The comparison is limited to microfracture and looks only at costs without considering quality-of-life.
Conclusion:
BST-CarGel potentially represents a cost-saving alternative for patients with knee cartilage injury by reducing the risk of clinical events through regeneration of chondral tissue with hyaline characteristics. Since the burden of this condition is high, both to the patient and society, an effective and economically viable alternative is of importance.
Introduction
Knee cartilage facilitates the movement of bones against each other whilst reducing friction. Following injury, cartilage has only limited capacity to repair itselfCitation1 and, left untreated, there is a long-term risk of developing secondary osteoarthritis. This causes disability and places a large socioeconomic burden on societyCitation2–6, often leading to requirement for a total knee replacement (TKR)Citation7.
Articular damage is a common cause of referral for orthopedic surgeryCitation8. Chondral lesions were identified in 63% of 31,516 patients undergoing knee arthroscopies in a retrospective review over a 4-year periodCitation2. These results are reflected in a review of 1000 knee arthroscopies, in which chondral lesions were identified in 61% of patientsCitation3, and an evaluation of 993 consecutive knee arthroscopies that identified articular cartilage pathology in 66% of patientsCitation9.
Treatment of cartilage damage aims to restore long-term painless joint motion, inducing repair of chondral tissue with hyaline characteristicsCitation10, which allows movement without frictionCitation7. Restoration of chondral tissue with hyaline characteristics may delay or prevent secondary osteoarthritis and the eventual need for TKR. Three types of cartilage repair surgery are currently available: cartilage/bone graft (for example, osteochondral allograft transplantation or osteochondral autologous transplantation [mosaicplasty]); cultured cell/tissue implantation (for example, autologous chondrocyte implantation [ACI]); bone marrow stimulation (BMS) to promote a healing response (for example, microfracture). Microfracture, mosaicplasty, ACI and osteochondral allograft transplantationCitation11 all relieve pain and improve joint function, but without complete restoration of the hyaline structure of the cartilageCitation12; tissue deterioration is often seen in the long-term, leading to a requirement for revision surgeryCitation13–16.
Microfracture, in which perforations are made in the sub-chondral bone plate to induce the formation of a blood clot to act as an optimal environment for healing and thus for new tissue formationCitation17, has become established as standard of care for chondral injuriesCitation4,Citation11,Citation18,Citation19 because it is less expensive than other interventions, quick and relatively unchallenging, technically. Good functional outcomes are consistently achieved up to 24-months post-procedureCitation7,Citation11,Citation18 and post-operative complications are generally rareCitation20–26. Microfracture can mostly be a short-term solution, since a variable amount of the new cartilage produced is similar to fibrocartilageCitation27, without the desirable hyaline characteristics necessary for a healthy joint, and thus has reduced resistance to wear, leading to functional deterioration over timeCitation11,Citation18,Citation22. Furthermore, inconsistencies in the quantity and quality of the stimulated blood clot can lead to highly variable outcomesCitation28–30.
BST-CarGel, a chitosan-based medical device, is designed to be used as an adjunct to BMS procedures, such as microfracture, to physically stabilise the blood clot via mixing with uncoagulated (autologous) peripheral whole bloodCitation30. The device acts to promote healing at early stages by increasing inflammatory and bone marrow-derived stromal cell (connective tissue) recruitment, vascularisation of repair tissue and intramembranous bone formation and bone re-modellingCitation29. As with microfracture, a post-surgical programme of rehabilitation is required to optimise clinical outcomesCitation17.
BST-CarGel as an adjunct to microfracture was compared with microfracture alone in an international, multi-centre, randomized, single-blind, controlled trialCitation31, in which patients were followed up for 12 months post-intervention. Compared with microfracture alone, the device was associated with statistically superior quantity (p = 0.011) and quality (p = 0.033) of repair tissue, alongside comparable safety outcomesCitation31. Clinical improvement, as demonstrated by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scales, was significant compared with baseline (p < 0.0001)Citation31; however, there were no statistically significant differences between the study groups at 12 months.
The objective of this analysis was to evaluate the economic relevance of the device as an adjunct to microfracture vs microfracture alone in articular cartilage repair surgery in the knee, in terms of resource use and costs incurred by patients, clinical outcomes (events) of the evaluated alternatives, and overall economic efficiency and relevance of the alternative. It was anticipated that use of the device could reduce healthcare resource use and costs, via improvements in the hyaline characteristics of the regenerated tissue compared with microfracture alone and through greater quantity and quality of the cartilage repair, which would lead to a treatment failure risk reduction.
Patients and methods
Description of decision analytic model
The economic evaluation was conducted via a linear decision tree model constructed in Microsoft Excel (). Patients enter the model with the requirement of cartilage repair for a lesion <7 cm2 in size, at which point the decision is made to implement treatment with either the device as an adjunct to microfracture or microfracture alone. Patients then possibly experience the risk of undesired clinical events following the initial surgical intervention. In the model it is assumed that a patient who is considered a treatment success does not consume any medical resource utilization or miss work days due to the initial cartilage repair, as validated by the Delphi panel. Those who experience treatment failure follow a cascade of events due to sub-optimal cartilage repair, in which the incremental risk is derived from the randomized, controlled trial (RCT) of BST-CarGel compared with microfracture alone in the repair of cartilage lesions in the kneeCitation31. These clinical events include conservative pain management, second cartilage repair (specifically ACI), conservative pain management due to failure of the second surgical intervention, and TKR due to failure of the second surgical intervention. Treatment patterns for clinical events are consistent between both arms of the model. Adverse events related to the initial surgery are not considered since the safety profile for the device as an adjunct to microfracture has been demonstrated to be similar at to that for microfracture alone at 12 monthsCitation31.
Figure 1. Graphic of the decision tree model used to infer the economic evaluation. Patients enter the model at the point of cartilage repair, which is undertaken with either the device as an adjunct to microfracture or microfracture alone and subsequently follow a cascade of events.
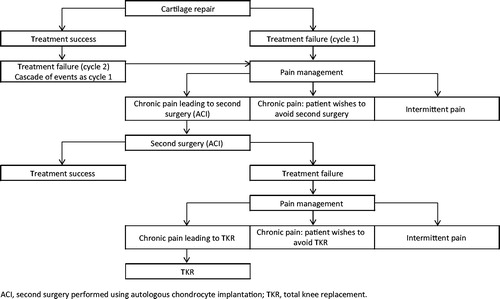
Treatment failure is assumed to occur in two ‘cycles’ (). As mentioned previously, initial cartilage repair with microfracture alone is intended to be a relatively short-term intervention, providing relief for 2–3 yearsCitation7,Citation11,Citation18; cycle 1 refers to treatment failure at this point. Some patients, however, may enjoy benefits of the initial intervention for 5 years or moreCitation18, before experiencing treatment failure in cycle 2. The total time-horizon for the model is 20 years, which is considered to be adequate to capture the incremental long-term risk of TKR.
Patient population considered in the analysis
The hypothetical cohort of patients modelled is assumed to take the characteristics of the subjects included in the RCT used to assess the risk of clinical events following initial intervention with either the device or microfracture aloneCitation31 (). The average age of patients entering the model is 35 years. Maximum lesion size for repair is 7 cm2; the RCT did allow lesions up to 10 cm2 in sizeCitation31 but no patient presented with a lesion larger than 7 cm2 so, therefore, the efficacy of the device in lesions greater than this cannot be said to have been demonstrated. Patients presented with a single lesion in the articular cartilage of the medial or lateral femoral condyle, classified as focal, full-thickness, grade 3 or 4 cartilage lesion (International Cartilage Repair Society score; Outerbridge score).
Table 1. Baseline characteristics of subjects treated with the device as an adjunct to microfracture and microfracture only in the RCT driving clinical outcomes in the decision tree modelCitation31.
Intervention and comparator
In the pivotal RCT, microfracture was performed arthroscopically and the device applied via a mini-arthrotomyCitation31; mixture volume per patient varied according to lesion size. Following initial intervention, all patients included in the RCT underwent a 12-week physiotherapy program, compliance with which was recordedCitation31 (see ). There were, therefore, no differences between study groups regarding quality of the surgical procedure and the rehabilitation program.
Perspective of the analysis
The economic evaluation was conducted from the perspective of the public healthcare provider and of society, and considered both direct medical costs and indirect costs (workdays lost). Germany was taken as the reference market for determining unit costs; however, input into model methodology, assumptions, and data was obtained from experts representing Germany, Italy, the UK, and Canada, so it is anticipated that outcomes and conclusions from the analysis are largely applicable to a wider audience across Europe and North America.
Discounting
Costs were discounted at a rate of 3% per annumCitation32.
Data sources
Incremental clinical outcomes from the RCT of the device vs microfracture aloneCitation31 were supplemented by information on treatment failure risk, treatment patterns, and costs, as determined by a literature review of both published and unpublished data. A Delphi panel of six internationally-recognized specialist orthopedic surgeons from across Europe and Canada was used to validate clinical assumptions, methodology, and model parameters.
Clinical outcomes related to initial intervention
Cartilage repair procedures aim to reduce pain and symptoms by replacing or regenerating articular tissue with characteristics close to those of native cartilageCitation15,Citation16, thus optimizing durability and function. Particular attributes of importance include components of the extracellular matrix, cartilage structure, cellular properties, and integration with surrounding bone and other native tissuesCitation33. Outcomes post-microfracture procedure have, accordingly, been demonstrated to be associated with the hyaline characteristics of the repaired chondral tissue; in terms of the quantity and quality of the repairCitation18,Citation34–36.
Functional and clinical outcomes correlate with the percentage of the lesion that is filled with repaired tissueCitation32,Citation37. Whilst complete lesion fill does not appear to be necessary for good short-term outcomes with microfracture (as demonstrated by consistent clinical improvements up to 2 years in the majority of patients), decrease in knee function at 24 months post-procedure has been primarily noted in patients in whom lesion fill was poorCitation37. A 2009 systematic review of the clinical evidence for microfracture supports this associationCitation18. Cartilage repair was evaluated with MRI in nine of the studies included in the review, across a total of 361 patients. The authors showed that the extent of lesion fill was demonstrated to correlate with functional outcomeCitation37–39.
In order to optimize clinical outcomes with microfracture, the procedure must be conducted to a consistent standard. There is wide variation between surgeons performing the microfracture procedure in relation to indications for surgery, surgical technique, post-operative rehabilitation and assessment of outcomeCitation40. In a recent study of Canadian orthopedic surgeons, 41% had no upper limit for body mass index (BMI) above which patients were not admitted for surgery, 31% did not remove calcified cartilage prior to creating holes, 89% did not use continuous passive motion (CPM) post-operatively, and 39% did not restrict weight-bearingCitation40. In general, outcomes achieved in trials are not borne out in clinical practice because the surgery is not always conducted according to good practiceCitation41. In order to optimize outcomes when using BST-CarGel, surgeons will be trained at an excellence center. This will help to guarantee consistent high-quality surgical technique and appropriate use of the intervention.
The improvement in clinical outcomes achieved after initial intervention with the device vs microfracture alone in the model pivots, therefore, on the following differentiating assumptions: (1) the device is associated with greater percentage lesion fill than microfractureCitation31; (2) the device promotes the generation of better quality tissue, with more hyaline characteristic cartilage than microfracture aloneCitation31; (3) treatment with the device is only implemented by surgeons trained in excellence centres, resulting in higher-quality procedures than are often seen with microfracture, in which important steps are often missedCitation40.
Percentage lesion fill <70% has been assumed in the analysis as the threshold for inferring treatment failure. This is based on the gradations of cartilage repair fill as reported by Mithoefer et al.Citation37 (good: 67–100% fill; moderate: 34–66% fill; poor: 0–33% fill) and validated by the Delphi panel. The incremental difference in the proportion of patients failing the threshold lesion fill rate of 70% for the device vs microfracture alone (determined by blinded three-dimensional quantitative MRI; 12-month dataCitation31 extrapolated to 36 months) for all lesion sizes, lesions ≥2 cm2 and lesions <2 cm2, is presented in . Incremental treatment failure risk reduction for the device vs microfracture alone is assumed to be greater in patients with large lesions, since the performance of microfracture in these patients is significantly reduced. The converse applies to patients with small lesions. Also presented in is the adjusted incremental treatment failure risk reduction applied in the model for each patient sub-group, taking into account the quantity and quality of generated cartilage and the training setting for surgeons undertaking the BST-CarGel procedure.
Table 2. The incremental difference in proportion of patients failing the threshold lesion fill rate assumed for treatment success (70%), and the resultant incremental treatment failure rates (at 3 years post-initial intervention), with the device vs microfracture alone, for all lesion sizes, lesions ≥2 cm2 and lesions <2 cm2.
For the base case patient population of those with all lesion sizes, the incremental risk of entering the cascade of events has been assigned at 20%, reflecting the incremental proportion of patients in the microfracture group who will receive pain management. The incremental treatment failure rate for microfracture alone vs the device is applied both at year 3 (cycle 1) and at year 5 (cycle 2). The incremental treatment failure rate is explored in sensitivity analysis by assuming alternative values of 25% and 15%.
Consistent post-initial intervention treatment failure risks are applied after both the device and microfracture alone and in cycle 1 and cycle 2. According to the cascade of events underlying assumptions, TKR is inferred to occur at year 10 for those failing initial intervention in cycle 1 and at year 12 for those failing in cycle 2.
Resource use
Intervention with the device was assumed to incur all surgery resource use associated with microfracture alone, along with an additional 30 minutes of surgery time.
Resource use associated with the inherent risk of entering the cascade of events is reported in . All resource use assumptions were based primarily on independent market researchCitation41 and validated by the Delphi panel. For those patients with chronic or intermittent pain not undergoing ACI, there is increased use of pain medications and a small proportion of patients will undergo lavage and debridement surgery.
Table 3. Resource use assumptions for all treatments post-failure of initial intervention, presented as a proportion of patients undergoing the intervention who incur the resource use (weighted risk) and the amount of resource use incurred (resource intensity). Assumptions based on independent market researchCitation41 and validated by the Delphi panel.
Unit costs
Unit costs applied to resource use in the analysis (in German Euros) are reported in .
Table 4. Unit costs for resource use applied in the economic analysis in Euros, using Germany as a reference.
Scenario analysis
Scenario analyses are conducted on lesion size considered (large, ≥2 cm2 and small, <2 cm2) and incremental treatment failure rate for the device vs microfracture alone (25% and 15%).
Results
Although results according to the societal perspective are reported here, it should be noted that indirect costs comprised only 5% of the incremental cost savings over the 20-year period.
All lesions sizes, incremental treatment failure of 20%
Incremental costs savings for BST-CarGel compared with microfracture alone, by cost component (clinical event), at time-horizons up to 20 years, are presented in and .
Figure 2. Cumulative incremental cost savings (€) for the device vs microfracture alone, at time-horizons up to 20 years (no cost savings are realized in years 1 and 2, since treatment failure is assumed to occur in year 3 in cycle 1 and year 5 in cycle 2). Costs are discounted at a rate of 3.0% per annum.
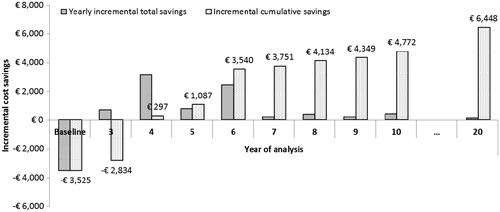
Table 5. Base case incremental cost savings (€) for the device vs microfracture alone, at time-horizons up to 20 years (no cost savings are realized in years 1 and 2, since treatment failure is assumed to occur in year 3 in cycle 1 and year 5 in cycle 2; ‘…’ indicates ongoing resource use between years 10 and 20). Results are presented for cycle 1 and cycle 2 and as yearly and cumulative incremental total cost savings. Costs are discounted at a rate of 3% per annum.
Although the device is associated with an initial incremental investment of €3525 over microfracture alone, the model infers that the reduced incremental treatment failure risk means that cost savings of €297 are realized by year 4 and, at 20 years post-initial intervention, the device is associated with a cumulative total incremental saving of €6448 vs microfracture alone. The main drivers of this financial benefit are risk reductions in the initial pain management (PM1) and the second surgery required in patients undergoing the device compared with those undergoing microfracture.
During cycle 1, the model infers that the device will generate incremental cost savings of €691 for pain management at year 3 (the point at which the first cycle treatment failure is assumed to occur in the model); at year 4 this incremental cost saving is reduced significantly since 75% of the pain management cohort will require a second surgical intervention (ACI). During cycle 2, the model infers that the device will generate incremental cost savings (€494) in year 5 (at which point the second cycle of treatment failure is assumed to occur in the model); at year 6 this incremental cost saving is reduced significantly since 75% of the pain management cohort will require a second surgical intervention (ACI). Over the 20-year time-horizon, incremental cost savings incurred by patients undergoing initial pain management post-microfracture in cycle 1 and cycle 2 (€3865) completely offset the initial investment in the device; this clinical event alone explains 39% of the avoided total costs with the device over microfracture alone (€6448 net cost savings +€3525 initial investment).
During cycle 1, the device is associated with a saving of €2977 in ACI costs vs microfracture at year 4. During cycle 2, the model predicts that the device could enable a saving related to ACIs avoided (€2245) in year 6. Over the 20-year time-horizon, incremental costs savings incurred by avoiding ACI post-microfracture in cycle 1 and cycle 2 (€5582) completely offset the initial investment in the device; this clinical event alone explains 56% of the avoided total costs with the device over microfracture alone (€6448 net cost savings +€3525 initial investment).
Scenario analyses
Incremental costs savings for the device compared with microfracture alone, at time-horizons up to 20 years, are presented in and , for each scenario analysis.
Figure 3. Cost savings (€) for the device vs microfracture alone, at 20 years for each scenario analysis. Costs are discounted at a rate of 3.0% per annum.
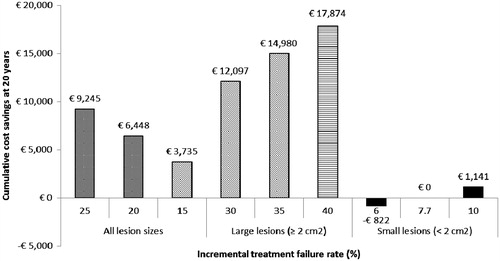
Table 6. Incremental cost savings (€) for the device vs microfracture alone, at time-horizons up to 20 years for each scenario analysis (no cost savings are realized in years 1 and 2, since treatment failure is assumed to occur in year 3 in cycle 1 and year 5 in cycle 2; ‘…’ indicates ongoing resource use between years 10 and 20). Results are presented as cumulative incremental cost savings. Costs are discounted at a rate of 3.0% per annum.
All lesions sizes, variable incremental treatment failure assumptions
As would be expected, for all lesion sizes, increasing the incremental treatment failure rate for microfracture alone vs the device from 20% to 25% results in greater total cost savings over the 20 year period (€9245 compared with €6448). As in the base case, the key drivers of the incremental cost savings are reductions in pain management and ACI required in patients undergoing the device compared with those undergoing microfracture in cycle 1; a financial benefit is first introduced in year 3 post-initial intervention. Conversely, if the incremental treatment failure rate for microfracture alone vs the device is reduced from 20% to 15%, achievement of a positive return on investment is delayed by 2 years and the 20-year cost saving is almost halved, to €3735. In this scenario, as before, the cost saving appears to be driven by reductions in pain management and ACI required in patients undergoing the device compared with those undergoing microfracture. The results of the scenario analysis indicate that the larger the increment in treatment failure between the groups, the larger the difference in financial impact becomes between cycles 1 and 2. Since the size of the cycle 2 cohort is dependent on the proportion of patients who have not failed in cycle 1, a higher rate of treatment failure in cycle 1 diminishes the weight of financial outcomes in cycle 2 on overall cost savings.
Large lesion size, variable incremental treatment failure risk
Total cost savings over 20 years for incremental treatment failure rates of 30%, 35%, and 40% are €12,097, €14,980, and €17,874, respectively. The device becomes a cost-saving alternative in year 4 in all three scenarios, as in the base case scenarios, mostly due to reductions in pain management and ACI required in patients undergoing the device compared with those undergoing microfracture in cycle 1. Total incremental cost savings for the device vs microfracture alone are at least twice as high for patients with large lesions, compared with those with lesions of all sizes. This is driven by the assumed improved performance for the device relative to microfracture alone when treating lesions ≥2 cm2, as validated by the Delphi panel.
Small lesion size, variable incremental clinical treatment failure risk
Small lesions (<2 cm2) are assumed to be associated with a reduced incremental treatment failure rate for microfracture alone vs the device, resulting in much more modest financial outcomes. For incremental treatment failures rates of 6.0%, 7.7%, and 10.0%, the 20 year budget impact for the device compared with microfracture alone is a cost increase of €822, cost neutrality, and a cost saving of €1141, respectively. The cost saving seen with the highest modeled incremental clinical outcome (10%) is achieved at the same time period (year 6) as the conservative base case scenario (15%), and, as such, appears to be driven by outcomes in cycle 1 slightly (11%) more than in cycle 2.
Discussion
Currently available surgical procedures for knee cartilage repair are associated with a variety of limitations and often present only short-term improvements in clinical and functional outcomes, due to the sub-optimal structure of the repaired cartilage. The device has demonstrated superior outcomes at 12 months compared with microfracture alone, the current standard of care, in terms of both quantity and quality of repaired tissue. This study aimed to demonstrate the economic value of the device as an adjunct to microfracture vs microfracture alone, in knee cartilage surgery. The results indicate that, when considering all lesion sizes, when it is inferred that the device could decrease the risk of treatment failure by 20% compared with microfracture alone, the initial investment of €3525 for the device is offset by year 4. The return on investment over a 20-year period is almost triple the incremental cost of the BST-CarGel procedure (net cost saving of €6448); over a 20-year period, almost €10,000 per patient could be avoided in resource utilization. Reducing the incremental treatment failure risk for microfracture alone vs the device to 15% still generates cost savings of €3735. The increased improvement in clinical outcomes seen with the device for large lesions is reflected in greater cumulative cost savings seen over 20 years in this sub-group of patients (€12,097–€17,874). In contrast, a decreased improvement in clinical outcomes vs microfracture of 6% in patients with small lesion sizes results in an overall cost increase over 20 years of €822.
The study is strengthened by the underlying evidence base: a pivotal RCT was supplemented by a wide-ranging literature search. All model parameters and assumptions were validated by a Delphi panel consisting of acknowledged experts from a range of European countries and Canada. Not only does this lend validity to the model structure, but it could allow the outcomes of the analysis to be generally applied to a range of markets and healthcare systems. The UK was the only country in which treatment patterns differed significantly from the other countries, due to guidelines issued by the National Institute for Health and Care Excellence (NICE). The UK experts included in the Delphi panel validated the underlying assumptions of the model (cascade of event, risks, and clinical assumptions) and concluded that UK treatment patterns vary widely in terms of resource utilization. All assumptions made were conservative where possible. Finally, the 20-year time-horizon allowed for all possible costs savings related to outcome of the initial intervention to be captured.
As with any economic evaluation, there were a number of limitations, which are recognized. Available clinical trial data for microfracture are limited by quality of study designs and, therefore, heterogeneity in their resultsCitation18. Improvement upon the existing evidence base will allow for more insightful comparisons with other therapeutic options for knee cartilage repair. In particular, long-term clinical data, charting the patient pathway post-initial intervention, are lacking, and this presents a key limitation to the current analysis. The model used to simulate long-term outcomes for this economic evaluation relies on assumptions, however conservative, and can only be strengthened by inclusion of clinical trial or real-world data.
It is recognized that external factors other than clinical efficacy may potentially influence the timing and probability of interventions further along the treatment pathway, such as TKR. Thus, whilst such a procedure could potentially be offset or delayed due to a more clinically-efficacious initial surgical procedure, in clinical practice it is possible that clinical and functional outcomes post-knee cartilage repair are two of a number of drivers.
Since the natural history of untreated chondral defects is itself unclearCitation42, there exists the risk that any studies of cartilage repair procedures may over- or under-estimate their relative clinical efficacy. This could be addressed by ensuring rigorous design of clinical trials of such treatments, including patient enrollment controlled by appropriate inclusion and exclusion criteria, independently-controlled randomization, consistently-performed surgical interventions, long-term follow-up, and validated data end-points and collection processes.
The comparator for the analysis is limited to microfracture. This was for two reasons: microfracture is known to be the standard of care first-line treatment option in this indication, and the pivotal RCT for the device uses microfracture as a comparator. Since other interventions are used in clinical practice, however, comparison with a range of techniques would allow the wider economic value of the device to be established.
The cascade of events post-initial intervention considered in the model did not include osteoarthritis, although this is a potential long-term outcome in clinical practice. This approach was taken because the Delphi panel could not agree on the risk reduction for osteoarthritis due to sub-optimal treatment with microfracture.
Finally, this analysis was limited to an assessment of resource use and costs only. It is recognized that an understanding of the impact of each intervention on health-related quality-of-life would enhance the economic evaluation of the device and allow this study to be compared with cost-utility analyses of similar therapies and those for different indications, in order to inform decision-makers more fully.
Conclusions
This analysis demonstrates that BST-CarGel could be a cost-saving alternative to microfracture alone over a relatively short period. This is due to greater improvements in the regeneration of chondral tissue with hyaline characteristics in patients requiring knee cartilage repair, as demonstrated by the clinical trialCitation31, which can reduce the risk of treatment failure and improve structural outcomes. The risk reduction in requirement for pain management and secondary surgical intervention resulting from use of the device as an adjunct to microfracture is enough to offset the initial investment in the supplementary product by year 4 post-procedure. The benefits of the device are even more pronounced in those patients with lesions of larger sizes (≥2 cm2). Since knee injuries represent a considerable burden to the patient and to society, and repair procedures can be limited in their effectiveness, the device could be a valuable addition to the currently available surgical options.
Transparency
Declaration of funding
Funding for the study was provided to the following by Piramal Life Sciences – Bio-Orthopaedics Division, Laval, Quebec, H7V 4B3, Canada
Declaration of financial/other relationships
Data 4 Actions, Laval, Quebec, Canada. Julie Frappier is a consultant to Piramal Health Sciences. Dr William Stanish is Medical Advisor for Piramal, as the Principal Investigator of the clinical study and follow-up. He is also a member of Piramal’s Scientific Advisory Board. Dr Mats Brittberg is Medical Advisor for Piramal, and is a member of Piramal’s Scientific Advisory Board. He is also a consultant to Sanofi Biosurgery, Anika Therapeutics, BMI Medical Implants, and owns stock in Neurovive. Dr Matthias Steinwachs is a Medical Advisor for Piramal, and is a member of its Scientific Advisory Board. Dr Alberto Restrepo and David Castelo have disclosed that they are employees of Piramal Life Sciences. Lydia Crowe is an employee of Abacus International, a company that was funded by Piramal to develop this study. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions: Julie Frappier was involved in the conception, design, lead authorship, analysis, and interpretation of data, and provided final approval of the manuscript submitted. Dr Stanish aided in data acquisition, provision of study subjects, manuscript revision, and final approval of the submitted paper. Dr Brittenberg and Dr Matthias Steinwatch contributed to the critical revision of the article and final approval of the version submitted. Lydia Crowe was involved in the conception and design, and aided in the drafting of this manuscript, providing technical and logistic support. She also provided assistance with the interpretation of the clinico-economic model used. Dr Alberto Restrepo contributed to the drafting of the manuscript, critical revision, data collection, and final approval of the submitted manuscript. David Castelo contributed to the drafting of the manuscript, provision of study materials, collection of clinical study data, administrative, and technical support.
Acknowledgments
The authors thank the following Principal Investigators for their participation at the Clinical Study Site for their help with the acquisition of data and the provision of study subjects to the Pivotal Clinical Study: Peter MacDonald, MD, Pan Am Clinic, Winnipeg, Manitoba, Canada; Nicholas Mohtadi, MD, University of Calgary Sports Medicine Center, Calgary, Alberta, Canada; Paul H. Marks, MD, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada; William Stanish, MD, Orthopaedic and Sport Medicine Clinic of Nova Scotia; Halifax, Nova-Scotia, Canada; Michel Malo, MD, Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada; Robert McCormack, MD, New West Sports Medicine, New Westminster, British-Columbia, Canada; Jacques Desnoyers, MD, Hôpital Charles LeMoyne, Greenfield Park, Québec, Canada; Stéphane Pelet, MD, Centre Hospitalier Affilié Universitaire de Québec, Québec, Québec, Canada; Don Johnson, MD, Sports Medicine Centre, Carleton University, Ottawa, Ontario, Canada; Jordan Leith, MD, Joint Preservation Centre of BC, Vancouver, British-Columbia, Canada; Paul Zalzal, MD, EntraLogix Clinical Group Inc., Newmarket, Ontario, Canada; Francisco Forriol, MD, FREMAP Centro de Prevención y Rehabilitación, Majadahonda, Madrid, Spain; Javier Vaquero, MD, Hospital General Universitario Gregorio Maraňón, Madrid, Spain; Santiago Bello, MD, Hospital Universitario La Paz, Madrid, Spain; Francisco Maculé, MD, Hospital Clinic i Provincial de Barcelona, Barcelona, Cataluña, Spain; Antonio Maestro, MD, Cirugia Ortopedica y Traumatologia, Medicina del Deporte, Gijón, Spain; Myung Chul Lee, MD, Seoul National University Hospital, Seoul, Korea; and Kyoung Ho Yoon, MD, Kyung Hee University Medical Center, Seoul, Korea.
The following is to acknowledge the participation of the Delphi panel who contributed to the health economics study, clinico-economic model of BST-CarGel for repair of focal articular cartilage lesions on the femoral condyle: Dr William Stanish (Principal Investigator of the pivotal trial), Orthopaedic and Sport Medicine Clinic of Nova Scotia, Dalhousie University, Halifax, Nova Scotia, Canada; Dr Patrick Lavigne, Arthroscopy/Sports Medicine, University of Montreal, Montreal, Quebec, Canada; Dr Philipp Niemeyer, Department Orthopädie und Traumatologie, Universitätsklinik Freiburg, Freiburg, Germany; Dr Elizaveta Kon, Nanobiotechnology Laboratory and Orthopaedic Clinic, Istituto Ortopedico Rizzoli, Bologna, Italy; Dr Asode Ananthram Shetty, Professor and Director of MIS Surgery, Faculty of Health and Social Sciences, Canterbury Christ Church University, Chatham Maritime, Kent, UK, and Senior Lecturer, Department of Surgery, King’s College London; Dr Alan Getgood, since 2012: Fowler Kennedy Sport Medicine Clinic, 3 M Centre, University of Western Ontario
London, Ontario, Canada, prior to 2012: University Hospitals Coventry and Warwickshire NHS Trust, Walsgrave, Coventry, UK.
References
- Bos P, van Melle M, van Osch G. Articular cartilage repair and the evolving role of regenerative medicine. Open Access Surg 2010;3:109-22
- Curl WW, Krome J, Gordon ES, et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456-60
- Hjelle K, Solheim E, Strand T, et al. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy 2002;18:730-4
- Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med 2000;133:321-8
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-56
- Merx H, Dreinhofer KE, Gunther KP. [Socioeconomic relevance of osteoarthritis in Germany]. Z Orthop Unfall 2007;145:421-9
- Gerlier L, Lamotte M, Wille M, et al. The cost utility of autologous chondrocytes implantation using ChondroCelect(R) in symptomatic knee cartilage lesions in Belgium. Pharmacoeconomics 2010;28:1129-46
- Vanlauwe J, Almqvist F, Bellemans J, et al. Repair of symptomatic cartilage lesions of the knee: the place of autologous chondrocyte implantation. Acta Orthop Belg 2007;73:145-58
- Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 2004;32:211-15
- Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med 2008;36:235-46
- inScience Communications. 2011. A systematic review of devices and techniques used for articular cartilage repair in the knee.
- Bugbee WD, Ammeen DJ, Engh GA. Does implant selection affect outcome of revision knee arthroplasty? J Arthroplasty 2001;16:581-5
- Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev 2010;16:305-29
- Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull 2008;87:77-95
- Kessler MW, Ackerman G, Dines JS, et al. Emerging technologies and fourth generation issues in cartilage repair. Sports Med Arthrosc 2008;16:246-54
- Lattermann C, Kang RW, Cole BJ. What's new in the treatment of focal chondral defects of the knee? Orthopedics 2006;29:898-903
- Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 2001;(391 Suppl):S362-9
- Mithoefer K, McAdams T, Williams RJ, et al. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 2009;37:2053-63
- Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 2002;15:170-6
- Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 2007;89:2105-12
- Mithoefer K, Williams RJ, 3rd, Warren RF, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am 2006;88(1 Suppl):294-304
- Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc 2005;13:213-21
- Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 2004;86A:455-64
- Marder RA, Hopkins G, Jr., Timmerman LA. Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy 2005;21:152-8
- Miller BS, Steadman JR, Briggs KK, et al. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg 2004;17:13-17
- Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 2003;19:477-84
- Clar C, Cummins E, McIntyre L, et al. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol Assess 2005;9:iii-iv, ix–x, 1–82
- Hoemann CD, Sun J, McKee MD, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthr Cartilage 2007;15:78-89
- Chevrier A, Hoemann CD, Sun J, et al. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthr Cartilage 2007;15:316-27
- Shive MS, Hoemann CD, Restrepo A, et al. BST-CarGel: in situ chondroinduction for cartilage repair. Operat Tech Orthop 2006;16:271-8
- Stanish WD, McCormack R, Forriol F, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am 2013;95:1640-50
- Marlovits S, Singer P, Zeller P, et al. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 2006;57:16-23
- Shive MS. BST-CarGel clinical summary. Internal document. 2011
- Domayer SE, Kutscha-Lissberg F, Welsch G, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome - preliminary results. Osteoarthr Cartilage 2008;16:903-8
- Eshed I, Trattnig S, Sharon M, et al. Assessment of cartilage repair after chondrocyte transplantation with a fibrin-hyaluronan matrix–correlation of morphological MRI, biochemical T2 mapping and clinical outcome. Eur J Radiol 2012;81:1216-23
- Brun P, Dickinson SC, Zavan B, et al. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther 2008;10:R132
- Mithoefer K, Williams RJ, 3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 2005;87:1911-20
- Kreuz PC, Erggelet C, Steinwachs MR, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy 2006;22:1180-6
- Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr Cartilage 2006;14:1119-25
- Theodoropoulos J, Dwyer T, Whelan D. Microfracture for knee chondral defects: a survey of surgical practice among Canadian orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc 2012;20:2430-7
- APTIS-Partners. BST-CarGel Market Opportunity Assessement: Final Report. 2009
- Harris JD, Siston RA, Pan X, et al. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am 2010;92:2220-33

