Abstract
In the past decade, extracellular vesicles (EVs) have been recognized as potent vehicles of intercellular communication, both in prokaryotes and eukaryotes. This is due to their capacity to transfer proteins, lipids and nucleic acids, thereby influencing various physiological and pathological functions of both recipient and parent cells. While intensive investigation has targeted the role of EVs in different pathological processes, for example, in cancer and autoimmune diseases, the EV-mediated maintenance of homeostasis and the regulation of physiological functions have remained less explored. Here, we provide a comprehensive overview of the current understanding of the physiological roles of EVs, which has been written by crowd-sourcing, drawing on the unique EV expertise of academia-based scientists, clinicians and industry based in 27 European countries, the United States and Australia. This review is intended to be of relevance to both researchers already working on EV biology and to newcomers who will encounter this universal cell biological system. Therefore, here we address the molecular contents and functions of EVs in various tissues and body fluids from cell systems to organs. We also review the physiological mechanisms of EVs in bacteria, lower eukaryotes and plants to highlight the functional uniformity of this emerging communication system.
Extracellular vesicles (EVs) are membrane-contained vesicles released in an evolutionally conserved manner by cells ranging from organisms such as prokaryotes to higher eukaryotes and plants (). The significance of EVs lies in their capacity to transfer information to other cells thereby influencing the recipient cell function. EV-mediated signals can be transmitted by all the different biomolecule categories – protein, lipids, nucleic acids and sugars – and the unique package of this information provides both protection and the option of simultaneous delivery of multiple different messengers even to sites remote to the vesicular origin.
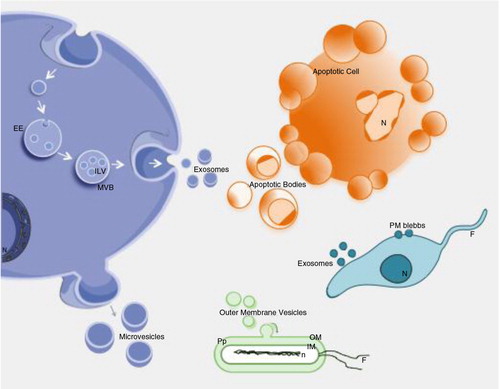
Fig. 1. Biogenesis and release of extracellular vesicles.
Extracellular vesicles can be broadly classified into 3 main classes: (a) Microvesicles/microparticles/ectosomes that are produced by outward budding and fission of the plasma membrane; (b) Exosomes that are formed within the endosomal network and released upon fusion of multi-vesicular bodies with the plasma membrane; and (c) Apoptotic bodies are released as blebs of cells undergoing apoptosis. Lower organisms, such as bacteria and parasites, are also able to secrete EVs. Outer membrane vesicles (OVM) are formed by outward bulging of the outer membrane of gram-negative bacteria. EE=early endosome; MVB=multi-vesicular body; ILV=intraluminal vesicles; N=Nucleus; OM=outer membrane; Pp=periplasm; IM=inner membrane; n=nucleoid; F=flagella.
While intensive investigation is targeted towards elucidating the role of EVs in intercellular communication in a range of pathological processes, research on EV-mediated maintenance of homeostasis and regulation of physiological functions remains less studied. Here, another significant role of EVs has emerged in the removal of unwanted molecular material as a means for cell maintenance. As a part of the European COST action initiative “Microvesicles and Exosomes in Disease and Health” (ME-HaD), here we aimed to review the current knowledge and understanding of the physiological roles of EVs in various tissues and cell systems of higher organisms, lower eukaryotes, bacteria and plants and show how this emerging data highlight the functional uniformity of this cellular communication system.
During the course of evolution, both prokaryotes and eukaryotes have developed elegant cell-to-cell communication strategies. These strategies have, for instance, helped bacteria to coordinate their social group activities by monitoring the environment and influencing the behaviour of other bacteria, a process known as quorum sensing (Citation1). These strategies also assist multi-cellular organisms to function as a system, for example, in pathogen interactions with hosts. Classically in cell biology, eukaryotic cells communicate with each other through direct interaction (juxtacrine signalling) and/or by secreting soluble factors such as hormones, growth factors and cytokines. These soluble factors can act on the cell itself (autocrine signalling) or have an impact on both neighbouring (paracrine signalling) and distant cells (endocrine signalling). The direct cell-to-cell signalling can be mediated by a membrane-anchored stimulus, deciphered by receptors located in other cells, or by junctional complexes including tight junctions, desmosomes, adherens and gap junctions. Interestingly, during the past decade, EVs have become recognized as potent vehicles of intercellular communication in different model systems (both prokaryotes and eukaryotes).
A brief history of EVs
The first observations of EVs and their relevance occurred somewhat simultaneously in various physiological settings without the realization that this form of function or communication is a universally shared cell biological property. Specifically, EVs were observed as procoagulant platelet-derived particles in normal plasma, originally reported in 1946 by Chargaff and West (Citation2) and referred to as “platelet dust” by Wolf in 1967 (Citation3). Early observations also included matrix vesicles identified during bone calcification by Anderson in 1969 (Citation4). In the 1970–1980s, separate independent EV observations included the release of plasma membrane vesicles from rectal adenoma microvillus cells (Citation5), reports on virus-like particles in human cell cultures and bovine serum (Citation6, Citation7) and the detection of vesicles, later termed prostasomes (Citation8), in seminal plasma (Citation9). Around the same time the first observations of tumour originating membrane fragments were made (Citation10), and they were also shown to be procoagulant (Citation11). In 1983, detailed ultrastructural studies showed that vesicles are also released by multi-vesicular bodies (MVBs) fusing with the cell membrane during the differentiation of immature red blood cells (Citation12–Citation14). More than a decade later, Raposo and colleagues demonstrated that these vesicles, then termed exosomes, isolated from Epstein–Barr virus-transformed B lymphocytes, were antigen-presenting and able to induce T cell responses (Citation15). In 2006–2007, with the discovery that EVs contain RNA, including microRNA, EVs acquired substantially renewed interest as mediators of cell-to-cell communication (Citation16, Citation17). Advancing on these pioneering studies, EVs have been isolated from most cell types and biological fluids such as saliva, urine, nasal and bronchial lavage fluid, amniotic fluid, breast milk, plasma, serum and seminal fluid (Citation18–Citation23) (see Functions of EVs present in body fluids section). An important step in the recent developments of the EV field has also been the enthusiastic collaborative work since 2011 by the members of the International Society of Extracellular Vesicles (ISEV: www.isev.org/), with the aim to unify the nomenclature and the methodologies of EVs.
The accumulating data have indicated that the contents, size and membrane composition of EVs are highly heterogeneous and dynamic and depend on the cellular source, state and environmental conditions. At present, at least 3 main subgroups of EVs have been defined (Citation24): (a) apoptotic bodies, (b) cellular microparticles/microvesicles/ectosomes and (c) exosomes (). Apoptotic bodies are released when plasma membrane blebbing occurs during apoptosis and are therefore excluded from this review. The second vesicle group comprises vesicles of different sizes that pinch directly off the plasma membrane. Finally, exosomes are intraluminal vesicles (ILVs) contained in MVBs, which are released to the extracellular environment upon fusion of MVBs with the plasma membrane. The biogenesis and secretion of EVs has recently been extensively reviewed elsewhere (Citation25).
Specific characteristics have been proposed for these subgroups of EVs in some instances, but currently there is still a lack of widely accepted specific markers to distinguish these populations (Citation26, Citation27). This may partly be explained by the lack of standardization of both isolation procedures and methods for the characterization of EV subgroups. In addition, isolation procedures typically do not unequivocally purify specific types of vesicles but, instead, yield complex mixtures. However, sub-fractionations of EV subgroups may potentially be achievable by the application of forms of affinity chromatography, employing antibodies against known or suspected EV surface markers (Citation28, Citation29), or using ligands (e.g. heparin) reactive with EV surfaces (Citation30). Other means of sub-fractionation being investigated include forms of charge separation or isoelectric focusing (Citation31, Citation32) or by size (along with other chemical characteristics) by field flow fractionation techniques (Citation33). As indicated above, the content of EV sub-fractions vary depending on the source of the EVs and their original isolation or enrichment techniques. So far, there are few studies detailing fractionation of EV subgroups with subsequent in-depth characterizations. To unify the nomenclature throughout this review we will, therefore, use the term EVs for all types of vesicles, but include the nomenclature used in the original work where it carries a specific significance for the context.
Molecular properties of EVs
Proteins and protein-associated functions of EVs
Proteomic studies of EVs released by primary cell cultures, cell lines, tissue cultures or isolated from biofluids have yielded extensive catalogues of the protein abundance in different types of EVs. Public on-line databases are available that catalogue EV-associated components. These include Vesiclepedia (www.microvesicles.org/) (Citation34), EVpedia (www.evpedia.info) (Citation35) and ExoCarta (www.exocarta.org) (Citation36).
EVs contain proteins that are considered to be pan-EV markers (i.e. common for most EVs), and their proteins and protein post-translational modifications that specifically reflect the vesicle localization, cellular origin and mechanism of secretion (Citation37–Citation40). In general, EVs are highly abundant in cytoskeletal-, cytosolic-, heat shock- and plasma membrane proteins, as well as in proteins involved in vesicle trafficking. Intracellular organelle proteins are less abundant. Proteomic profiles obtained have been found to be highly dependent on how EVs were isolated. Different methods yield EVs and EV sub-fractions of variable homogeneity, which makes it difficult to extrapolate findings between different proteomic studies of EVs.
While protein profiles may be characteristic of different EV subgroups, there is, nevertheless, no single marker that can uniquely identify EVs. These vesicles are best isolated, defined and characterized based on multiple techniques. These include isolation by differential ultracentrifugation, density gradient centrifugation (sucrose or iodixanol gradients), filtration and size-exclusion chromatography. Due to the small differences in physical properties and composition, discrimination between different EV subgroups after their cellular release remains difficult. Furthermore, the same cell type may secrete different subgroups of vesicles depending on environmental factors (e.g. oxygen tension), cell topography (e.g. from basolateral or apical cell surfaces) (Citation41) or activating stimulus (e.g. apoptosis or autophagy) (Citation42). In addition, the protein contents of the same EV subgroups are regulated based on activatory stimulus (Citation43). Further, a given cell may contain different types of MVBs characterized by differential exosome content (Citation44, Citation45). Characterization of EV protein content is commonly conducted by, for example, immunoblotting, immuno-gold labelling combined with electron microscopy and antibody-coupled bead flow cytometry analysis. Proteins enriched in EV sub-populations that are often used as markers (although not necessarily specific) include tetraspanins (CD9, CD63, CD81 and CD82), 14-3-3 proteins, major histocompatibility complex (MHC) molecules and cytosolic proteins such as specific stress proteins (heat shock proteins; HSPs), Tsg101 and the Endosomal Sorting Complex Required for Transport (ESCRT-3) binding protein Alix (Citation46). Tetraspanins CD9, CD63 and CD81 were previously considered to be specific markers for exosomes; however, these proteins have now also been observed in apoptotic bodies and microvesicles (Citation41, Citation47). Conversely, some studies indicate that CD63 (and Tsg101) are only present in certain EV subgroups (Citation48). Overall, CD9 and CD81 belong to the top 200 most frequently identified EV proteins (Citation35). A consensus on isolation procedures and additional experimental data are required to determine if there are indeed specific proteins to be associated with specific EV-subgroups (Citation41).
Protein glycosylation and lectins
The first comprehensive insight into the glycome of EVs was obtained by lectin-microarray analysis of EVs from T cells. Their glyco-pattern was found to be distinct from that of the parent cell membrane (Citation49). EVs were enriched in highly mannosylated epitopes, including complex N-glycans, N-acetyl lactosamine, sialylated and fucosylated epitopes, while blood group antigens A/B were excluded. The same distinctions from parent cell membranes were found in the EVs from a series of human cell lines (T cells, melanoma and colon cancer) (Citation50). Lectin-binding patterns were found to be conserved in all the EVs examined, although binding of a given lectin was associated with different proteins. Glycosylation was found to be different between exosomes and apoptotic bodies (Citation37). Several studies reported changes in the glycosylation patterns of EVs in pathological conditions including ovarian cancer (Citation37), classical galactosaemia (Citation51) and polycystic kidney disease (Citation52), pointing out the important role of glycosylation in EV (patho) physiology.
Studies using classical biochemical techniques and proteomic profiling of EVs have revealed the presence of several glycan-binding proteins. These may be particularly relevant to which cells EVs will be targeted and how they interact with those target cells. As an example, the C-type P-selectin (CD62), which is present on the surface of EVs released from activated platelets, allows EVs to bind to target cells via its classical P-selectin glycoprotein ligand-1 (PSGL-1) ligand (Citation53). Also, B cell-derived EVs were found to be enriched with α2,3-linked sialic acid allowing their capture by sialoadhesin (CD169, Siglec1) on macrophages (Citation54). Proteomic profiling of EVs derived from human plasma revealed 9 lectins including collectin sub-family member 10 (COLEC10), ficolin 1, 2 and 3 precursors, mannose-binding lectin serine protease 1 and 2 precursors (Citation55). The presence of osteosarcoma amplified-9 endoplasmic reticulum lectin and mannose-binding lectins in saliva (Citation56), plasma (Citation55) and urine (Citation18, Citation38) EVs has been reported. Intelectin-1, a galactofuranose-binding lectin, was found in the urinary EVs (Citation56). The lectin galactose binding protein-3 (LGALS3BP), that binds galectin 3, was predominantly found in EVs derived from prostate (Citation57) and ovarian cancer cell lines (Citation58).
Galectins are a family of soluble lectins characterized by their affinity for beta-galatosides in the absence of divalent cations. EVs derived from bladder cancer (Citation59) were reported to carry galectin-1 and galectin-3; the latter was also detected in EVs derived from saliva (Citation60), parotid gland (Citation56), conditioned medium from the human colon cancer cell line LIM1215 (Citation28), urine (Citation18, Citation38) and plasma (Citation55). Galectin-4 has been detected in EVs secreted by human colorectal cell line HT 29 (Citation61) and colon tumour cell line LIM1215 (Citation28), while galectin-5 on the surface of EVs from reticulocytes was found to be crucial for EV uptake by macrophages (Citation62). Finally, galectin-7 has been detected in EVs derived from human parotid saliva (Citation56).
The importance of glyco-interactions in EVs sorting and EVs effect on target cells is supported by recent studies (Citation63, Citation64). Moreover, surface glycosylation patterns may be important for the EV uptake by recipient cells (Citation37, Citation50) (Citation62), which has been shown to be dependent on heparin sulphate proteoglycans (Citation65) so that it can be inhibited by heparin addition (Citation30).
Molecule sorting to EVs
The common protein signature of different kinds of EVs, which is likely to be crucial for their function and may relate to their biogenesis, may also be connected to membrane curvature (). Membrane constituents are more or less free to move laterally over the membrane, so molecules with a given effective shape will accumulate in regions that are energetically favourable (Citation66), determining the local membrane composition and its curvature (i.e. shape). Curvature-based sorting of proteins (Citation67, Citation68) and lipids (Citation69, Citation70) has been studied in artificial and eukaryotic membranes and it has been established that bacteria are capable of sorting macromolecules to distinct sub-cellular domains (Citation71, Citation72).
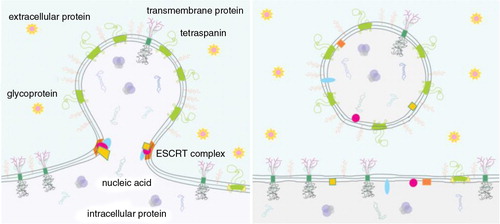
Fig. 2. Curvature sorting mechanism.
In the process of budding, membrane constituents redistribute to regions with fitting membrane curvature to minimize membrane free energy. Redistribution of membrane constituents is then reflected in the pinched off vesicles. As examples, this scheme indicates tetraspanins, ESCRT (Endosomal Sorting Complex Required for Transport) complexes, anonymous integral membrane proteins of a given type, glycoproteins and proteins that are preferentially located in the cell interior and exterior. ESCRT complex favours the neck region of the bud and is disintegrated after the vesicle pinches off. The content that is enclosed by the vesicle membrane becomes mobile and may reach distant cells.
This self-consistent mechanism of the curvature sorting of membrane constituents (Citation73) begins in the parent cell during the membrane budding. It largely determines the shape, size and composition of the EV and consequently influences their physiological role. The mechanism is non-specific; it takes place in all membrane types and applies to vesicles formed either inside the MVB or by budding from the plasma membrane. Thus, this mechanism implies that several structural components are shared among different kinds of vesicles.
Some membrane constituents such as lectins (Citation50) and tetraspanin-enriched microdomains (Citation74, Citation75) have already been reported to play a crucial role in the concentration of EV protein components and, at the same time, in the recruitment of structural and shaping components. Curvature-induced sorting of membrane constituents and their direct interactions may lead to the formation of lateral microdomains with specific composition such as tetraspanin-enriched microdomains (Citation76) and membrane rafts (Citation77) (). Tetraspanins have been proposed to induce membrane curvature (Citation78) and incorporation of the membrane receptors into tetraspanin-enriched microdomains has been shown to be relevant for their routing towards exosomes (Citation74, Citation75) (Citation79). Analysis of ganglioside GM1 and the cytosolic protein content of erythrocyte membrane buds and the released vesicles have shown a redistribution of these molecules with respect to the parent cell membrane. This indicated that entire microdomains may be sorted to relatively flat membrane regions or to highly curved ones (that eventually become EVs), depending on their intrinsic molecular shape and/or interactions between the microdomain elements (Citation73). Among the curvature-favouring structural components, the BAR (Bin/Amphiphysin/Rvs) domain-containing proteins were shown to drive the formation of tubular and vesicular membrane structures (Citation80, Citation81). The ESCRT proteins seem to favour the neck region of the forming EVs (Citation82, Citation83), where they play an important role in the fission of membrane buds (Citation84, Citation85). Besides the morphological arrangement of membranes to induce the formation of ILVs in MVBs (Citation86), the ESCRT complex recruits exosomal cargo components through the binding to ubiquitinilated proteins. Incorporation of a given protein into EVs may depend on the site of vesicle generation (plasma membrane versus MVB) and follow either an ESCRT-dependent or -independent pathway. Intraluminal components of the EV membrane, for example, cytoskeletal adaptor molecules, may also have a role in both editing and maintaining the morphology of the vesicles. The post-synaptic density protein, disc-large, zonulin I (PDZ) protein syntenin was reported to be necessary for the formation of MVB ILVs and, therefore, exosomes (Citation87, Citation88). Proteins of the ERM (Ezrin, Radixin and Moesin) family are highly enriched in EVs and have been linked to different components within the tetraspanin-enriched microdomains (Citation74, Citation89). Insertion into membrane microdomains may also influence the degree of oligomerization, which may also function as a targeting mechanism (Citation90, Citation91). All these studies suggest that local protein and, as described below, also lipid sorting within the membrane is closely connected to the formation and identity of EVs.
Uptake mechanisms
Due to their extensive and variable protein content, EVs may be considered as vectorial signalosomes (Citation92). The protein composition of EVs may determine their functionality in several different ways. Surface-exposed receptors and ligands are responsible for biodistribution, for the binding of EVs to target cells or to the extracellular matrix. Subsequently, EVs may trigger intracellular signalling pathways through a simple interaction with the surface receptors or ligands of target cells or by undergoing internalization. In addition, EVs may induce changes in the cell phenotype by transfer to the target cell of functionally active receptors such as CCR5 (Citation93), EGFRvIII (Citation94) or MET (Citation95).
EV uptake by target cells appears to depend on the type of recipient cells. In most instances, EV uptake seems to occur through phagocytosis (Citation65, Citation96) and its extent may depend upon the phagocytic capabilities of the recipient cell (Citation97). Macropinocytosis may represent an alternative way through which EVs may transfer their content (Citation98–Citation100). As membrane fusion requires a similar fluidity between the 2 fusing membranes, and both EVs and plasma membranes display the same fluidity at pH 5.0 (Citation101, Citation102) but not at neutral pH (which makes the membrane more rigid) (Citation103), the direct fusion of EVs with the plasma membrane may be limited to acidic pH conditions such as those found inside a tumour (Citation99). It is noteworthy that MVBs have a pH of ~5, and that the fusion of the ILVs to the MVB-limiting membrane (i.e. back fusion) has also been reported to occur (Citation104). The key influence of the microenvironment's pH suggests that the differences in the electrostatic charges between EVs and the plasma membrane of the cells should be considered in relation to the physiological roles of EVs.
It is conceivable, therefore, that when a functional molecule is delivered by EVs it may be more active than in its soluble form. One clear example of this is the ligands for death receptors, which are more functional when expressed on a membrane than in their soluble form (Citation105, Citation106). Furthermore, proteomic analyses have revealed that both cell surface-anchored and soluble matrix metalloproteinases are present in EVs from cell cultures and body fluids (Citation107). Some of these metalloproteinases were proteolytically active, suggesting that they may alter the EV content; directly interact or cleave extracellular matrix proteins; or shed membrane-anchored receptors from target cells.
Biodistribution and targeting
The steady-state level of EVs in circulation reflects a balance between the EV generation and their clearance. Independent studies indicate that the half-life of purified exogenous EVs, artificially introduced into circulation, is very short. Biotinylated rabbit EVs were cleared in rabbit circulation in ~10 min (Citation108). EVs from splenocyte supernatants (Citation54), red blood cell-derived EVs (Citation109) and EVs from B16 melanoma cells (Citation110) all showed a clearance of more than 90% after 30 min. However, human platelet concentrate-derived EVs remained in the circulation with a half-life of 5.5 hour (Citation111). As EVs may show protection from complement-mediated lysis due to expression of glycosylphosphatidylinositol (GPI)-anchored CD55 and CD59 (Citation112), their clearance from circulation is most likely due to retention and uptake in target organs. Indeed, a biodistribution study with red blood cell-derived EVs showed an uptake by the liver (44.9%), bone (22.5%), skin (9.7%), muscle (5.8%), spleen (3.4%), kidney (2.7%) and lung (1.8%) (Citation109). In contrast, melanoma-derived EVs were mainly taken up by lungs and spleen (Citation110). Biodistribution of EVs most probably depends on the parent cell source, as well as the availability of different target cell types to internalize the circulating EVs. More detailed studies comparing different injection sites, donor cells and healthy and disease conditions are necessary to establish the clearance and the organ uptake of the various EV populations.
Targeting by specific adhesion molecules determines EV biodistribution. Sialic-acid-binding immunoglobulin lectins (siglec) are expressed on a variety of leukocytes, and CD169 (sialoadhesin) preferentially binds to α2,3-linked sialic acids which decorate proteins on the surface of EVs. Indeed, B cell-derived EVs are captured by CD169-expressing macrophages in both spleen and lymph nodes (Citation54). In CD169 knock-out mice, EV access to the lymphoid system is dysregulated, resulting in aberrant trafficking of EVs into the splenic red pulp or lymph node cortex (Citation54). Another insight into the potential influence of saccharides came from the finding that EV uptake by dendritic cells (DCs) was reduced in the presence of D-mannose or D-glucosamine (Citation113), suggesting an EV uptake mechanism is based on the C-type lectin interaction. In addition, β-galactosides on reticulocyte EV surfaces may be involved in their uptake by macrophages through interaction with galectin-5 (Citation62). However, sugars do not seem to play a significant role in the EV interaction and their uptake, at least in the in vitro studies with the SKOV-3 ovarian carcinoma cell line (Citation37), suggesting cell- or condition-specific differences in the uptake mechanisms.
Lactadherin (also known as milk fat globule-epidermal growth factor 8) binds phosphatidylserine (PS) on the surface of apoptotic cells and platelet-derived EVs (Citation114, Citation115). Upon binding, a conformational change exposes the Arg–Gly–Asp (RGD) motif, which then binds to αvβ3 and αvβ5 integrins, subsequently promoting EVs phagocytosis by macrophages (Citation115). Thus, lactadherin bridges the binding of PS-positive EVs to the splenic macrophages, enabling their removal from the circulation (Citation114). Similarly, developmental endothelial locus-1 (Del-1) also mediates binding to both PS on the platelet-derived EVs and αvβ3 integrin on endothelial cells (ECs) (Citation116). Both the interaction and the capture of EVs by cell surfaces are highly facilitated by the reciprocal expression of intercellular adhesion molecules, such as ICAM-1 and LFA-1 integrin (Citation117–Citation119).
Interaction with membrane receptors
EVs can interact with target cells through a ligand-to-receptor interaction. Specific EV proteins such as MHC I and II (Citation119–Citation124), transferrin receptors (Citation125) and tetraspanins (Citation74, Citation75) are active in the downstream signalling pathways of target cells by triggering, for example, integrins and calcium signalling (Citation126), mitogen-activated protein kinase (MAPK) activation (Citation125) or natural killer group 2D (NKG2D) signalling (Citation127, Citation128).
Among ligand-to-receptor interactions, noteworthy are those between some HSPs, such as HSP60 and HSP70, and a number of membrane receptors present mainly on immune cells, such as CD14, CD91, Toll-like receptor (TLR)-2, TLR-4 and LOX-1 (Citation129), as well as CD94/CD56 (Citation130). In particular, some HSPs such as HSPs 27, 60, 70 and 90 can be intracellularly redistributed from their canonical sites to plasma membrane, lipid rafts and MVBs in some pathological conditions such as cancer. In turn, they are secreted via EVs in which they are localized at membrane level (Citation31, Citation32) (Citation131, Citation132). As a consequence, their binding to these receptors may be of relevance for the interaction between EVs and target cells during these diseases.
It is, however, likely that the enrichment in signalling molecules alone is insufficient for facilitating the signalling functions of EVs. In fact, EVs also contain active lipolytic moieties, such as phospholipases, leading to the formation of bioactive lipid mediators (fatty acids and prostaglandins), which may interact with peripheral G-protein-coupled receptors and the nuclear receptors in target cells (Citation133).
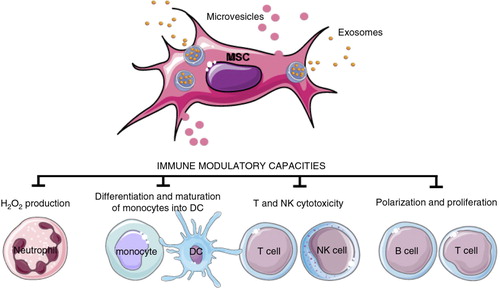
A clear example of the functional role of EVs ligands for membrane receptors is the presence of ligands for death receptors in EVs. It has been shown that human natural killer (NK) cells release EVs that express both NK cell markers and cytotoxic molecules such as FasL and perforin (Citation134). Incidentally, this is also evident for other cytotoxic cells as well (Citation135). These EVs were released in the extracellular milieu and could be detected in the circulation. The NK-derived EVs are fully active in inducing cell death of target cells. Moreover, human tumour cells release EVs expressing ligands for death receptors, including FasL and TNF-related apoptosis-inducing ligand (TRAIL). These EV-associated ligands are fully functional in inducing death receptor-mediated cell death. Intriguingly, the FasL- and TRAIL-bearing EVs released by malignant tumour cells may participate in lysing lymphocytes that should kill the tumour cells, while being unable to trigger cell death in the EV-releasing parent tumour cells (Citation136, Citation137).
EV-associated cytokines
Besides mediating exchange of intercellular information by their surface molecules, EVs have been shown to be carriers of important soluble mediators, such as cytokines. For cytokines that lack an N-terminal signal peptide, release by EVs represents a form of leaderless secretion. Examples of EV-associated or -secreted cytokines are given in Table .
Table I. Examples of EV-associated cytokines
The best-known example of the involvement of EVs in the cytokine transport is interleukin 1β (IL-1β). IL-1β is not only released by cells upon the fusion of secretory lysosomes with the plasma membrane, but it is also secreted by EVs (Citation138, Citation139). Once IL-1β-containing EVs are secreted, their cytokine cargo is released into the extracellular space upon binding of ATP to P2X7R on the EVs (Citation140). Another member of the IL-1 family, IL-1α, has been found in EC-derived apoptotic bodies both in its precursor and mature forms (Citation141).
Similar to IL-1β, the leaderless cytokine IL-18, which is also secreted upon inflammasome activation, was shown to associate with EVs shed from the surface of macrophages (Citation142). Macrophage migration inhibitory factor (MIF) (Citation143) and IL-32 (Citation144) represent other examples of EV-associated cytokines undergoing an unconventional secretion in the absence of a signal peptide. Membrane-bound tumour necrosis factor (TNF) was demonstrated to be secreted by EVs (Citation145), mast cells release vesicular IL-6 upon IL-1 stimulation (Citation146), while platelets liberate vascular endothelial growth factor (VEGF)-containing EVs (Citation147). VEGF was also shown to be present in tumour-shed EVs, and it was released from EVs in a bioactive form only at acidic pH characteristic for the tumour microenvironment (Citation148).
Chemokines constitute a highly significant and distinct category of cytokines. Among chemokines, IL-8 (CXCL8) and fractalkine (CX3CL1) were found to be associated with EVs (Citation149, Citation150), while EVs from heat-stressed tumour cells were associated with CCL2, CCL3, CCL4, CCL5 and CCL20 (Citation151).
Regarding regulatory cytokines, thymus-derived EVs were shown to induce regulatory T cells via vesicle-associated Transforming Growth Factor (TGF) β (Citation152). Also, tumour-derived EVs were found to use a TGFβ-mediated mechanism to induce regulatory T cells (Citation153, Citation154) and myeloid suppressor cells (Citation155). While the nature of the cytokine association with the various EVs in general is poorly understood, the role of heparan sulphate proteoglycans in tethering TGF-beta to the vesicle membrane, and its functional handover to recipient cells, has been reported (Citation156, Citation157). However, in fact, no systematic studies have been conducted to determine the complete spectrum of EV-associated cytokines. Furthermore, the extent to which vesicular localization of cytokines affects conventional cytokine measurements remains a key issue that has yet to be addressed.
RNA composition
Extracellular RNA exists in different forms. It may be enclosed in EVs, bound in protein complexes or exist in freely circulating form. The presence of functional RNA in EVs was first described in 2006 for murine stem cell-derived EVs (Citation17) and in 2007 for murine mast cell-derived EVs taken up by human mast cells (Citation16). While cellular mRNA varies in size from 400 to 12,000 nucleotides (nt), RNA detected in EVs has a predominant size of <700 nt (Citation158, Citation159). EVs, however, do contain intact mRNA (Citation160), mRNA fragments (Citation159), long non-coding RNA (Citation161, Citation162), miRNA (Citation163, Citation164), piwi-interacting RNA (Citation161), ribosomal RNA (rRNA) (Citation161) and fragments of tRNA-, vault- and Y-RNA (Citation165, Citation166). Most studies report absence or minor amounts of ribosomal 18S and 28S in EVs, as opposed to their abundant intracellular presence (e.g. 16,47,161,166). Some studies do, however, report of a substantial proportion of rRNA (~87%) in this EV sub-group (Citation167) and others have reported large amounts of rRNA fragments based on next-generation sequencing (Citation168). Thus, variability can exist depending on the EV source and the methodology used to obtain the data. Verification of the intraluminal localization of RNA in EVs, rather than in free circulating form, is mostly conducted by RNaseA treatment of EV (Citation47, Citation169). However, some studies have reported that protein interaction with Ago2 may also provide resistance to RNaseA (Citation170), so that a pre-treatment with proteinase K, which renders AGO–RNA complexes susceptible to RNAse degradation, should also be performed (Citation171).
An enrichment of 3′UTR mRNA fragments, rather than intact mRNA molecules, in EVs has been reported (Citation159). As the 3′UTR contains multiple sites for regulatory miRNA binding, this suggests that the RNA of EVs may compete with cellular RNA for binding of miRNAs or RNA-binding proteins in the recipient cells so as to regulate stability and translation (Citation159). The release of specific RNA molecules may also have intrinsic effects on the regulation of gene expression in the parental cells (Citation172).
MicroRNAs (miRNAs) are ~21 nt regulatory molecules that are transcribed as hairpin precursors (pri-miRNAs), cleaved by Dicer (into pre-miRNAs), bound by Argonaute proteins (Ago) and loaded into the miRNA-induced silencing complex (miRISC) for mRNA target regulation. miRNAs are secreted both in EVs and in a non-vesicular form. When released as soluble protein-complexes molecules, miRNAs have been detected in complexes with the Ago2 protein or high-density lipoprotein (HDL) (Citation173–Citation175).
Some studies report absence of miRISC complex proteins (including Ago2) in the exosomes sub-group of EVs (Citation39), whereas others report Ago2 presence (Citation170). In this regard, it has been proposed that RISC proteins in EVs could process precursor microRNAs (pre-miRNAs) into mature miRNAs inducing cell-independent microRNA biogenesis (Citation176).
The relatively decreased levels of mRNA targets of exocytosed miRNAs have been observed (Citation39, Citation172) (Citation177). Together, these observations indicate that miRNA loading into EVs can occur independent of mRNA target engagement and by a mechanism different from the Ago2-complexed miRNA secretion. The observation that miRISCs accumulate at sites of MVBs suggests that a regulatory circuit of miRISC activity and/or miRNA exosome loading may exist (Citation177).
Mechanisms that control RNA-sorting to EVs
Since the discovery of RNA in EVs (Citation16, Citation17) (Citation178), increasing evidence suggests that RNAs are not passively loaded into EVs, but that certain populations of RNAs become enriched in EVs compared to parental cells. Although this enrichment could occur because of a size restriction, there is a specific repertoire of miRNAs selectively exported to EVs even among small RNA species, whereas other miRNAs are usually excluded (Citation164, Citation166) (Citation179, Citation180), indicating that an active sorting mechanism occurs at RNA level. An enrichment of RNA containing specific nucleotide motifs has been documented in EVs (Citation181, Citation182). Furthermore, the expression of cellular miRNAs or miRNA target sequences can modulate the presence of miRNAs in exosomes (Citation183).
The loading of miRNAs into EVs has been shown to be controlled by heterogeneous nuclear ribonucleoprotein (hnRNP) A2B1 (Citation182). hnRNPs are a family of ubiquitous protein with roles in RNA trafficking and function. hnRNPA2B1 recognizes the EXOmotif (GGAG tetranucleotide) in miRNAs and controls the loading of these miRNAs into EVs. hnRNPA2B1 inside the EVs is sumoylated; this post-translational modification is necessary for the loading of miRNAs into EVs. mRNA species also show a selective enrichment into EVs. Evidence suggest that a consensus sequence within the 3′UTR of a number of mRNAs enriched in EVs may act as a zipcode sequence that targets mRNAs into EVs, similar to the EXOmotif of miRNAs. This zipcode consists of a 25 nt sequence which contains a short CTGCC core domain on a stem-loop structure and carries a miR-1289 binding site (Citation184). It has also been proposed that the addition of non-templated nucleotides to the 3′end of the microRNA may contribute to direct microRNA sorting into EVs (Citation185).
Biological significance of the horizontal transfer of mRNAs
The first experimental evidence that EVs can transfer intact, functional mRNAs to recipient cells was gained by the finding that the treatment of murine bone marrow mononuclear cells with embryonic stem cell-derived EVs enriched in Oct4 mRNA resulted in the increase of Oct4 protein expression in the bone marrow cells, while pre-treating the EVs with RNAse abrogated this effect (Citation17). In turn, it was thereafter demonstrated by incubating human mast cells with mouse mast cell-derived EVs that the murine mRNAs could be transferred to the recipient cells by EVs where they were translated into murine proteins, although the EVs themselves did not have functional machinery for the protein synthesis (Citation16). Yet another study showed that endothelial progenitor cell-derived EVs could transfer functional mRNAs to microvascular ECs thus triggering neoangiogenesis (Citation186). The transfer of functional mRNA was proved by generating cells that express GFP mRNA but had not detectable levels of the protein in their EVs, yet ECs treated with these EVs started to express GFP protein (Citation186). Taken together, these studies provide a solid basis for the concept that EVs transfer functional mRNAs that can be internalized and translated in the recipient cells.
mRNA-containing EVs also have been shown to enhance cell survival and repair of tissues under various stress conditions (Citation187–Citation189). Human mesenchymal stem cell-derived EVs were found to contain 239 mRNAs, most of which are involved in cell differentiation, transcription, cell proliferation and immune regulation (Citation188). Two of these were shown to be internalized and translated into full-length proteins in murine kidney epithelial cells in vitro and in vivo, thus demonstrating the feasibility of a horizontal transfer of mRNAs in this experimental setting (Citation187, Citation188). Treatment of mice with these EVs protected against glycerol or cisplatin-induced kidney injury (Citation187, Citation188). Interestingly, the mRNA content of EVs is modulated by the physiological state of the cell and stress conditions and may play a role in the maintenance of tissue homeostasis and synchronizing the functional state of cells. For instance, the mRNA content was found to differ significantly between EVs derived from mast cells grown under oxidative stress and normal conditions (Citation189). EVs released under oxidative stress enhanced the ability of untreated mast cells to handle H2O2-induced oxidative stress, while the exposure to UV-light eliminated the protective effect (Citation189). Likewise, the EV-containing mRNAs were found to be regulated by growth factor stimulation of cardiomyocytes (Citation190) and by hypoxia in glioma cells resulting in the expression of a variety of hypoxia-induced mRNAs and proteins in hypoxic glioma-derived EVs (Citation191). Regarding the synchronization of the functional status of cells, EVs derived from large adipocytes have been shown to transfer specific mRNAs involved in fatty acid esterification and lipid droplet biogenesis to small adipocytes, where they stimulated lipid synthesis and storage (Citation192).
All these effects are at least partially mediated by the horizontal transfer of RNAs. However, currently it is difficult to distinguish between the effects triggered by mRNAs and various non-coding RNAs that are abundant components of exosomal RNAs (Citation161) and to assess the extent to which individual mRNAs contribute to these effects. Furthermore, it is not yet clear what proportion of a cell's transcriptome in EVs consists of intact mRNAs that can be translated in the recipient cells and which mRNA fragments may play regulatory roles (Citation159, Citation161).
miRNA-based functions
Accumulating evidence indicates that the incorporation of miRNAs in EVs allows those miRNAs to circulate in the blood while avoiding degradation from blood RNAse activity. Selective disposal of some miRNAs in EVs has been also suggested to be a rapid way of regulating gene expression during, for example, lymphocyte activation, as when prompted by vaccination (Citation193), or as a mechanism of tumour suppressor miRNA removal in cancer (Citation172). A comprehensive list of miRNAs found incorporated into EVs is available in the miRandola database (www.atlas.dmi.unict.it/mirandola/index.html) (Citation194) and other databases such as EVpedia or Vesiclepedia. Such miRNAs may be secreted by a range of cells, such as immune cells (Citation164), stem cells (Citation195), blood cells (Citation196) or adipocytes (Citation192, Citation197), and growing evidence indicates that they may have important physiological roles [reviewed in Refs. (Citation198–Citation202)]. At least for some cell types, miRNAs may be transferred within EVs to neighbouring cells, where they alter the gene expression and phenotype of the recipient cells.
EV-mediated transfer of miRNAs has been shown to have immunological relevance (Citation203). For example, an antigen-driven unidirectional transfer of some miRNAs (such as miR-335) from T cells to antigen-presenting cells (APCs), mediated by CD63+ EVs, has been demonstrated to occur during immune synapses formation. The transferred miRNAs were shown to modulate gene expression in recipient cells (Citation164). It has been described that EVs released by different effector T-cell subsets (Th1, Th2 and Treg) have different miRNA signatures (Citation204). The authors identified exosome-shuttled specific miRNAs transferred from Treg that suppress pathogenic Th1 cells and prevented inflammation. Similarly, mast cell-derived EVs have been shown to contain miRNAs (Citation205) and macrophage-derived microvesicles transfer miR-223 and induce differentiation of naive monocytes, suggesting that an amplification loop – mediated by EVs – may exist to enhance immune function (Citation206). Interestingly, high expression levels of immune-related miRNAs (such as miR-181a and miR-17) in CD63+ EVs were detected in human milk during the first 6 months of lactation (Citation207). Deep sequencing technology has identified many miRNAs in human breast milk EVs with an abundance of immune-related miRNAs. This suggests that these EV miRNAs are transferred from the mother's milk to the infant, possibly having an essential role in the development of the infant immune system (Citation208). Placenta-specific miRNAs are also packaged into EVs and may mediate cross-talk between the feto-placental unit and the mother during pregnancy [reviewed in Ref. (Citation209)].
Evidence suggests that miRNAs transported by EVs also have a physiological role in ECs. For example, the efficacy of islet transplantation in type 2 diabetes patients is often limited by poor graft vascularization. However, EVs derived from the endothelial progenitor cells activate an angiogenic programme in the islet endothelium, mediated by the pro-angiogenic miR-126 and miR-296, and were shown to be crucial for transplanted islet engraftment and survival (Citation210). During atherosclerosis, EC-derived apoptotic bodies enriched in miR-126 are generated and transfer paracrine “alarm signals” to recipient vascular cells, inducing CXCL12-dependent vascular protection (Citation211). Blood cell-derived EVs, containing miR-150 (more abundant in atherosclerotic patients) have been shown to enter endothelial HMEC-1 cells, delivering miR-150, which reduced c-Myb expression and enhanced cell migration of HMEC-1 cells (Citation179). In turn, EC-derived EVs transferred miR-143 and miR-145 to smooth muscle cells, inducing an atheroprotective phenotype (Citation212).
Although investigations are yet in their infancy, there are reports showing the relevance of miRNA transfer in several physiological settings. For instance, the transport of miRNAs in EVs seems to function as a neuron-to-astrocyte communication pathway in the central nervous system (CNS) (Citation213). Other examples are EV-mediated transfer of miRNAs during muscle cell differentiation (Citation214), follicular maturation (Citation215) or osteogenic differentiation of human bone marrow-derived mesenchymal stem cells (Citation216). In addition, in stem cells, miR-126 in EVs has been implicated in the regulation of hematopoietic stem/progenitor cell trafficking between the bone marrow and peripheral sites (Citation217). In addition, EVs from embryonic stem cells were reported to have an abundant quantity of miRNAs which could be transferred to mouse embryonic fibroblasts in vitro (Citation218). Interestingly, EVs derived from preosteoblasts were found to influence embryonic stem cell differentiation and 20% of the examined miRNAs in the EV cargo were increased more than twofold when compared with the preosteoblast cells (Citation219). Despite the emerging evidence that miRNAs transported in EVs may be responsible for intercellular communication, it is yet to be determined if the amounts of miRNAs required to produce that effect are adequate to confer relevant paracrine and/or endocrine effects with regards to physiological impact in vivo, and how common this process is in vivo [reviewed in Ref. (Citation220)].
DNA content of EVs
In contrast to RNA, the presence of DNA in EVs has so far been less explored despite the early concept of the presence of oncogenic DNA in apoptotic bodies (Citation221). Mitochondrial DNA (mtDNA), single-stranded DNA, double-stranded DNA (dsDNA) and oncogene amplifications (i.e. c-Myc) have been detected in EVs (Citation222–Citation226). Migration of mtDNA may take place via EVs and, hence, EVs may represent an alternative pathway through which altered mtDNA can enter into other cells, favouring the diffusion of various pathologies (Citation223). Tumour EVs carry DNA that reflects the genetic status of the tumour, including amplification of the oncogene c-Myc (Citation222). Furthermore, DNA transfer into target fibroblasts was achieved by EVs, where EVs stained for DNA were seen in the fibroblast cytosol and even in the nuclei (Citation225). The presence of dsDNA representing the genomic DNA was detected in EVs reflecting the mutational status of parental tumour cells (Citation224, Citation226) (Citation227). It was also shown that different EV subgroups carried different DNA cargos (Citation227). The fact that EV-carried DNA can be used to identify mutations present in the parental tumour cells illustrates its significant potential as a translational biomarker, but the physiological significance of the DNA cargo in EVs is currently unknown.
Lipids in EVs
The metabolomic analyses on EVs reported so far have been focused on lipids, which are emerging as very important players for the physiological functions of these vesicles (Table ). The first studies addressing the lipid composition of EVs date from more than 2 decades ago and were performed on prostate-derived EVs (termed prostasomes) found in seminal fluid (Citation228, Citation229). An increasing number of studies providing lipidomic data sets of EVs from cell lines and biological fluids of multiple species are summarized in Table . Several specific lipids have been suggested to play a role in the formation and function of EVs. Lipids have been included in the EV databases such as Vesiclepedia (Citation34) and EVpedia (Citation35), and specific reviews on EV lipids are also available (Citation104, Citation230–Citation232). Although differences in the lipid composition of EVs derived from different sources have already been found, EVs are generally enriched in sphingomyelin, cholesterol, PS and glycosphingolipids compared to their parent cells (Citation232). EVs from placenta also contain an elevated proportion of sphingomyelin and cholesterol; sphingomyelin/phosphatidylcholine ratio showed a unique reversal of ratio (3:1), compared to that normally found in human cells or plasma (Citation233). The characteristic lipid composition of the EV bilayer probably contributes to the stability that they show in different extracellular environments. Therefore, knowledge about the specific lipids that confer the stability of EVs may be used to improve liposomal drug delivery systems (Citation231, Citation234).
Table II. Lipidomic studies on EVs
Lipids sorting and the role of lipids in EV biogenesis and release
Lipids are not randomly included into EVs but, similarly to other biomolecules, they are specifically sorted. EV membranes are enriched in cholesterol and sphingomyelin, suggesting that EV membranes may contain cholesterol/sphingolipid-enriched membrane domains similar to raft domains (detergent-resistant membranes) (Citation235–Citation237). Cholesterol and long saturated fatty acids of sphingolipids enable tighter lipid packaging of lipids than the phospholipids, with mainly unsaturated acyl chains found in other regions of the membrane. The high content in sphingolipids and cholesterol provides structural rigidity to EVs and an elevated resistance to physicochemical changes.
Several lipids have been suggested to be involved in and/or regulate EV formation/release. Cholesterol has been shown to regulate EV release (Citation236, Citation238) (Citation239). Interestingly, cholesterol is also important for the release of several enveloped viruses, including influenza virus and HIV-1, which select membrane rafts and tetraspanin-enriched microdomains as budding platforms to exit from the host cells. In addition, membrane microdomain-associated proteins, which are critical determinants of host–viral interactions, are the most likely key determinants of EVs–target cell interactions. Ceramide, formed by the action of neutral sphingomyelinase 2 on sphingomyelin, has also been proposed to be involved in the formation of ILVs within the MVBs (Citation240). In addition, other lipids such as lysobisphosphatidic acid (LBPA) and phosphatidic acid have been suggested to be involved in the biogenesis of EVs (Citation232, Citation241).
Lipid-dependent functions of EVs
Besides the essential structural role of lipids in formation of EV membranes, bioactive lipids, such as eicosanoids, fatty acids and cholesterol (Citation232), can be transferred between cells by EVs. Vesicle-bound lysophosphatidylcholine has been proposed to play a role in the maturation of DCs and triggers lymphocyte chemotaxis via the G protein-coupled receptor (Citation103). In addition, vesicle-bound prostaglandins triggered prostaglandin-dependent intracellular signalling pathways within target cells (Citation133) and EV lipids impacted Notch signalling and induced cell death in pancreatic tumoural cells (Citation242). The angiogenic activity of tumour-derived EVs in vitro and in vivo was found to be mediated mainly by sphingomyelin (Citation243). EVs lipids may also play a role in reproduction. It has been suggested that seminal EVs interact with sperm cells and transfer to them particular lipids such as cholesterol that are fundamental for the capacitation process (Citation244, Citation245).
Lipidomics and complete lipid profiles of EVs have become an interesting research area in the dissection of the biology of EVs; however, only a handful of lipidomes have been described to date. Since lipids are essential structural and functional constituents of EVs, additional lipidomic studies of EVs from different cell types and body fluids are required to elucidate the role of lipids in the biogenesis and biological functions of EVs. Furthermore, given the absence of information related to other metabolites distinct from lipids, metabolomic studies should also be extended to non-lipid analytes to obtain a more comprehensive picture of the small molecule composition and function of EVs.
Physiological functions of EVs in mammals
Functions of EVs present in body fluids
Body fluid-derived EVs are a mixture of vesicles originating from different sources such as the cells found in the body fluids and/or the cells lining the cavities of extruded body fluids (). The lipid membrane of EVs encapsulates and protects their contents from the degrading enzymes present in the body fluids and thus, protects them as a source of physiological and pathological information, which can be sent over a distance. Here, we summarize the physiological role of EVs in various body fluids and relate their presence with physiological functions.
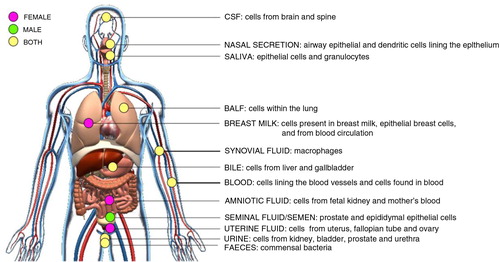
Fig. 3. Schematic of in vivo-derived EVs isolated from body fluids.
Cells from different human tissues of the body communicate through the secretion of EVs into proximal body fluids. EVs contain proteins, lipids and RNA molecules that may affect the physiology of cells bathed in or lining these body fluids. Highlighted here are the body fluids where EVs have been identified and their possible cellular origin. Pink spots represent body fluids, which are only present in females. Green spots represent body fluids, which are only present in male. Yellow spots represent body fluids present in both female and male. CSF=cerebrospinal fluid; BALF=bronchoalveolar lavage fluid.
EVs in urine
The existence of lipid membranes in urine was first described in the early 1990s (Citation246). It was hypothesized that these membranes were derived from intracellular vesicles that were somehow released into the urine (Citation247). However, it was as recent as 2004 that urinary EVs were first depicted as such (Citation18) and it has now been estimated that only about 3% of the total urinary protein content is derived from EVs.
An extensive description of urinary-derived EV content has been reviewed elsewhere (Citation248). The urinary EV cargo suggests that cells along the renal epithelium, extending from the glomerular podocytes (Citation249, Citation250) through the proximal tubule, the thick ascending limb of Henle, the distal convoluted tubule and the collecting duct, are releasing EVs into urine (Citation18, Citation38). CD24, which is expressed both by tubule cells and podocytes, has been proposed as a suitable urinary EV marker (Citation251). It is noteworthy that urinary EVs may not only come from the kidney but also from the ureters, the transitional epithelium of the urinary bladder, the urethra (Citation252–Citation254), and from the prostate epithelial cells, especially when a prostate massage is performed (Citation255). Analysis of the RNA content from urinary EVs showed that the majority of RNA within EVs is rRNA, while only 5% of the total RNA aligned to protein coding genes and splice sites. Exploration of these coding genes revealed that the entire genitourinary system might be mapped within EVs, which may play an emerging role in cell regulation (Citation167).
The role of urinary EVs as a reservoir of biomarkers and as potential mediators of intrarenal signalling has been suggested (Citation256). Initially, it was thought that the main physiological role of urinary EVs was the disposal of senescent proteins and lipids from cells (Citation257). Due to the fact that excretion via EVs probably requires a significant amount of energy, it has been proposed that EVs are preserved through evolution, due to their impact in other different physiological functions (Citation258, Citation259). It is possible that EVs represent a mechanism for cell-to-cell signalling along the nephron, through secretion and reuptake of their content such as proteins, mRNAs and miRNAs that can affect the function of the recipient cell (Citation258). The vasopressin-regulated water channel aquaporin-2 (AQP2), an apical Na+ transporter protein, is predominantly excreted via urinary EVs from renal collecting duct cells (Citation18, Citation247) (Citation260). Thus, EVs apparently trigger AQP2 trafficking towards the apical plasma membrane where they fuse, thereby increasing water permeability across the nephron. Other Na+ transporter proteins expressed along the renal tubule, as well as their activators, were also detected in urinary EVs (Citation57, Citation261–Citation263). Moreover, it has been speculated that Tamm–Horsfall protein (THP), an abundant polymeric protein in normal urine, has a role on limiting EVs fusion with cells in downstream nephron segments (Citation257). An additional role for EVs in kidney physiology seems to be is through direct actions of EV-resident proteins in the renal tubule lumen (Citation257), such as the angiotensin-converting enzyme (Citation18, Citation38), which could have a role in the renin–angiotensin system hence playing a role in water (fluid) balance. Urinary EVs are described as enriched in innate immune proteins, such as antimicrobial proteins and peptides and bacterial and viral receptors. This suggests a new role for urinary EVs as innate immune effectors that contribute to host defence within the urinary tract (Citation264). Finally, it has been proposed that urinary EVs exposing tissue factor (TF) could provide additional sources of TF which could boost coagulation and haemostasis, thus reducing blood loss and contributing to host defence by reducing the risk of microorganisms entering the body through urinary and urethral epithelia (Citation265).
EVs in saliva
EVs from saliva contain proteins (Citation56, Citation266) (Citation267) and several different RNA species (Citation20, Citation268–Citation271) which can be internalized by oral keratinocytes and macrophages (Citation268, Citation271) and alter their protein expression. This suggests that saliva-derived EVs are biologically active (Citation268). As salivary gland epithelial cells in culture release EVs and epithelial cell markers can be detected on saliva-derived EVs (Citation56, Citation272), it is likely that these cells are the source of the EVs found in saliva (Citation273). In addition to epithelial cell markers, the granulocyte marker CD66b has also been identified on saliva-derived EVs (Citation272), suggesting that saliva-derived EVs are mainly from epithelial cells and granulocyte origin. Two types of EVs have been identified in saliva, that is, 1 population that is heterogeneous in their size (30–250 nm), and 1 population that is homogeneous in their size (20–80 nm). The protein and RNA contents of these 2 populations are dissimilar (Citation266, Citation269).
EVs isolated from saliva of healthy subjects have been shown to contain TF and CD26. CD26 is a protein that can cleave several different peptides, and saliva-derived EVs have been shown to cleave substance P and chemokines (Citation60, Citation266). TF may initiate blood coagulation and, interestingly, saliva EVs induced clotting of vesicle-free plasma (Citation272). It has, therefore, been suggested that EVs could be an important part of the process during which humans and animals lick a bleeding wound to promote coagulation and the subsequent wound healing.
EVs in synovial fluid
Improved flow cytometric assessment of EVs has revealed that synovial fluid – a clear fluid secreted by membranes in joint cavities, tendon sheaths and bursae – which functions as a lubricant, has a distinct EV signature (Citation274). Synovial fluid-derived EVs have mainly been examined in subjects with autoimmune diseases such as rheumatoid arthritis and juvenile idiopathic arthritis. Initial studies on synovial EVs showed the accumulation and distribution of citrullinated proteins via these specific EVs, suggesting an important role in autoimmune mechanisms since citrullinated peptides are specific autoantigens in rheumatoid arthritis (Citation275). Synovial fluid-derived EVs have been observed, by immunoelectron microscopy, to be associated with IgG and IgM immune complexes (Citation275). Moreover, they bear functional integrins, capable of mediating anchorage to cell-surface adhesion molecules. Therefore, they may represent a novel mode of delivering autoantigens at distances beyond that of direct cell-to-cell contact (Citation275). Furthermore, a mammalian nuclear DEK-phosphoprotein was observed in EVs from synovial macrophages indicating the involvement of EVs in joint inflammatory processes (Citation276).
EVs in bile
Bile is a fluid that helps with digestion and the breaking down of fats into fatty acids, which then can be taken up by the cells in the digestive tract. Bile consists mainly of cholesterol, bile acids and bilirubin. EVs were found in the bile of bile duct ligated rats, suggesting the existence of biliary EVs in vivo (Citation277). Recently this was confirmed after identifying EVs by their size, morphology and markers such as CD63 and Tsg101 in this body fluid. Furthermore, the same authors observed that biliary EVs were involved in cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia (Citation278, Citation279).
EVs in cerebrospinal fluid
Cerebrospinal fluid (CSF) has been described to have many functions as an intermediary between blood and brain for the transport of nutrients and growth factors, and as a buffer for the brain to protect both the brain tissue and the large vessels supplying brain circulation. CSF is also involved in the elimination of toxins and other metabolic by-products (Citation280). Because of the potential importance of EVs in the context of the CNS and neurological diseases, the presence of EVs in human CSF is of much interest. Numerous studies have demonstrated the presence of EVs in CSF of humans (Citation281, Citation282) that carry signalling and intracellular proteins (Citation283). EVs have been proposed to neutralize the synaptic-plasticity disrupting activities of amyloid β-protein (Aβ) in vivo, primarily via the sequestration of Aβ oligomers by exosomal surface proteins, such as PrPC. These indicate a protective role of EVs against Aβ accumulation (Citation284).
EVs in bronchoalveolar fluid
EVs in bronchoalveolar lavage fluid (BALF) are released by cells residing in the lung and contain MHC class I and II, CD54, CD63 and the co-stimulatory molecule CD86 (Citation285). The presence of RNA and miRNA in those EVs has also been documented (Citation286).
So far, the main role described for EVs in BALF points to immunity in the lung as a response to different stimuli (Citation287, Citation288). BALF-derived EVs may act as signal conveyors for nanoparticles (Citation289), pathogens (Citation290) and allergen-induced systemic immune responses (Citation291–Citation294). Upon exposure to magnetic iron oxide nanoparticles, secretion of EVs was shown to increase in a dose-dependent manner in BALF of BALB/c mice, and the EVs were quickly eliminated from alveoli into systemic circulation and transferred their signals to the immune system, producing maturation of DCs and activation of splenic T cells (Citation289). Further supporting the role of EVs in the immune response towards allergens and exogenous infections, BALF EVs were shown to express the scavenger receptor CD36, which has been implicated in bacterial recognition (Citation295). Moreover, EVs isolated from the BALF of mice infected with M. bovis BCG had mycobacterial pathogen-associated molecular patterns (PAMPs) and were immune stimulatory (Citation290).
EVs in nasal fluid
EVs have also been detected in the nasal secretions of healthy humans. These vesicles were of the size of exosomes and had surface markers considered to be enriched in exosomes such as Tsg101, CD63, CD9 and CD81 (Citation23). Although, the functional significance of nasal EVs has to be further investigated, they may, similarly to the EVs from the lung, have immune modulatory effects. Within the field of vaccine development, the intranasal distribution of EVs for systemic delivery of drugs is under intense investigation as theses vesicles could have therapeutic effects in the brain, lungs and intestines (Citation296–Citation298).
EVs in uterine fluid
EVs from the uterine fluid (also known as uterosomes) as well as EVs from the oviductal luminal fluid (also known as oviductosomes) have been described as a way of protein trafficking, which may play an important role in the sperm capacitation and fertilization (Citation299, Citation300). Although experimental data exist in murine models only, plasma membrane calcium-transporting ATPase 4 (PMCA4) protein – which is transported through EVs within the uterine fluid during oestrus – is likely to be key to the maintenance of Ca2+ homeostasis and sperm viability during their storage in the oviduct and during capacitation and the acrosome reaction (Citation299). Furthermore, acquisition of sperm adhesion molecule (SPAM1)1 protein, localized outer surface of EVs, has been suggested to be an important prerequisite for sperm maturation and capacitation in the male and female reproductive tracts (Citation300).
In addition, EVs present in uterine fluid may directly transfer information, such as miRNAs [hsa-miR200c, hsa-miR17 and hsa-miR106a (Citation301)] or proteins [CD52 (Citation302) and leukaemia inhibitor factor (LIF) (Citation303, Citation304)] contributing to the endometrial-embryo cross-talk essential for the embryo implantation process.
EVs in amniotic fluid
In 2007, EVs were detected in the amniotic fluid of laboratory mice and 4 samples from women undergoing routine amniocentesis (Citation251). It has been speculated that the origin source of the amniotic fluid-derived EVs could be from both mother and foetus. The foetal kidney releases EVs that contain specific markers, such as AQP2, CD24 and annexin-I, to the foetal urine; which is a major constituent of amniotic fluid. A second fraction of EVs expressing annexin-I and HSP70, but not CD24, might originate from the maternal side (Citation251). EVs from amniotic fluid have been suggested to regulate the immune response in order to maximize foetal survival during pregnancy. In this process, HSP72 was indicated as an important factor (Citation305), as it modulates intra-amniotic cytokine production (Citation306). Supporting an immune role of EVs, EVs from the amniotic fluid were shown to be captured by human monocytic THP-1 cells and to stimulate cytokine release and NFkB/STAT3 activation in a TLR-dependent manner (Citation307).
EVs in breast milk
Breast milk is a complex body fluid, rich in immunological components that affect the development of the infant's immune system. It has been shown that human breast milk contains EVs (Citation271), but their origin is uncertain. It has been established that breast milk EVs differ from DC EVs, and it has been suggested that they may originate from other cells present in breast milk, from epithelial breast cells or even from other compartments of the body that can reach breast milk through the blood circulation (Citation308). Interestingly, high expression levels of immune-related miRNAs (such as miR-181a and miR-17) in CD63+ EVs were detected in human milk during the first 6 months of lactation. Deep sequencing technology has identified many miRNAs in human breast milk EVs with an abundance of immune-related miRNAs, suggesting that such EV miRNAs are transferred from the mother's milk to the infant, possibly having an essential role in the development of the infant immune system (Citation208). In fact, human breast milk EVs have the potential to influence the immune system of the infant at the protein level. Milk EV preparations have been shown to inhibit anti-CD3 and anti-PHA-induced (activated T cell) cytokine production and increase the number of a specific group of T regulatory cells (Citation308, Citation309). The isolation of EVs from breast milk and their participation in the development and maturation of the neonate's immune system has also been described in other species (Citation207, Citation310–Citation314).
EVs in blood
The first report of the existence of EVs in blood was almost 70 years ago and was later described as platelet “dust” (Citation2, Citation3). In addition, in the mid-1970s, vesicles of approximately 55 nm were identified in bovine, lamb and porcine serum (Citation6, Citation7). In the 1980s, the release of transferrin receptor-containing EVs during the maturation of reticulocytes was demonstrated (12, 13). Since this early work on blood-derived EVs, it has been recommended by some that EV studies should preferably be conducted on plasma (Citation46), since plasma is the physiological fluid of blood and EVs may potentially be generated in serum after the blood collection during blood clotting (Citation46, Citation315) (Citation316). Conversely, due to the many large proteins present in plasma, other researchers have found EV isolation from serum to be more reproducible (Citation317).
Although plasma-derived EVs are a mixture of vesicles from the cells lining the blood vessels and the different cells found in blood, the largest individual population of EVs in plasma is positive for platelet specific markers (such as, CD41a, CD61 and GPIb) and are considered now to be ~25% of the total blood EVs (Citation318), in contrast to the previous notion of 70–80% (Citation21, Citation53) (Citation196, Citation272) (Citation319, Citation320). However, it has also been suggested that the platelet-marker-positive EVs in plasma from healthy subjects are mostly derived from megakaryocytes (Citation321). As platelets are induced to form EVs by different activatory mechanisms, they may provide a versatile way for the platelet to participate in various physiological maintenance functions from haemostasis to immunity and development (Citation43, Citation322). Finally, preparations of plasma-derived EVs may also include cell organelles, such as mitochondria (Citation323).
The protein and RNA content of plasma-derived EVs, as well as the number of EVs present, has been shown to be altered by several pathological states, suggesting that blood can also harbor an EV population derived, for example, from tumours (Citation95, Citation324). In addition, altered physiological status (such as pregnancy) is reflected in the number and origin of circulating EVs (Citation309, Citation325). The physiological functions of plasma-derived EVs, such as in vascular biology, coagulation and the maternal–foetal communication, will be later described in more detail (see EVs in Vascular Biology and EV functions related to pregnancy sections).
EVs in faeces
The existence of faeces’ EVs has been described (Citation326). Faeces contain bacterial EVs, which seem to have both local and systemic pro-inflammatory effects. The group of Dr. Yong Song Gho presented in the last (ISEV 2014) meeting (Citation327) the physiological role of faeces EVs by using a murine model. Peritoneally injected faecal EVs were reported to induce a dose-dependent peritoneal and systemic inflammation in the mice. In addition, EV uptake by macrophages induced a significant release of TNF-α and IL-6. These results are in accordance with other studies demonstrating that EVs derived from gram-negative intestinal Escherichia coli (E. coli) induce vascular inflammation in vivo (Citation328). This suggests that EVs from the gut microbiota may have the capacity to induce systemic inflammatory responses. More studies are needed to further demonstrate the physiological role of faecal EVs. The role of bacteria-derived EVs will be discussed in more detail in the Bacterial EVs section.
EVs in seminal plasma
Mammalian seminal plasma contains multiple types of EVs that originate from the epididymal duct and the male accessory glands (Citation329–Citation331). Interestingly, seminal plasma was one of the first biofluids in which EVs were characterized (Citation9). These EVs were first termed prostasomes since they were thought to be a specific product from the prostate (Citation8). However, it is now known that seminal plasma EVs originate from distinct sources within the male reproductive tract and, therefore, even if the prostate is the major contributor to seminal plasma EVs, the term prostasome should not be used to refer to all EVs found in seminal plasma. Similarly to EVs in other body fluids, seminal plasma EVs contain a characteristic sets of proteins, lipids and RNA molecules (Citation22, Citation228) (Citation229, Citation332) (Citation333), but there also exist differences in the composition of the EVs released by the different organs of the male reproductive tract. For example, prostasomes, which have been characterized by comprehensive proteomic approaches (Citation22, Citation332), contain prostate specific proteins such as prostatic acid phosphatase, prostate specific antigen or prostate stem cell antigen. Moreover, different types of EVs may be released by the same organ. It has been shown that seminal plasma from vasectomized men contains at least 2 different subgroups of EVs that have prostate specific markers (Citation334), but that differ in size, density and lipid and protein content (Citation335). It is also noteworthy that the EV composition of seminal plasma of different mammals is likely different, as the presence and functions of the accessory sex glands vary between species. For physiological functions of seminal plasma EVs, see EVs in male reproduction section.
EVs in vascular biology
Coagulation
So far, one of the best characterized physiological roles of EVs is their capacity to enhance coagulation and thus participate in haemostasis (). Further, the procoagulant capacity of EVs seems to be amplified in several pathological processes, for example, in EVs generated by cancer cells (Citation11). Since the first descriptions of a procoagulant factor in plasma (Citation2, Citation3), speculation about the significance of EVs during the various spatio-temporal phases of coagulation has been ongoing [reviewed in more detail in Ref. (Citation336)]. The physiological relevance of EVs in coagulation is supported by clinical disorders in which microvesiculation is impaired resulting in bleeding tendency (Citation337–Citation339); the most studied of which is Scott syndrome, a severe bleeding disorder with a reduced procoagulant effect of platelets (Citation339). In this disorder, an impaired phospholipid scramblase activity has been demonstrated, leading to reduced PS exposure, decreased release of procoagulant vesicles and low prothrombinase activity (Citation340). Recently, a defect in the gene encoding TMEM16F, a Ca2+-gated ion channel and a Ca2+-dependent phospholipid scramblase, was identified for Scott syndrome (Citation341), helping to explain the relationship of lipid bilayer changes with the vesicle formation. The physiologically relevant procoagulant role of EVs is supported by a study of sedentary men in which increased formation of procoagulant platelet-derived EVs during hypoxic exercise training enhanced in vitro thrombin generation (Citation342). Furthermore, the addition of exogenous platelet EVs to a flow model of circulation induced thrombosis (Citation343). The procoagulant activity of EVs seems to be predominantly exerted by the larger-sized EV populations from different cellular sources rather than exosomes (Citation53, Citation102), but contrasting evidence has been presented particularly in regard of the TF+ EVs (Citation344), and as reviewed in Ref. (Citation345). Most importantly, procoagulant EVs were also reported to be functional in other body fluids such as in saliva and urine of healthy subjects (Citation265, Citation272).
Fig. 4. EVs in coagulation.
Haemostasis: Originating from various sources (monocytes, endothelial cells, platelets), procoagulant (tissue factor (TF)-EVs and phosphatidylserine (PS)-bearing EVs) and anticoagulant, as well as pro-fibrinolytic EVs may circulate at low levels in normal, healthy blood, contributing to the maintenance of the homeostatic balance in blood coagulation. Up-regulated coagulation or thrombosis: Various clinical conditions (cancer, cardiovascular diseases, inflammation, diabetes, sepsis and others) may trigger the coagulation system, activating circulating monocytes and platelets, making endothelial cells procoagulant and resulting in increased generation of procoagulant EVs, particularly TF–EVs, thus leading to a hypercoagulable condition with thrombotic events, hallmarked with fibrin formation and platelet entrapment (thrombus formation).
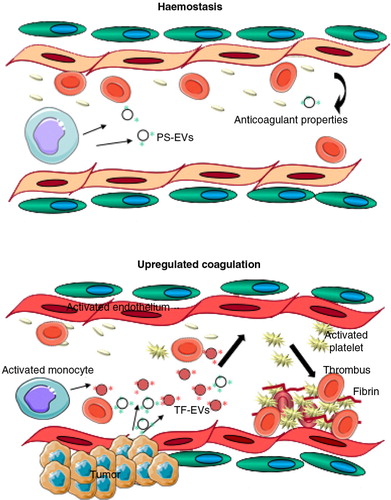
Assigning a defined procoagulant role for EVs in physiology is not only complicated by the lack of studies addressing normal physiological status of healthy humans, but also by the presence of EVs from several cellular sources (particularly in blood) and the spatio-temporal complexity of the coagulation process itself. Cellular interactions and cooperation of EV populations from several cellular sources are likely occurring under the various phases of coagulation (Citation346) (). This hampers the analysis of the cellular origin of the procoagulant EVs. In addition to platelets, various leukocyte populations [for review, see (Citation336)], red cells (Citation347), ECs (Citation336) and even megakaryocytes (Citation321) may participate in forming procoagulant EVs. Moreover, the so-called “haemostatic ring,” that is, smooth muscle cells (Citation336, Citation348) may act as a source of procoagulant EVs. Activated monocytes (Citation336, Citation349) (Citation350) shed TF+ EVs (Citation351, Citation352) and neutrophils may also contribute (Citation353). The presence or absence of TF in EVs can be regarded as the major determinant of the procoagulant potential of an EV-population (Citation336).
EV-mediated transfer of TF in circulation may also be relevant. Monocyte-derived TF+ EVs were reported to bind activated P-selectin-expressing platelets via PSGL-1 and to fuse with them, leading to enhanced TF-FVIIa activity (Citation236). A similar transfer of TF may also operate with other cells within the circulation and, in addition, uptake of heterogenic EVs may promote TF expression by other cells as, for example, neutrophil-derived EVs were reported to induce TF expression by ECs (Citation354). While a low concentration of TF+ EVs may play a role in normal haemostasis and clot formation, to what extent this notion holds true in small vessels–as opposed to large vessels–remains unclear (Citation336). Increased circulating levels of procoagulant, particularly TF+ EVs, have also been associated with pathological states. In diseases such as cancer (Citation355, Citation356) or acute coronary heart syndrome (Citation357), the thromboembolic risk mediated by EVs may be enhanced. Altered coagulation states have also been observed under normal physiological conditions. This may include a transient hypercoagulable state during a healthy pregnancy, whereas pre-eclampsia is characterized by an increased thrombotic tendency which may depend on the increased levels of TF-expressing placenta-derived EVs in the circulation (Citation358–Citation360).
The exact role of EVs in the balance between coagulation and anticoagulation remains unclear, as the predominantly procoagulant role of EVs has been challenged by observations that EVs may also harbour anticoagulant and fibrinolytic properties. ECs, as well as monocytes, express tissue factor pathway inhibitor (TFPI) (Citation361). TFPI inhibits the TF-Factor VIIa complex in a Factor Xa-dependent manner, in addition to inhibiting Factor Xa (Citation361). TF+ EVs and TFPI+ EVs have been detected in normal pregnancies, but their presence was found to be increased in gestational vascular complications (Citation362). Furthermore, platelet activation and the released EVs also induced Factor Va inactivation by activated protein C and TFPI expression (Citation363). This supports the hypothesis that EV sub-populations may have different pro- or anticoagulant properties (Citation53). The role of EVs during the different phases of haemostasis and coagulation may depend on both the quality and the quantity of circulating EVs. An inverse correlation between the EV number and their capacity to form both thrombin and thrombin–antithrombin complexes in plasma in healthy individuals has been shown (Citation364). This may suggest that EVs mainly execute an anticoagulant role, as the low amount of thrombin induced by EVs activates protein C that, in turn, inactivates Factor Va (Citation364). Finally, fibrinolytic activity may also be another physiological counterpart of the procoagulant effect of EVs in circulation, as EVs may generate and disseminate plasmin activity due to plasminogen activators carried by EVs from ECs and leukocytes, but not by EVs of platelet or erythrocyte origin (Citation365).
In summary, cell-derived EVs from multiple sources, with various procoagulant, anticoagulant and fibrinolytic properties, are physiological constituents of the blood and other body fluids in healthy individuals. At the moment, the highly variable normal circulating concentrations and compositions of EVs reported in the literature [for review (Citation366, Citation367)] hamper data interpretation. Therefore, defined standards, protocols for pre-analytical sample preparation, as well as optimized EV analyses, are required before a complete understanding of EV function in coagulation and fibrinolysis can be reached.
Reticulocyte maturation
Exosomes, in their strict sense, were originally termed and described in reticulocytes where they allow remodelling of the plasma membrane in the maturation to erythrocytes (Citation13, Citation368). Reticulocytes are immature erythroid cells generated through enucleation of precursors in the bone marrow. Remarkably, during their maturation to erythrocytes, reticulocytes selectively remove proteins, noticeably the transferrin receptor, as well as membrane-associated enzymes, through the formation of reticulocyte-derived exosomes, rex (Citation14, Citation369). Studies of rex have revealed that segregation of proteins into endosomal membranes occurs via different routes involving endosomal sorting complex, lipid rafts and lectin clustering (Citation370). Of particular interest is the secretion of rex through a non-classical secretion mechanism involving the tumour suppressor-activated pathway 6 (TSAP6) (Citation371). Thus, functional studies with TSAP6 KO mice demonstrated delayed reticulocyte maturation and retention of transferrin receptor leading to microcytic anaemia, strongly indicating possible links of red blood cell disorders and rex secretion. In addition, an increasing number of studies have identified proteins with other relevant biological activities found in these EVs (Citation372). For instance, it has now been established that rex contain α4β1 integrin, suggesting that removal of this adhesion molecule from mature erythrocytes likely avoids pathological processes due to aberrant cyto-adherence (Citation373). Similarly, they have been shown to contain AQP1, indicating that extracellular osmotic conditions can induce release of this protein and suggesting a role for its release during the maturation of erythrocytes (Citation374). In addition, galectin-5 is also partially sorted out in rex, likely contributing to the clearing of specific glycoproteins from the reticulocyte membrane (Citation62). The first proteomics analysis of rex isolated by ultracentrifugation from rat reticulocytes identified close to 1,000 proteins revealing canonical exosomal proteins, but also missing rex-protein markers identified by other biochemical analyses (Citation375). Altogether, these data clearly indicate that reticulocyte-derived EVs are a selective cargo-disposal pathway for destruction of obsolete proteins in the differentiation of reticulocytes into erythrocytes. Yet, rex were also shown to be involved in induction of reticulocitemia, antigen presentation and modulation of immune responses (Citation376). Clearly, further studies on rex might reveal critical insights into erythropoiesis and into novel therapeutics for red blood cell diseases.
Function related to the vascular biology
Leukocyte- and platelet-derived EVs are believed to be responsible for the delivery of pro-angiogenic factors at sites of angiogenic sprouts (Citation377). EVs derived from mononuclear blood cells are reportedly involved in horizontal mRNA transfer and induce pro-angiogenic effects in vitro and in vivo (Citation186). These results are complemented by a study where human EC network formation was improved by supernatants derived from T cells and monocytes co-cultured under pro-angiogenic conditions (Citation378). EC stress influenced the protein and RNA content of EC-derived EV, which would interact with macrophages in vascular niches to promote vascular growth (Citation379, Citation380). Endothelial as well as tumour cells secrete delta-like 4 (Dll4). The blockade of Dll4 inhibited tumour growth by inducing a form of non-productive angiogenesis, poor perfusion and hypoxia (Citation381). EV-associated Dll4 did not require direct cell contact to alter Notch signalling and may thus promote the transformation of ECs into tip cells, angiogenic sprouting and increased vessel density (Citation382). Moreover, EVs derived from mesenchymal stem cells (MSC), incubated under varying oxygen tensions, increased EC migration and proliferation in vitro and regulated vascular adaptation to hypoxia in vivo (Citation383). Hypoxia-cued EVs from multiple myeloma cells were identified to accelerate angiogenesis by targeting the FIH-1/HIF-1 signalling pathway via miR-135b (Citation384). Current research activities focus on extracellular miRNAs, vesicular transport of angiogenesis-associated miRNAs and signalling networks to generate a more detailed understanding of spatio-temporal interactions and regulatory signalling cascades in vascular homeostasis (Citation380, Citation385–Citation387). In this regard, EV-mediated protection against endothelial apoptosis depended on annexin-I/PS receptor function in target ECs and the transfer of miRNA-126 promoted endothelial repair (Citation388, Citation389). EV-mediated cross-talk between ECs depends on miR-214 and was shown to activate an angiogenic programme in target cells, while EC senescence was suppressed (Citation390). Increased understanding of the role of EVs in vascularization has opened up the potential use of EVs in therapeutics, with emerging concepts focused on the development of EVs for pro- or anti-angiogenic therapies used for organ regeneration or cancer treatments, respectively.
EV in the immune system
Innate immunity
The innate immune system is the first mechanism of vertebrate defense against pathogen infection. EVs act on the innate immune system as paracrine messengers and have been mainly described as pro-inflammatory mediators () inducing or propagating inflammatory signals during infections (Citation391, Citation392), sepsis (Citation393, Citation394), chronic inflammatory diseases, such as RA (Citation395, Citation396), atherosclerosis (Citation397), type 2 diabetes (Citation398) and pre-eclampsia (Citation399). In patients with autoimmune diseases (Citation400–Citation403) or with renal (Citation404) and vascular (Citation405) diseases, increased levels of EVs carrying complement components were detected and associated with complement activation. However, a strong anti-inflammatory therapy suppressed inflammation in patients with RA but not the number of EVs associated with complement activation, suggesting that inflammation may not be the main underlying cause of EV release (Citation403).
Fig. 5. Physiological role of EVs related to cells of the innate immune system.
Activated macrophages release EVs that contain cytokines, miR-223 and carry out lateral transfer of receptors influencing myeloid cell proliferation and differentiation. Neutrophilic granulocytes (PMN) produce different types of EVs, depending on the type of stimulus. Neutrophil-derived EVs counteract the activation of immune cells or inhibit bacterial growth directly. EVs containing HSP-70 activate NK cells to combat tumour cells. DC=dendritic cell; NK=natural killer; NKG2D=natural killer group 2D; HSP=heat shock protein.
In addition to promoting inflammation, EVs released by innate immune cells can also contribute to negative regulation of inflammation (), for example, by inducing TGFβ secretion or by regulating endogenous pro-resolving lipid mediators (Citation406).
Various cell types of the innate immune system have been shown to release EVs (seeInnate immunity section). The cargo of these EVs and the type of recipient cell determine their functional effects. EV components that were demonstrated to affect the innate immune response include agonists of pattern recognition receptors (e.g. the TLRs), endogenous pro-inflammatory ligands such as high-mobility group box 1 protein [HMGB1 (Citation407)], membrane phospholipids (Citation408), miRNAs (Citation409), DNA (Citation410), fibronectin (Citation411) and several PAMPs, including lipoarabinomannan and glycopeptidolipids (Citation290, Citation412) (Citation413). In addition, cytokines found to be associated with EVs included IL-1β (Citation391, Citation395) (Citation414), IL-1α (Citation141), TNFα (Citation415), TGFβ (Citation416). Furthermore, it has been demonstrated that EVs present in urine contain viral receptors and anti-microbial proteins and peptides that could inhibit the growth of pathogenic and commensal E. coli and induce bacterial lysis (Citation264).
These data indicate that the role of EVs in innate immunity is complex and that the role of systemically released EVs is unclear. However, several studies have addressed the composition and function of EV isolated from in vitro from cultured innate immune cells.
Monocytes/macrophages: EVs released from monocytes/macrophages can exert several different functions. First, these EV were shown to cause inflammation-induced programmed cell death in vascular smooth muscle cells via transfer of functional pyroptotic caspase-1 (Citation417). Subsequently, it was shown that macrophage-derived EVs could induce differentiation of naïve monocyte recipient cells to macrophages (Citation206). The macrophage-derived vesicles contained high levels of the miRNA molecule miR-223, which is an important regulator of myeloid cell proliferation and differentiation. In addition, EVs released by macrophages contain MHC class II and co-stimulatory molecules and, similar to DC-derived EVs, can play a role in antigen presentation (Citation418, Citation419). Nevertheless, most studies focused on the function of EV released by microbially infected macrophages.
Microbial infection of macrophages (Citation290, Citation413) (Citation416) was shown to modify their EV contents and to promote the release of EVs that stimulate pro-inflammatory responses in resting macrophages (Citation412, Citation413). EV released from macrophages infected with Mycobacterium tuberculosis were shown to contain mycobacterial products, including cell wall components such as the glycolipid virulence factor lipoarabinomannan (Citation420). Upon co-incubation with DC, these EV were able to induce antigen-specific T cell proliferation and may, therefore, be regarded as amplifiers of the immune response in situations where the initial number of bacteria is still low. Macrophages treated in vitro with EVs from Mycobacterium-tuberculosis-infected cells secreted pro-inflammatory cytokines and chemokines, which could subsequently lead to disruption of the integrity of respiratory epithelial cell monolayers (Citation420, Citation421). These pro-inflammatory cytokines and chemokines could stimulate migration of other immune cells to the site of infection in vivo (Citation420). In addition to bacterial pathogens, macrophages that are infected with the immune-modulatory Leishmania parasite were shown to secrete EVs containing the immune-modulatory Leishmania surface protein GP63 (Citation422). These EVs may deliver immune down-modulating signals to bystander macrophages to evade immune attack. Other immune down-modulatory effects of macrophage-derived EV include the inhibition of complement activation and induction of TGFβ release (Citation416).
PMN: Polymorphonuclear neutrophils (PMN) represent the most abundant cell type involved in innate immunity. These have been shown to generate large EVs, termed ectosomes, at the plasma membrane. Both the composition and the biological properties of these EVs depended on the type of stimulation that the producing cells undergo (Citation423, Citation424).
PMN-derived EVs induced the secretion of the anti-inflammatory cytokine TGFβ from monocytes or DCs and decreased the release of the inflammatory cytokines IL-8, IL-6 and TNFα (Citation406, Citation425). They also promoted the phagocytosis of apoptotic PMN and the release of pro-resolving mediators from macrophages (Citation424). The anti-inflammatory protein annexin A1 from PMN-EVs impaired the adhesion of leukocytes to ECs (Citation426), while EVs produced during the process of PMN extravasation seemed to enhance the endothelial barrier function (Citation427). PMN-EVs may also display a pro-thrombotic function by the encapsulation of platelet-activating factor, combined with the exposure of activated Mac-1 (CD11c/CD18) integrin (Citation428, Citation429) and TF (Citation353).
Similarly to the macrophage-derived EVs, a special type of PMN-derived EVs could mediate direct anti-bacterial effects, which were selective to specific bacterial strains (Citation423). The anti-microbial effect of these neutrophil-derived microvesicles was of a bacteriostatic, rather than bactericidal, nature and is thereby different from the action of neutrophil extracellular traps (NETs) (Citation430). Instead, bacterial growth was inhibited by formation of large aggregates between bacteria and EVs which relied on continuous remodelling of the actin cytoskeleton and on the exposition of Mac-1 integrin.
NK cells: As important cells of the innate immune system, NK cells keep the homeostatic balance, combat viral invasion and prevent survival or spreading of malignantly transformed cells. NK cells have generally been considered as components of the first early innate immune defense line, as they do not require “priming,” that is, previous contact with the invading organisms. The old idea that NK cells would kill any target lacking self–MHC class I molecules (the “missing self” hypothesis) has been reconsidered in the past decade (Citation431). It is now clear that NK cells have multiple inhibitory receptors that recognize self-MHC class I (e.g. killing inhibitory receptors) and activating receptors (e.g. NKG2D) (Citation432). The balance between activating and inhibitory signals determines whether or not NK cells become activated.
NK cells could be activated by HSP0 present as soluble or membrane-bound protein in EVs (Citation433). Release of HSP70-containing EVs by tumour cells activated NK cells, which, via recognition of stress-inducible NKG2D ligands on tumour cells, can reduce tumour growth (Citation434, Citation435). Furthermore, EV-associated Bcl2-associated gene 6 (BAG-6), which is required for the protein stabilization and accumulation of HSP70 upon heat shock, can activate NK cells (Citation436).
On the other hand, NK cell function can be downregulated by EVs containing the NKG2D ligands MICA/B (MHC class I-related chains [MIC] A/B (Citation127, Citation437) (Citation438). Treatment of NK cells with EVs containing MICA*008 not only downregulated NKG2D expression, but also provoked a marked reduction in NK cytotoxicity independent of NKG2D ligand expression by the target cells (Citation439), thus providing a mechanism for tumour immune escape.
Finally, human NK cells themselves constitutively release EVs. Although the release of EVs by NK cells may be independent of their activation status (Citation134, Citation440), the composition of these EVs can change depending on the environmental factors. NK cell-derived EVs exhibited cytotoxic activity against tumour cells and activated immune cells (Citation134, Citation440). Taken together, both NK cell-derived EVs and stimulation of NK cells by EVs released by stressed cells or tumour cells can play a role in immune regulation.
Besides the above-described roles of innate immune cell-derived EV in regulation of inflammatory processes, EVs have also been implicated in resolution of inflammation, which is important for the maintenance of tissue homeostasis. Resolution is a biochemically active process that involves the local and temporal biosynthesis of pro-resolving lipid mediators or anti-inflammatory proteins, for which EVs were identified as important regulators (Citation424, Citation441). Self-limited acute inflammation temporally generated leukocyte-derived EVs with pro-resolving lipid mediators in vivo (Citation441). In this context, EVs enriched in resolvin D1 or lipoxin A4 analogues were shown to protect against inflammation in the temporomandibular articular joint (Citation441).
Mast cell-derived EVs: Mast cells are highly versatile cells strategically located at tissues facing the environment, but also in spleen and lymph nodes. Besides their role in IgE-mediated allergic reactions, mast cells contribute by secreting a plethora of immune-modulatory mediators to innate immunity, chronic inflammation and regulation of adaptive immunity (Citation442). Although much is known about the secretion of soluble mediators from secretory granule stores via IgE cross-linking, the release and physiological role of mast cell-derived EVs in immune modulation is rather obscure (Citation443). Mast cell-derived EVs have been reported to contain immune-modulatory proteins, for example, MHC II, LFA-1, ICAM-1, HSPs and the high-affinity IgE receptor (Citation444, Citation445), and were able to target other mast cells; induce DC maturation and deliver antigens for cross-presentation; and induce B- and T-cell activation (Citation16, Citation445). Although the molecular mechanisms behind these processes are largely unknown, the finding that mast cell-derived EVs could functionally transfer RNAs to recipient cells was of great importance (Citation16).
Acquired immunity
Capture of EVs by APCs: modulating the immune response
Antigen-presenting cells, such as DCs, macrophages and B cells, are key players in the translation of information from innate to adaptative immune responses through the capture and processing of antigens and presentation of these antigens using MHC molecules, together with co-stimulatory signals ().
Fig. 6. EVs in the immune system: antigen presentation and acquired immunity.
EVs may have a role in both the origin and progress of the acquired immune response, acting at different levels and on different cells. This figure summarizes how EVs are involved in this process. APC=antigen-presenting cell; Treg=regulatory T cell; NK=natural killer; MHC=major histocompatibility complex.
EVs released by any cell type can function as a source of antigens for APCs. EVs released by a given tissue can harbour antigens signalling the presence of infection/inflammation or malfunctioning of that given organ or tissue. Consequently, such EVs can induce immunogenic or tolerogenic responses as necessary. Several studies addressed the requirements of EV capture by APCs. Integrins and adhesion molecules on EVs and their lipid content may facilitate their attachment and fusion with the plasma membrane of “acceptor” cells. In DCs, internalization of EVs was shown to be an active process (inhibited by cytochalasin D, EDTA or low temperatures, among others) and involved the action of integrins (CD51, CD61, CD11a), CD54, PS and MFGE8 (Citation96). Recently, the participation of sugar domains in EV capture has also been proposed. The capture of Jurkat cell-derived EVs by mature DCs (mDCs) was almost completely inhibited by blocking Siglec-1, a sugar-binding lectin (Citation446). Consistent with this observation, mouse plasmacytoid DCs (which express Siglec-H) were able to capture EVs in vivo (Citation447). Other sugar-binding proteins involved in capture of APC-derived EV include sialoadhesin (CD169) on lymph node macrophages that binds to α2,3-linked sialic acids on the surface of B cell-derived EVs (Citation54) and galectin-5, a β-galactoside-binding lectin on macrophages, which participates in the capture of erythrocyte-EVs (Citation62).
EVs captured by APCs can both convey stimulatory or down-regulatory signals to these cells and contribute to antigen presentation. Although initial studies indicated that internalization of blood-borne allogeneic EVs by splenic DCs did not affect DC maturation (Citation96), other reports have shown that the cellular source and molecular composition of EVs determine how the EV affect the function of immune cells (Citation448).
Several lines of evidence indicate that antigens carried to APCs via EVs can be used to activate antigen-specific T cell responses. Circulating EVs transporting alloantigens, for example, activated anti-donor CD4+ T cells after being captured by splenic DCs (Citation449). Furthermore, EVs from intestinal epithelium bearing exogenous peptides in MHC II interacted preferentially with DCs, potentiating peptide presentation to T cells (Citation450). In the context of microbial infections, EVs derived from Toxoplasma gondii were transported to the spleen, where these EVs elicited a systemic and protective Th1 immune response (Citation451). Furthermore, EVs released by ECs infected with cytomegalovirus could carry virus-derived antigens to DCs, which, in turn, activated specific CD4+ T cells (Citation452). Antigen delivery via EV released by tumour cells could either potentiate the anti-tumour immune response or inhibit this response, for example, by preventing T cell or DC activation (Citation44, Citation453) (Citation454).
Release of EVs by APCs: another way to present antigens: Besides presentation of EV-associated antigens acquired from non-immune cells, APCs themselves also release EVs containing peptide-MHC I or II complexes and co-stimulatory molecules, which can contribute to antigen presentation (Citation15, Citation455) (Citation456). APCs release EVs in a constitutive manner, but this secretion seems to be increased after stimulation, such as following TLR ligation on DCs (Citation457) or BCR cross-linking in B cells (Citation458). Maturation of APCs can lead to increased levels of co-stimulatory molecules on the released EVs (Citation459, Citation460). EVs secreted by DCs and B cells could stimulate primed CD4+ T cells or cognate T cell clones. In contrast, APC-EV-mediated stimulation of naive CD4+ T cells required bystander mDC or B cells (Citation285, Citation461) (Citation462). Besides carrying MHC-peptide complexes, B cell-derived EVs were shown to carry a whole antigen, which was bound to EVs via surface Ig (Citation462). Several lines of evidence have indicated that mDC-derived EVs are able to elicit potent immune responses, whilst immature DC-derived EVs promote tolerance. Examples of immune-activating effects of mDC-derived EVs include the induction of T-cell proliferation and anti-microbial responses by EVs bearing pathogen-peptide-MHC-II complexes derived from bacterially infected DCs (Citation463, Citation464) and induction of T cell proliferation and secretion of IL-5 and IL-13 by B-cell EVs loaded with a birch allergen (Citation465). On the other hand, EVs released by CD95L- or IL-10- expressing immature DCs have been associated with tolerogenic effects, such as the promotion of graft survival (Citation466) and reduction of the inflammatory response in a model of arthritis (Citation467, Citation468).
T cell-derived EVs: EVs released by T cells can be targeted to many different cell types thereby inducing a wide variety of immune regulatory effects ranging from immune activation to immune suppression (Citation469). Although T cells release EVs constitutively, TCR triggering and intracellular calcium stimulation increased EV secretion (Citation470). Activated T cells can produce immune-regulatory EVs that carry MHC, TCR, APO2 ligand, FasL (Citation471) and NKG2D ligands and, for example, inhibit NK cytotoxicity (Citation472), block T cell stimulation (Citation473), promote T cells apoptosis (Citation474) and down-modulate the T cell stimulatory capacity of antigen-presenting cells (Citation475); thereby contributing to dampen immune responses. Furthermore, T regulatory cells produced EVs expressing CD73 that contributed to their suppressive role (Citation476) and prolonged allograft survival in a model of kidney transplantation (Citation477). Besides these immune suppressive effects, T cell-derived EVs were implicated in RANTES (CCL5)-dependent induction of T cell proliferation (Citation478) and in the promotion of immunogenicity via gene regulation in targeted APCs (Citation164). Furthermore, T cell-derived EVs could activate mast cells resulting in degranulation, IL8 and IL24 induction (Citation479, Citation480).
Although EVs can be transferred between cells at a distance, the lytic synapse between CD8+ cytotoxic T cells and infected target cells or tumour cells (Citation481), as well as the immune synapse between T cells and antigen-presenting cells (Citation201), provides a specialized platform for the efficient transfer of EVs. During the formation of these synapses, intercellular compartments containing vesicles move towards the target cells in order to confine EV release towards the synaptic cleft.
MSC-EVs: After the description of the multi-lineage potential of human mesenchymal stem/stromal cells (Citation482), MSCs have emerged as one of the most intensively studied adult stem cell population so far (Citation483). Compelling evidence indicates that in addition to their multipotent capabilities, MSCs can modulate both innate and adaptive immune responses and their therapeutic use in many immunologically related diseases have been promising (Citation370, Citation484) (Citation485). Although some pre-clinical in vivo studies implied that MSCs home into various tissues, especially into sites of injury and inflammation or into tumours, (Citation486–Citation489), other studies suggest that migration of MSCs into damaged tissues may not be required for MSCs to exert their therapeutic functions. According to novel findings, MSCs mediate their clinical effects in a paracrine manner rather than by cellular interactions (Citation490–Citation492). In this context, part of this paracrine/endocrine mechanism of MSCs is thought to be mediated by the MSC-derived EVs (Citation493) (). MSC-derived EVs have been shown to mediate immune suppressive effects, enforce M2 macrophage polarization and drive Treg cell induction (Citation494). Furthermore, it was demonstrated that MSC-EVs suppressed T-cell proliferation and enhanced the proliferation of the regulatory CD4+CD25+FOXP3+ T-cell population in vitro upon co-culturing with PBMCs (Citation495–Citation497). Strikingly, in an individual treatment attempt of a steroid-resistant grade IV graft-versus-host disease patient, MSC-EVs suppressed the symptoms over time without revealing any side effects; similarly to that previously reported for MSC administration (Citation495, Citation498) (Citation499). Additional examples in which MSC-EVs modulated immune responses in a similar manner to MSCs have come from studies using a murine model of kidney ischaemia and reperfusion (I/R) injury (Citation188, Citation195) (Citation500–Citation502). Here, MSCs as well as their EVs were found to protect I/R kidneys by promoting epithelial cell survival (Citation503–Citation505), most likely during the early phase rather than in the late phase of kidney I/R injury (Citation496). Studies in I/R kidney, as well as in affected cardiac and lung tissues, point towards innate immune system regulating activities of MSC-EVs rather than Treg cell-dependent mechanisms (Citation493, Citation496) (Citation506). In summary, although the exact mode of action needs to be unravelled, evidence suggests that MSC may mediate their beneficial therapeutic impact by means of MSC-EVs. As non-replicating units that can be sterilized by filtration, MSC-EVs provide several mayor advantages for the clinical application compared to MSCs. This enhances the relevance of MSC-EVs qualifies them as a very promising tool for future regenerative and immune modulating therapies.
EV functions related to pregnancy
Successful pregnancy relies on the immunological communication between the foetus and the mother. Direct cell–cell interactions, EVs and soluble mediators are all involved in these communication pathways, both at the fetomaternal interface and/or at a systemic level (Citation507, Citation508) (). The presence of trophoblast cells in maternal blood was shown in the early 1990s (Citation509) and, subsequently, circulating trophoblast-derived membrane particles [syncytiotrophoblast (STB) microvillous membranes; STB microvillous membrane fragments; placental debris] were reported in plasma of pregnant women (Citation510, Citation511). STB-derived vesicles, both MVB-derived exosomes and syncytial membrane-released microvesicles/microparticles (STBMs), are simultaneously present in the maternal blood throughout pregnancy and influence the maternal immune system. The role of EVs and their relationship with pregnancy complications have been investigated by several research groups (Citation309, Citation325) (Citation512, Citation513). Both the phenotypic composition and number of circulating EVs were reported to be altered in the plasma of pregnant women compared to non-pregnant healthy individuals (Citation514–Citation516). Increased amounts of circulating CD14+ (monocyte-derived) EVs and reduced numbers of CD41+/CD42+ (platelet-derived) EVs were detected during pregnancy. In contrast, there was no difference in the number of T- and B cell-derived EVs (Citation517).
Fig. 8. Summary of functions of EVs released by syncytiotrophoblasts.
Syncytiotrophoblasts and EVs secreted by them (STMBs) control both maternal adaptive and innate immunity during pregnancy, mainly via their non-classical MHC I molecules (HLA–E, HLA–F, HLA–G) expression. STMB play a key role in the induction of local immune tolerance. Cell surface or STMB-bound non-classical MHC–I molecules inhibit the killer activity of NK cells through inhibitory receptors, suppress CTL activity and regulate the local CD4+ Treg cell differentiation. STMB can also act on innate immunity via the modification of neutrophil function and NET formation. uNK=uterine natural killer cells; Tc=cytotoxic T cell; Tact=activated T cells; Treg=regulatory T cell; Th=T helper cell; PMN=polymorphonuclear granulocyte, neutrophil.
As previously outlined, EVs found in uterine fluid have been associated with sperm capacitation, fertilization (Citation299, Citation300) and with embryo implantation (Citation301–Citation304). EVs have been shown to play an immune regulatory role whereby they may protect the semi-allogenic foetus. The production of different types of vesicles by STBs is limited by maternal conditions. On the other hand, while STBM were found to be immunoactivating, procoagulant and anti-angiogenic, exosomes had immunosuppressive properties (Citation512). STBM may interact with neighbouring cells including lymphocytes, monocytes, neutrophils, platelets and ECs exerting a wide range of effects. STBM bound to circulating peripheral T lymphocytes inducing STAT3 phosphorylation in T cells (Citation517, Citation518). Furthermore, STBM affected local cytokine production and regulated cytokine responsiveness of neighbouring immune cells. They modified the IL-6 sensitivity of CD4+ lymphocytes by the downregulation of their IL-6Rα expression, which may be important in the development and maintenance of local immune tolerance (Citation519). EVs were reported to induce pro-inflammatory response via their danger molecules including HMGB1 and HSP-70 (Citation512, Citation520) (Citation521), and to mediate procoagulant activity by their surface expression of TF. Their Flt-1 and endoglin contents contributed to endothelial dysfunction (Citation522). In contrast, STB-derived exosomes induced apoptosis in T lymphocytes through the downregulation CD3-zeta chain expression (Citation518) and downregulated cytotoxic activity by inducing apoptosis via FasL/TRAIL (Citation523) or by inhibiting the NK cell cytotoxic activity via NKG2D receptor–ligand interaction (Citation524). Secretion of other immunomodulatory molecules, such as PD-L1/B7-H1/CD274 on exosomes has also been reported (Citation525). Thus, these EVs may have a role in the maintenance of successful pregnancy through downregulation of T cell activity (Citation309). STBM production seems to somewhat dominate over exosome secretion with a net effect of a transient slightly inflammatory, hypercoagulative state balanced by the counteraction of exosomes. However, if the STBM production were highly enhanced compared to exosome secretion, pathology occurs, being the most classical example pre/eclampsia (Citation526, Citation527).
During normal pregnancy, the transient hypercoagulable state is well balanced. In contrast, pre-eclampsia is characterized by excessive platelet activation, endothelial damage and dysfunction and an increased tendency to thrombosis. STBM express TF and the expression levels are higher on pre-eclamptic vesicles (Citation360). The increased numbers of circulating STBM in the plasma of pre-eclamptic women (along with the higher TF expression) are proposed to comprise a substantial intravascular pro-thrombotic stimulus in these patients (Citation358–Citation360).
In summary, interactions of STB-derived EVs with cells affect local angiogenesis; modulate the differentiation and activity of immune cells at the fetomaternal interface; have a direct effect on coagulation; and regulate local and systemic inflammatory responses. On the basis of these findings, STB-derived EVs may offer novel diagnostic possibilities in the monitoring of pregnancy progression and may also be considered in novel fertilization strategies.
EVs in male reproduction
The multiple functions of EVs in semen physiology are based on their ability to transfer molecules either to sperm cells or to immune cells within the female reproductive tract. Sperm cells come in contact with several types of EVs, which can then promote their fertilizing ability by modifying their molecular composition and behaviour (). After sperm cells leave the testis, they recruit membrane (P34H, ADAM7) and cytosolic (aldose reductase and sorbitol dehydrogenase) constituents from epididymosomes, that is, EVs released into the epididymal duct by direct fission from the plasma membrane of epididymal cells (Citation528, Citation529). Sperm cells later come in contact with prostasomes. Prostasomes have been proposed to play a role in the regulation of capacitation and acrosome exocytosis (Citation530, Citation531). These are a complex series of biochemical and biophysical changes that sperm cells undergo in the female genital track to acquire fertilization properties and reach the oocyte (Citation530). Both capacitation and the acrosome reaction involve protein phosphorylation, changes in cytosolic levels of Ca2+ and cyclic nucleotides, cholesterol transfer and remodelling of the sperm plasma membrane domains (Citation532). The function of prostasomes in these processes is complex and both inhibitory (Citation533) and stimulatory effects have been reported (Citation534). In a unifying model (Citation535) it was proposed that prostasomes bind to sperm cells in the uterus early during capacitation, inhibiting premature progression to late capacitation events. Prostasomes may then “piggy-back” onto the sperm cell's surface until approaching the oocyte–cumulus complex in the oviduct. There, prostasomes may fuse with sperm cells, stimulating late capacitation events and, ultimately, allowing the acrosome reaction.
Fig. 9. EVs in the male reproductive tract and seminal plasma.
The epithelial cells of the epididymis produce a population of EVs that are thought to bud directly from the plasma membrane. These vesicles, called epididymosomes, fuse with sperm cells to transfer proteins that contribute to the maturation of sperm cells. Epithelial cells of the prostate also secrete EVs. These vesicles, sometimes termed prostasomes, have been suggested to originate largely from MVB. Prostasomes are thought to interact with sperm cells in the female reproductive tract to facilitate them reaching the oocyte.
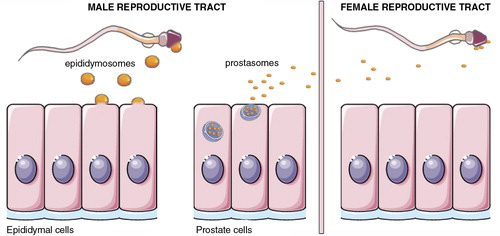
Another main function of prostasomes is to protect sperm cells from the female immune system on their way to the ovum (Citation536). Macrophages, neutrophil granulocytes and NK cells may all attack sperm cells (Citation537, Citation538). Human prostasomes were able to inhibit phagocytosis by monocytes and neutrophil granulocytes (Citation539, Citation540), as well as interfere with lymphocyte proliferation. Also NK cell activity was shown to be inhibited by prostasomes and a role for the prostasomal protein CD48 (which is the ligand for activating NK cell receptor 2B4) was proposed (Citation541). Furthermore, prostasomes may also protect spermatozoa against the complement pathway via their constituents CD59 and CD46 (Citation542, Citation543). Finally, EVs in human semen inhibited replication of sexually transmitted retroviruses, but not herpes simplex viruses, in target cells (Citation544).
In conclusion, EVs in seminal plasma play an important role in fertilization by protecting sperm cells in the female genital tract and by regulating their motility and maturation. However, the composition of these EVs is complex and the specific molecular processes and mechanisms by which EVs help sperm cells in vivo on their way to the oocyte have yet to be elucidated.
EVs function related to the embryonic development
EVs may also contribute to intercellular communication during embryonic development. Here, the current evidence of EV functions in central processes of embryogenesis, such as morphogenetic gradients formation, cell migration and the development of cellular polarity are reviewed.
EVs and morphogens gradients
One of the central processes of embryogenesis is the coordination of cell positioning and fate acquisition in response to the morphogens gradient (Citation545). Certain features of morphogen distribution, for example, specificity towards certain target cells, dynamics of the distribution over long distances and the formation of intra- and extracellular gradients, could not be explained by the widely accepted model of gradient formation by passive diffusion. The first hypothesis of an active morphogen transport in Drosophila was suggested to be mediated by means of membrane fragments called “argosomes” (Citation546, Citation547). Later on, argosomes were defined as exogenously derived lipoprotein particles enriched with GPI-linked proteins that support the transport of morphogens through the epithelium (Citation548). The function of EVs as one of the secretion routes for functional Wnt proteins in vivo was demonstrated by showing that Wnt proteins were co-segregated from the supernatants of mammalian and Drosophila cells, within the 100,000×g pellets, and were located on the outer membrane of EVs of approximately 80 nm diameter which also harboured CD63 and CD81 tetraspanins (Citation549). Shuttle of functional Wnt proteins to MVBs required ESCRT and was mediated by their cargo receptor Evi Ykt6 protein, which regulates early/recycling endosomes (Citation549). Other lipid-modified morphogens such as Hedgehog have also been shown to be secreted in an ESCRT-dependent manner (Citation550) in EVs travelling along cytonemes in order to create a gradient in the tissue (Citation551). These data suggest that the distribution of morphogens during embryogenesis is a complex process combining different routes, such as passive diffusion of soluble proteins and the active transport by lipoprotein particles or by EVs. It remains to be established how the sorting of morphogens between these routes is regulated and what the specific impact of each particular route is in the embryonic development.
EVs and tissue polarity
The contribution of EVs to the regulation of cell polarity and developmental tissue patterning was initially suggested by Lakkarajau and Rodriguez-Boulan (Citation552). However, until now only a few instances of indirect evidence support the involvement of EVs in the regulation of cell polarity during embryo- and organogenesis. For instance, vesicular traffic was required for the asymmetrical distribution of adenylyl cyclase during collective head-to-tail cell migration in Dictyostelium discoideum during which adenylyl cyclase accumulated in the back of the cells within multi-vesicular bodies, which then could be released as EVs and be tracked to the direction of cell migration (Citation553). In polarized cells, EVs derived from the apical and basal membrane differed in their content (Citation554). Proteins involved in the regulation of cell polarity, such as Rab11 (mediating exocytosis) and syntaxin-3 (essential for establishing the polarized epithelium) were located on the EVs and played important roles in the regulation of vesicle release (Citation555, Citation556). In addition, Notch signalling, essential for the determination of cell polarization, could be modulated through delta-like 4 located on the EVs, supporting potential impact of EVs on cell polarity (Citation557).
In conclusion, EVs are likely to be involved in the regulation of main routes of embryonic development, including the regulation of morphogen gradients, collective cell migration and tissue polarity. However, this still remains an emerging field with many unanswered questions, which need further investigation.
EV function related to tissue repair
Phenotype change and cellular plasticity are potential driving factors in tissue regeneration and are being increasingly explained by cellular communication via EV-dependent delivery of RNA species (Citation558). Stem cell niches are considered areas of microenvironmental influence providing environmental aspects such as oxygenation, position, mechano-transduction, soluble factors and EVs mediating cell–cell communication. It is likely that regeneration is, in part, regulated by EV vesicle transfer. Phenotype modulation with the expression of epithelial prosurfactant B in marrow cells by lung-derived EVs and engraftment of marrow cells after lung injury has been shown as a novel mechanism for lung repair (Citation559). A report about the anti-apoptotic and cardio-protective effects of human embryonic stem cell-derived mesenchymal stem cell conditioned medium in a porcine model of ischaemia–reperfusion injury resulting in a 60% reduction in infarct size initiated an intensive search for the responsible paracrine factors (Citation500). Fractionation studies revealed that the cardio-protective effect of conditioned medium was exclusively exerted by the >1,000 kDa fraction with sizes ranging from 100 to 220 nm; indicating that the active agent was a complex of multiple components that might include proteins and lipids (Citation500). A subsequent study, using a myocardial ischaemia–reperfusion injury mouse model, confirmed that EVs were indeed the large complexes responsible for the cardio-protection (Citation493). The evidence that tissue regeneration triggered by exogenous (stem) cells might depend on the release of paracrine factors delivered by EVs, rather than on stem cell trans-differentiation per se was further supported by a report on hepatic regeneration (Citation560). EVs from human liver stem cells accelerated morphological and functional recovery in a rat model of 70% hepatectomy and mediated de novo expression of human argonaute proteins in rat hepatocytes. This is considered as definitive proof of horizontal mRNA transfer between human and rat cells (Citation560). EVs derived from human adult MSCs have been found to protect against ischaemia–reperfusion kidney injury and enhanced survival in a model of lethal acute kidney injury (Citation187, Citation501). In addition, in a clinical model of acute myocardial infarction, lung resident MSCs efficiently exerted pro-regenerative functions (Citation490). Confirming the paracrine nature of the MSCs supporting activity, the supernatants of in vitro expanded MSCs have been shown as sufficient in reducing myocardial infarct sizes (Citation500). Furthermore, the protective effect of EVs derived from human peripheral blood-derived cells cultured under angiogenic conditions was attributed to a miRNA-dependent reprogramming of resident renal cells and was reversible with RNase or siRNA treatment (Citation210).
EVs in bone calcification
From a historical perspective, investigations of the transformation of cartilage into mineralized bone via extracellular TFs dates back to at least the 1920s. The early work of Anderson (Citation4, Citation561) supported the hypothesis presented by Robison (Citation562) that enzymes involved in ossification are secreted from cells within the calcifying tissue. First important results included the definite distinction of extracellular matrix vesicles (matrix EVs) from lysosomes. From the presence of lipids at the calcification sites, the existence of membranous structures was extrapolated, and membrane-bounded vesicles were indeed demonstrated in various mineralizing tissues by electron microscopy. As a consequence, it was postulated that matrix EVs contain enzymes that mediate a local increase in orthophosphate and drive the formation of hydroyxapatite at the sites of matrix EVs accumulation or release (Citation561). Essentially, the purification strategy for matrix EVs by sequential differential centrifugation has not changed since 1970 (Citation561), with the 100,000×g pellet containing the highest activity. Matrix EVs were later identified as carriers of morphogenetic information and some of the molecular details of bone morphogenetic protein (BMP) transfer and VEGF, or bone sialoprotein (BSP) delivery, were elucidated. Matrix EVs were found to stimulate expression of alkaline phosphatase via BMPs, while VEGF may promote capillary invasion of the growth plate (Citation563). Notably in this latter study, the first report of physiological mineralization via matrix EVs with an initial release of BMPs into the matrix and a subsequent breakdown of the matrix EV membrane following mineral initiation was described.
This seminal work has fundamentally influenced our view of matrix EVs and much of the initially described characteristics have stood the test of time. A current model proposes matrix EVs to originate from the plasma membrane of mineral-forming cells [see also (Citation564)] and to induce calcification during endochondral bone formation. Indeed, the proposed biogenesis of matrix EVs from apical microvilli (Citation565), as well as the lipid and protein content of matrix EVs, strongly suggests a plasma membrane-derived origin. However, in several studies, vesicle biogenesis-related proteins were identified on the surface of matrix EVs derived from aberrantly calcifying cells that point to an endosomal origin.
Cardiovascular calcification and bone remodelling share some fundamental regulatory principles and the EVs involved are apparently differentially loaded with specific cargo whose sorting and packaging is largely influenced by the cellular context [reviewed in Ref. (Citation566)]. It seems that matrix EVs, under pathological conditions, may act as intercellular signalling modules in a manner similar to exosomes rather than as “extracellular nucleation” sites under physiological conditions. Considering the polarized release of matrix EVs into the extracellular matrix and the proposed mode of action as a nucleation site for calcification within the extracellular matrix, the repertoire of proteins that are found in matrix EVs appears both necessary and sufficient for these duties.
EVs function related to liver homeostasis
The liver is essential for metabolism and is involved in the synthesis and clearance of blood and bile components, storage and mobilization of lipids and carbohydrates and response to external (e.g. diet, drugs) and internal (e.g. endotoxins) stresses (Citation567). Although this organ is formed mostly by hepatocytes, it also contains other non-parenchymal immune and non-immune cells that need to communicate with each of them in order to elicit a proper response to specific hepatic stimuli and insults. The resident liver tissue macrophages (Kupffer cells), NK cells, T cells and B cells are all members of the hepatic immune system and are all important mediators in inflammation (Citation568). Among non-immune cells, the stellate cells, also known as Ito cells, are involved in angiogenesis (Citation569), inflammation and fibrosis processes. All these cellular populations, with their diverse physiological processes, must be strictly coordinated to keep the liver healthy and, subsequently, to maintain the right homeostasis of the body. Increasing evidence supports the idea that EVs mediate part of the intercellular communication among different cell types. For example, it has been shown that primary cultured hepatocytes are able to secrete EVs that, based on density, structure and composition, show many exosomal features (Citation570). In addition, a comprehensive proteomic study of these hepatocyte-derived EVs revealed the presence of several members of cytochrome P450, Uridinediphosphate–Glucuronosyl–Transferase (UGTs) and Glutathione S-Transferase (GST) protein families, supporting a role of these vesicles in the metabolism of endogenous and xenobiotic compounds (Citation570, Citation571). Recently, the RNAs present in these vesicles were characterized, showing that EVs from hepatocytes were able to activate stellate cells to mediate a response to liver damage, in an RNA-dependent manner (Citation572). Moreover, EVs derived from a sub-population of pluripotent/multipotent resident liver cells were shown to accelerate the morphological and functional recovery of liver in partially hepatectomized rats (Citation560). This effect was lost when EVs were treated with RNase, suggesting that RNA was also involved in the process (Citation560). Other hepatic cell types, such as cholangiocytes, can also secrete EVs (Citation277) and, by means of transmission electron microscopy, EVs present in the bile duct were shown to interact with the primary cilia of cholangiocytes (Citation573), supporting a role in intercellular communication in this cellular system. Biliary EVs secreted by cholangiocytes contribute to the inactivation of ERK kinase signalling (Citation278), a pathway associated with cholangiocyte proliferation (Citation574). Cholangiocytes and myofibroblastic hepatic stellate cells released EVs containing active Hedgehog ligands in response to platelet-derived growth factor, which, in the acceptor cells, activated Hedgehog signals that may stimulate angiogenesis (Citation277).
Although studies on hepatic EVs have been limited to date, they support an important role of these vesicles in maintaining liver homeostasis. Further research in other liver resident cells (e.g. hepatic sinusoidal cells) and studies involving the co-culture of combinations of different cell types in controlled conditions are required to further unravel the physiological role of the network of EVs established in the liver.
EVs in the nervous system
The major task of the nervous system is the integration of incoming information and generation of an output, coordinating the functions of the different organs and tissues in the body. Systemic signal processing not only is achieved by synaptic cross-talk among electrically active neurons, but also depends on non-synaptic neuronal interaction and intense communication between neurons and glial cells. Recent research provides compelling evidence that the exchange of EVs may be a common mode of neural cell communication. Cultured neurons and the different types of glial cells release EVs [reviewed in Ref. (Citation575–Citation578)]. Furthermore, EVs of distinct size and origin can be detected in the CSF (see EVs in Cerebrospinal Fluid section), the drainage system of the brain (Citation281, Citation282). Several studies suggest that EVs possess the ability to cross the blood–brain barrier in both directions, although the route of transfer remains unclear (Citation324, Citation579) (Citation580). EVs were shown to enter the brain parenchyma at the choroid plexus and to mediate folate import into the brain (Citation581). Notably, inflammatory conditions, often associated with a leaky blood–brain barrier, facilitated the entry of peripheral EVs into the brain resulting in genetic modulation of the target cells of the CNS (Citation582). EVs released from neurons have been implicated in the transfer of biomolecules across synapses and were suggested to mediate synaptic plasticity in vertebrates and invertebrates. In rodents, glutamatergic synaptic activity triggered the release of EVs largely from somato-dendritic (post-synaptic) sites (Citation575, Citation583). These EVs contained neurotransmitter receptor subunits, which led to the suggestion that release of EVs may affect the local elimination of these receptors from post-synapses and, thus, may modulate synaptic strength as part of a process termed homeostatic synaptic scaling. Moreover, neuronal EV released in activity-dependent fashion carried the synaptic-plasticity-associated protein MAP1b and miRNAs (Citation584) and preferentially interacted with target neurons at pre-synaptic terminals (Citation575, Citation583) (Citation585). At the Drosophila larval neuromuscular junction, EVs modulated synapse expansion by mediating the transmission of Wnt-signalling molecules, as well as the transfer of synaptotagmin 4 from pre-synaptic motor neurons to post-synaptic muscles (Citation586, Citation587). In Caenorhabditis elegans, it was demonstrated that EV budding from the cilia of sensory neurons mediated communication even between different animals and influenced mating related behaviour (Citation588).
Moreover, all types of macroglia and microglia secrete EVs in the form of exosomes or microvesicles. Microglia, which are phagocytic cells contributing to CNS tissue homeostasis, respond to ATP-mediated P2X7 receptor activation by shedding EVs from their plasma membrane. Intriguingly, microglia-derived EVs appeared to modulate neurotransmission at excitatory glutamatergic as well as inhibitory GABA-ergic synapses largely by lipid-mediated signalling (Citation578, Citation589) (Citation590). In addition, microglial EVs were proposed to propagate inflammation in the CNS, since they carried the pro-inflammatory cytokine IL-1β and were increased in CSF during inflammation (Citation591). Conversely, microglia mediated the immunologically silent clearance of EVs originating from other CNS cells (such as oligodendrocytes); at least under physiological conditions (Citation98, Citation592).
Moreover, it has been demonstrated that EVs may participate in reciprocal communication between myelinating oligodendrocytes and neurons. Electrically active neurons could trigger the release of oligodendroglial EVs by neurotransmitter signalling and, furthermore, internalized these EVs by endocytosis (Citation593). Hence, target neurons directly modulated the availability of oligodendroglial EVs, following the principle of “delivery on demand.” Neurons that received oligodendroglial EVs were more resistant to cell stress, indicating that oligodendroglial EVs provided trophic support to target neurons and mediate neuroprotection. Consistently, oligodendroglial EVs activated pro-survival signalling pathways and modulated gene expression in target neurons (Citation594). Likewise, Schwann cells in the peripheral nervous system secreted EVs that were internalized by axons, providing local axonal support. Schwann cell-derived EVs were able to enhance axonal regeneration after nerve damage (Citation595). EVs that enter the CNS from the periphery may also exhibit regenerative functions: MSC-EVs were shown to transfer neuroregenerative miRNAs to astrocytes and neurons in a rat model of stroke (Citation596). Furthermore, serum EVs harvested from youthful mice were suggested to enhance myelination as well as remyelination (Citation597).
In summary, EVs have been established as novel players in neural cell communication with versatile physiological implications, both in the developing and the adult nervous system. The role of EV-mediated horizontal transfer of RNA in intercellular gene regulation and the local phenotypic adaptation of neural cells remains an exciting open question in neuroscience. Furthermore, substantial efforts are being invested into deciphering the putative role of EVs in the spreading of neuropathological agents in neurodegenerative diseases as well as in promoting the growth of brain tumours [reviewed in Ref. (Citation598, Citation599)].
EVs in lower organisms
Parasites have plagued humans throughout the world for more than 150,000 years (Citation600). It is currently believed that there are close to 400 species affecting humans, of which approximately 90 are responsible for great clinical burden and death (Citation601). The use of secretion systems is an essential biological process exploited by pathogenic microorganisms to promote survival. In this context, the study of EVs released by pathogens is a new and exciting field that may realistically contribute to a better understanding of the pathogenic process (Citation602, Citation603) () and provide alternate control strategies for the 2 major groups of parasites, the helminths (worms) and the parasitic protozoa (Citation604–Citation606) (). The half-life of these EVs can vary, they can either be quickly broken down, existing only in the immediate space of the pathogen; or, they can persist appearing in many biological fluids such as urine or blood (Citation100). This potential for persistency enhances their capacity to interact with target cells in ways impossible for free soluble molecules functioning as extensions of the pathogen (Citation602). Moreover, their membranous nature enables their fusion with/uptake by target cells, potentiating the horizontal transfer of cargo molecules including proteins and RNA (Citation100). These pathogen-derived EVs, therefore, have the potential to mimic the characteristics of the host EVs.
Fig. 10. EVs in parasitic diseases.
Secretion of EVs has been described for both helminths and parasitic protozoa. In helminths, they serve as mechanism for protein and miRNA export and host manipulation. In parasitic protozoa from the kinetoplastids family, EVs released by Leishmania spp. are able to induce specific recruitment of neutrophils to the site of infection. They are also taken up by phagocytic cells, enabling the delivery of immunomodulatory proteins contributing to the creation of a permissive environment for the infection. In T. cruzi, EVs contribute to the stabilization of the C3 convertase disturbing the functioning of the complement system. Regarding Apicomplexa in malaria, circulating levels of EVs rise during human infections and in rodent models, while exosomes derived from reticulocytes induced protection upon immunization in a murine model. Also, exosomes from malarial infections were able to induce parasite sexual development. Other obligate intracellular parasitic protozoa are Toxoplasma gondii and Trichomonas vaginalis. EVs isolated from dendritic cells and primed with Toxoplasma antigens conferred protection upon immunizations being a proof-of-concept of EVs as therapeutics agents. In trichomoniasis EVs increased virulence by inducing parasite attachment to cervical epithelium, thus facilitating host cell colonization.
Helminths
Helminths can be divided into 2 major groups known as the nematodes (roundworms) and the Platyhelminthes (flatworms), this latter composed of cestoda (tapeworms) and trematoda (flukes). Together, these are responsible for a large burden of disease and socio-economic losses, as hundreds of millions of people–mostly in areas of extreme poverty–are infected (Citation600). Early reports suggesting the existence of EVs in helminths came from TEM studies of tegument of flukes Schistosoma mansoni and Fasciola hepatica (Citation607, Citation608). Later, protein analysis of the tegument of Schistosoma spp. revealed the presence of typical “exosome proteins,” suggesting that helminths could actively secrete EVs (Citation609, Citation610). Recently, the existence of exosome-like EVs in the parasitic intestinal trematode Echinostoma caproni and the liver fluke Fasciola hepatica has been confirmed (Citation611). This report constitutes the first description of EVs in parasitic helminths, identifying 51 and 79 parasitic proteins from E. caproni and F. hepatica, respectively. More than half of the proteins identified had previously been described in the secretome of other parasitic trematodes (Citation612). These data suggest that EVs may constitute the primary mechanism for protein export in trematodes.
In contrast to trematodes, little is known about the presence of EVs in parasitic nematodes. Yet, preliminary studies have identified EVs in the parasitic nematode Heligmosomoides polygyrus, exhibiting immunomodulatory activity (Citation613, Citation614). Recently, the presence of “atypical secreted” proteins, including 14-3-3 and serpin, was described in the Ascaris suum larval proteome, suggesting that they were secreted in EVs (Citation615).
As highlighted in the RNA composition section, EVs are also gaining attention since they act as a novel RNA shuttle mechanisms capable of signalling messages to other cells and as new powerful diagnostic markers of disease (Citation16). miRNAs were isolated from EVs from the parasitic trematode Dicrocoelium dendriticum (Citation616). In addition, H. polygyrus derived miRNAs and Y RNAs were shown to be transported into mammalian host cells via EVs, where they regulated host genes associated with immunity and inflammation and suppressed the innate type 2 response in vivo (Citation616, Citation617) suggesting that this may be a common feature for parasitic helminths (Citation618). The function and diagnostic potential of such RNAs needs further investigation.
Parasitic protozoa
Close to 70 species of parasitic protozoa affect hundreds of millions of humans annually causing a wide spectrum of poverty-related diseases such amoebiasis, malaria, African and American trypanosomiasis and leishmaniasis. As in helminths, research on EVs in parasitic protozoa is gaining attention, especially in host–parasite interactions (Citation604–Citation606). For this reason, we briefly discuss EVs in the context of 2 major groups, that is, kinetoplastids and apicomplexa.
Kinetoplastids
Trypanosoma cruzi and Trypanosoma brucei: Trypanosomes is a complex group of unicellular parasitic protozoa belonging to the order kinetoplastida, which often require intermediate hosts to complete their complex life cycle (Citation619). In humans, trypanosomes cause a variety of diseases such sleeping sickness caused by Trypanosoma brucei (T. brucei) and Chagas disease caused by Trypanosoma cruzi (T. cruzi). The first description of the shedding of EVs from trypanosomes was elegantly shown by TEM studies of T. cruzi where the release of 20–80 nm EVs containing parasite antigens was evident (Citation620). The proteomics analyses of EVs from T. cruzi have expanded the list of known parasite proteins, including members of the transialidase multigene family, proteases and cruzipain, among many others (Citation621). Similar to T. cruzi, T. brucei actively secretes EVs containing parasite proteins that are apparently involved in intercellular communication with the host (Citation622). Interestingly, a significant proportion of proteins in the secretome lack a transit peptide, suggesting that they are not secreted through a classical sorting pathway. To clarify this, EVs were isolated and characterized from secreted material as well as from infected rat sera, confirming an active exocytosis process beyond the flagellar pocket (Citation622). The secretion of proteins via the EV pathway may have several advantages for trypanosomes, such as delivering an avalanche of new epitopes to overwhelm the host immune system or to establish a communication link between parasites as a survival strategy. Also in EVs derived from T. cruzi, proteins associated with virulence where also detected (Citation621, Citation623) (Citation624) as a clear indication of the potential of these EVs as immunomodulatory agents. Moreover, T. cruzi were found to induce EV release from infected blood cells. Those EVs formed a complex with the complement C3 convertase on the T. cruzi surface, leading to its stabilization and inhibition and resulting in increased parasite survival (Citation416).
Leishmania spp Leishmania are the etiological agents of leishmaniasis. This parasite adapted to survive and proliferate in the shadow of the immune system thriving in the inhospitable environment of the macrophage phagolysosome. Recent emphasis has been given to the possible role of EVs in this process (Citation625). Leishmania EVs were originally reported in L. donovani promastigotes grown in CM (Citation626). The size, density and protein content of recovered EVs are consistent with their identification as exosomes (Citation626). Nonetheless, the detection of EVs of larger size with protein content not traditionally associated with exosomes suggests the existence of different types of EVs (Citation627). The release of EVs seems to be constitutive, being detected in culture supernatant of logarithmic and stationary promastigotes in axenic growth (Citation627). Moreover, physiological stress conditions such as temperature shift to 37°C (Citation626, Citation628), acidic pH (Citation626), death-inducing agents (Citation627) and starvation (Citation629) are capable of increasing EVs release in vitro. Therefore, the constitutive nature of EVs release and their involvement in the response to external stimuli suggests that they might be involved in significant biological processes that are still unreported (Citation627). In fact, most of the reports on Leishmania EVs are related to their involvement in the infectious process, overlooking their possible roles in housekeeping, communication, death and differentiation.
The capacity of Leishmania EVs to function as extensions of the parasite enabling close and long-range immunomodulation was shown in vitro. The EVs from L. donovani inhibit pro-inflammatory cytokine production (TNF-α), while promoting immunosuppressive cytokine production (IL-10) in human monocytes (Citation630). Interestingly, consistent with an immunosuppressive profile, it was shown that EVs treatment also hampers the in vitro differentiation of naïve CD4 T cells into IFN-γ Th1 cells in a cargo-dependent manner (Citation630). Moreover, the few reports that addressed the in vivo properties of these EVs also seem to be consistent with a permissive infection (Citation630). The vesicle components responsible for these immunomodulatory properties are, understandably, subjects of great interest as Leishmania EVs are capable of delivering their content into target cells (Citation626). Interestingly, all the known Leishmania proteins shown to translocate into the macrophage cytosol were found in Leishmania EVs cargo (Citation626, Citation627). The possibility of virulence factor delivery mediated by EVs was clearly shown using a protease (GP63) that is associated with direct modulation of host signalling in the precocious stages of infection (Citation631, Citation632). Using EVs recovered from L. major gp63 -/-, it was demonstrated that their immunomodulatory capacities were substantially reduced (Citation633). Moreover, GP63 delivery by EVs was also associated with in vivo and in vitro downregulation of specific miRNAs in hepatocytes, facilitating liver infection (Citation634). The capacities of EVs to influence the outcome of the infection might not be restricted to the delivery of virulence factors. In fact, EVs are also capable of cell-specific recruitment (Citation633), possibly contributing to the cellular environment in the initial inoculum. Therefore, as consequence of the delivery of immunomodulatory molecules and also the direct interaction with target cells, Leishmania EVs are expected to be significant players in the precocious steps of infection enabling a permissive environment for the parasite.
Apicomplexa
Apicomplexa is one of the largest groups of parasitic protozoa with more than 5,000 species, including human parasites such as Plasmodium spp. (malaria), Cryptosporidium spp. and Toxoplasma spp. in humans, and animal parasites such as Babesia spp. in cattle and Eimeria spp. in poultry. Apicomplexa are characterized by having a unique organelle of algal origin known as the apicoplast. Studies on EVs in Apicomplexa have started to shed light into the complex signalling pathways mediated by these vesicles, which act as intercellular communicators between hosts and parasites.
Malaria is the most prevalent parasite worldwide and responsible for close to 300 million clinical cases and 1 million deaths annually; mostly in children under 5 years old. Both exosomes and microvesicles have previously been described in human and rodent malaria parasites. Specifically, EVs have been detected in the peripheral blood of P. falciparum as well as P. vivax patients and seem to be involved in systemic inflammation (Citation635–Citation637). In the case of P. falciparum, the presence of EVs was associated with severe malaria suggesting that they play a role in malaria pathogenesis (Citation636). Interest in the studies of EVs in malaria received further impetus after it was demonstrated that EVs derived from reticulocytes in a rodent malaria model contained parasite proteins and were able to modulate induced protective immune responses upon a lethal challenge (Citation376). More recently, 2 independent studies have demonstrated that EVs secreted by P. falciparum-infected red blood cells act as intercellular communicators (Citation638, Citation639). Remarkably, both studies suggest that the transference of exosome-like vesicles and microvesicles to other infected red blood cells induces gametocytogenesis, providing the parasite a route to escape a hostile environment.
In addition to malaria, studies have reported on EVs of other apicomplexa and unicellular parasites such as Cryptosporidium parvum (Citation640) and T. gondii (Citation641) (obligate opportunistic intracellular pathogens recognized as a major cause of diarrhoea in AIDS and immunodepressed patients), Giardia lamblia (Citation642) (responsible for diarrheal illness) and Trichomonas vaginalis (Citation624) (causing the most prevalent sexually transmitted disease).
In summary, although study on the molecular composition and function of EVs in parasites is a young research field, the data gathered so far from different host–parasite interactions, clearly indicates the role of EVs in intercellular communication, immune evasion mechanisms and establishment of chronic infections. In turn, these data will hopefully bring new insights into the pathophysiology of human parasitic diseases to guide rationale efforts in developing novel control strategies, implemented as new diagnostics, treatment tools and vaccines (Citation618).
Bacterial EVs
EVs are produced by both gram-negative and gram-positive bacteria (Citation643). Gram-negative bacteria possess 2 phospholipid membranes. The inner (cytoplasmic) membrane is the main diffusion barrier, but the cells are protected by the additional peptidoglycan cell wall situated in the periplasma and the outer membrane. The outer membrane is the interface of the gram-negative bacteria with its environment and the extracellular lipid leaflet contains membrane lipids with extensive sugar modifications, the lipopolysaccharides (LPS). By an outward budding of the outer membrane, EVs are produced with a diameter in the range of 20–250 nm (). The molecular mechanism of vesiculation is not completely understood, but it appears to be an essential and conserved biological process of gram-negative bacteria (Citation644). During the genesis of outer membrane vesicles (OMV), periplasmatic components are entrapped into their lumen. Analysis of OMV suggests that their protein and lipid composition is not random (Citation645), so that the cargo of these OMV (either in the lumen or attached on the outside) is regulated by mechanisms not yet understood.
Fig. 11. Biogenesis of an outer membrane vesicle (OMV).
Initialized by outward bulging of the outer membrane, vesicles bud off the gram-negative cell and are then termed OMVs. The membrane of the vesicle is derived from the outer membrane and the lumen contains proteins and other biomolecules from the periplasma.
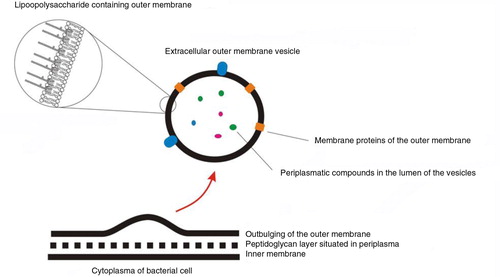
In contrast to gram-negative bacteria, gram-positive bacteria are surrounded only by a single membrane. Although the mechanism of vesicle formation is currently unclear, the membrane and the lumen of vesicles of gram-positive bacteria are thought to be derived from the cytoplasmic membrane and the cytoplasm, respectively (Citation646).
These distinct bacterial secretion pathways enable cells to deliver a multitude of effector molecules at a higher concentration over long distances while encased in a protective membranous sphere (Citation644, Citation647–Citation649). The bacterial EV content may be delivered into animal, plant and bacterial cells by membrane fusion and/or internalization and its delivery can be targeted by molecules attached at the outside of the vesicles.
Bacterial EVs perform several functions, including molecular transport, mediation of stress response, biofilm formation and the influence on hosts, which are described in more detail in the following sections.
Transport & delivery
OMVs have been shown to act as trafficking vehicles for hydrophobic molecules such as the Pseudomonas signalling molecule PQS, thereby playing important roles in intra-species cell–cell communications and quorum sensing (Citation650, Citation651). In addition, OMVs could facilitate the horizontal transfer of antibiotic resistance between bacteria (Citation652, Citation653) in 2 different ways, that is, by intra- or inter-species transfer of resistance proteins (i.e. β-lactamase) to neighbouring cells (Citation654, Citation655) or by lateral transfer of resistance genes following the fusion of the OMVs with the recipient cell membrane (Citation656–Citation658). Our current knowledge of bacterial OMVs is focused on the pathogenic bacteria, which can specifically deliver toxins and virulence factors to the eukaryotic target cells by OMVs. It was recently shown that the gram-positive bacterium Bacillus anthracis produced EVs containing anthrax toxin (Citation659).
In contrast, the physiological roles of commensal-derived OMVs remain yet largely unexplored. One member of human microbiota, Bacteroides fragilis, was shown to secrete polysaccharide A capsular antigen (PSA)-containing OMVs that could trigger a TLR2 mediated signalling in DCs, resulting in production of the immunoregulatory cytokine IL-10 which resulted in the maturation of T regulatory cells (Citation660), implicating OMV as important mediators in the establishment of mutualism.
Stress response
OMV production is an indispensable bacterial survival strategy in response to environmental stress. Stressors such as heat shock can lead to the accumulation of misfolded proteins within a cell, thus promoting toxicity. Misfolded proteins may be sequestered in OMVs and exported out of the cell (Citation661). OMVs may also act as decoys to protect bacteria from complement components (Citation662), long-chain alcohols, metal chelators (Citation663) and anti-microbial peptides (Citation664).
Biofilm
Biofilm formation is an alternative bacterial survival strategy that enables protection and persistence of a bacterial community. In biofilms, bacteria live as a multi-cellular community in an extracellular matrix. OMVs play a prominent role in the formation and maintenance of this extracellular matrix (Citation665). Biofilm OMVs mediate adhesive interactions contributing to the biofilm formation and stability, the nutrient delivery to the cells embedded deep within the matrix and the long-range transport of effector molecules/neutralizing agents beyond the matrix boundaries, contributing to virulence and antibiotic resistance (Citation663, Citation665–Citation669). OMVs can also function as offensive tools, lysing competitor bacteria (Citation670, Citation671). Such a strategy would be particularly suitable for adaptation to new ecological niches and is also followed by other organisms (as described above for parasites).
Interaction with the host
Both symbiotic and pathogenic bacteria can interact with human cells by means of OMVs. In particular, the human bacterial flora consists mainly of anaerobic bacteria that, in healthy individuals, create a symbiotic relationship with the host, since these bacteria play important physiologic roles such as repression of the growth of pathogenic microorganisms, differentiation of the epithelial barrier, regulation of nutrition and metabolic functions and stimulation of innate immunity functions. Collectively, the human bacterial flora colonizes some organs of the human body; primarily conjunctiva, oropharyngeal cavity, upper respiratory tract, gastrointestinal and urogenital organs and skin, where they exert their functions by interacting with different cell types. Although the first barrier of cells with bacteria are the epithelial tissues, the OMVs produced by these bacteria may “cross” this epithelial barrier and interact with some of the connective tissue cells, primarily immune cells (Citation672). Hence, in both the epithelium and the connective tissue of skin and mucosa there are heterogeneous populations of cells that are putative targets and effectors of OMVs. However, a comprehensive view of how the OMVs of the bacterial flora interact with human cells is not yet available, and so far, there have been no published studies about the effects of OMVs in some anatomical locations, such as the skin and conjunctival, urethral, vaginal and large bowel mucosae. Here, we have summarized the few studies that have addressed the direct OMV interaction with human cells (Table ). This table highlights the current knowledge – and also lack of knowledge – on the physiologic role of OMVs produced by bacteria found in humans and should encourage further studies in this field.
Table III. A summary of 14 papers selected from 180 Pubmed papers published between 1991 and 2013, on the subject of “outer membrane vesicles (OMVs),” published in international journals that studied, using either in vivo or in vitro models, the direct effects on OMVs on human cells
EVs derived from virus-infected cells
Several lines of evidence indicate that viruses can modify both the quality and the quantity of EVs released by infected cells and that these EVs can be beneficial for either the host or the pathogen [reviewed in Ref. (Citation673)]. Epstein Barr virus (EBV), herpes simplex virus and HIV belong to the most intensively studied viruses with respect to EV release. EVs released by EBV-infected B cells were shown to deliver the EBV latent membrane protein 1 (LMP1) protein to surrounding uninfected cells, thereby contributing to virus latency and tumour progression (Citation674, Citation675). In addition, EVs derived from EBV-infected cells were shown to carry EBV-encoded mature miRNAs in their lumen (Citation676, Citation677). Upon EV-mediated delivery to uninfected cells, EBV miRNAs were shown to be functional and to mediate translational repression of their target genes (Citation676). In addition, the EBV-encoded miRNA miR-BART15 was released in the EVs from EBV-infected B cells which inhibited the NLRP3 component of the inflammasome in EV-targeted uninfected cells (Citation678). HIV has also been shown to encode its own miRNAs. A prominent HIV miRNA, the trans-activation response element (TAR), was found to be present in EVs secreted from HIV-infected cells. Exposing uninfected cells to EVs derived from HIV-infected cells resulted in an increased susceptibility to HIV infection, through TAR-regulated inhibition of apoptosis in the recipient cells (Citation679). Also, the presence of HIV co-receptors in EVs derived from HIV-infected cells may confer an increased susceptibility to infection in recipient cells that are otherwise non-permissive (Citation93). Infection of hepatocytes with hepatitis C virus led to the incorporation and export of viral genomic and sub-genomic RNA sequences in EVs. Targeting of these EVs to non-permissive plasmacytoid DCs induced a strong interferon response in these cells, which contributes to the antiviral response (Citation680).
Interestingly, viral genomes from coxsackie virus B1, Hepatitis A and C viruses were shown to be packaged in EVs for release in the extracellular milieu [reviewed in Ref. (Citation681)]. Based on these observations, it can be hypothesized that EVs could serve as vehicles that mediate intercellular transmission of non-enveloped viruses. The use of cellular secretion and vesicular trafficking and targeting pathways may allow viruses to disseminate and gain access to a pool of potential target cells that are otherwise non-permissive for virus entry, while escaping immune surveillance (Citation682).
Function of EVs in plants
To date, the pathway of EV release has not been directly dissected in plants. Indirect evidence does exist, however, that vesicle exocytosis is involved in normal plant physiology ().
Fig. 12. Putative role of EVs in the pathogen attack response in plants.
Schematic representation of a plant cell penetrated by hyphae from powdery mildew fungus. Pathogen attack results in transport of defence compounds to the attack location near the plasma membrane by polarization of the cytoskeleton. Two routes of vesicle secretion have been identified: (a) Golgi-derived vesicle secretion relying on SNARE complex formation at the plasma membrane and (b) Fusion of MVBs with the plasma membrane leading to release of intraluminal vesicles (exosomes) into the paramural space (in between the cell wall and plasma membrane). Cross-talk may exist between MVBs and the trans Golgi network/early endosomes in sorting proteins for MVB-mediated degradation or recycling/exosome secretion to the plasma membrane. Plasmodesmata connect the paramural space across cell walls and may facilitate transport of cargo released from vesicles over longer distances. [Figure adapted from (Citation714, Citation715)].
![Fig. 12. Putative role of EVs in the pathogen attack response in plants.Schematic representation of a plant cell penetrated by hyphae from powdery mildew fungus. Pathogen attack results in transport of defence compounds to the attack location near the plasma membrane by polarization of the cytoskeleton. Two routes of vesicle secretion have been identified: (a) Golgi-derived vesicle secretion relying on SNARE complex formation at the plasma membrane and (b) Fusion of MVBs with the plasma membrane leading to release of intraluminal vesicles (exosomes) into the paramural space (in between the cell wall and plasma membrane). Cross-talk may exist between MVBs and the trans Golgi network/early endosomes in sorting proteins for MVB-mediated degradation or recycling/exosome secretion to the plasma membrane. Plasmodesmata connect the paramural space across cell walls and may facilitate transport of cargo released from vesicles over longer distances. [Figure adapted from (Citation714, Citation715)].](/cms/asset/6a9113b0-79ec-4013-b61e-9bdf8f24049a/zjev_a_11815543_f0012_ob.jpg)
Apoplastic fluid of sunflower seeds was reported to contain 50–200 nm phospholipid-containing vesicles of exosomal appearance (Citation683). The protein profile of these EVs was distinct from that of whole sunflower extract and also contained a protein showing 68% identity with human Rab11a GTPase, a mammalian exosome protein identified in several proteomic studies (Citation92) which promotes the docking and the fusion of MVBs with the plasma membrane (Citation556, Citation684). Rab11 GTPases were also involved in secretion and recycling of cell wall components in tomato (Citation685) and polarized pollen tube growth in tobacco (Citation686). Here, coordinated Rab11 GTPase activity was required for essential membrane trafficking, protein secretion and cell wall modification during fruit ripening.
Direct evidence for fusion of MVBs with the plant plasma membrane has so far come primarily from studies of fungal infection. Vesicle-like bodies or granules containing anti-microbial compounds were shown to aggregate directly beneath sites of fungal attack, for example, upon attempted entry of pathogenic powdery mildew fungus Blumeria graminis f.sp. hordei (Bgh) into barley leaves (Citation687). By TEM, the outer membrane of MVBs was observed to be attached to the plasma membrane. The observation led to the proposal that MVB-mediated exocytosis of vesicles into the paramural space (situated between the plasma membrane and cell wall) of plant cells occurs analogous to animal exosome secretion; presumably such EVs participate in the defense response to pathogens.
Evidence also exists that vesicular exocytosis may be involved in the double fertilization process of Arabidopsis (Citation688). During fertilization, one sperm fuses with the egg cell, whereas a second sperm cell fuses with the central cell. An egg cell-specific transcript, EC-1, is accumulated in spherical vesicle structures within the unfertilized egg cell. Upon sperm arrival, EC-1-containing vesicles are exocytosed. This exocytosis is a prerequisite for the second-round fertilization of the central cell and, hence, reproductive success. Furthermore, a cross-kingdom communication between plants and mammals may be facilitated by EVs. Rice-derived miR168 were reported to be elevated in Chinese individuals and this miRNA was shown to enter the circulation of rice-fed mice enclosed in EVs. Upon circulation, miR168 could down-regulate low-density lipoprotein receptor adaptor protein 1 (LDLRAP1) in mouse liver with concomitant increase in plasma low-density lipoprotein (LDL) levels (Citation689). A different study, however, found little evidence for general uptake of dietary plant xeno-miRNAs in the blood stream of pig-tailed macaques (Citation690). Future studies may help to establish how widespread the phenomenon of EV-mediated cross-kingdom communication is.
Concluding remarks
The data obtained so far and reviewed here has already established EVs as novel players in various cell communication systems. These EVs have displayed versatile physiological functions and are likely to be involved in the regulation of main routes of signalling and intercellular information transfer between different cell types, possibly even cross-kingdom. Thus, in further developing the great scope of physiological cell system compositions and dynamics – previously understood to be comprised of different tissue stroma, body fluids and soluble mediators – the presence and functional modalities of EVs should now be considered and paradigms redefined. The biology of EVs – their molecular composition and function, targeting and uptake mechanisms – is still a young research field, to some extent awaiting new technological advances for the isolation and characterization of complex mixtures including very small vesicles. Despite this, research to date has clearly highlighted the role of EV-mediated horizontal transfer of bioactive proteins, lipids and nucleic acids in gene regulation, local phenotypic adaptation and immune evasion, undoubtedly opening exciting perspectives for clinical and therapeutic advances. More extensive research to establish a deeper understanding of the physiological relevance of EVs in different homeostatic changes is now warranted.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study. For funding, we acknowledge the European Network on Microvesicles and Exosomes in Health & Disease (ME-HaD; BM1202 www.cost.eu/COST_Actions/bmbs/Actions/BM1202).
Acknowledgements
The authors wish to thank Dr R Simpson and Dr D Taylor for critical reading of the manuscript and acknowledge the Horizon 2020 European Cooperation in Science and Technology programme and its support of our European Network on Microvesicles and Exosomes in Health & Disease (ME-HaD; BM1202 www.cost.eu/COST_Actions/bmbs/Actions/BM1202).
References
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Ann Rev Cell Dev Biol. 2005; 21: 319–46.
- Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946; 166: 189–97.
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967; 13: 269–88.
- Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969; 41: 59–72.
- De Broe M, Wieme R, Roels F. Letter: membrane fragments with koinozymic properties released from villous adenoma of the rectum. Lancet. 1975; 2: 1214–15.
- Benz EW Jr., Moses HL. Small, virus-like particles detected in bovine sera by electron microscopy. J Natl Cancer Inst. 1974; 52: 1931–4.
- Dalton AJ. Microvesicles and vesicles of multivesicular bodies versus “virus-like” particles. J Natl Cancer Inst. 1975; 54: 1137–48.
- Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res. 1982; 10: 253–7.
- Ronquist G, Brody I, Gottfries A, Stegmayr B. An Mg2+ and Ca2+-stimulated adenosine triphosphatase in human prostatic fluid: part I. Andrology. 1978; 10: 261–72.
- Taylor DD, Homesley HD, Doellgast GJ. Binding of specific peroxidase-labeled antibody to placental-type phosphatase on tumor-derived membrane fragments. Cancer Res. 1980; 40: 4064–9.
- Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AMet al. Tumor shedding and coagulation. Science. 1981; 212: 923–4.
- Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984; 35: 256–63.
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983; 33: 967–78.
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987; 262: 9412–20.
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJet al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996; 183: 1161–72.
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654–9.
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak Pet al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006; 20: 847–56.
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004; 101: 13368–73.
- Lasser C, Eldh M, Lotvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012; 3037.
- Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011; 9: 86.
- Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005; 17: 879–87.
- Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009; 69: 159–67.
- Lasser C, O'Neil SE, Ekerljung L, Ekstrom K, Sjostrand M, Lotvall J. RNA-containing exosomes in human nasal secretions. Am J Rhinol Allergy. 2011; 25: 89–93.
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013; 2 20389, doi: http://dx.doi.org/10.3402/jev.v2i0.20389.
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Ann Rev Cell Dev Biol. 2014; 30: 255–89.
- Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJet al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013; 13: 3354–64.
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2013; 56: 293–304.
- Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010; 9: 197–208.
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008; 110: 13–21.
- Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, Van Noorden CJet al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 2013; 115: 343–51.
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DAet al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009; 23: 1541–57.
- Epple LM, Griffiths SG, Dechkovskaia AM, Dusto NL, White J, Ouellette RJet al. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS One. 2012; 7: e42064.
- Kang D, Oh S, Ahn SM, Lee BH, Moon MH. Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. J Proteome Res. 2008; 7: 3475–80.
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase Pet al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012; 10: e1001450.
- Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013; 2 20384, doi: http://dx.doi.org/10.3402/jev.v2i0.20384.
- Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012; 1 18374, doi: http://dx.doi.org/10.3402/jev.v1i0.18374.
- Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011; 11: 108.
- Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta Ret al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009; 20: 363–79.
- Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KAet al. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and non-metastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics. 2014; 14: 699–712.
- Ostergaard O, Nielsen CT, Iversen LV, Jacobsen S, Tanassi JT, Heegaard NH. Quantitative proteome profiling of normal human circulating microparticles. J Proteome Res. 2012; 11: 2154–63.
- Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013; 12: 587–98.
- Pallet N, Sirois I, Bell C, Hanafi LA, Hamelin K, Dieude Met al. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics. 2013; 13: 1108–20.
- Aatonen MT, Öhman T, Nyman TA, Laitinen S, Grönholm M, Siljander PR. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014; 3 24692, doi: http://dx.doi.org/10.3402/jev.v3.24692.
- Bobrie A, Thery C. Exosomes and communication between tumours and the immune system: are all exosomes equal?. Biochem Soc Trans. 2013; 41: 263–7.
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch Pet al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013; 126: 5553–65.
- Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall Jet al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013; 2 20360, doi: http://dx.doi.org/10.3402/jev.v2i0.20360.
- Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani Iet al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013; 2 http://dx.doi.org/10.3402/jev.v2i0.20677.
- Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013; 2 20424, doi: http://dx.doi.org/10.3402/jev.v2i0.20424.
- Krishnamoorthy L, Bess JW Jr., Preston AB, Nagashima K, Mahal LK. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat Chem Biol. 2009; 5: 244–50.
- Batista BS, Eng WS, Pilobello KT, Hendricks-Munoz KD, Mahal LK. Identification of a conserved glycan signature for microvesicles. J Proteome Res. 2011; 10: 4624–33.
- Staubach S, Schadewaldt P, Wendel U, Nohroudi K, Hanisch FG. Differential glycomics of epithelial membrane glycoproteins from urinary exovesicles reveals shifts toward complex-type N-glycosylation in classical galactosemia. J Proteome Res. 2012; 11: 906–16.
- Gerlach JQ, Kruger A, Gallogly S, Hanley SA, Hogan MC, Ward CJet al. Surface glycosylation profiles of urine extracellular vesicles. PLoS One. 2013; 8: e74801.
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999; 94: 3791–9.
- Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014; 123: 208–16.
- Looze C, Yui D, Leung L, Ingham M, Kaler M, Yao Xet al. Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochem Biophys Res Commun. 2009; 378: 433–8.
- Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JEet al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J Proteome Res. 2009; 8: 1304–14.
- Hosseini-Beheshti E, Pham S, Adomat H, Li N, Tomlinson Guns ES. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol Cell Proteomics. 2012; 11: 863–85.
- Escrevente C, Grammel N, Kandzia S, Zeiser J, Tranfield EM, Conradt HSet al. Sialoglycoproteins and N-glycans from secreted exosomes of ovarian carcinoma cells. PLoS One. 2013; 8: e78631.
- Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth Jet al. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010; 9: 1324–38.
- Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008; 31: 1059–62.
- Choi DS, Park JO, Jang SC, Yoon YJ, Jung JW, Choi DYet al. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics. 2011; 11: 2745–51.
- Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJet al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010; 115: 696–705.
- Liang Y, Eng WS, Colquhoun DR, Dinglasan RR, Graham DR, Mahal LK. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J Biol Chem. 2014; 289: 32526–37.
- Menck K, Scharf C, Bleckmann A, Dyck L, Rost U, Wenzel D et al. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J Mol Cell Biol. 2015;7:143–53
- Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA. 2013; 110: 17380–5.
- Kralj-Iglic V. Stability of membranous nanostructures: a possible key mechanism in cancer progression. Int J Nanomedicine. 2012; 7: 3579–96.
- Capraro BR, Yoon Y, Cho W, Baumgart T. Curvature sensing by the epsin N-terminal homology domain measured on cylindrical lipid membrane tethers. J Am Chem Soc. 2010; 132: 1200–1.
- Hsieh WT, Hsu CJ, Capraro BR, Wu T, Chen CM, Yang Set al. Curvature sorting of peripheral proteins on solid-supported wavy membranes. Langmuir. 2012; 28: 12838–43.
- Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost Jet al. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci USA. 2009; 106: 5622–6.
- van Meer G, Vaz WL. Membrane curvature sorts lipids. Stabilized lipid rafts in membrane transport. EMBO Rep. 2005; 6: 418–19.
- Huang KC, Ramamurthi KS. Macromolecules that prefer their membranes curvy. Mol Microbiol. 2010; 76: 822–32.
- Ramamurthi KS. Protein localization by recognition of membrane curvature. Curr Opin Microbiol. 2010; 13: 753–7.
- Kralj-Iglič V, Veranič P. Curvature-induced sorting of bilayer membrane constituents and formation of membrane rafts. Adv Planar Lipid Bilayers Liposomes. 2006; 5: 129–49.
- Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid Fet al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013; 288: 11649–61.
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg Met al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010; 70: 1668–78.
- Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009; 19: 434–46.
- Hagerstrand H, Mrowczynska L, Salzer U, Prohaska R, Michelsen KA, Kralj-Iglic V et al. Curvature-dependent lateral distribution of raft markers in the human erythrocyte membrane. Mol Membrane Biol. 2006; 23: 277–88.
- Bari R, Guo Q, Xia B, Zhang YH, Giesert EE, Levy Set al. Tetraspanins regulate the protrusive activities of cell membrane. Biochem Biophys Res Commun. 2011; 415: 619–26.
- Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014; 5: 442.
- Arkhipov A, Yin Y, Schulten K. Four-scale description of membrane sculpting by BAR domains. Biophys J. 2008; 95: 2806–21.
- Yin Y, Arkhipov A, Schulten K. Simulations of membrane tubulation by lattices of amphiphysin N-BAR domains. Structure. 2009; 17: 882–92.
- Hanson PI, Shim S, Merrill SA. Cell biology of the ESCRT machinery. Curr Opin Cell Biol. 2009; 21: 568–74.
- Metcalf D, Isaacs AM. The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochem Soc Trans. 2010; 38: 1469–73.
- Fyfe I, Schuh AL, Edwardson JM, Audhya A. Association of the endosomal sorting complex ESCRT-II with the Vps20 subunit of ESCRT-III generates a curvature-sensitive complex capable of nucleating ESCRT-III filaments. J Biol Chem. 2011; 286: 34262–70.
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009; 458: 172–7.
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Ann Rev Cell Dev Biol. 2012; 28: 337–62.
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts Aet al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012; 14: 677–85.
- Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale Net al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014; 5: 3477.
- Sala-Valdes M, Ursa A, Charrin S, Rubinstein E, Hemler ME, Sanchez-Madrid Fet al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem. 2006; 281: 19665–75.
- Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007; 5: e158.
- Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011; 286: 14383–95.
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012; 40: D1241–4.
- Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak Jet al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000; 6: 769–75.
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha Aet al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008; 10: 619–24.
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno Get al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012; 18: 883–91.
- Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GDet al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004; 104: 3257–66.
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LFet al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010; 11: 675–87.
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti Met al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011; 124: 447–58.
- Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito Aet al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009; 284: 34211–22.
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009; 9: 581–93.
- Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar Det al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004; 572: 11–14.
- Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JFet al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004; 380: 161–71.
- Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007; 89: 205–12.
- van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biology. 2006; 16: 514–21.
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998; 4: 31–6.
- Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana Aet al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998; 187: 1205–13.
- Shimoda M, Khokha R. Proteolytic factors in exosomes. Proteomics. 2013; 13: 1624–36.
- Rand ML, Wang H, Bang KW, Packham MA, Freedman J. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J Thromb Haemost. 2006; 4: 1621–3.
- Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, van den Bos AGet al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005; 105: 2141–5.
- Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai Tet al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013; 165: 77–84.
- Rank A, Nieuwland R, Crispin A, Grutzner S, Iberer M, Toth Bet al. Clearance of platelet microparticles in vivo. Platelets. 2011; 22: 111–16.
- Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003; 33: 522–31.
- Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007; 120: 90–102.
- Dasgupta SK, Abdel-Monem H, Niravath P, Le A, Bellera RV, Langlois Ket al. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009; 113: 1332–9.
- Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009; 182: 581–7.
- Dasgupta SK, Le A, Chavakis T, Rumbaut RE, Thiagarajan P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012; 125: 1664–72.
- Nolte-‘t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009; 113: 1977–81.
- Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007; 179: 1489–96.
- Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21: 575–81.
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal Met al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004; 101: 9683–8.
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002; 3: 893–905.
- Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006; 107: 102–8.
- Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003; 15: 446–55.
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002; 3: 321–30.
- Calzolari A, Raggi C, Deaglio S, Sposi NM, Stafsnes M, Fecchi Ket al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. 2006; 119: 4486–98.
- Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004; 18: 977–9.
- Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008; 180: 7249–58.
- Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault Set al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009; 4: e4942.
- Macario AJ, Cappello F, Zummo G, Conway de Macario E. Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Ann N Y Acad Sci. 2010; 1197: 85–93.
- Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LAet al. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003; 8: 348–60.
- Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DFet al. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS One. 2012; 7: e42008.
- Merendino AM, Bucchieri F, Campanella C, Marciano V, Ribbene A, David Set al. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010; 5: e9247.
- Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse Met al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010; 51: 2105–20.
- Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro Fet al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012; 189: 2833–42.
- Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Qet al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012; 188: 5954–61.
- Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho Pet al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002; 195: 1303–16.
- Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina Pet al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005; 128: 1796–804.
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001; 15: 825–35.
- Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007; 179: 1913–25.
- Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio Eet al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007; 109: 3856–64.
- Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CAet al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1 alpha. Proc Natl Acad Sci USA. 2011; 108: 20684–9.
- Gulinelli S, Salaro E, Vuerich M, Bozzato D, Pizzirani C, Bolognesi Get al. IL-18 associates to microvesicles shed from human macrophages by a LPS/TLR-4 independent mechanism in response to P2X receptor stimulation. Eur J Immunol. 2012; 42: 3334–45.
- Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis. 2005; 35: 1–10.
- Hasegawa H, Thomas HJ, Schooley K, Born TL. Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine. 2011; 53: 74–83.
- Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang Set al. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006; 176: 7385–93.
- Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher Wet al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003; 171: 4830–6.
- Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004; 124: 376–84.
- Taraboletti G, D'Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi Det al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006; 8: 96–103.
- Baj-Krzyworzeka M, Weglarczyk K, Mytar B, Szatanek R, Baran J, Zembala M. Tumour-derived microvesicles contain interleukin-8 and modulate production of chemokines by human monocytes. Anticancer Res. 2011; 31: 1329–35.
- Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IEet al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008; 112: 5026–36.
- Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011; 186: 2219–28.
- Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang Xet al. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008; 181: 5242–8.
- Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010; 5: e11469.
- Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007; 67: 7458–66.
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang Jet al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009; 124: 2621–33.
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010; 70: 9621–30.
- Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman Ret al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015; 34: 290–302.
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010; 38: 215–24.
- Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biol Direct. 2013; 8: 12.
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski Pet al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006; 55: 808–18.
- Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du Met al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013; 14: 319.
- Kogure T, Yan IK, Lin WL, Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013; 4: 261–72.
- Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth Det al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010; 5: e13515.
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MAet al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011; 2: 282.
- Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012; 40: 10937–49.
- Nolte-‘t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Research. 2012; 40: 9272–85.
- Miranda KC, Bond DT, Levin JZ, Adiconis X, Sivachenko A, Russ Cet al. Massively parallel sequencing of human urinary exosome/microvesicle RNA reveals a predominance of non-coding RNA. PLoS One. 2014; 9: e96094.
- Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. Peer J. 2013; 1: e201.
- Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014; 3 23743, doi: http://dx.doi.org/10.3402/jev.v3.23743.
- Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen Xet al. Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One. 2012; 7: e46957.
- Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014; 3 24783, doi: http://dx.doi.org/10.3402/jev.v3.24783.
- Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson Bet al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014; 74: 5758–71.
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DFet al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011; 108: 5003–8.
- Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011; 39: 7223–33.
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011; 13: 423–33.
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal Aet al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014; 26: 707–21.
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009; 11: 1143–9.
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006; 20: 1487–95.
- Zhang Y, Liu D, Chen X, Li J, Li L, Bian Zet al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010; 39: 133–44.
- Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014; 28: 3–13.
- Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics. 2011; 12(Suppl 3): S18.
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces Net al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013; 4: 2980.
- Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle Ret al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014; 8: 1432–46.
- Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EPet al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012; 1: e10.
- Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MA, Sadek P, Sie Det al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014; 8: 1649–58.
- Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone Let al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007; 110: 2440–8.
- Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone Let al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012; 7: e33115.
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino Fet al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009; 20: 1053–67.
- Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas Met al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010; 5: e15353.
- Gennebäck N, Hellman U, Malm L, Larsson G, Ronquist G, Waldenström Aet al. Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes. J Extracell Vesicles. 2013; 2 20167, doi: http://dx.doi.org/10.3402/jev.v2i0.20167.
- Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner Met al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013; 110: 7312–17.
- Muller G, Schneider M, Biemer-Daub G, Wied S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. 2011; 23: 1207–23.
- de Candia P, Torri A, Gorletta T, Fedeli M, Bulgheroni E, Cheroni Cet al. Intracellular modulation, extracellular disposal and serum increase of MiR-150 mark lymphocyte activation. PLoS One. 2013; 8: e75348.
- Russo F, Di Bella S, Nigita G, Macca V, Lagana A, Giugno Ret al. miRandola: extracellular circulating microRNAs database. PLoS One. 2012; 7: e47786.
- Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono Let al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010; 5: e11803.
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu Let al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008; 3: e3694.
- Ogawa R, Tanaka C, Sato M, Nagasaki H, Sugimura K, Okumura Ket al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010; 398: 723–9.
- Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012; 22: 125–32.
- Hu G, Drescher KM, Chen XM. Exosomal miRNAs: biological properties and therapeutic potential. Front Genet. 2012; 3: 56.
- Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012; 21: R125–34.
- Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012; 13: 328–35.
- Katsuda T, Ikeda S, Yoshioka Y, Kosaka N, Kawamata M, Ochiya T. Physiological and pathological relevance of secretory microRNAs and a perspective on their clinical application. Biol Chem. 2014; 395: 365–73.
- Fernandez-Messina L, Gutierrez-Vazquez C, Rivas-Garcia E, Sanchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. 2015; 107: 61–77.
- Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova Tet al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014; 41: 89–103.
- Ekström K, Valadi H, Sjöstrand M, Malmhäll C, Bossios A, Eldh Met al. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles. 2012; 1 18389, doi: http://dx.doi.org/10.3402/jev.v1i0.18389.
- Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte Ket al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013; 121: 984–95.
- Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010; 1: 7.
- Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Qet al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012; 8: 118–23.
- Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: placenta-specific microRNAs in exosomes – good things come in nano-packages. Placenta. 2014; 35(Suppl): S69–73.
- Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MCet al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012; 21: 1305–20.
- Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke Bet al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009; 2: ra81.
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AMet al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012; 14: 249–56.
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky Set al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013; 288: 7105–16.
- Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon Aet al. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle. 2014; 13: 78–89.
- Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa Met al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014; 102: 1751–61, e1.
- Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BSet al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014; 9: e114627.
- Salvucci O, Jiang K, Gasperini P, Maric D, Zhu J, Sakakibara Set al. MicroRNA126 contributes to granulocyte colony-stimulating factor-induced hematopoietic progenitor cell mobilization by reducing the expression of vascular cell adhesion molecule 1. Haematologica. 2012; 97: 818–26.
- Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NBet al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009; 4: e4722.
- Nair R, Santos L, Awasthi S, von Erlach T, Chow LW, Bertazzo Set al. Extracellular vesicles derived from preosteoblasts influence embryonic stem cell differentiation. Stem Cells Dev. 2014; 23: 1625–35.
- Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012; 37: 460–5.
- Holmgren L, Szeles A, Rajnavolgyi E, Folkman J, Klein G, Ernberg Iet al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999; 93: 3956–63.
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XOet al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011; 2: 180.
- Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010; 117: 1–4.
- Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva Bet al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014; 24: 766–9.
- Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012; 7: e34653.
- Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem Biophys Res Commun. 2014; 451: 295–301.
- Lazaro-Ibanez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido Aet al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate. 2014; 74: 1379–90.
- Arienti G, Carlini E, Polci A, Cosmi EV, Palmerini CA. Fatty acid pattern of human prostasome lipid. Arch Biochem Biophys. 1998; 358: 391–5.
- Arvidson G, Ronquist G, Wikander G, Ojteg AC. Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta. 1989; 984: 167–73.
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013; 13: 1554–71.
- Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012; 7: 1525–41.
- Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim Biophys Acta. 2014; 1841: 108–20.
- Baig S, Lim JY, Fernandis AZ, Wenk MR, Kale A, Su LLet al. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta. 2013; 34: 436–42.
- Smyth TJ, Redzic JS, Graner MW, Anchordoquy TJ. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim Biophys Acta. 2014; 1838: 2954–65.
- Biro E, Akkerman JW, Hoek FJ, Gorter G, Pronk LM, Sturk Aet al. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J Thromb Haemost. 2005; 3: 2754–63.
- Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005; 106: 1604–11.
- Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011; 3: a004697.
- Llorente A, van Deurs B, Sandvig K. Cholesterol regulates prostasome release from secretory lysosomes in PC-3 human prostate cancer cells. Eur J Cell Biol. 2007; 86: 405–15.
- Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005; 27: 375–87.
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland Fet al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008; 319: 1244–7.
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure Jet al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004; 303: 531–4.
- Beloribi S, Ristorcelli E, Breuzard G, Silvy F, Bertrand-Michel J, Beraud Eet al. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells. PLoS One. 2012; 7: e47480.
- Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002; 62: 6312–17.
- Palmerini CA, Cametti C, Sennato S, Gaudino D, Carlini E, Bordi Fet al. Role of cholesterol, DOTAP, and DPPC in prostasome/spermatozoa interaction and fusion. J Membr Biol. 2006; 211: 185–90.
- Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013; 146: R21–35.
- Sato S, Zhu XL, Sly WS. Carbonic anhydrase isozymes IV and II in urinary membranes from carbonic anhydrase II-deficient patients. Proc Natl Acad Sci USA. 1990; 87: 6073–6.
- Kanno K, Sasaki S, Hirata Y, Ishikawa S, Fushimi K, Nakanishi Set al. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N Engl J Med. 1995; 332: 1540–5.
- Dear JW, Street JM, Bailey MA. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics. 2013; 13: 1572–80.
- Hara M, Yanagihara T, Hirayama Y, Ogasawara S, Kurosawa H, Sekine Set al. Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol. 2010; 41: 1265–75.
- Prunotto M, Farina A, Lane L, Pernin A, Schifferli J, Hochstrasser DFet al. Proteomic analysis of podocyte exosome-enriched fraction from normal human urine. J Proteomics. 2013; 82: 193–229.
- Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert Set al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007; 72: 1095–102.
- Dimov I, Jankovic Velickovic L, Stefanovic V. Urinary exosomes. ScientificWorldJournal. 2009; 9: 1107–18.
- Gonzales P, Pisitkun T, Knepper MA. Urinary exosomes: is there a future?. Nephrol Dial Transplant. 2008; 23: 1799–801.
- Moon PG, You S, Lee JE, Hwang D, Baek MC. Urinary exosomes and proteomics. Mass Spectrom Rev. 2011; 30: 1185–202.
- Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XOet al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009; 100: 1603–7.
- Fang DY, King HW, Li JY, Gleadle JM. Exosomes and the kidney: blaming the messenger. Nephrology (Carlton). 2013; 18: 1–10.
- van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011; 80: 1138–45.
- Knepper MA, Pisitkun T. Exosomes in urine: who would have thought …?. Kidney Int. 2007; 72: 1043–5.
- Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008; 8: 4083–99.
- Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs Pet al. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA. 2006; 103: 18344–9.
- McKee JA, Kumar S, Ecelbarger CA, Fernandez-Llama P, Terris J, Knepper MA. Detection of Na(+) transporter proteins in urine. J Am Soc Nephrol. 2000; 11: 2128–32.
- Esteva-Font C, Wang X, Ars E, Guillen-Gomez E, Sans L, Gonzalez Saavedra Iet al. Are sodium transporters in urinary exosomes reliable markers of tubular sodium reabsorption in hypertensive patients?. Nephron Physiol. 2010; 114: 25–34.
- Olivieri O, Chiecchi L, Pizzolo F, Castagna A, Raffaelli R, Gunasekaran Met al. Urinary prostasin in normotensive individuals: correlation with the aldosterone to renin ratio and urinary sodium. Hypertens Res. 2013; 36: 528–33.
- Hiemstra TF, Charles PD, Gracia T, Hester SS, Gatto L, Al-Lamki Ret al. Human urinary exosomes as innate immune effectors. J Am Soc Nephrol. 2014; 25: 2017–27.
- Kleinjan A, Boing AN, Sturk A, Nieuwland R. Microparticles in vascular disorders: how tissue factor-exposing vesicles contribute to pathology and physiology. Thromb Res. 2012; 130(Suppl 1): S71–3.
- Ogawa Y, Miura Y, Harazono A, Kanai-Azuma M, Akimoto Y, Kawakami Het al. Proteomic analysis of two types of exosomes in human whole saliva. Biol Pharm Bull. 2011; 34: 13–23.
- Xiao H, Wong DT. Proteomic analysis of microvesicles in human saliva by gel electrophoresis with liquid chromatography-mass spectrometry. Anal Chim Acta. 2012; 723: 61–7.
- Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010; 5: e8577.
- Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull. 2013; 36: 66–75.
- Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012; 7: e30679.
- Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios Aet al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011; 9: 9.
- Berckmans RJ, Sturk A, van Tienen LM, Schaap MC, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011; 117: 3172–80.
- Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005; 52: 1517–21.
- Gyorgy B, Szabo TG, Turiak L, Wright M, Herczeg P, Ledeczi Zet al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 2012; 7: e49726.
- Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006; 54: 3809–14.
- Mor-Vaknin N, Kappes F, Dick AE, Legendre M, Damoc C, Teitz-Tennenbaum Set al. DEK in the synovium of patients with juvenile idiopathic arthritis: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum. 2011; 63: 556–67.
- Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WKet al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009; 136: 320–30 e2.
- Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TVet al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010; 299: G990–9.
- Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TVet al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013; 304: G1013–24.
- Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays. 2005; 27: 262–74.
- Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil Det al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005; 118: 2849–58.
- Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TSet al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012; 10: 5.
- Zappaterra MD, Lisgo SN, Lindsay S, Gygi SP, Walsh CA, Ballif BA. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res. 2007; 6: 3537–48.
- An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O'Dowd STet al. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol Brain. 2013; 6: 47.
- Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund Aet al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003; 22: 578–83.
- Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JLet al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013; 131: 894–903.
- Torregrosa Paredes P, Esser J, Admyre C, Nord M, Rahman QK, Lukic Aet al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012; 67: 911–19.
- Esser J, Gehrmann U, D'Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius Aet al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010; 126: 1032–40, 40 e1-4.
- Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012; 8: 404–12.
- Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007; 110: 3234–44.
- Almqvist N, Lonnqvist A, Hultkrantz S, Rask C, Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008; 125: 21–7.
- Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013; 131 1194–203, 203 e1–14.
- Shin TS, Kim JH, Kim YS, Jeon SG, Zhu Z, Gho YSet al. Extracellular vesicles are key intercellular mediators in the development of immune dysfunction to allergens in the airways. Allergy. 2010; 65: 1256–65.
- Villalba M, Rodriguez R, Batanero E. The spectrum of olive pollen allergens. From structures to diagnosis and treatment. Methods. 2014; 66: 44–54.
- Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000; 165: 1259–65.
- Prado N, Canamero M, Villalba M, Rodriguez R, Batanero E. Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol Immunol. 2010; 47: 2148–51.
- Camacho AI, de Souza J, Sanchez-Gomez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011; 29: 8222–9.
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RCet al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011; 19: 1769–79.
- Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca(2+)-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One. 2013; 8: e80181.
- Griffiths GS, Galileo DS, Reese K, Martin-Deleon PA. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol Reprod Dev. 2008; 75: 1627–36.
- Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CLet al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 2013; 8: e58502.
- Kumamoto K, Yang XZ, Hasegawa A, Komori S, Koyama K. CD52 expression is induced in the mouse uterus at the time of embryo implantation. J Reprod Immunol. 2009; 82: 32–9.
- Kabir-Salmani M, Nikzad H, Shiokawa S, Akimoto Y, Iwashita M. Secretory role for human uterodomes (pinopods): secretion of LIF. Mol Hum Reprod. 2005; 11: 553–9.
- Aghajanova L, Altmae S, Bjuresten K, Hovatta O, Landgren BM, Stavreus-Evers A. Disturbances in the LIF pathway in the endometrium among women with unexplained infertility. Fertil Steril. 2009; 91: 2602–10.
- Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski Det al. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol. 2008; 79: 12–17.
- Jean-Pierre C, Perni SC, Bongiovanni AM, Kalish RB, Karasahan E, Ravich Met al. Extracellular 70-kd heat shock protein in mid-trimester amniotic fluid and its effect on cytokine production by ex vivo-cultured amniotic fluid cells. Am J Obstet Gynecol. 2006; 194: 694–8.
- Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp Cet al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via TLR signaling. J Biol Chem. 2013; 288: 36691–702.
- Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman Met al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007; 179: 1969–78.
- Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006; 176: 1534–42.
- Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics. 2012; 75: 1486–92.
- Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Purification of RNA from milk whey. Methods Mol Biol. 2013; 1024: 191–201.
- Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell. 2013; 4: 197–210.
- Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang Xet al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS One. 2012; 7: e43691.
- Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J. 2013; 12: 103.
- George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982; 60: 834–40.
- Gemmell CH, Sefton MV, Yeo EL. Platelet-derived microparticle formation involves glycoprotein IIb-IIIa. Inhibition by RGDS and a Glanzmann's thrombasthenia defect. J Biol Chem. 1993; 268: 14586–9.
- Hill AF, Pegtel DM, Lambertz U, Leonardi T, O'Driscoll L, Pluchino Set al. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesicles. 2013; 2 doi: http://dx.doi.org/10.3402/jev.v2i0.22859.
- Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet Set al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014; 12: 614–27.
- Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg Let al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses – novel mechanisms for host–microbe interactions in atopic eczema. PLoS One. 2011; 6: e21480.
- Ren Y, Yang J, Xie R, Gao L, Yang Y, Fan Het al. Exosomal-like vesicles with immune-modulatory features are present in human plasma and can induce CD4+ T-cell apoptosis in vitro. Transfusion. 2011; 51: 1002–11.
- Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli Eet al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009; 113: 1112–21.
- Aatonen M, Gronholm M, Siljander PR. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost. 2012; 38: 102–13.
- Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger Jet al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014; 124: 2173–83.
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves Met al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008; 10: 1470–6.
- Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006; 56: 345–55.
- Koga Y, Yasunaga M, Moriya Y, Akasu T, Fujita S, Yamamoto Set al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol. 2011; 2: 215–22.
- Park K-S, Kim J-W, Lee J, Kim OY, Jang SC, Kim SRet al. Induction of peritoneal and sepsis-like systemic inflammation by bacteria-free extracellular vesicles from faeces. J Extracell Vesicles. 2014; 3 doi: http://dx.doi.org/10.3402/jev.v3.24214 [PubMed Abstract].
- Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DKet al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013; 8: e76520.
- Sahlen G, Nilsson O, Larsson A, Carlsson L, Norlen BJ, Ronquist G. Secretions from seminal vesicles lack characteristic markers for prostasomes. Ups J Med Sci. 2010; 115: 107–12.
- Saez F, Frenette G, Sullivan R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl. 2003; 24: 149–54.
- Frenette G, Girouard J, Sullivan R. Comparison between epididymosomes collected in the intraluminal compartment of the bovine caput and cauda epididymidis. Biol Reprod. 2006; 75: 885–90.
- Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DRet al. Proteomic analysis of human prostasomes. Prostate. 2003; 56: 150–61.
- Brouwers JF, Aalberts M, Jansen JW, van Niel G, Wauben MH, Stout TAet al. Distinct lipid compositions of two types of human prostasomes. Proteomics. 2013; 13: 1660–6.
- Aalberts M, Sostaric E, Wubbolts R, Wauben MW, Nolte-‘t Hoen EN, Gadella BMet al. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta. 2013; 1834: 2326–35.
- Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TAet al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012; 86: 82.
- Owens AP, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011; 108: 1284–97.
- Castaman G, Yu-Feng L, Rodeghiero F. A bleeding disorder characterised by isolated deficiency of platelet microvesicle generation. Lancet. 1996; 347: 700–1.
- Stormorken H, Sjaastad O, Langslet A, Sulg I, Egge K, Diderichsen J. A new syndrome: thrombocytopathia, muscle fatigue, asplenia, miosis, migraine, dyslexia and ichthyosis. Clin Genet. 1985; 28: 367–74.
- Weiss HJ, Vicic WJ, Lages BA, Rogers J. Isolated deficiency of platelet procoagulant activity. Am J Med. 1979; 67: 206–13.
- Toti F, Satta N, Fressinaud E, Meyer D, Freyssinet JM. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996; 87: 1409–15.
- Malvezzi M, Chalat M, Janjusevic R, Picollo A, Terashima H, Menon AKet al. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat Commun. 2013; 4: 2367.
- Chen YW, Chen YC, Wang JS. Absolute hypoxic exercise training enhances in vitro thrombin generation by increasing procoagulant platelet-derived microparticles under high shear stress in sedentary men. Clin Sci (Lond). 2013; 124: 639–49.
- Suades R, Padro T, Vilahur G, Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb Haemost. 2012; 108: 1208–19.
- Davila M, Robles-Carrillo L, Unruh D, Huo Q, Gardiner C, Sargent ILet al. Microparticle association and heterogeneity of tumor-derived tissue factor in plasma: is it important for coagulation activation?. J Thromb Haemost. 2014; 12: 186–96.
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012; 64: 676–705.
- Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011; 9: 2251–61.
- Jy W, Johansen ME, Bidot C Jr., Horstman LL, Ahn YS. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb Haemost. 2013; 110: 751–60.
- Schecter AD, Spirn B, Rossikhina M, Giesen PL, Bogdanov V, Fallon JTet al. Release of active tissue factor by human arterial smooth muscle cells. Circ Res. 2000; 87: 126–32.
- Egorina EM, Sovershaev MA, Osterud B. Regulation of tissue factor procoagulant activity by post-translational modifications. Thromb Res. 2008; 122: 831–7.
- Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb haemost. 2008; 100: 878–85.
- Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries Eet al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003; 197: 1585–98.
- Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege Vet al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994; 153: 3245–55.
- Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RGet al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000; 95: 930–5.
- Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999; 274: 23111–18.
- Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012; 122: 2331–6.
- Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013; 122: 1873–80.
- Matsumoto N, Nomura S, Kamihata H, Kimura Y, Iwasaka T. Increased level of oxidized LDL-dependent monocyte-derived microparticles in acute coronary syndrome. Thromb Haemost. 2004; 91: 146–54.
- Markiewicz M, Richard E, Marks N, Ludwicka-Bradley A. Impact of endothelial microparticles on coagulation, inflammation, and angiogenesis in age-related vascular diseases. J Aging Res. 2013; 2013: 734509.
- VanWijk MJ, Boer K, Berckmans RJ, Meijers JC, van der Post JA, Sturk Aet al. Enhanced coagulation activation in preeclampsia: the role of APC resistance, microparticles and other plasma constituents. Thromb Haemost. 2002; 88: 415–20.
- Gardiner C, Tannetta DS, Simms CA, Harrison P, Redman CW, Sargent IL. Syncytiotrophoblast microvesicles released from pre-eclampsia placentae exhibit increased tissue factor activity. PLoS One. 2011; 6: e26313.
- Osterud B. Tissue factor/TFPI and blood cells. Thromb Res. 2012; 129: 274–8.
- Aharon A, Katzenell S, Tamari T, Brenner B. Microparticles bearing tissue factor and tissue factor pathway inhibitor in gestational vascular complications. J Thromb Haemost. 2009; 7: 1047–50.
- Steppich B, Mattisek C, Sobczyk D, Kastrati A, Schomig A, Ott I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb Haemost. 2005; 93: 35–9.
- Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001; 85: 639–46.
- Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez de Lizarrondo Set al. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012; 97: 1864–72.
- Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011; 105: 396–408.
- Hargett LA, Bauer NN. On the origin of microparticles: from “platelet dust” to mediators of intercellular communication. Pulm Circ. 2013; 3: 329–40.
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983; 97: 329–39.
- Vidal M. Exosomes in erythropoiesis. Transfus Clin Biol. 2010; 17: 131–7.
- Blanc L, Vidal M. Reticulocyte membrane remodeling: contribution of the exosome pathway. Curr Opin Hematol. 2010; 17: 177–83.
- Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JCet al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008; 15: 1723–33.
- Blanc L, Vidal M. [Secreted exosomes from reticulocytes express an eat-me signal]. Med Sci (Paris). 2008; 24: 462–3.
- Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000; 267: 583–90.
- Blanc L, Liu J, Vidal M, Chasis JA, An X, Mohandas N. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume. Blood. 2009; 114: 3928–34.
- Carayon K, Chaoui K, Ronzier E, Lazar I, Bertrand-Michel J, Roques Vet al. Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem. 2011; 286: 34426–39.
- Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One. 2011; 6: e26588.
- Rhee JS, Black M, Schubert U, Fischer S, Morgenstern E, Hammes HPet al. The functional role of blood platelet components in angiogenesis. Thromb Haemost. 2004; 92: 394–402.
- Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch Wet al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007; 25: 1746–52.
- de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma Get al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012; 1 doi: http://dx.doi.org/10.3402/jev.v1i0.18396.
- Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp Cell Res. 2013; 319: 1626–34.
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NWet al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006; 444: 1032–7.
- Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CEet al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010; 116: 2385–94.
- Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell Met al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013; 8: e68451.
- Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014; 124: 3748–57.
- Martinez MC, Andriantsitohaina R. Microparticles in angiogenesis: therapeutic potential. Circ Res. 2011; 109: 110–19.
- Finn NA, Searles CD. Intracellular and extracellular miRNAs in regulation of angiogenesis signaling. Curr Angiogenes. 2012; 4: 299–307.
- Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (Review). Int J Mol Med. 2013; 32: 763–7.
- Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MUet al. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012; 32: 1925–35.
- Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting Set al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013; 128: 2026–38.
- van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PMet al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013; 121: 3997–4006, S1–15.
- Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JPYet al. Monocytic microparticles activate endothelial cells in an IL-1 beta-dependent manner. Blood. 2011; 118: 2366–74.
- Oehmcke S, Morgelin M, Malmstrom J, Linder A, Chew M, Thorlacius Het al. Stimulation of blood mononuclear cells with bacterial virulence factors leads to the release of pro-coagulant and pro-inflammatory microparticles. Cell Microbiol. 2012; 14: 107–19.
- Mastronardi ML, Mostefai HA, Meziani F, Martinez MC, Asfar P, Andriantsitohaina R. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med. 2011; 39: 1739–48.
- Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BRH. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg. 2012; 73: 401–6.
- Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt MEet al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010; 327: 580–3.
- Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey Let al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013; 5: 235–49.
- Hoyer FF, Giesen MK, Franca CN, Lutjohann D, Nickenig G, Werner N. Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice. J Cell Mol Med. 2012; 16: 2777–88.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444: 860–7.
- Holder BS, Tower CL, Jones CJP, Aplin JD, Abrahams VM. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol Reprod. 2012; 86:103, 1–7
- Stahl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011; 117: 5503–13.
- Nielsen CT, Ostergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand Bet al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012; 64: 1227–36.
- Biro E, Nieuwland R, Tak PP, Pronk LM, Schaap MCL, Sturk Aet al. Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis. 2007; 66: 1085–92.
- van Eijk IC, Tushuizen ME, Sturk A, Dijkmans BA, Boers M, Voskuyl AEet al. Circulating microparticles remain associated with complement activation despite intensive anti-inflammatory therapy in early rheumatoid arthritis. Ann Rheum Dis. 2010; 69: 1378–82.
- Renner B, Klawitter J, Goldberg R, McCullough JW, Ferreira VP, Cooper JEet al. Cyclosporine induces endothelial cell release of complement-activating microparticles. J Am Soc Nephrol. 2013; 24: 1849–62.
- Yin W, Ghebrehiwet B, Peerschke EIB. Expression of complement components and inhibitors on platelet microparticles. Platelets. 2008; 19: 225–33.
- Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004; 104: 2543–8.
- Schiller M, Heyder P, Ziegler S, Niessen A, Classen L, Lauffer Aet al. During apoptosis HMGB1 is translocated into apoptotic cell-derived membraneous vesicles. Autoimmunity. 2013; 46: 342–6.
- Thomas LM, Salter RD. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J Immunol. 2010; 185: 3740–9.
- Fabbri M, Paone A, Calore F, Galli R, Croce CM. A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol. 2013; 10: 169–74.
- Schiller M, Parcina M, Heyder P, Foermer S, Ostrop J, Leo Aet al. Induction of type I IFN is a physiological immune reaction to apoptotic cell-derived membrane microparticles. J Immunol. 2012; 189: 1747–56.
- Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory Il-1 beta production by macrophages. Am J Reprod Immunol. 2011; 66: 259–69.
- Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007; 282: 25779–89.
- O'Neill HC, Quah BJC. Exosomes secreted by bacterially infected macrophages are proinflammatory. Sci Signal. 2008; 1: pe8.
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli Met al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005; 174: 7268–77.
- Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol. 2009; 175: 696–705.
- Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI. Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol. 2012; 188: 1942–52.
- Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009; 4: e7140.
- Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez Get al. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009; 182: 5052–62.
- Teo BHD, Wong SH. MHC class II-associated invariant chain (Ii) modulates dendritic cells-derived microvesicles (DCMV)-mediated activation of microglia. Biochem Biophys Res Commun. 2010; 400: 673–8.
- Singh PP, Smith VL, Karakousis PC, Schorey JS. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J Immunol. 2012; 189: 777–85.
- Walters SB, Kieckbusch J, Nagalingam G, Swain A, Latham SL, Grau GEet al. Microparticles from mycobacteria-infected macrophages promote inflammation and cellular migration. J Immunol. 2013; 190: 669–77.
- Hassani K, Olivier M. Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis. 2013; 7: e2185.
- Timar CI, Lorincz AM, Csepanyi-Komi R, Valyi-Nagy A, Nagy G, Buzas EIet al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013; 121: 510–18.
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012; 120: e60–72.
- Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol. 2008; 180: 817–24.
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008; 112: 2512–19.
- Lim K, Sumagin R, Hyun YM. Extravasating neutrophil-derived microparticles preserve vascular barrier function in inflamed tissue. Immune Netw. 2013; 13: 102–6.
- Watanabe J, Marathe GK, Neilsen PO, Weyrich AS, Harrison KA, Murphy RCet al. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem. 2003; 278: 33161–8.
- Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DIet al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008; 112: 2327–35.
- Timar CI, Lorincz AM, Ligeti E. Changing world of neutrophils. Pflugers Arch. 2013; 465: 1521–33.
- Caligiuri MA. Human natural killer cells. Blood. 2008; 112: 461–9.
- Fernandez-Messina L, Reyburn HT, Vales-Gomez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012; 3: 299.
- Kim HP, Morse D, Choi AM. Heat-shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets. 2006; 10: 759–69.
- Elsner L, Flugge PF, Lozano J, Muppala V, Eiz-Vesper B, Demiroglu SYet al. The endogenous danger signals HSP70 and MICA cooperate in the activation of cytotoxic effector functions of NK cells. J Cell Mol Med. 2010; 14: 992–1002.
- Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller Het al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007; 179: 5523–33.
- Binici J, Koch J. BAG-6, a jack of all trades in health and disease. Cell Mol Life Sci. 2014; 71: 1829–37.
- Baginska J, Viry E, Paggetti J, Medves S, Berchem G, Moussay Eet al. The Critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol. 2013; 4: 490.
- Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013; 41: 245–51.
- Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez Met al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010; 70: 481–9.
- Fais S. NK cell-released exosomes: natural nanobullets against tumors. Oncoimmunology. 2013; 2: e22337.
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011; 186: 5543–7.
- Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction?. Immunity. 2012; 37: 13–24.
- Shefler I, Salamon P, Hershko AY, Mekori YA. Mast cells as sources and targets of membrane vesicles. Curr Pharm Des. 2011; 17: 3797–804.
- Carroll-Portillo A, Surviladze Z, Cambi A, Lidke DS, Wilson BS. Mast cell synapses and exosomes: membrane contacts for information exchange. Front Immunol. 2012; 3: 46.
- Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier Get al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003; 170: 3037–45.
- Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson Eet al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012; 10: e1001448.
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JMet al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012; 119: 756–66.
- Zech D, Rana S, Buchler MW, Zoller M. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012; 10: 37.
- Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJet al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008; 180: 3081–90.
- Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave Cet al. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007; 132: 1866–76.
- Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004; 72: 4127–37.
- Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J Immunol. 2009; 182: 1548–59.
- Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen Tet al. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005; 11: 7554–63.
- Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy Ket al. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett. 2012; 148: 34–8.
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli Pet al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999; 147: 599–610.
- Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008; 3: e2461.
- Obregon C, Rothen-Rutishauser B, Gitahi SK, Gehr P, Nicod LP. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006; 169: 2127–36.
- Rialland P, Lankar D, Raposo G, Bonnerot C, Hubert P. BCR-bound antigen is targeted to exosomes in human follicular lymphoma B-cells. Biol Cell. 2006; 98: 491–501.
- Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol. 2006; 177: 3757–62.
- Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux Fet al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005; 106: 216–23.
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002; 3: 1156–62.
- Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009; 113: 2673–83.
- Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 2010; 28: 5785–93.
- Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu Ket al. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun. 2010; 78: 5116–25.
- Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius Aet al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007; 120: 1418–24.
- Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003; 76: 1503–10.
- Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007; 179: 2242–9.
- Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash Jet al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005; 174: 6440–8.
- Nolte-‘t Hoen EN, Wauben MH. Immune cell-derived vesicles: modulators and mediators of inflammation. Curr Pharm Des. 2012; 18: 2357–68.
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo Get al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002; 168: 3235–41.
- Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo Vet al. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011; 18: 1161–73.
- Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011; 6: e16899.
- Busch A, Quast T, Keller S, Kolanus W, Knolle P, Altevogt Pet al. Transfer of T cell surface molecules to dendritic cells upon CD4+ T cell priming involves two distinct mechanisms. J Immunol. 2008; 181: 3965–73.
- Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde Met al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001; 167: 6736–44.
- Xie Y, Zhang H, Li W, Deng Y, Munegowda MA, Chibbar Ret al. Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. J Immunol. 2010; 185: 5268–78.
- Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler Ret al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013; 43: 2430–40.
- Yu X, Huang C, Song B, Xiao Y, Fang M, Feng Jet al. CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol. 2013; 285: 62–8.
- Wahlgren J, Karlson Tde L, Glader P, Telemo E, Valadi H. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS One. 2012; 7: e49723.
- Shefler I, Salamon P, Reshef T, Mor A, Mekori YA. T cell-induced mast cell activation: a role for microparticles released from activated T cells. J Immunol. 2010; 185: 4206–12.
- Shefler I, Pasmanik-Chor M, Kidron D, Mekori YA, Hershko AY. T cell-derived microvesicles induce mast cell production of IL-24: relevance to inflammatory skin diseases. J Allergy Clin Immunol. 2014; 133 217–24 e1-3.
- Peters PJ, Geuze HJ, Van der Donk HA, Slot JW, Griffith JM, Stam NJet al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989; 19: 1469–75.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JDet al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143–7.
- Gortner L, Felderhoff-Muser U, Monz D, Bieback K, Kluter H, Jellema Ret al. Regenerative therapies in neonatology: clinical perspectives. Klin Padiatr. 2012; 224: 233–40.
- Dalal J, Gandy K, Domen J. Role of mesenchymal stem cell therapy in Crohn's disease. Pediatr Res. 2012; 71: 445–51.
- MacDonald GI, Augello A, De Bari C. Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011; 63: 2547–57.
- Kerkela E, Hakkarainen T, Makela T, Raki M, Kambur O, Kilpinen Let al. Transient proteolytic modification of mesenchymal stromal cells increases lung clearance rate and targeting to injured tissue. Stem Cells Transl Med. 2013; 2: 510–20.
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009; 4: 206–16.
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CPet al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008; 14: 181–7.
- Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003; 101: 2999–3001.
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BLet al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG–6. Cell Stem Cell. 2009; 5: 54–63.
- Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PAet al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007; 1: 129–37.
- Zanotti L, Sarukhan A, Dander E, Castor M, Cibella J, Soldani Cet al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia. 2013; 27: 500–3.
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TSet al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010; 4: 214–22.
- Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cell secretes immunologically active exosomes. Stem Cells Dev. 2014; 23: 1233–44.
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TRet al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014; 28: 970–3.
- Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen Set al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013; 2 http://dx.doi.org/10.3402/jev.v2i0.21927.
- Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012; 147: 47–54.
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel Met al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363: 1439–41.
- Kaipe H, Erkers T, Sadeghi B, Ringden O. Stromal cells-are they really useful for GVHD?. Bone Marrow Transplant. 2014; 49: 737–43.
- He J, Wang Y, Sun S, Yu M, Wang C, Pei Xet al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton). 2012; 17: 493–500.
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta Cet al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. NephrolDial Transplant. 2011; 26: 1474–83.
- Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Yet al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013; 4: 34.
- Togel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010; 6: 179–83.
- Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RBet al. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol. 2006; 177: 3380–7.
- Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002; 90: 133–8.
- Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011; 7: 105–15.
- Oreshkova T, Dimitrov R, Mourdjeva M. A cross-talk of decidual stromal cells, trophoblast, and immune cells: a prerequisite for the success of pregnancy. Am J Reprod Immunol. 2012; 68: 366–73.
- Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. 2013; 19: 548–56.
- Chua S, Wilkins T, Sargent I, Redman C. Trophoblast deportation in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1991; 98: 973–9.
- Smarason AK, Sargent IL, Starkey PM, Redman CW. The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br J Obstet Gynaecol. 1993; 100: 943–9.
- Cockell AP, Learmont JG, Smarason AK, Redman CW, Sargent IL, Poston L. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br J Obstet Gynaecol. 1997; 104: 235–40.
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000; 21: 597–602.
- Frangsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod. 2005; 11: 35–41.
- Carp H, Dardik R, Lubetsky A, Salomon O, Eskaraev R, Rosenthal Eet al. Prevalence of circulating procoagulant microparticles in women with recurrent miscarriage: a case-controlled study. Hum Reprod. 2004; 19: 191–5.
- Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent ILet al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006; 27: 56–61.
- Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007; 76: 61–7.
- Pap E, Pallinger E, Falus A, Kiss AA, Kittel A, Kovacs Pet al. T lymphocytes are targets for platelet- and trophoblast-derived microvesicles during pregnancy. Placenta. 2008; 29: 826–32.
- Gercel-Taylor C, O'Connor SM, Lam GK, Taylor DD. Shed membrane fragment modulation of CD3-zeta during pregnancy: link with induction of apoptosis. J Reprod Immunol. 2002; 56: 29–44.
- Pállinger E, Kiss A, Pap E, Tóth S, Falus A. BeWo-derived microvesicles modulate T cell differentiation by the downregulation of IL-6Ralpha expression on CD4+T lymphocytes. Journal of extracellular vesiclses. 2012; 1(Suppl 1): 18180.
- Askelund KJ, Chamley LW. Trophoblast deportation part I: review of the evidence demonstrating trophoblast shedding and deportation during human pregnancy. Placenta. 2011; 32: 716–23.
- Pantham P, Askelund KJ, Chamley LW. Trophoblast deportation part II: a review of the maternal consequences of trophoblast deportation. Placenta. 2011; 32: 724–31.
- Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One. 2013; 8: e56754.
- Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013; 191: 5515–23.
- Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov Vet al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009; 183: 340–51.
- Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam Met al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012; 33: 982–90.
- Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GPet al. Review: does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012; 33(Suppl): S48–54.
- Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014; 72: 440–57.
- Girouard J, Frenette G, Sullivan R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int J Androl. 2011; 34: e475–86.
- Schwarz A, Wennemuth G, Post H, Brandenburger T, Aumuller G, Wilhelm B. Vesicular transfer of membrane components to bovine epididymal spermatozoa. Cell Tissue Res. 2013; 353: 549–61.
- Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007; 59: 286–92.
- Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010; 56: 334–48.
- Guraya SS. Cellular and molecular biology of capacitation and acrosome reaction in spermatozoa. Int Rev Cytol. 2000; 199: 1–64.
- Pons-Rejraji H, Artonne C, Sion B, Brugnon F, Canis M, Janny Let al. Prostasomes: inhibitors of capacitation and modulators of cellular signalling in human sperm. Int J Androl. 2011; 34: 568–80.
- Palmerini CA, Saccardi C, Carlini E, Fabiani R, Arienti G. Fusion of prostasomes to human spermatozoa stimulates the acrosome reaction. Fertil Steril. 2003; 80: 1181–4.
- Aalberts M, Stout TA, Stoorvogel W. Prostasomes: extracellular vesicles from the prostate. Reproduction. 2013; 147: R1–14.
- Ronquist G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J Intern Med. 2012; 271: 400–13.
- Matthijs A, Engel B, Woelders H. Neutrophil recruitment and phagocytosis of boar spermatozoa after artificial insemination of sows, and the effects of inseminate volume, sperm dose and specific additives in the extender. Reproduction. 2003; 125: 357–67.
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005; 206: 306–35.
- Skibinski G, Kelly RW, Harkiss D, James K. Immunosuppression by human seminal plasma – extracellular organelles (prostasomes) modulate activity of phagocytic cells. Am J Reprod Immunol. 1992; 28: 97–103.
- Kelly RW. Immunosuppressive mechanisms in semen: implications for contraception. Hum Reprod. 1995; 10: 1686–93.
- Tarazona R, Delgado E, Guarnizo MC, Roncero RG, Morgado S, Sanchez-Correa Bet al. Human prostasomes express CD48 and interfere with NK cell function. Immunobiology. 2011; 216: 41–6.
- Babiker AA, Ronquist G, Nilsson UR, Nilsson B. Transfer of prostasomal CD59 to CD59-deficient red blood cells results in protection against complement-mediated hemolysis. Am J Reprod Immunol. 2002; 47: 183–92.
- Kitamura M, Namiki M, Matsumiya K, Tanaka K, Matsumoto M, Hara Tet al. Membrane cofactor protein (CD46) in seminal plasma is a prostasome-bound form with complement regulatory activity and measles virus neutralizing activity. Immunology. 1995; 84: 626–32.
- Madison MN, Roller RJ, Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology. 2014; 11: 102.
- Osterfield M, Kirschner MW, Flanagan JG. Graded positional information: interpretation for both fate and guidance. Cell. 2003; 113: 425–8.
- Greco V, Hannus M, Eaton S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001; 106: 633–45.
- Erickson JL. Formation and maintenance of morphogen gradients: an essential role for the endomembrane system in Drosophila melanogaster wing development. Fly (Austin). 2011; 5: 266–71.
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005; 435: 58–65.
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012; 14: 1036–45.
- Matusek T, Wendler F, Poles S, Pizette S, D'Angelo G, Furthauer Met al. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014; 516: 99–103.
- Gradilla AC, Gonzalez E, Seijo I, Andres G, Bischoff M, Gonzalez-Mendez Let al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun. 2014; 5: 5649.
- Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008; 18: 199–209.
- Kriebel PW, Barr VA, Rericha EC, Zhang G, Parent CA. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J Cell Biol. 2008; 183: 949–61.
- van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan Net al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001; 121: 337–49.
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002; 115: 2505–15.
- Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005; 6: 131–43.
- Hermle T, Guida MC, Beck S, Helmstadter S, Simons M. Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J. 2013; 32: 245–59.
- Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008; 4: 137–47.
- Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KAet al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007; 25: 2245–56.
- Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella Det al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010; 14: 1605–18.
- Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970; 67: 1513–20.
- Robison R. The possible significance of hexosephosphoric esters in ossification. Biochem J. 1923; 17: 286–93.
- Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab. 2008; 26: 514–19.
- Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 2009; 1790: 1592–8.
- Thouverey C, Strzelecka-Kiliszek A, Balcerzak M, Buchet R, Pikula S. Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like Saos-2 cells. J Cell Biochem. 2009; 106: 127–38.
- Goettsch C, Hutcheson JD, Aikawa E. MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circ Res. 2013; 112: 1073–84.
- Shirakami Y, Lee SA, Clugston RD, Blaner WS. Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta. 2012; 1821: 124–36.
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008; 48: 322–35.
- Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007; 131: 1728–34.
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle Met al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008; 7: 5157–66.
- Conde-Vancells J, Gonzalez E, Lu SC, Mato JM, Falcon-Perez JM. Overview of extracellular microvesicles in drug metabolism. Expert Opin Drug Metab Toxicol. 2010; 6: 543–54.
- Royo F, Schlangen K, Palomo L, Gonzalez E, Conde-Vancells J, Berisa Aet al. Transcriptome of extracellular vesicles released by hepatocytes. PLoS One. 2013; 8: e68693.
- Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006; 291: G500–9.
- Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope Aet al. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest. 2008; 118: 3714–24.
- Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet Set al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013; 41: 241–4.
- Frühbeis C, Frohlich D, Kuo WP, Kramer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013; 7: 182.
- Koles K, Budnik V. Exosomes go with the Wnt. Cell Logist. 2012; 2: 169–73.
- Prada I, Furlan R, Matteoli M, Verderio C. Classical and unconventional pathways of vesicular release in microglia. Glia. 2013; 61: 1003–17.
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011; 29: 341–5.
- Wood MJ, O'Loughlin AJ, Samira L. Exosomes and the blood-brain barrier: implications for neurological diseases. Ther Deliv. 2011; 2: 1095–9.
- Grapp M, Wrede A, Schweizer M, Huwel S, Galla HJ, Snaidero Net al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun. 2013; 4: 2123.
- Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Turco DDet al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014; 12: e1001874.
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon Get al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011; 46: 409–18.
- Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CVet al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014; 42: 9195–208.
- Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014; 3 doi: http://dx.doi.org/10.3402/jev.v3.24722.
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi Net al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009; 139: 393–404.
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara Met al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013; 77: 1039–46.
- Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DHet al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014; 24: 519–25.
- Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone Let al. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015; 16: 213–20.
- Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta Cet al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012; 31: 1231–40.
- Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini Fet al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol. 2012; 72: 610–24.
- Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012; 287: 10977–89.
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab ASet al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013; 11: e1001604.
- Frohlich D, Kuo WP, Fruhbeis C, Sun JJ, Zehendner CM, Luhmann HJet al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans Roy Soc Lond B Biol Sci. 2014; 369:20130510
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013; 61: 1795–806.
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Yet al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013; 31: 2737–46.
- Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014; 62: 284–99.
- Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012; 3: 228.
- Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases?. Front Physiol. 2012; 3: 124.
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008; 118: 1311–21.
- Cox FE. History of human parasitology. Clin Microbiol Rev. 2002; 15: 595–612.
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010; 74: 81–94.
- Schertzer JW, Whiteley M. Bacterial outer membrane vesicles in trafficking, communication and the host-pathogen interaction. J Mol Microbiol Biotechnol. 2013; 23: 118–30.
- Barteneva NS, Maltsev N, Vorobjev IA. Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol. 2013; 3: 49.
- Torrecilhas AC, Schumacher RI, Alves MJ, Colli W. Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect. 2012; 14: 1465–74.
- Marcilla A, Martin-Jaular L, Trelis M, de Menezes-Neto A, Osuna A, Bernal Det al. Extracellular vesicles in parasitic diseases. J Extracell Vesicles. 2014; 3 doi: http://dx.doi.org/10.3402/jev.v3.25040 [PubMed CentralFull Text].
- Senft AW, Philpott DE, Pelofsky AH. Electron microscope observations of the integument, flame cells, and gut of Schistosoma mansoni. J Parasitol. 1961; 47: 217–29.
- Threadgold LT. The ultrastructure of the “cuticle” of Fasciola hepatica. Exp Cell Res. 1963; 30: 238–42.
- Mulvenna J, Moertel L, Jones MK, Nawaratna S, Lovas EM, Gobert GNet al. Exposed proteins of the Schistosoma japonicum tegument. Int J Parasitol. 2010; 40: 543–54.
- Schulte L, Lovas E, Green K, Mulvenna J, Gobert GN, Morgan Get al. Tetraspanin-2 localisation in high pressure frozen and freeze-substituted Schistosoma mansoni adult males reveals its distribution in membranes of tegumentary vesicles. Int J Parasitol. 2013; 43: 785–93.
- Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez MTet al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012; 7: e45974.
- Toledo R, Bernal MD, Marcilla A. Proteomics of foodborne trematodes. J Proteomics. 2011; 74: 1485–503.
- Coakley G, Simbari F, McSorley H, Maizels R, Buck A. Secreted exosomes from Heligmosomoides polygyrus modulate cellular responses of the murine host. J Extracell Vesicles. 2014; 3 24214, doi: http://dx.doi.org/10.3402/jev.v3.24214.
- Maizels RM, Hewitson JP, Murray J, Harcus YM, Dayer B, Filbey KJet al. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol. 2012; 132: 76–89.
- Wang T, Van Steendam K, Dhaenens M, Vlaminck J, Deforce D, Jex ARet al. Proteomic analysis of the excretory-secretory products from larval stages of Ascaris suum reveals high abundance of glycosyl hydrolases. PLoS Negl Trop Dis. 2013; 7: e2467.
- Bernal D, Trelis M, Montaner S, Cantalapiedra F, Galiano A, Hackenberg Met al. Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. J Proteomics. 2014; 105: 232–41.
- Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan Tet al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014; 5: 5488.
- Montaner S, Galiano A, Trelis M, Martin-Jaular L, Del Portillo HA, Bernal Det al. The role of extracellular vesicles in modulating the host immune response during parasitic infections. Front Immunol. 2014; 5: 433.
- Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJet al. The trypanosomiases. Lancet. 2003; 362: 1469–80.
- Goncalves MF, Umezawa ES, Katzin AM, de Souza W, Alves MJ, Zingales Bet al. Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol. 1991; 72: 43–53.
- Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez Aet al. Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res. 2013; 12: 883–97.
- Geiger A, Hirtz C, Becue T, Bellard E, Centeno D, Gargani Det al. Exocytosis and protein secretion in Trypanosoma. BMC Microbiol. 2010; 10: 20.
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida ICet al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008; 7: 58–67.
- Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JAet al. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog. 2013; 9: e1003482.
- Lambertz U, Silverman JM, Nandan D, McMaster WR, Clos J, Foster LJet al. Secreted virulence factors and immune evasion in visceral leishmaniasis. J Leukoc Biol. 2012; 91: 887–99.
- Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang Cet al. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010; 123: 842–52.
- Santarem N, Racine G, Silvestre R, Cordeiro-da-Silva A, Ouellette M. Exoproteome dynamics in Leishmania infantum. J Proteomics. 2013; 84: 106–18.
- Hassani K, Antoniak E, Jardim A, Olivier M. Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signalling and function. PLoS One. 2011; 6: e18724.
- Garcia-Silva MR, Cura das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello Cet al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res. 2014; 113: 285–304.
- Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly Iet al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol. 2010; 185: 5011–22.
- Halle M, Gomez MA, Stuible M, Shimizu H, McMaster WR, Olivier Met al. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J Biol Chem. 2009; 284: 6893–908.
- Jaramillo M, Gomez MA, Larsson O, Shio MT, Topisirovic I, Contreras Iet al. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe. 2011; 9: 331–41.
- Hassani K, Shio MT, Martel C, Faubert D, Olivier M. Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS One. 2014; 9: e95007.
- Ghosh J, Bose M, Roy S, Bhattacharyya SN. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe. 2013; 13: 277–88.
- Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher Tet al. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010; 6: e1000744.
- Nantakomol D, Dondorp AM, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes Vet al. Circulating red cell-derived microparticles in human malariam. The Journal of infectious diseases. 2011; 203: 700–6.
- Campos FM, Franklin BS, Teixeira-Carvalho A, Filho AL, de Paula SC, Fontes CJet al. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J. 2010; 9: 327.
- Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug Met al. Cell–cell communication between malaria parasites promotes sexual differentiation via exosome-like vesicles. Cell. 2013; 153: 1120–33.
- Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev Iet al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13:521–34
- Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu Get al. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013; 9: e1003261.
- Pope SM, Lässer C. Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracell Vesicles. 2013; 2 http://dx.doi.org/10.3402/jev.v2i0.22484.
- Wampfler PB, Tosevski V, Nanni P, Spycher C, Hehl AB. Proteomics of secretory and endocytic organelles in Giardia lamblia. PLoS One. 2014; 9: e94089.
- Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim Set al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009; 9: 5425–36.
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Ann Rev Microbiol. 2010; 64: 163–84.
- Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002; 32: 1–13.
- MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012; 163: 607–18.
- Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004; 23: 4538–49.
- Manning AJ, Kuehn MJ. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microbiol Biotechnol. 2013; 23: 131–41.
- Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson Jet al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003; 115: 25–35.
- Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005; 437: 422–5.
- Mashburn-Warren L, McLean RJ, Whiteley M. Gram-negative outer membrane vesicles: beyond the cell surface. Geobiology. 2008; 6: 214–19.
- Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Hoiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J Antimicrob Chemother. 2000; 45: 9–13.
- Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol. 2006; 61: 839–46.
- Schaar V, Paulsson M, Morgelin M, Riesbeck K. Outer membrane vesicles shield Moraxella catarrhalis beta-lactamase from neutralization by serum IgG. J Antimicrob Chemother. 2013; 68: 593–600.
- Schaar V, Uddback I, Nordstrom T, Riesbeck K. Group A streptococci are protected from amoxicillin-mediated killing by vesicles containing beta-lactamase derived from Haemophilus influenzae. J Antimicrob Chemother. 2014; 69: 117–20.
- Dorward DW, Garon CF, Judd RC. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989; 171: 2499–505.
- Rumbo C, Fernandez-Moreira E, Merino M, Poza M, Mendez JA, Soares NCet al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011; 55: 3084–90.
- Yaron S, Kolling GL, Simon L, Matthews KR. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl Environ Microbiol. 2000; 66: 4414–20.
- Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci USA. 2010; 107: 19002–7.
- Shen G, Zhou E, Alspaugh JA, Wang P. Wsp1 is downstream of Cin1 and regulates vesicle transport and actin cytoskeleton as an effector of Cdc42 and Rac1 in Cryptococcus neoformans. Eukaryot Cell. 2012; 11: 471–81.
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007; 63: 545–58.
- Grenier D, Belanger M. Protective effect of Porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun. 1991; 59: 3004–8.
- Baumgarten T, Sperling S, Seifert J, von Bergen M, Steiniger F, Wick LYet al. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol. 2012; 78: 6217–24.
- Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011; 11: 258.
- Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006; 188: 5945–57.
- Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia.”. Infect Immun. 2006; 74: 5023–8.
- Schooling SR, Hubley A, Beveridge TJ. Interactions of DNA with biofilm-derived membrane vesicles. J Bacteriol. 2009; 191: 4097–102.
- Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai Ket al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009; 9: 197.
- Yu Q, Griffin EF, Moreau-Marquis S, Schwartzman JD, Stanton BA, O'Toole GA. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J Antimicrob Chemother. 2012; 67: 2673–81.
- Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996; 178: 2767–74.
- Li Z, Clarke AJ, Beveridge TJ. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J Bacteriol. 1998; 180: 5478–83.
- Zhang G, Ducatelle R, Pasmans F, D'Herde K, Huang L, Smet Aet al. Effects of Helicobacter suis gamma-glutamyl transpeptidase on lymphocytes: modulation by glutamine and glutathione supplementation and outer membrane vesicles as a putative delivery route of the enzyme. PLoS One. 2013; 8: e77966.
- Wurdinger T, Gatson NN, Balaj L, Kaur B, Breakefield XO, Pegtel DM. Extracellular vesicles and their convergence with viral pathways. Adv Virol. 2012; 2012: 767694.
- Meckes DG Jr., Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA. 2010; 107: 20370–5.
- Flanagan J, Middeldorp J, Sculley T. Localization of the Epstein-Barr virus protein LMP 1 to exosomes. J Gen Virol. 2003; 84: 1871–9.
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JLet al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010; 107: 6328–33.
- Canitano A, Venturi G, Borghi M, Ammendolia MG, Fais S. Exosomes released in vitro from Epstein-Barr virus (EBV)-infected cells contain EBV-encoded latent phase mRNAs. Cancer Lett. 2013; 337: 193–9.
- Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IBet al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012; 189: 3795–9.
- Narayanan A, Iordanskiy S, Das R, Van Duyne R, Santos S, Jaworski Eet al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013; 288: 20014–33.
- Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer Cet al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012; 12: 558–70.
- Nour AM, Modis Y. Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol. 2014; 24: 449–54.
- Izquierdo-Useros N, Naranjo-Gomez M, Archer J, Hatch SC, Erkizia I, Blanco Jet al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009; 113: 2732–41.
- Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009; 583: 3363–6.
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Yet al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012; 287: 16820–34.
- Lu C, Zainal Z, Tucker GA, Lycett GW. Developmental abnormalities and reduced fruit softening in tomato plants expressing an antisense Rab11 GTPase gene. Plant Cell. 2001; 13: 1819–33.
- de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005; 17: 2564–79.
- An Q, Huckelhoven R, Kogel KH, van Bel AJ. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006; 8: 1009–19.
- Sprunck S, Rademacher S, Vogler F, Gheyselinck J, Grossniklaus U, Dresselhaus T. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science. 2012; 338: 1093–7.
- Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Yet al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012; 22: 107–26.
- Witwer KW, McAlexander MA, Queen SE, Adams RJ. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: limited evidence for general uptake of dietary plant xenomiRs. RNA Biol. 2013; 10: 1080–6.
- Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase.”. J Cell Physiol. 1989; 140: 455–62.
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer Jet al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003; 278: 10963–72.
- Laulagnier K, Vincent-Schneider H, Hamdi S, Subra C, Lankar D, Record M. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol Dis. 2005; 35: 116–21.
- Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012; 189: 744–54.
- Vallejo MC, Nakayasu ES, Longo LV, Ganiko L, Lopes FG, Matsuo ALet al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 2012; 7: e39463.
- Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski Aet al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta. 2013; 1831: 1302–9.
- Arienti G, Polci A, De Cosmo A, Saccardi C, Carlini E, Palmerini CA. Lipid fatty acid and protein pattern of equine prostasome-like vesicles. Comp Biochem Physiol B Biochem Mol Biol. 2001; 128: 661–6.
- Piehl LL, Cisale H, Torres N, Capani F, Sterin-Speziale N, Hager A. Biochemical characterization and membrane fluidity of membranous vesicles isolated from boar seminal plasma. Anim Reprod Sci. 2006; 92: 401–10.
- Weerheim AM, Kolb AM, Sturk A, Nieuwland R. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal Biochem. 2002; 302: 191–8.
- Del Boccio P, Raimondo F, Pieragostino D, Morosi L, Cozzi G, Sacchetta Pet al. A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: application to RCC urinary exosomes. Electrophoresis. 2012; 33: 689–96.
- Schaar V, de Vries SP, Perez Vidakovics ML, Bootsma HJ, Larsson L, Hermans PWet al. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell Microbiol. 2011; 13: 432–49.
- Bomberger JM, Ye S, Maceachran DP, Koeppen K, Barnaby RL, O'Toole GAet al. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 2011; 7: e1001325.
- Bomberger JM, Ely KH, Bangia N, Ye S, Green KA, Green WRet al. Pseudomonas aeruginosa Cif Protein Enhances the Ubiquitination and Proteasomal Degradation of the Transporter Associated with Antigen Processing (TAP) and Reduces Major Histocompatibility Complex (MHC) Class I Antigen Presentation. J Biol Chem. 2014; 289: 152–62.
- Bartruff JB, Yukna RA, Layman DL. Outer membrane vesicles from Porphyromonas gingivalis affect the growth and function of cultured human gingival fibroblasts and umbilical vein endothelial cells. J Periodontol. 2005; 76: 972–9.
- Vidakovics ML, Jendholm J, Morgelin M, Mansson A, Larsson C, Cardell LOet al. B cell activation by outer membrane vesicles – a novel virulence mechanism. PLoS Pathog. 2010; 6: e1000724.
- Nakao R, Takashiba S, Kosono S, Yoshida M, Watanabe H, Ohnishi Met al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. 2014; 16: 6–16.
- Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen Set al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012; 80: 31–42.
- Sharpe SW, Kuehn MJ, Mason KM. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect Immun. 2011; 79: 4361–9.
- Ismail S, Hampton MB, Keenan JI. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect Immun. 2003; 71: 5670–5.
- Chitcholtan K, Hampton MB, Keenan JI. Outer membrane vesicles enhance the carcinogenic potential of Helicobacter pylori. Carcinogenesis. 2008; 29: 2400–5.
- Mullaney E, Brown PA, Smith SM, Botting CH, Yamaoka YY, Terres AMet al. Proteomic and functional characterization of the outer membrane vesicles from the gastric pathogen Helicobacter pylori. Proteomics Clin Appl. 2009; 3: 785–96.
- Parker H, Chitcholtan K, Hampton MB, Keenan JI. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect Immun. 2010; 78: 5054–61.
- Elmi A, Watson E, Sandu P, Gundogdu O, Mills DC, Inglis NFet al. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect Immun. 2012; 80: 4089–98.
- Dormann P, Kim H, Ott T, Schulze-Lefert P, Trujillo M, Wewer Vet al. Cell-autonomous defense, re-organization and trafficking of membranes in plant–microbe interactions. New Phytol. 2014; 204: 815–22.
- Huckelhoven R. Transport and secretion in plant–microbe interactions. Curr Opin Plant Biol. 2007; 10: 573–9.