Abstract
Therapeutic properties of antibodies strongly depend on the composition of their glycans. Most of the currently approved antibodies are produced in mammalian cell lines, which yield mixtures of different glycoforms that are close to those of humans, but not fully identical. Glyco-engineering is being developed as a method to control the composition of carbohydrates and to enhance the pharmacological properties of mAbs. The recent approval in Japan of mogamulizumab (POTELIGEO®), the first glyco-engineered antibody to reach the market, is a landmark in the field of therapeutic antibodies. Mogamulizumab is a humanized mAb derived from Kyowa Hakko Kirin’s POTELLIGENT® technology, which produces antibodies with enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) activity. The approval was granted April 30, 2012 by the Japanese Ministry of Health, Labour and Welfare for patients with relapsed or refractory CCR4-positive adult T-cell leukemia-lymphoma.
At least 15 glyco-engineered antibodies are currently being evaluated in clinical studies. The next approval of a glyco-engineered antibody is likely to be obinutuzumab (GA101), the Roche-Glycart antibody that is currently in Phase 3 clinical trials. GA101 is a third-generation, humanized, glyco-engineered anti-CD20 IgG1 mAb that is undergoing evaluation for the potential treatment of B cell malignancies. GA101 induces 5- to 100-fold greater ADCC than observed upon treatment with rituximab. Another promising application of the Roche-Glycart technology is GA201 (RG7160), an epidermal growth factor receptor (EGFR)-targeting antibody, that could be indicated for the nearly 40% of colorectal patients with KRAS mutation who do not respond to cetuximab and panitumumab.Citation1 GA201 is a dual-acting, humanized, IgG1 mAb that has been designed to provide enhanced ADCC activity and increased immune response in combination with signaling inhibition. Notably, there are a plethora of alternative production systems for glyco-optimized proteins, including yeast, duck, rat, algae, moss, and tobacco. Last but not least, biobetter antibody versions of trastuzumab, cetuximab, rituximab and infliximab derived from these technologies are also in development.
Current production systems for approved IgGs
Chinese hamster ovary cells (CHO) and mouse myeloma cells (NS0, SP2/0) have become the gold-standard mammalian host cells for the production of therapeutic antibodies and Fc-fusion proteins that have already reached the market.Citation2 Of the 28 mAbs marketed in the United States or European Union, 43% are produced in CHO cells, 50% in mouse-derived cells (18% in NS0, 25% in SP2/0 and 7% in hybridomas) and 7% in E. coli (non-glycosylated Fab).Citation3 Most of these cell lines have been adapted to grow in suspension culture and are well-suited for reactor culture, scale-up and large volume production (up to 20,000 L), with a productivity ranging from 1 to 8 g/L. Such manufacturing scales are essential features for supplying antibodies used in chronic diseases for the world-wide market. Blockbuster antibodies are currently produced at a multi-ton scale per year. The main glycoforms of antibodies and other glycoproteins produced in these mammalian cell line systems are close to the human ones. But minor, non-human glycoforms also exist; these may be immunogenic, resulting in faster clearance if present in large amounts.
Antibody glycosylation in human sera vs. recombinant mAbs from CHO, NS0, or SP2/0
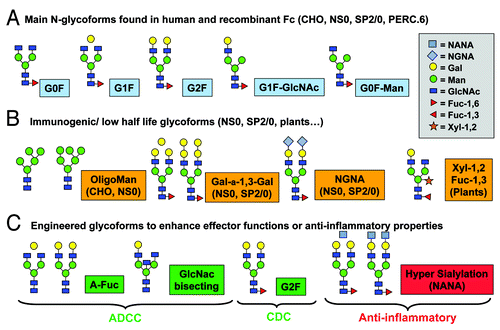
The glycoforms identified on IgGs produced from CHO cells are close to human ones except for the third GlcNac bisecting arm, which represents ~10% of human IgGs glycoforms, and very low amounts of terminal N-acetylneuraminic acid (NANA; ).Citation4 Murine NS0 or SP2/0 cells produce mAbs exhibiting small amounts of glycoforms with additional Gal α-1,3-gal and different sialic acids such as N-glycolylneuraminic acid (NGNA) instead of NANA. NGNA is the predominant sialic acid present in glycoproteins produced by mouse cells, but it appears only as traces in glycoproteins expressed from CHO cells ().Citation5 NGNA is reported to be immunogenic in human, but, from a practical standpoint, the amount present in most of the NS0-produced mAbs is generally very low in the Fc part (~1–2%). No serious adverse events linked to these glycoforms were reported for the marketed NS0- and SP2/0-produced mAbs, e.g., palivizumab, which was first approved in 1998. The same stands for the mouse Gal α-1,3-gal residue, which is generally a very minor glycoform (2 – 4%) on Asn297.Citation5 A notable exception is cetuximab, which contains a second N-glycosylation site in its Fab portion on heavy chain Asn88. For the marketed version of cetuximab produced in SP2/0 cells, at least 21 different glycoforms were identified with ~30% capped by at least one Gal α-1,3-gal residue, 12% capped by a NGNA residue and traces of oligomannose.Citation6 Importantly, both Gal α-1,3-gal and NGNA were found only in the Fab moieties in contrast to the Fc fragment, for which only typical IgGs G0F, G1F and G2F glycoforms were identified. In a recent report on cetuximab-induced anaphylaxis, pre-existing IgEs specific for this galactose-α-1,3-gal epitope were detected in patients treated with cetuximab.Citation7-Citation9 Using a solid phase immunoassay, these IgEs were found to bind to SP2/0-produced cetuximab and F(ab)2 fragment, and not to the Fc fragment. Interestingly, no IgE immunoreactivity was found against a version of cetuximab produced in CHO (CHO-C225), which represents a simple way to produce a biobetter version of cetuximab.Citation10,Citation11
Effect of glycosylation on immunogenicity or clearance
High mannose-type N-glycans contain from five to nine mannose residues and are found on antibodies produced in mammalian cells,Citation12 yeast,Citation13 insect cellsCitation14 and plants,Citation15 but only at a very low level in normal human antibodies.Citation16 High mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans.Citation17,Citation18 Several other glycoforms containing fucose or xylose moieties characteristic of mice, yeast or plant-derived glycoproteins are highly immunogenic in mammals (). As a consequence, only mammalian-based production systems are used for the manufacturing of approved biopharmaceuticals, which need proper glycosylation. Nevertheless, tremendous efforts are developed both in academic labs and in industry to engineer the glycosylation pathways of mammalian cells, yeasts, insect cells and plants to allow the production of recombinant proteins exhibiting human-like glycosylation.
Glyco-engineered antibodies in CHO cells with enhanced ADCC
ADCC is an important effector function, especially for human IgG1 mAbs developed in oncology, when the major goal is to selectively destroy tumor cells.Citation19 The presence of a bisecting N-acetylglucosamine (GlcNAc) associated with the depletion in fucose residues (e.g., by genetic knockdown of α-1,6-fucosyltransferase) from oligosaccharides in the conserved attachment region to Fcγ receptors results in an up to 100-fold increase in ADCC activity.Citation20 The current CHO cell lines are not suitable for the production of completely defucosylated antibodies as they retain a high level of intrinsic α-1,6-fucosyltransferase (FUT8) enzyme activity, which is responsible for the core fucosylation of N-linked oligosaccharides.Citation21 Kyowa Kirin Hakko has established a FUT8 knockout CHO cell lines by gene targeting using a homologous recombination technique.Citation22 Except for the complete depletion of FUT8 expression, the properties of the established FUT8 knockout CHO cells were unaltered from those of the parent cells in terms of morphology, growth kinetics, and productivity (POTELLIGENT® technology). Recombinant DNA-based glyco-engineering for increased antibody effector function was also achieved by overexpression of heterologous β1,4-N-acetylglucosaminyltransferase III (GnT-III) in antibody-producing cells,Citation20 which is the Glycart-Roche technology. GnT-III catalyzes the addition of a bisecting GlcNAc to N-linked oligosaccharides. Once GnT-III adds a bisecting GlcNAc to an oligosaccharide, other central reactions of the biosynthetic pathway such as core-fucosylation and conversion of hybrid to complex glycans are blocked. Overexpression of GnTIII in antibody producing cells results in the formation of bisected, non-fucosylated oligosaccharides linked to the antibodies that mediate increased ADCC.
Cytotoxic enhancement for glyco-engineered mAbs with a bisecting GlcNAc or a depletion of Fucose was not only demonstrated for CHO cells but also for a plethora of alternative systems like yeasts, baculovirus -infected insect cells, avian cells, YB2/0 rat cells, aquatic plants, moss and tobacco as illustrated in and briefly discussed below.
Table 1. Selected antibody glyco-engineering technologies (biobetter or next-generation mAbs)
Glyco-engineered antibodies with humanized glycoforms in other heterologous expression systems
Pichia pastoris (GlycoFi technology)
GlycoFi’s glyco-engineering technology allows the generation of yeast strains capable of replicating the most essential steps of the N-glycosylation pathway found in mammals.Citation13 Merck acquired GlycoFi in 2006 to synergize GlycoFi’s yeast glyco-engineering know-how and patent portfolio with Merck’s expertise in large-scale production of biologicals (e.g., Gardasil® human papillomavirus vaccine is produced in Saccharomyces cerevisiae) to produce enhanced biopharmaceuticals or follow-on biologicals with lower costs-of-goods than can be attained with mammalian cell lines. The glyco-engineering technology of the Pichia pastoris N-glycosylation pathway developed by GlycoFi allows producing human proteins with complex N-glycosylation modifications that are similar to the ones performed in human. Moreover, more homogeneous glycosylation patterns are observed, as opposed to the large heterogeneity of glycan moieties that are found naturally in mammals or in other production systems such as CHO and NS0 cell lines. These properties, which are positive attributes when considering industrialization of the manufacturing process, makes Pichia a very promising expression system to produce large-scale batches of therapeutics at a lower cost.
S. pombe and S. cerevisiae (Glycode technology)
Glycode develops yeast strains that are deficient in high-mannose-type glycosylation, and that express, upon stable integration, all enzymes needed to perform hybrid and complex-type N-glycosylation. Up to 30 different yeast strains that perform various steps of the mammalian glycosylation pathway are available. The feasibility of their technology was exemplified by the production of recombinant erythropoietin (EPO). An important advantage of this technology is based on the stability of the glyco-engineered strains. The selection of the desired knockout and knock-in yeast strains is based on auxotrophy selectable markers, which might be more stable than resistance markers, classically used by others during scale-up and manufacturing process.
Filamentous fungi (Aspergillus niger and nidulans)
were also glyco-engineered by a approach similar to the one applied in Pichia pastoris by deletion of genes coding for fungal glycosylation enzymes and introduction of genes necessary to produced humanized complex N-glycans.
Duck embryonic stem cells (EB66 cell line, Vivalis technology)
Antibodies produced in EB66 cells display a naturally reduced fucose content resulting in enhanced ADCC activity.Citation23 A comparative N-linked oligosaccharide analysis of CHO- and EB66-produced rituximab was performed by mass spectrometry, as well as in vitro ADCC assays.
Plants
are another attractive production system for recombinant proteins.Citation24 A major concern is the presence of β-1,2 xylose (not present in human glycans) and α-1,3 fucose sugars (instead of α-1,6 fucose), which are allergenic epitopes in human. The first generation of plant-derived antibodies (“plantibodies”) were investigated in early clinical trials a decade ago for topical applications (e.g., genital herpes, dental carries), but development of them was terminated. More recently, controlled glycosylation of anti-rabies antibodies was achieved in tobacco plants by expression of human light and heavy chains genetically fused to a Lys-Asp-Glu-Leu (“KDEL”) sequence at the C-terminal parts. Interestingly, this signal peptide allows the retention of the glycoproteins in the endoplasmic reticulum and the biosynthesis of mainly oligomannose variants free of β-1,2 xylose and α-1,3 fucose.
Moss (Physcomitrella patens)
is alternatively proposed as culture system for production of mAbs in photo-bioreactors. Non-immunogenic and ADCC-improved glycan patterns were obtained by targeted gene replacements of two moss enzymes (xylosyltransferase and fucosyltransferase) to block the processing of the corresponding non-mammalian sugar moieties.
Lemna minor
expression system (LEX, an aquatic plant) enables rapid clonal expansion and secretion of mAbs at high yields (at least up to 300 g scale) with full containment and no risk of transmission of mammalian pathogens. To avoid the expression of immunogenic plant glycans, co-expression with single RNAi transcript to silence α-1,3 fucosyltransferase and β-1,2 xylosyltransferase was performed and shown to be stable at least for 3 y. As a proof-of-concept, increased ADCC activity and Fcγ RIIIa binding was demonstrated for an anti-CD30 mAb compared with the same antibody produced in CHO, as well as for an anti-CD20 mAb (BLX-300).Citation25
Glyco-engineered antibodies with enhanced CDC
Gramer et al. recently reported data showing that the combination of uridine, manganese chloride, and galactose is useful for specifically affecting antibody galactosylation correlated with CDC activity.Citation26 This was demonstrated in a GS-CHO fed-batch process with minimal impact on other glycoforms and other product quality attributes. The level of galactosylation could be controlled in a first cell line from 3% to 23% by varying the concentration of these additives, and in the second cell line from 5% to 29%. This approach enabled design of a tailored process by adding the appropriate amount of chemicals to the culture medium to enhance the CDC type of effector functions.
Glyco-engineered antibodies with enhanced inflammatory properties
Sialylated glycans are known to be species-characteristic and essential components of glycoproteins as illustrated, for example, by the switch in specificity of avian influenza viruses hemagglutinins to human flu viruses (α-2–3 sialyl to α-2–6). Nevertheless, in contrast to other circulating glycoproteins (e.g., EPO, human IgGs are poorly sialylated. The same observation was reported for recombinant antibodies produced in eukaryotic cells. Interestingly, it was recently shown that antibody sialylation could suppress inflammation and reduce cytoxicity through the engagement of its Fc fragment with different Fc gamma receptors as demonstrated by Nimmerjahn and Ravetsch in several papers. For this purpose, in vitro desialylation was achieved by antibody incubation with neuraminidase and the anti-inflammatory properties of the IgGs were lost. On the other hand, over-sialylated antibodies were obtained by affinity-chromatography purification with agarose-bound lectins and shown to have enhanced anti-inflammatory activities. Alternatively, terminally sialylated recombinant antibodies could be obtained in engineered yeast and for this purpose the GlycoFi technology looks very promising.Citation27 Higher-level antibody sialylation is associated with reduced ADCC, which is another indication of the pharmacological importance of these residues, as well as of the fine structural tuning of glycosylation, which can be achieved by the GlycoFi or SiaMedExpress technologies.
Glyco-engineered antibodies in clinical trials
To our knowledge, a total of 16 mAbs derived from four different glyco-engineering approaches have entered clinical studies (). One mAb derived from Kyowa Hakko Kirin’s POTELLIGENT® technology has been approved for marketing and six POTELLIGENT®-derived mAbs are in clinical studies. Mogamulizumab (POTELIGEO®) was approved in Japan in March 2012 as a treatment for patients with relapsed or refractory CCR4-positive T cell leukemia-lymphoma. Kyowa Hakko Kirin is also evaluating mogamulizumab in patients with peripheral T-cell lymphoma (PTCL) or cutaneous T-cell lymphoma. The mAb is licensed to Amgen for development in non-cancer indications. In December 2011, Amgen initiated a Phase 1 study of mogamulizumab in adults with asthma.
Table 2. Glyco-engineered antibodies in clinical study
Of the six POTELLIGENT®-derived mAbs in clinical studies, three (benralizumab, MEDI-551, BIW-8962) have advanced to Phase 2 studies and three (KHK2898, KHK2804, KHK2866) are in Phase 1 studies. Benralizumab is an IgG1k mAb that targets interleukin (IL)-5 receptor α chain; it is undergoing evaluation as a treatment for asthma and for moderate-to-severe chronic obstructive pulmonary disease and sputum eosinophilia. MEDI-551, which targets CD19 on B cells, is currently in a Phase 1/2 study of patients with scleroderma, a Phase 2 study in adults with diffuse large B cell lymphoma, a Phase 2 study in adults with chronic lymphocytic leukemia, and a Phase 1 study in adults with advanced B cell malignancies. A Phase 1/2 study [NCT01585766] of MEDI-551 in adults with relapsing-remitting multiple sclerosis was planned but not yet recruiting patients as of mid-May 2012. BIW-8962 was undergoing evaluation as monotherapy in a Phase1/2 study [NCT00775502] of patients with previously treated multiple myeloma, but the study was terminated due to lack of efficacy. The three mAbs at Phase 1 are undergoing evaluation as therapy for patients with advanced solid tumors.
Three GlycoMabTM-derived mAbs (obinutuzumab, GA201, RG7116) are in clinical study. Obinutuzumab, which targets CD20, is undergoing evaluation in four Phase 3 studies, four Phase 2 studies, and two Phase 1 studies, all of which include patients with hematological malignancies. The anti-EGFR GA201 is currently being evaluated in a Phase 2 study of patients with non-small cell lung cancer, a Phase 2 study of patients with colorectal cancer and a Phase 1 study of patients with head and neck squamous cell carcinoma. RG7116, which targets human epidermal growth factor receptor (HER)-3, is in a Phase 1 dose-escalation study in patients with HER3-positive solid tumors.
Three GlycoExpressTM-derived mAbs (GT-MAB2.5GEX, GT-MAB5.2GEX, GT-MAB7.3GEX) are in Phase 1 studies. The safety and tolerability of GT-MAB2.5GEX, which targets MUC1, is being evaluated in a dose escalation study in patients with advanced MUC1-positive solid malignancies. Anti-EGFR GT-MAB5.2GEX and anti-HER2 GT-MAB7.3GEX are undergoing evaluation in Phase 1 studies of patients with EGFR-positive and HER2-positive solid tumors, respectively. The estimated study completion date for all three of these Phase 1 studies is June 2012.
Three mAbs produced in YB2/0 cells, and therefore with low fucose content, are currently in Phase 2 clinical studies. The safety and effectiveness of ecromeximab, developed by Kyowa Hakko and licensed by Life Science Pharmaceuticals, is being evaluated in a Phase 2 study of patients with metastatic melanoma. LFB is developing two low-fucose mAbs, roledumab and ublituximab (EMABLING technology). Anti-rhesus (Rh) D roledumab was evaluated in a Phase 2 study [NCT00952575] designed to demonstrate the ability of LFB-R593 to effectively eliminate exogenously-administered RhD-positive red blood cells from the circulation of an RhD-negative individual, thereby preventing RhD-alloimmunization. Ublituximab, which targets CD20, was evaluated in a Phase 1 study [NCT01098188] of patients with chronic lymphocytic leukemia. TG Therapeutics, Inc. licensed the worldwide commercial rights to ublituximab in March 2012.
Future of glyco-engineering
Research done during the 1990s and 2000s on the glyco-engineering of antibodies has yielded a wide variety of approaches to production and a growing pipeline of these molecules. Thus, the technology is now delivering on the promise of therapeutic mAbs with improved properties compared with first-generation versions. With one glyco-engineered mAb approved, at least 15 in the clinic and many more in preclinical development, the future of glyco-engineering looks bright indeed.
References
- Paz-Ares LG, Gomez-Roca C, Delord JP, Cervantes A, Markman B, Corral J, et al. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol 2011; 29:3783 - 90; http://dx.doi.org/10.1200/JCO.2011.34.8888; PMID: 21900113
- Beck A, Wagner-Rousset E, Bussat MC, Lokteff M, Klinguer-Hamour C, Haeuw JF, et al. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr Pharm Biotechnol 2008; 9:482 - 501; http://dx.doi.org/10.2174/138920108786786411; PMID: 19075687
- Reichert JM. Marketed therapeutic antibodies compendium. MAbs 2012; 4:413 - 5; http://dx.doi.org/10.4161/mabs.19931; PMID: 22531442
- Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys 2012; In press http://dx.doi.org/10.1016/j.abb.2012.03.021; PMID: 22465822
- Raju TS, Jordan RE. Galactosylation variations in marketed therapeutic antibodies. MAbs 2012; 4:385 - 91; http://dx.doi.org/10.4161/mabs.19868; PMID: 22531450
- Sundaram S, Matathia A, Qian J, Zhang J, Hsieh MC, Liu T, et al. An innovative approach for the characterization of the isoforms of a monoclonal antibody product. MAbs 2011; 3:505 - 12; http://dx.doi.org/10.4161/mabs.3.6.18090; PMID: 22123057
- Mariotte D, Dupont B, Gervais R, Galais MP, Laroche D, Tranchant A, et al. Anti-cetuximab IgE ELISA for identification of patients at a high risk of cetuximab-induced anaphylaxis. MAbs 2011; 3:396 - 401; http://dx.doi.org/10.4161/mabs.3.4.16293; PMID: 21654207
- Daguet A, Watier H. 2nd Charles Richet et Jules Héricourt workshop: therapeutic antibodies and anaphylaxis; May 31-June 1, 2011, Tours, France. MAbs 2011; 3:417 - 21; http://dx.doi.org/10.4161/mabs.3.5.17485; PMID: 21857165
- Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, et al. Anti-galactose-α-1,3-galactose IgE from allergic patients does not bind α-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol 2011; 29:574 - 6; http://dx.doi.org/10.1038/nbt.1912; PMID: 21747378
- Wang C, He X, Zhou B, Li J, Li B, Qian W, et al. Phase 1 study of anti-epidermal growth factor receptor monoclonal antibody in patients with solid tumors. MAbs 2011; 3:67 - 75; http://dx.doi.org/10.4161/mabs.3.1.14021; PMID: 21051930
- Beck A, -Cianférani S, Van Dorsselaer A. Biosimilar, biobetter and next generation antibody characterization by mass spectrometry. Anal Chem 2012; In press http://dx.doi.org/10.1021/ac3002885; PMID: 22510259
- Pacis E, Yu M, Autsen J, Bayer R, Li F. Effects of cell culture conditions on antibody N-linked glycosylation-what affects high mannose 5 glycoform. Biotechnol Bioeng 2011; In press http://dx.doi.org/10.1002/bit.23200; PMID: 21557201
- Beck A, Cochet O, Wurch T. GlycoFi’s technology to control the glycosylation of recombinant therapeutic proteins. Expert Opin Drug Discov 2010; 5:95 - 111; http://dx.doi.org/10.1517/17460440903413504
- Cérutti M, Golay J. Lepidopteran cells, an alternative for the production of recombinant antibodies?. MAbs 2012; 4:294 - 309; http://dx.doi.org/10.4161/mabs.19942; PMID: 22531440
- Fischer R, Schillberg S, Hellwig S, Twyman RM, Drossard J. GMP issues for recombinant plant-derived pharmaceutical proteins. Biotechnol Adv 2012; 30:434 - 9; http://dx.doi.org/10.1016/j.biotechadv.2011.08.007; PMID: 21856403
- Goetze AM, Schenauer MR, Flynn GC. Assessing monoclonal antibody product quality attribute criticality through clinical studies. MAbs 2010; 2:500 - 7; http://dx.doi.org/10.4161/mabs.2.5.12897; PMID: 20671426
- Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011; 21:949 - 59; http://dx.doi.org/10.1093/glycob/cwr027; PMID: 21421994
- Alessandri L, Ouellette D, Acquah A, Rieser M, LeBlond D, Saltarelli M, et al. Increased serum clearance of oligomannose species present on a human IgG1 molecule. MAbs 2012; In press
- Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 2010; 10:345 - 52; http://dx.doi.org/10.1038/nri2747; PMID: 20414207
- Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999; 17:176 - 80; http://dx.doi.org/10.1038/6179; PMID: 10052355
- Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci 2009; 100:1566 - 72; http://dx.doi.org/10.1111/j.1349-7006.2009.01222.x; PMID: 19538497
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 2004; 87:614 - 22; http://dx.doi.org/10.1002/bit.20151; PMID: 15352059
- Olivier S, Jacoby M, Brillon C, Bouletreau S, Mollet T, Nerriere O, et al. EB66 cell line, a duck embryonic stem cell-derived substrate for the industrial production of therapeutic monoclonal antibodies with enhanced ADCC activity. MAbs 2010; 2:405 - 15; PMID: 20562528
- Saint-Jore-Dupas C, Faye L, Gomord V. From planta to pharma with glycosylation in the toolbox. Trends Biotechnol 2007; 25:317 - 23; http://dx.doi.org/10.1016/j.tibtech.2007.04.008; PMID: 17493697
- Gasdaska JR, Sherwood S, Regan JT, Dickey LF. An afucosylated anti-CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity and B-cell depletion and lower complement-dependent cytotoxicity than rituximab. Mol Immunol 2012; 50:134 - 41; http://dx.doi.org/10.1016/j.molimm.2012.01.001; PMID: 22305040
- Gramer MJ, Eckblad JJ, Donahue R, Brown J, Shultz C, Vickerman K, et al. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnol Bioeng 2011; 108:1591 - 602; http://dx.doi.org/10.1002/bit.23075; PMID: 21328321
- Hamilton SR, Davidson RC, Sethuraman N, Nett JH, Jiang Y, Rios S, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science 2006; 313:1441 - 3; http://dx.doi.org/10.1126/science.1130256; PMID: 16960007
- Lugovskoy AA, Reichert JM, Beck A. 7th Annual European Antibody Congress 2011: November 29-December 1, 2011, Geneva, Switzerland. MAbs 2012; 4:134 - 52; http://dx.doi.org/10.4161/mabs.4.2.19426; PMID: 22453093
- Decker EL, Reski R. Glycoprotein production in moss bioreactors. Plant Cell Rep 2012; 31:453 - 60; http://dx.doi.org/10.1007/s00299-011-1152-5; PMID: 21960098
- Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. MAbs 2009; 1:230 - 6; http://dx.doi.org/10.4161/mabs.1.3.8328; PMID: 20065644
- Oflazoglu E, Audoly LP. Evolution of anti-CD20 monoclonal antibody therapeutics in oncology. MAbs 2010; 2:14 - 9; http://dx.doi.org/10.4161/mabs.2.1.10789; PMID: 20081379
- Zhang N, Liu L, Dumitru CD, Cummings NR, Cukan M, Jiang Y, et al. Glycoengineered Pichia produced anti-HER2 is comparable to trastuzumab in preclinical study. MAbs 2011; 3:289 - 98; http://dx.doi.org/10.4161/mabs.3.3.15532; PMID: 21487242
- Sehn LH, Assouline SE, Stewart DA, Mangel J, Gascoyne RD, Fine G, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood 2012; 119:5118 - 25; http://dx.doi.org/10.1182/blood-2012-02-408773; PMID: 22438256