Abstract
The polarization and migration of eukaryotic cells are fundamental processes for the development and maintenance of a tissue. These aspects gain especial interest when it comes to stem and progenitor cells in the way that their manipulation might open new avenues in regenerative therapy. In recent years, novel biological facets of migrating hematopoietic stem cells were revealed by several groups including ours. Among these features, the polarization of their membranous (proteins and lipids) and cytoplasmic constituents, which leads to the formation of a specialized sub-cellular structure located at the rear pole – the uropod – has gained increasing interest. In a new study we have demonstrated that such phenomena involve a coordinated mechanism between Rho GTPase signaling and the microtubule network. Specifically, our results based on the use of synthetic inhibitors and RNA interference suggest that the activity of RhoA and its effector ROCK I is indispensable for cell polarization and the active reorganization of microtubules that are required for migration.
Understanding the cellular and molecular trafficking mechanisms that regulate the migration of hematopoietic stem and progenitor cells (HSPCs) throughout the development of an organism and later on its homeostasis are important not only from a biological standpoint, but also with regard to therapeutic purposes. For instance, bone marrow transplantation is one recognized procedure for treating hematological diseases. However, the accurate mechanism underlying the migration and engraftment of HSPCs into the bone-marrow niche is not fully characterized. In order to gain novel insights we have developed an ex vivo co-culture system consisting of human HSPCs from healthy donors growing on primary human multipotent mesenchymal stromal cells (MSCs) as feeder cell layer (for cell isolation and culture conditions see literatures).Citation1–Citation3 Such cellular system reproduces numerous characteristics found within bone marrow cavitiesCitation4 including adhesive interactionsCitation5 and the essential chemotactic axisCitation6 based on the G-protein-coupled receptor CXCR4, which is expressed by HSPCs and its chemokine ligand CXCL12 (alias stromal cell-derived factor-1α; SDF-1α) secreted by MSCs.Citation1,Citation7
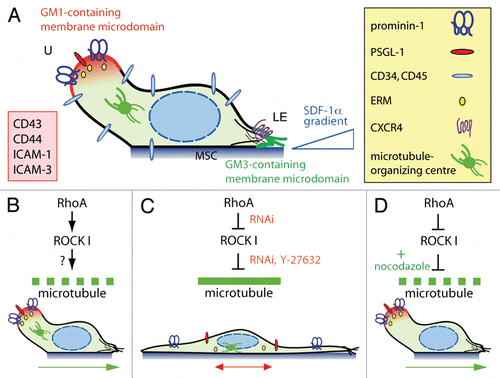
Under these conditions, HSPCs display various morphologically identifiable types of plasma membrane protrusions.Citation1 Interestingly, in migrating HSPCs a noteworthy protrusion called uropod is formed at the rear pole ().Citation1,Citation8 Like in leukocytes (e.g., T cells), the uropod might play a role in intercellular adhesion, communication and motility.Citation9,Citation10 Numerous proteins with adhesive properties are found therein including P-selectin glycoprotein ligand-1 (PSGL-1; ).Citation11,Citation12 The presence of the stem cell marker CD133 (prominin-1),Citation13–Citation15 a 5-span transmembrane glycoprotein that binds plasma membrane cholesterol and associates with a specific membrane microdomain (lipid raft),Citation16 was instructive with regard to its membrane organization. Indeed, a membrane microdomain enriched in ganglioside GM1 is concentrated in the uropod,Citation10 and its spatiotemporal regulation might engage molecules such as flotillins.Citation17 From the cytoplasmic side, certain proteins of the ezrin/radixin/moesin (ERM) family might link membrane proteins (e.g., PSGL-1) via their juxta-membrane domains to the underlying actin cytoskeleton.Citation18 The microtubule-organizing centre is found at the base of the uropod.Citation12 At the front pole, a migrating HSPC exhibits a lamellipodium, which concentrates CXCR4 at its tips in agreement with a chemotactic role.Citation11 As reported for T cells, a distinct membrane microdomain based on ganglioside GM3 instead of GM1 is found therein ().Citation11,Citation19 From both morphological and phenotypical angles, the migrating HSPC develops thus a highly polarized structure that underlies coordinated but opposite actions at both cell sides. While retracting the uropod, the cell extends its lamellipodium at the leading edge. As a result, a net cell movement can be achieved by continuous attachment to and de-adhesion from the substratum at the front and rear pole, respectively ( and green arrow).
From a mechanistical and/or biochemical perspective, our recent study has focused on the implication of Rho GTPase signaling pathway in these orchestrated processes ().Citation12 It is known that Rho GTPases such RhoA, Rac and Cdc42 are key players in cell polarity and migration by modulating cytoskeletal dynamics.Citation20 As the most important downstream effectors of RhoA, Rho-associated coiled-coil protein kinases (ROCK) are implicated in various cellular functions such as actin organization and transformation. Using Rho kinase inhibitor Y-27632 and RNA interference (RNAi) directed against either RhoA or ROCK I we demonstrated that both proteins are indispensable for the polarization of HSPCs, and hence their migration. For instance, the use of the synthetic drug resulted in the complete loss of the uropod and the formation of two to three long and thin plasma membrane protrusions (). Narrowed lamellipodia were formed at the tip of those protrusions rather than close to the cell body, as in untreated cells ().Citation12 Such a drastic morphological alteration was followed at the molecular level by a redistribution of plasma membrane (prominin-1 and PSGL-1) and cytoskeleton (ezrin, an ERM protein) constituents of the uropod. The asymmetric distribution of microtubule-organizing centre was also lost ().Citation12 As a functional consequence, Y-27632-treated cells displayed a net impairment of migration as evaluated by time-lapse video microscopy and Transwell-filter assay.Citation12 Specifically, Y-27632-treated cells showed a defect in retracting the long plasma membrane protrusion located at the rear pole and frequently changed their directional movement by 180°, suggesting a perturbation in the front-rear orientation mediated by CXCR4/SDF-1α axis ( and red double-headed arrow). All the characteristics described above for Y-27632-treated cells were reproduced upon the use of RNAi-mediated knockdown of RhoA and ROCK I, thus confirming the direct implication of Rho GTPase signaling pathway in the polarization and migration of HSPCs (). These outcomes appeared highly specific since the knockdown of ROCK II did not provide such radical effects.
Surprisingly, our study also revealed that the defect in cell polarization including the formation of the uropod could be fully rescued by the nocodazole-mediated depolymerization of the microtubule network (). Not only was the polarized morphology of RhoA/ROCK I-deficient HSPCs restored, but also their locomotion. The actin depolymerization triggered by latrunculin B did not produce such reversible effects.Citation12 The precise way that RhoA/ROCK I signaling contributes to the microtubule instability at the uropod cortex is currently unknown. Nevertheless our experiments with nocodazole/RNAi seem to exclude a feedback loop involving microtubule-associated guanine nucleotide exchange factor (GEF)-H1, which activates RhoACitation21 upon its release from microtubules after the disruption of the latter. However such phenomenon might occur in a natural context. The potential direct or indirect targets of ROCK remain to be identified, but they might engage enzymes that mediate tubulin detyrosination and acetylation, e.g., histone deacetylase 6, the activity of which is modulated by RhoA/ROCK.Citation22,Citation23 Active crosstalk between players at the leading edge and the uropod as well as a dynamic balance of the actomyosin and microtubule systems also need to be considered.Citation24 The lack of the front-rear orientation of RhoA/ROCK I-deficient HSPCs and altered lamellipodia are consistent with it.Citation12 Microtubule-destabilizing protein stathmin/OP18 might participate in these biochemical reactions via Rac/Cdc42,Citation25 and the regulation of Rac by ROCK via the filamin A-binding RhoGTPase-activating protein reveals the complexity of the system.Citation26 Similarly, members of the ERM protein family such as ezrin or moesin might be involved as well in the integrity of the uropod, and it might be more than a coincidence that these adaptor proteins, which are also potential substrates of ROCK, are playing an active role in membrane microdomain dynamics.Citation27
Lastly, it is noteworthy that comparable data showing the implication of RhoA and microtubule network in the migration of T cells were recently reported independentlyCitation28,Citation29 indicating that our current observations might extend to cells of hematopoietic origin in general. Further studies based on a quantitative proteomic approach should lead to an exhaustive list of ROCK I substrates involved in these processes, which might represent potential therapeutic targets in the development of new strategies to improve the efficiency of bone marrow transplantation.
Abbreviations
| ERM | = | ezrin/radixin/moesin |
| HSPCs | = | hematopoietic stem and progenitor cells |
| MSCs | = | multipotent mesenchymal stromal cells |
| PSGL-1 | = | P-selectin glycoprotein ligand-1 |
| ROCK | = | Rho-associated coiled-coil protein kinase |
| RNAi | = | RNA interference |
| SDF-1α | = | stromal cell-derived factor-1α |
Figures and Tables
Figure 1 RhoA/ROCK I pathway and remodeling of microtubule network underlie the polarization and migration of HSPCs. (A) A migrating HSPC growing on MSC displays a polarized morphology with the formation of a uropod (U) at the rear pole and a leading edge (LE) at the front. Both types of plasma membrane protrusions contain a specific ganglioside-based membrane microdomain—the uropod being enriched in GM1 (red) whereas the leading edge in GM3 (green). In addition to prominin-1, a plethora of cell adhesion molecules (inset) including PSGL-1 are concentrated in the uropod whereas the chemokine receptor CXCR4 is found at the leading edge consistent with its sensory role towards an SDF-1α gradient.Citation6 ERM proteins seem to be actively involved in the subcellular localization of some uropod-associated membrane proteins.Citation18 Other molecules such as CD34 and CD45 are evenly distributed. The microtubule-organizing centre is found between the nucleus and the uropod. (B) The activity (↓) of RhoA and its downstream effector ROCK I contributes to the formation of the uropod, and hence polarization and migration of HSPCs. The downstream target(s) remain to be identified (?), but it might engage a protein involved in microtubule destabilization (dashed green line). (C) Inhibition (⊥) of ROCKs using Y-27632 or the specific knockdown of ROCK I or its upstream regulator RhoA by means of RNAi results in an elongated morphology where the uropod is lost. Both membrane (prominin-1, PSGL-1) and cytoplasmic (ezrin) proteins are redistributed. These cells display an impairment of migration caused by microtubule stability (solid green line). (D) In RhoA/ROCKI-deficient HSPCs, the addition (+) of nocodazole restores their proper polarization and migration highlighting the implication of unidentified microtubule-destabilizing proteins. Green and red arrows indicate the direction of migration.
Acknowledgements
We thank C.A. Fargeas for critically reading the manuscript. This work was supported by grant from Deutsche Forschungsgemeinschaft (SFB 655 B3, SFB/TRR83 No.6).
Addendum to:
References
- Freund D, Bauer N, Boxberger S, Feldmann S, Streller U, Ehninger G, et al. Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells—effects on proliferation and clonogenicity. Stem Cells Dev 2006; 15:815 - 829
- Freund D, Oswald J, Feldmann S, Ehninger G, Corbeil D, Bornhäuser M. Comparative analysis of proliferative potential and clonogenicity of MACS-immunomagnetic isolated CD34+ and CD133+ blood stem cells derived from a single donor. Cell Prolif 2006; 39:325 - 332
- Freund D, Fonseca AV, Janich P, Bornhäuser M, Corbeil D. Differential expression of biofunctional GM1 and GM3 gangliosides within the plastic-adherent multipotent mesenchymal stromal cell population. Cytotherapy 2010; 12:131 - 142
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466:829 - 834
- Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA 1998; 95:14423 - 14428
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4 and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 2000; 95:3289 - 3296
- Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K, Bornhäuser M, et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells-modeling the niche compartments in vitro. Haematologica 2010; 95:542 - 550
- Bauer N, Fonseca AV, Florek M, Freund D, Jászai J, Bornhäuser M, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissue Organs 2008; 188:127 - 138
- Sánchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol 2009; 10:353 - 359
- Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol 2009; 11:303 - 305
- Giebel B, Corbeil D, Beckmann J, Höhn J, Freund D, Giesen K, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood 2004; 104:2332 - 2338
- Fonseca AV, Freund D, Bornhäuser M, Corbeil D. Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J Biol Chem 2010; 285:31661 - 31671
- Fargeas CA, Fonseca AV, Huttner WB, Corbeil D. Prominin-1 (CD133): from progenitor cells to human diseases. Future Lipidology 2006; 1:213 - 225
- Corbeil D, Fargeas CA, Huttner WB. Rat prominin, like its mouse and human orthologues, is a pentaspan membrane glycoprotein. Biochem Biophys Res Commun 2001; 285:939 - 944
- Kania G, Corbeil D, Fuchs J, Tarasov KV, Blyszczuk P, Huttner WB, et al. The somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells 2005; 23:791 - 804
- Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett 2007; 581:1783 - 1787
- Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol 2010; 191:771 - 781
- Serrador JM, Urzainqui A, Alonso-Lebrero JL, Cabrero JR, Montoya MC, Vicente-Manzanares M, et al. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur J Immunol 2002; 32:1560 - 1566
- Gómez-Móuton sC, Abad J, Mira E, Lacalle R, Gallardo E, Jimenez-Baranda S, et al. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA 2001; 98:9642 - 9647
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9:690 - 701
- Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell 2008; 19:2147 - 2153
- Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 2002; 21:6820 - 6831
- Ling L, Lobie PE. RhoA/ROCK activation by growth hormone abrogates p300/histone deacetylase 6 repression of Stat5-mediated transcription. J Biol Chem 2004; 279:32737 - 32750
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol 2007; 9:299 - 309
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem 2001; 276:1677 - 1680
- Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol 2006; 8:803 - 814
- Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol 2006; 6:625 - 633
- Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS ONE 2010; 5:8774
- Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol 2010; 190:553 - 563
