Abstract
The polymorphic fungus Candida albicans is a member of the normal human microbiome. In most individuals, C. albicans resides as a lifelong, harmless commensal. Under certain circumstances, however, C. albicans can cause infections that range from superficial infections of the skin to life-threatening systemic infections. Several factors and activities have been identified which contribute to the pathogenic potential of this fungus. Among them are molecules which mediate adhesion to and invasion into host cells, the secretion of hydrolases, the yeast-to-hypha transition, contact sensing and thigmotropism, biofilm formation, phenotypic switching and a range of fitness attributes. Our understanding of when and how these mechanisms and factors contribute to infection has significantly increased during the last years. In addition, novel virulence mechanisms have recently been discovered. In this review we present an update on our current understanding of the pathogenicity mechanisms of this important human pathogen.
Introduction
The total number of eukaryotic species on Earth has recently been estimated at 8.7 million, with fungi making up approximately 7% (611,000 species) of this number.Citation1 Of all fungi, only around 600 species are human pathogens.Citation2 This relatively small group encompasses fungi that cause relatively mild infections of the skin (e.g., dermatophytes and Malassezia species), fungi that cause severe cutaneous infections (e.g., Sporotrix schenkii) and fungi that have the potential to cause life-threatening systemic infections (e.g., Aspergillus fumigatus, Cryptococcus neoformans, Histoplasma capsulatum, and Candida albicans). Indeed, Candida spp are the fourth most common cause of hospital-acquired systemic infections in the United States with crude mortality rates of up to 50%.Citation3,Citation4 C. albicans can cause two major types of infections in humans: superficial infections, such as oral or vaginal candidiasis, and life-threatening systemic infections (for a comprehensive description of C. albicans infections see the second edition of Candida and CandidiasisCitation5).
C. albicans and to a lesser extent other Candida species are present in the oral cavity of up to 75% of the population.Citation6 In healthy individuals this colonization generally remains benign. However, mildly immunocompromised individuals can frequently suffer from recalcitrant infections of the oral cavity. These oral infections with Candida species are termed “oral candidiasis” (OC).Citation6 Such infections are predominantly caused by C. albicans and can affect the oropharynx and/or the esophagus of persons with dysfunctions of the adaptive immune system. Indeed, HIV is a major risk factor for developing OC. Further risk factors for developing OC include the wearing of dentures and extremes of age.Citation7
It is estimated that approximately 75% of all women suffer at least once in their lifetime from vulvovaginal candidiasis (VVC), with 40–50% experiencing at least one additional episode of infection.Citation8,Citation9 A small percentage of women (5–8%) suffer from at least four recurrent VVC per year.Citation10 Predisposing factors for VVC are less well defined than for OC and include diabetes mellitus, use of antibiotics, oral contraception, pregnancy and hormone therapy.Citation11 Despite their frequency and associated morbidity, superficial C. albicans infections are non-lethal. In stark contrast, systemic candidiasis is associated with a high crude mortality rate, even with first line antifungal therapy.Citation3,Citation4,Citation12 Both neutropenia and damage of the gastrointestinal mucosa are risk factors for the development of experimental systemic (disseminated) candidiasis.Citation13 Further risk factors include central venous catheters, which allow direct access of the fungus to the bloodstream, the application of broad-spectrum antibacterials, which enable fungal overgrowth, and trauma or gastrointestinal surgery, which disrupts mucosal barriers.Citation14
During both superficial and systemic infection, C. albicans relies on a battery of virulence factors and fitness attributes. The major factors and fitness traits are discussed below.
Pathogenicity Mechanisms
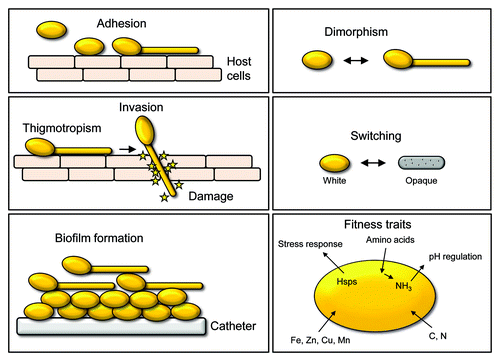
The ability of C. albicans to infect such diverse host niches is supported by a wide range of virulence factors and fitness attributes. A number of attributes, including the morphological transition between yeast and hyphal forms, the expression of adhesins and invasins on the cell surface, thigmotropism, the formation of biofilms, phenotypic switching and the secretion of hydrolytic enzymes are considered virulence factors. Additionally, fitness attributes include rapid adaptation to fluctuations in environmental pH, metabolic flexibility, powerful nutrient acquisition systems and robust stress response machineries ().Citation15
Figure 1. An overview of selected C. albicans pathogenicity mechanisms. Yeast cells adhere to host cell surfaces by the expression of adhesins. Contact to host cells triggers the yeast-to-hypha transition and directed growth via thigmotropism. The expression of invasins mediates uptake of the fungus by the host cell through induced endocytosis. Adhesion, physical forces and secretion of fungal hydrolases has been proposed to facilitate the second mechanism of invasion, i.e., fungal-driven active penetration into host cells by breaking down barriers. The attachment of yeast cells to abiotic (e.g., catheters) or biotic (host cells) surfaces can give rise to the formation of biofilms with yeast cells in the lower part and hyphal cells in the upper part of the biofilm. Phenotypic plasticity (switching) has been proposed to influence antigenicity and biofilm formation of C. albicans. In addition to these virulence factors, several fitness traits influence fungal pathogenicity. They include a robust stress response mediated by heat shock proteins (Hsps); auto-induction of hyphal formation through uptake of amino acids, excretion of ammonia (NH3) and concomitant extracellular alkalinization; metabolic flexibility and uptake of different compounds as carbon (C) and nitrogen (N) sources; and uptake of essential trace metals, e.g., iron (Fe), zinc (Zn), copper (Cu) and manganese (Mn).
Polymorphism
C. albicans is a polymorphic fungus that can grow either as ovoid-shaped budding yeast, as elongated ellipsoid cells with constrictions at the septa (pseudohyphae) or as parallel-walled true hyphae.Citation16 Further morphologies include white and opaque cells, formed during switching, and chlamydospores, which are thick-walled spore-like structures.Citation17 While yeast and true hyphae are regularly observed during infection and have distinct functions (as discussed below), the role of pseudohyphae and switching in vivo is rather unclear and chlamydospores have not been observed in patient samples.Citation18,Citation19
A range of environmental cues affect C. albicans morphology. For example, at low pH (< 6) C. albicans cells predominantly grow in the yeast form, while at a high pH (> 7) hyphal growth is induced.Citation20 Indeed, a number of conditions, including starvation, the presence of serum or N-acetylglucosamine, physiological temperature and CO2 promote the formation of hyphae.Citation21 Morphogenesis has also been shown to be regulated by quorum sensing, a mechanism of microbial communication.Citation22 In C. albicans, the main quorum sensing molecules include farnesol, tyrosol and dodecanol.Citation23-Citation25 Due to quorum sensing, high cell densities (> 107 cells ml−1) promote yeast growth, while low cell densities (< 107 cells ml−1) favor hyphal formation.
The transition between yeast and hyphal growth forms is termed dimorphism and it has been proposed that both growth forms are important for pathogenicity.Citation26 The hyphal form has been shown to be more invasive than the yeast form.Citation16 On the other hand the smaller yeast form is believed to represent the form primarily involved in dissemination.Citation27
Mutants that are unable to form hyphae under in vitro conditions are generally attenuated in virulence.Citation28 However, hypha formation is linked to the expression of a subset of genes encoding virulence factors that are not involved in hyphal formation per se. Such hypha-associated proteins include the hyphal wall protein Hwp1, the agglutinin-like sequence protein Als3, the secreted aspartic proteases Sap4, Sap5 and Sap6 and the hypha-associated proteins Ece1 and Hyr1. Deletion of HGC1, which encodes a hypha-specific G1 cyclin-related protein, results in cells that grow normally in the yeast form but fail to produce hyphae. Nevertheless, the hgc1Δ/Δ mutant cells still express at least four hypha-associated genes (HWP1, ECE1, HYR1 and ALS3).Citation29,Citation30 The finding that an hgc1Δ/Δ mutant was attenuated in a mouse model of systemic infection, supported the view that hyphal formation per se is an important virulence attribute.Citation29
Adhesins and invasins
C. albicans has a specialized set of proteins (adhesins) which mediate adherence to other C. albicans cells to other microorganisms, to abiotic surfaces and to host cells.Citation31,Citation32 Arguably the best studied C. albicans adhesins are the agglutinin-like sequence (ALS) proteins which form a family consisting of eight members (Als1–7 and Als9). The ALS genes encode glycosylphosphatidylinositol (GPI)-linked cell surface glycoproteins. Of the eight Als proteins, the hypha-associated adhesin Als3 is especially important for adhesion.Citation33-Citation35ALS3 gene expression is upregulated during infection of oral epithelial cells in vitro and during in vivo vaginal infection.Citation36-Citation39 Another important adhesin of C. albicans is Hwp1, which is a hypha-associated GPI-linked protein.Citation33,Citation40,Citation41 Hwp1 serves as a substrate for mammalian transglutaminases and this reaction may covalently link C. albicans hyphae to host cells. An hwp1Δ/Δ mutant displayed reduced adherence to buccal epithelial cells and displayed attenuated virulence in a mouse model of systemic candidiasis.Citation40,Citation42,Citation43
Hwp1 and Als3 were also demonstrated to contribute to biofilm formation by acting as complementary adhesins.Citation44
Morphology-independent proteins can also contribute to adhesion. These include GPI-linked proteins (Eap1, Iff4 and Ecm33), non-covalent wall-associated proteins (Mp65, a putative β-glucanase, and Phr1, a β-1,3 glucanosyl transferase), cell-surface associated proteases (Sap9 and Sap10) and the integrin-like surface protein Int1.Citation38,Citation45
C. albicans is a remarkable pathogen as it can utilize two different mechanisms to invade into host cells: induced endocytosis and active penetration.Citation37,Citation38,Citation45,Citation46 For induced endocytosis, the fungus expresses specialized proteins on the cell surface (invasins) that mediate binding to host ligands (such as E-cadherin on epithelial cellsCitation34 and N-cadherin on endothelial cellsCitation47), thereby triggering engulfment of the fungal cell into the host cell. Indeed, even killed hyphae are taken up, indicating that induced endocytosis is a passive process that does not require the activities of viable fungal cells.Citation46,Citation48 Two invasins have been identified so far, namely Als3 (which also functions as an adhesin, see above) and Ssa1.Citation34,Citation49 Ssa1 is a cell-surface expressed member of the heat shock protein 70 (Hsp70) family. Both als3Δ/Δ and ssa1Δ/Δ mutants exhibited reduced epithelial adherence and invasion, and reduced virulence in a murine model of oropharyngeal candidiasis.Citation38,Citation49 Als3 and Ssa1 bind to host E-cadherin and likely induce endocytosis by a clathrin-dependent mechanism; however, macropinocytosis has also been implicated in C. albicans induced endocytosis.Citation34,Citation46 In contrast, active penetration is a fungal-driven process and requires viable C. albicans hyphae.Citation36,Citation46 It is still unclear exactly which factors mediate this second route of invasion into host cells. Fungal adhesion and physical forces are believed to be crucial.Citation36 Secreted aspartic proteases (Saps) have also been proposed to contribute to active penetration. Lipases and phospholipases, on the other hand, have not been shown to contribute to this process.Citation38,Citation46
In summary, invasion into host cells by C. albicans relies on two likely complementary mechanisms: induced endocytosis mediated by Als3 and Ssa1 and active penetration mediated by yet undefined molecular mechanisms.
Biofilm formation
A further important virulence factor of C. albicans is its capacity to form biofilms on abiotic or biotic surfaces. Catheters, dentures (abiotic) and mucosal cell surfaces (biotic) are the most common substrates.Citation50 Biofilms form in a sequential process including adherence of yeast cells to the substrate, proliferation of these yeast cells, formation of hyphal cells in the upper part of the biofilm, accumulation of extracellular matrix material and, finally, dispersion of yeast cells from the biofilm complex.Citation51 Mature biofilms are much more resistant to antimicrobial agents and host immune factors in comparison to planktonic cells.Citation50,Citation51 The factors responsible for heightened resistance include the complex architecture of biofilms, the biofilm matrix, increased expression of drug efflux pumps and metabolic plasticity.Citation50 Dispersion of yeast cells from the mature biofilm has been shown to directly contribute to virulence, as dispersed cells were more virulent in a mouse model of disseminated infection.Citation52 The major heat shock protein Hsp90 was recently identified as a key regulator of dispersion in C. albicans biofilms.Citation53 In addition, Hsp90 was also required for biofilm antifungal drug resistance.Citation53
Several transcription factors control biofilm formation. These include the transcription factors Bcr1, Tec1 and Efg1.Citation50 In a recent study, Nobile et al. investigated the transcriptional network regulating biofilm formation and identified further, previously unknown regulators of biofilm production.Citation54 These novel factors include Ndt80, Rob1 and Brg1. Deletion of any of these regulators (BCR1, TEC1, EFG1, NDT80, ROB1 or BRG1) resulted in defective biofilm formation in in vivo rat infection models.Citation54
Extracellular matrix production is controlled by additional factors. The zinc-responsive transcription factor Zap1 negatively regulates β-1,3 glucan, the major component of biofilm matrix.Citation55 Glucoamylases (Gca1 and Gca2), glucan transferases (Bgl2 and Phr1) and the exo-glucanase, Xog1, are positive regulators of β-1,3 glucan production.Citation55,Citation56 While expression of GCA1 and GCA2 are controlled by Zap1, the enzymes Bgl2, Phr1 and Xog1 function independently of this key negative regulator.Citation56 Biofilms formed by mutants lacking BGL2, PHR1 or XOG1 were shown to be more susceptible to the antifungal agent, fluconazole, both in vitro and in vivo.Citation56 Furthermore, recent studies indicate that C. albicans biofilms are resistant to killing by neutrophils and do not trigger production of reactive oxygen species (ROS).Citation57 Evidence suggests that β-glucans in the extracellular matrix protect C. albicans from these attacks.Citation57
Contact sensing and thigmotropism
An important environmental cue that triggers hypha and biofilm formation in C. albicans (see above) is contact sensing. Upon contact with a surface, yeast cells switch to hyphal growth.Citation58 On certain substrates, such as agar or mucosal surfaces, these hyphae can then invade into the substratum. Contact to solid surfaces also induces the formation of biofilms.Citation58 On surfaces with particular topologies (such as the presence of ridges) directional hyphal growth (thigmotropism) may occur.Citation59
Brand et al. demonstrated that thigmotropism of C. albicans hyphae is regulated by extracellular calcium uptake through the calcium channels Cch1, Mid1 and Fig1.Citation59 Additional mechanisms include the polarisome Rsr1/Bud1-GTPase module.Citation60 Brand et al. also provided evidence that C. albicans thigmotropism is required for full damage of epithelial cells and normal virulence in mice.Citation61
Therefore, the correct sensing and response to both abiotic (biofilm formation) and biotic (invasion) surfaces is important for pathogenicity.
Secreted hydrolases
Following adhesion to host cell surfaces and hyphal growth, C. albicans hyphae can secrete hydrolases, which have been proposed to facilitate active penetration into these cells.Citation62 In addition, secreted hydrolases are thought to enhance the efficiency of extracellular nutrient acquisition.Citation63 Three different classes of secreted hydrolases are expressed by C. albicans: proteases, phospholipases and lipases.
The family of secreted aspartic proteases (Saps) comprises ten members, Sap1–10. Sap1–8 are secreted and released to the surrounding medium, whereas Sap9 and Sap10 remain bound to the cell-surface.Citation63-Citation65 Sap1–3 have been shown to be required for damage of reconstituted human epithelium (RHE) in vitro, and for virulence in a mouse model of systemic infection.Citation66,Citation67 However, the relative contribution of Saps to C. albicans pathogenicity is controversial. Recent results indicate that Saps are not required for invasion into RHE and that Sap1–6 are dispensable for virulence in a mouse model of disseminated candidiasis.Citation68,Citation69
However, the observed expansion of Sap-encoding genes in C. albicans compared with its less pathogenic relatives suggests a role for these proteases in virulence.Citation70 Indeed, the large size of the Sap family itself makes it likely that a certain degree of functional redundancy may exist.
The family of phospholipases consists of four different classes (A, B, C and D).Citation71 Only the five members of class B (PLB1–5) are extracellular and may contribute to pathogenicity via disruption of host membranes.Citation72 Both plb1Δ/Δ and plb5Δ/Δ mutants have been shown to be attenuated in virulence in a mouse model of systemic infection.Citation73,Citation74
The third family of secreted hydrolases, the lipases, consists of 10 members (LIP1–10).Citation75,Citation76 A lip8Δ/Δ mutant had reduced virulence in a mouse model of systemic infection, supporting a role for these extracellular hydrolases in C. albicans pathogenicity.Citation77
pH-sensing and regulation
In the human host, C. albicans is exposed to a surrounding pH ranging from slightly alkaline to acidic.Citation78 Additionally, depending on the host niche, the environmental pH can be very dynamic. Therefore, C. albicans must be able to adapt to changes in pH.Citation78 The pH of human blood and tissues is slightly alkaline (pH 7.4), while the pH of the digestive tract ranges from very acidic (pH 2) to more alkaline (pH 8), and the pH of the vagina is around pH 4.Citation78 Neutral to alkaline pH can cause severe stress to C. albicans, including malfunctioning of pH-sensitive proteins, and impaired nutrient acquisition (as a consequence of a disrupted proton gradient).Citation78 Among the first proteins identified as being important for adaptation to changing pH were the two cell wall β-glycosidases Phr1 and Phr2.Citation79 PHR1 is expressed at neutral-alkaline pH. In contrast, PHR2 is mainly expressed at acidic pH.Citation80 Correspondingly, Phr1 is required for systemic infections, and Phr2 is essential for infections of the vagina.Citation81
C. albicans senses pH via the Rim101 signal transduction pathway. In this pathway, environmental pH is gauged by the plasma membrane receptors Dfg16 and Rim21.Citation78 Activation of these receptors leads to induction of a signaling cascade, finally leading to activation of the major pH-responsive transcription factor Rim101 via its proteolytic cleavage. Rim101 then enters the nucleus and mediates pH-dependent responses.Citation78 A dfg16Δ/Δ mutant had reduced virulence in a mouse infection model of systemic candidiasis,Citation82 and a rim20Δ/Δ mutant had attenuated virulence in a mouse corneal infection model.Citation83,Citation84 Finally, a rim101Δ/Δ mutant had reduced virulence in both a systemic mouse model of hematogenously disseminated candidiasisCitation85 and a murine model of oropharyngeal candidiasis.Citation86 Together these data demonstrate that the Rim101 pathway, and pH sensing in general, are critical for C. albicans virulence.
C. albicans is not only able to sense and adapt to environmental pH, but can also modulate extracellular pH, actively alkalinizing its surrounding environment under nutrient starvation and, thereby, autoinducing hypha formation.Citation87,Citation88 The molecular mechanisms underlying this are beginning to be uncovered and appear to involve the uptake of amino acids and probably other amine-containing molecules, such as polyamines, in the absence of glucose. C. albicans then cleaves these substrates intracellularly with the urea amidolyase Dur1,2, and exports the resulting ammonia through the Ato (ammonia transport outward) export proteins. The extrusion of ammonia leads to an alkalinization of the extracellular milieu, which in turn promotes hyphal morphogenesis.Citation87 Hyphal formation itself is considered a key virulence factor of C. albicans as non-filamentous mutants are attenuated in virulence (see above).Citation28 Therefore, C. albicans senses, adapts to and, strikingly, also actively modulates extracellular pH. All these features contribute to its remarkable capacity to co-exist as a commensal, and to prevail as a fungal pathogen in humans.
Metabolic adaptation
Nutrition is a central and fundamental prerequisite for survival and growth of all living organisms. Metabolic adaptability mediates the effective assimilation of alternative nutrients in dynamic environments.Citation89 This metabolic flexibility is particularly important for pathogenic fungi during infection of different host niches.Citation90,Citation91 Glycolysis, gluconeogenesis, and starvation responses are all thought to contribute to host colonization and pathogenesis, but their specific contribution may be highly niche-specific and is still only partially understood. In healthy individuals C. albicans is predominantly found as part of the gastrointestinal microbiome. Although the concentration of nutrients in this environment can be naturally high, growth of the fungus is believed to be controlled through competition with other members of the intestinal microbial flora.Citation90 During disseminated candidiasis in susceptible individuals, C. albicans gains access to the bloodstream. Blood is relatively rich in glucose (6–8 mM),Citation90 the preferred nutrient source of most fungi. However, phagocytic cells (macrophages and neutrophils) can efficiently phagocytose C. albicans. Once inside a macrophage or neutrophil, however, the nutritional environment completely changes for the fungus. Not only does the phagocyte produce highly reactive intermediates like ROS, reactive nitrogen species (RNS) and antimicrobial peptides (AMPs), it also restricts the availability of nutrients, thereby creating an environment of nutrient starvation.Citation92 Prompt and efficient metabolic plasticity is therefore required for adaptation of C. albicans to such a hostile host milieu. Inside macrophages, the fungus initially switches from glycolysis to gluconeogenesis and a starvation response (activation of the glyoxylate cycle). Lipids and amino acids are proposed to serve as nutrient sources within macrophages.Citation93
In addition to metabolic flexibility, the fungus has also evolved ways to escape from macrophages by inhibiting the production of antimicrobial effectors and inducing hyphal formation. Hyphae formed inside phagocytic cells can pierce through the host immune cell by mechanical forces and can permit escape.Citation93,Citation94 During systemic candidiasis, fungal cells can disseminate to virtually every organ within the human host, each with potentially different availability of nutrients. In the liver for example, C. albicans has access to large quantities of glycogen, the main storage molecule of glucose. The brain has high concentrations of glucose and vitamins as potential nutrient sources.Citation91 In other tissues, C. albicans faces relatively poor glucose concentrations and uses alternative metabolic pathways to utilize host proteins, amino acids, lipids and phospholipids. The fungus can use secreted proteases (see above) to hydrolyse host proteins. It was recently shown that adaptation to different nutrient sources by C. albicans not only promotes survival and growth, but also affects virulence.Citation95 Growth on alternative carbon sources, such as lactate or amino acids, rendered the fungus more resistant to environmental stresses and increased its virulence potential in both a mouse model of systemic candidiasis, and a murine vaginal infection model.Citation95 Furthermore, the glyoxylate cycle has been shown to be required for full virulence in C. albicans.Citation96 Uptake of amino acids, and likely also polyamines, affects the virulence of C. albicans by allowing the fungus to autoinduce hypha formation through extracellular alkalinization ().Citation87,Citation88
In summary, during infection the main nutrient sources for C. albicans are likely to be host-derived glucose, lipids, proteins and amino acids, depending on the anatomical niche. Besides being able to use these different nutrients individually, the ability of C. albicans to rapidly and dynamically respond to host and pathogen-induced changes in micro-environmental nutrient availability contributes to its success as a pathogen.
Environmental stress response
A robust stress response contributes to the survival and virulence of C. albicans by facilitating the adaptation of the fungus to changing conditions and protecting it against host-derived stresses. Phagocytic cells of the immune system produce oxidative and nitrosative stresses. pH-stress occurs, for example, in the gastrointestinal and urogenital tract.Citation89 Stress-responsive regulatory pathways, as well as downstream targets, were shown to be essential not only for efficient stress adaptation, but also for full virulence of the fungus.Citation89 In fact, several mutants lacking genes encoding regulators of stress response or detoxifying enzymes are attenuated in virulence. Cellular responses to stresses include heat shock-, osmotic-, oxidative- and nitrosative-stress responses.Citation89
The heat shock response is mediated by heat shock proteins (see below) which act as molecular chaperones to prevent deleterious protein unfolding and aggregation. Additionally, thermal stress leads to trehalose accumulation in C. albicans, which is thought to act as a “chemical chaperone” by stabilizing proteins prone to unfolding.Citation89 However, the exact function of trehalose accumulation following thermal insults remains unknown.
The osmotic stress response results in intracellular accumulation of the compatible solute glycerol to counteract loss of water due to the outward-directed chemical gradient. Glycerol biosynthesis is mediated by the glycerol 3-phosphatase Gpp1 and the glycerol 3-phosphate dehydrogenase Gpd2.Citation36 Both gpp1Δ/Δ and gpd2Δ/Δ mutants were shown to have reduced capacity to damage oral epithelial cells in vitro. However, this was probably due to an inability to generate hyphal turgor pressure and mechanical forces (see above) rather than heightened sensitivity to osmotic stress in this infection model.Citation36
Reactive oxygen species (ROS), such as peroxide, superoxide anions, and hydroxyl radicals, induce an oxidative stress response.Citation89 Catalase Cta1 and superoxide dismutases, Sod1 and Sod5, are crucial for efficient detoxification of ROS in C. albicans, and are required for full virulence in mouse models of systemic candidiasis.Citation97-Citation99
Neutrophils also produce reactive nitrogen species (RNS), which induce a nitrosative stress response in phagocytosed C. albicans cells. The major protein implicated in detoxification of RNS is the flavohemoglobin-related protein Yhb1. Deletion of Yhb1 renders C. albicans cells sensitive to RNS and attenuates virulence in a mouse model of systemic candidiasis.Citation100
In fungi, environmental signals, including stress signals, are sensed and transmitted by mitogen-activated protein (MAP) kinase pathways through sequential phosphorylation events.Citation101 The three main MAP kinase signaling pathways in C. albicans are the Mkc1-, Hog1- and Cek1-MAP kinase pathway.Citation101
The Mkc1 (MAP kinase from C. albicans) pathway is primarily involved in maintaining cellular integrity, cell wall biogenesis, invasive growth under embedded conditions and biofilm formation.Citation101 Mkc1 is activated upon oxidative and osmotic stress conditions.
The Hog1 (High osmolarity glycerol response) pathway mediates the response to osmotic, oxidative and thermal stress, morphogenesis and cell wall formation.Citation101 Under osmotic stress, activated Hog1 leads to glycerol accumulation.Citation101
The Cek1 (Candida ERK-like kinase) pathway mediates filamentation, mating and likely also adaptation to thermal stress.Citation101,Citation102 Mutants in all three pathways (mkc1∆, hog1∆ or cek1∆) were all attenuated in virulence in mouse infection models, highlighting the importance of the stress response during infection.Citation103-Citation105
In addition to the sequential cascade of activation in the three MAP kinase pathways, environmental signals also trigger crosstalk between these pathways. For example, activated Hog1 both represses the Cek1-pathway and activates the Mkc1 pathway.Citation101 Moreover, certain signals, like oxidative or osmotic stress are sensed by more than one pathway. This interweaved MAP kinase sensing network probably engenders fine-tuning of a robust adaptive response.
Heat shock proteins
The heat shock response is a conserved reaction of living organisms to stressful conditions such as high temperature, starvation and oxidative stress.Citation106,Citation107 Such stresses can induce protein unfolding and nonspecific protein aggregation, ultimately leading to cell death. In order to prevent this detrimental fate, cells produce heat shock proteins (Hsps).Citation106 These specialized proteins act as chaperones and prevent protein unfolding and aggregation by binding to their clients and stabilizing them.Citation108 Six major Hsps have been identified in C. albicans: Hsp104, Hsp90, Hsp78, two Hsp70 proteins (Ssa1 and Ssa2) and Hsp60. HSP104 encodes a Hsp required for proper biofilm formation, and virulence in a Caenorhabditis elegans infection model.Citation109 Hsp90 is a major Hsp in C. albicans and regulates drug resistance, morphogenesis, biofilm formation and virulence.Citation53,Citation110-Citation113 HSP78 encodes an uncharacterized Hsp that is transcriptionally upregulated in response to phagocytosis by macrophages.Citation93 The two C. albicans Hsp70 family members, Ssa1 and Ssa2 (stress-70 subfamily A), are expressed on the cell surface and function as receptors for antimicrobial peptides, e.g., Ssa2 binds histatin 5.Citation114-Citation116 An ssa2Δ/Δ mutant had increased resistance to histatin 5, but was dispensable for virulence in mouse models of disseminated and oropharyngeal candidiasis.Citation49,Citation114,Citation116 Ssa1 also acts as an invasin.Citation49 An ssa1Δ/Δ mutant had attenuated virulence in mouse models of both disseminated and oropharyngeal candidiasis.Citation49 Finally, HSP60 encodes a putative mitochondrial Hsp of unknown function. An hsp60Δ/HSP60 heterozygous mutant has increased sensitivity to elevated temperatures, indicating that Hsp60 might be required for thermal stress tolerance.Citation117
Expression of Hsps is mainly controlled by the transcription factor heat shock factor 1 (Hsf1).Citation118,Citation119 Hsf1 is phosphorylated in response to heat stress and induces transcription of Hsp-encoding genes via binding to heat shock elements (HSEs) in their promoters.Citation119 C. albicans Hsf1 is essential for viability and a mutant that is unable to activate Hsf1 displays attenuated virulence in a mouse model of systemic candidiasis.Citation15
Small heat shock proteins
In addition to the above mentioned heat shock proteins, six small Hsps (sHsps) have also been identified in C. albicans.Citation120 sHsps are low-molecular-mass chaperones that prevent protein aggregation.Citation121,Citation122 Upon heat, or other forms of stress, cells express sHSPs which transition from an oligomeric to a multimeric state and bind aggregated proteins.Citation123 In these chaperone-aggregate complexes, client proteins are held ready for disaggregation and refolding by other major Hsps, such as Hsp104.Citation124
C. albicans is predicted to encode six sHsps: Hsp31, Hsp30, Hsp21, two Hsp12 proteins and Hsp10.Citation120 As yet only Hsp12 and Hsp21 have been investigated. Hsp12 is expressed in response to different stresses, including heat shock and oxidative stress. Deletion of both HSP12 genes did not influence virulence of C. albicans in a Drosophila infection model.Citation125 It should be noted, however, that the fly infection experiment was performed at 30°C, and it remains to be investigated how the mutant behaves at physiological temperature in a mammalian host.
We recently investigated the function of Hsp21 and showed that this sHsp is crucial for regulation of intracellular levels of trehalose. Deletion of HSP21 resulted in impaired thermotolerance, enhanced sensitivity toward oxidative stress, and strongly attenuated virulence in a mouse model of systemic candidiasis.Citation102 Importantly, Hsp21 is not found in humans. These results indicate that sHsps can act as virulence factors and might represent attractive drug targets.
Metal acquisition
Trace metals are essential for the growth and survival of all living organisms including humans, animals, plants, bacteria and fungi. Among the most important metals are iron, zinc, manganese and copper, all of which are essential for the proper function of a large number of proteins and enzymes. Pathogenic microorganisms, as well as their respective hosts, have evolved elaborate mechanisms to acquire or restrict access to these metals.Citation126
To date, the most widely investigated transition metal with regard to pathogenesis is iron. C. albicans acquires this metal by different strategies, including a reductive system, a siderophore uptake system and a heme-iron uptake system.Citation127 The reductive system mediates iron acquisition from host ferritin, transferrin or the environment. The adhesin and invasin Als3 (see above) was shown to be the receptor for ferritin.Citation30 Despite the fact that an als3Δ/Δ mutant had normal virulence in a mouse infection model of disseminated candidasis,Citation128 deletion of ALS3 resulted in reduced capacity to damage oral epithelial host cells in vitro, suggesting that Als3-mediated iron acquisition from host ferritin contributes to iron acquisition depending on the stage of the infection.Citation30,Citation35 Although C. albicans does not synthesize its own siderophores, the fungus uses an uptake system to steal iron from siderophores produced by other microorganisms, also known as xeno-siderophores. The only described siderophore transporter in C. albicans is Sit1. A sit1Δ/Δ mutant exhibited normal virulence in a mouse model of disseminated candidiasis. However, the mutant was strongly impaired in its capacity to damage ex vivo human keratinocyte tissue.Citation129 Finally, the heme-iron uptake system promotes iron acquisition from hemoglobin and heme-proteins and is mediated by the heme-receptor gene family members RBT5, RBT51, CSA1, CSA2 and PGA7 (RBT6).Citation127,Citation130 An rbt5Δ/Δ mutant has normal virulence in mice, however, this may be due to functional redundancy.Citation127,Citation131 The role of the other four heme-binding proteins (Rbt51, Csa1, Csa2 and Pga7) in virulence has not yet been investigated.
Zinc is the second most abundant metal in most living organisms.Citation126 Our group has recently uncovered a previously undescribed mechanism of zinc acquisition by C. albicans.Citation132 The fungus secretes the zinc-binding protein Pra1 (pH-regulated antigen 1), which, analogous to siderophore-mediated iron acquisition, acts as a zincophore by binding extracellular zinc and re-associating with the fungal cell. Re-association of Pra1 is mediated by the zinc transporter Zrt1.Citation132 Despite enhanced virulence of a pra1Δ/Δ mutant in mice,Citation133 deletion of PRA1 strongly reduces the capacity of C. albicans to damage endothelial cells in vitro in the absence of exogenous zinc, suggesting that zinc acquisition plays an important role during certain steps of infection.Citation132 Copper and manganese are also essential for fungal growth; however, the mechanisms by which C. albicans acquires these metals is currently poorly understood. A putative manganese transporter, Ccc1,Citation120 and a copper transporter, Ctr1,Citation134 have been identified, although their roles in virulence have not yet been determined. Therefore, future studies are required to elucidate the role of these other essential metals in C. albicans pathogenicity.
Conclusions
Understanding the pathogenicity mechanisms that C. albicans uses during infection is crucial for the development of new antifungal therapies and diagnostics. Classically, antifungal drugs were designed to exert fungicidal activities, i.e., to kill the pathogenic microorganism. Recently however, specifically targeting virulence factors has been proposed as a new and promising antifungal strategy.Citation135 Several virulence factors, such as dimorphism, the secretion of proteases and the expression of adhesins and invasins, have been suggested as attractive targets,Citation26,Citation135 and recent investigations have further broadened our understanding of the C. albicans factors and activities which contribute to virulence. The heat shock response, including major and small heat shock proteins, has emerged as a promising drug target. Specifically, those Hsps which are unique to fungi and do not occur in humans (for example Hsp21) represent good candidates for specific drug targets. Another avenue of research is the interplay between host nutritional immunity and fungal nutrient acquisition systems. In particular, interfering with the iron, zinc, manganese or copper homeostasis mechanisms of pathogenic microorganisms may represent promising therapeutic strategies. As our detailed understanding of fungal pathogenicity mechanisms improves, the potential for developing novel therapeutic and diagnostic strategies expands.
| Abbreviations: | ||
| AMP | = | antimicrobial peptide |
| Hsp | = | heat shock protein |
| sHsp | = | small Hsp |
| RHE | = | reconstituted human oral epithelium |
| RNS | = | reactive nitrogen species |
| ROS | = | reactive oxygen species |
Acknowledgments
Our work on C. albicans pathogenicity mechanisms was supported as follows: F.L.M. and B.H. were supported by the International Leibniz Research School for Microbial and Biomolecular Interactions (ILRS) as part of the excellence graduate school Jena School for Microbial Communication (JSMC). D.W. and B.H. were supported by the ERA-NET PathoGenoMics Program (Candicol; BMBF 0315 901 B). B.H. was also supported by the Center for Sepsis Control and Care (CSCC; BMBF 01EO1002) and the Deutsche Forschungsgemeinschaft (DFG Hu 528/15, 16 and 17). We apologize to all colleagues in the field of Candida albicans research whose work we were unable to cite due to space limitations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on Earth and in the ocean?. PLoS Biol 2011; 9:e1001127; http://dx.doi.org/10.1371/journal.pbio.1001127; PMID: 21886479
- Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science 2012; 336:647; http://dx.doi.org/10.1126/science.1222236; PMID: 22582229
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1 - 53; http://dx.doi.org/10.3109/10408410903241444; PMID: 20088682
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133 - 63; http://dx.doi.org/10.1128/CMR.00029-06; PMID: 17223626
- Calderone RA, Clancy CJ. Candida and Candidiasis: ASM Press, Washington, DC, 2012.
- Ruhnke M. Skin and mucous membrane infections. In: Calderone RA, ed. Candida and Candidiasis: ASM Press, Washington, DC, pp. 307-325., 2002.
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr., Calandra TF, Edwards JE Jr., et al, Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503 - 35; http://dx.doi.org/10.1086/596757; PMID: 19191635
- Hurley R, De Louvois J. Candida vaginitis. Postgrad Med J 1979; 55:645 - 7; http://dx.doi.org/10.1136/pgmj.55.647.645; PMID: 523355
- Sobel JD. Vulvovaginal candidosis. Lancet 2007; 369:1961 - 71; http://dx.doi.org/10.1016/S0140-6736(07)60917-9; PMID: 17560449
- Foxman B, Marsh JV, Gillespie B, Sobel JD. Frequency and response to vaginal symptoms among white and African American women: results of a random digit dialing survey. J Womens Health 1998; 7:1167 - 74; http://dx.doi.org/10.1089/jwh.1998.7.1167; PMID: 9861594
- Fidel PL Jr.. History and new insights into host defense against vaginal candidiasis. Trends Microbiol 2004; 12:220 - 7; http://dx.doi.org/10.1016/j.tim.2004.03.006; PMID: 15120141
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 2007; 45:321 - 46; http://dx.doi.org/10.1080/13693780701218689; PMID: 17510856
- Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 2008; 4:e35; http://dx.doi.org/10.1371/journal.ppat.0040035; PMID: 18282097
- Spellberg B, Marr K, Filler SG. Candida: What Should Clinicians and Scientists Be Talking About? In: Calderone RA, Clancy, C.J., ed. Candida and Candidiasis: ASM Press, Washington, DC, pp. 225-242., 2012.
- Nicholls S, MacCallum DM, Kaffarnik FA, Selway L, Peck SC, Brown AJ. Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans.. Fungal Genet Biol 2011; 48:297 - 305; http://dx.doi.org/10.1016/j.fgb.2010.08.010; PMID: 20817114
- Berman J, Sudbery PE. Candida Albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet 2002; 3:918 - 30; http://dx.doi.org/10.1038/nrg948; PMID: 12459722
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans.. Trends Microbiol 2004; 12:317 - 24; http://dx.doi.org/10.1016/j.tim.2004.05.008; PMID: 15223059
- Staib P, Morschhäuser J. Chlamydospore formation in Candida albicans and Candida dubliniensis--an enigmatic developmental programme. Mycoses 2007; 50:1 - 12; http://dx.doi.org/10.1111/j.1439-0507.2006.01308.x; PMID: 17302741
- Soll DR. Why does Candida albicans switch?. FEMS Yeast Res 2009; 9:973 - 89; http://dx.doi.org/10.1111/j.1567-1364.2009.00562.x; PMID: 19744246
- Odds FC. Candida and Candidosis. second ed. Bailliere Tindall, London, United Kingdom, 1988.
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol 2011; 9:737 - 48; http://dx.doi.org/10.1038/nrmicro2636; PMID: 21844880
- Albuquerque P, Casadevall A. Quorum sensing in fungi--a review. Med Mycol 2012; 50:337 - 45; http://dx.doi.org/10.3109/13693786.2011.652201; PMID: 22268493
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 2001; 67:2982 - 92; http://dx.doi.org/10.1128/AEM.67.7.2982-2992.2001; PMID: 11425711
- Chen H, Fujita M, Feng Q, Clardy J, Fink GR. Tyrosol is a quorum-sensing molecule in Candida albicans.. Proc Natl Acad Sci U S A 2004; 101:5048 - 52; http://dx.doi.org/10.1073/pnas.0401416101; PMID: 15051880
- Hall RA, Turner KJ, Chaloupka J, Cottier F, De Sordi L, Sanglard D, et al. The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans.. Eukaryot Cell 2011; 10:1034 - 42; http://dx.doi.org/10.1128/EC.05060-11; PMID: 21666074
- Jacobsen ID, Wilson D, Wächtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 2012; 10:85 - 93; http://dx.doi.org/10.1586/eri.11.152; PMID: 22149617
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2003; 2:1053 - 60; http://dx.doi.org/10.1128/EC.2.5.1053-1060.2003; PMID: 14555488
- Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell 1997; 90:939 - 49; http://dx.doi.org/10.1016/S0092-8674(00)80358-X; PMID: 9298905
- Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J 2004; 23:1845 - 56; http://dx.doi.org/10.1038/sj.emboj.7600195; PMID: 15071502
- Almeida RS, Brunke S, Albrecht A, Thewes S, Laue M, Edwards JE, et al. the hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog 2008; 4:e1000217; http://dx.doi.org/10.1371/journal.ppat.1000217; PMID: 19023418
- Garcia MC, Lee JT, Ramsook CB, Alsteens D, Dufrêne YF, Lipke PN. A role for amyloid in cell aggregation and biofilm formation. PLoS One 2011; 6:e17632; http://dx.doi.org/10.1371/journal.pone.0017632; PMID: 21408122
- Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol 2006; 60:5 - 15; http://dx.doi.org/10.1111/j.1365-2958.2006.05072.x; PMID: 16556216
- Zordan R, Cormack B. Adhesins on Opportunistic Fungal Pathogens. In: Calderone RA, Clancy, C.J., ed. Candida and Candidiasis: ASM Press, Washington, DC, pp 243-259, 2012.
- Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 2007; 5:e64; http://dx.doi.org/10.1371/journal.pbio.0050064; PMID: 17311474
- Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, et al. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS One 2012; 7:e33362; http://dx.doi.org/10.1371/journal.pone.0033362; PMID: 22428031
- Wächtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One 2011; 6:e17046; http://dx.doi.org/10.1371/journal.pone.0017046; PMID: 21407800
- Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol 2007; 9:2938 - 54; http://dx.doi.org/10.1111/j.1462-5822.2007.01009.x; PMID: 17645752
- Naglik JR, Moyes DL, Wächtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect 2011; 13:963 - 76; http://dx.doi.org/10.1016/j.micinf.2011.06.009; PMID: 21801848
- Cheng G, Wozniak K, Wallig MA, Fidel PL Jr., Trupin SR, Hoyer LL. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect Immun 2005; 73:1656 - 63; http://dx.doi.org/10.1128/IAI.73.3.1656-1663.2005; PMID: 15731066
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 1999; 283:1535 - 8; http://dx.doi.org/10.1126/science.283.5407.1535; PMID: 10066176
- Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis 2002; 185:521 - 30; http://dx.doi.org/10.1086/338836; PMID: 11865405
- Sundstrom P, Cutler JE, Staab JF. Reevaluation of the role of HWP1 in systemic candidiasis by use of Candida albicans strains with selectable marker URA3 targeted to the ENO1 locus. Infect Immun 2002; 70:3281 - 3; http://dx.doi.org/10.1128/IAI.70.6.3281-3283.2002; PMID: 12011025
- Sundstrom P. Adhesion in Candida spp. Cell Microbiol 2002; 4:461 - 9; http://dx.doi.org/10.1046/j.1462-5822.2002.00206.x; PMID: 12174081
- Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, et al. Complementary adhesin function in C. albicans biofilm formation. Curr Biol 2008; 18:1017 - 24; http://dx.doi.org/10.1016/j.cub.2008.06.034; PMID: 18635358
- Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol 2010; 12:273 - 82; http://dx.doi.org/10.1111/j.1462-5822.2009.01412.x; PMID: 19919567
- Dalle F, Wächtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 2010; 12:248 - 71; http://dx.doi.org/10.1111/j.1462-5822.2009.01394.x; PMID: 19863559
- Phan QT, Fratti RA, Prasadarao NV, Edwards JE Jr., Filler SG. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 2005; 280:10455 - 61; http://dx.doi.org/10.1074/jbc.M412592200; PMID: 15632157
- Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, E Edwards J, et al. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 2005; 7:499 - 510; http://dx.doi.org/10.1111/j.1462-5822.2004.00476.x; PMID: 15760450
- Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 2010; 6:e1001181; http://dx.doi.org/10.1371/journal.ppat.1001181; PMID: 21085601
- Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog 2012; 8:e1002585; http://dx.doi.org/10.1371/journal.ppat.1002585; PMID: 22496639
- Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 2011; 9:109 - 18; http://dx.doi.org/10.1038/nrmicro2475; PMID: 21189476
- Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 2010; 6:e1000828; http://dx.doi.org/10.1371/journal.ppat.1000828; PMID: 20360962
- Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, Lopez-Ribot JL, et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog 2011; 7:e1002257; http://dx.doi.org/10.1371/journal.ppat.1002257; PMID: 21931556
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans.. Cell 2012; 148:126 - 38; http://dx.doi.org/10.1016/j.cell.2011.10.048; PMID: 22265407
- Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault JS, Nantel A, et al. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol 2009; 7:e1000133; http://dx.doi.org/10.1371/journal.pbio.1000133; PMID: 19529758
- Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, et al. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog 2012; 8:e1002848; http://dx.doi.org/10.1371/journal.ppat.1002848; PMID: 22876186
- Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, et al. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J Infect Dis 2012; In press http://dx.doi.org/10.1093/infdis/jis607; PMID: 23033146
- Kumamoto CA. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol 2008; 6:667 - 73; http://dx.doi.org/10.1038/nrmicro1960; PMID: 18679170
- Brand A, Shanks S, Duncan VM, Yang M, Mackenzie K, Gow NA. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol 2007; 17:347 - 52; http://dx.doi.org/10.1016/j.cub.2006.12.043; PMID: 17275302
- Brand A, Gow NA. Mechanisms of hypha orientation of fungi. Curr Opin Microbiol 2009; 12:350 - 7; http://dx.doi.org/10.1016/j.mib.2009.05.007; PMID: 19546023
- Brand A, Vacharaksa A, Bendel C, Norton J, Haynes P, Henry-Stanley M, et al. An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans.. Eukaryot Cell 2008; 7:712 - 20; http://dx.doi.org/10.1128/EC.00453-07; PMID: 18281602
- Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, et al. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One 2012; 7:e36952; http://dx.doi.org/10.1371/journal.pone.0036952; PMID: 22606314
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 2003; 67:400 - 28; http://dx.doi.org/10.1128/MMBR.67.3.400-428.2003; PMID: 12966142
- Taylor BN, Hannemann H, Sehnal M, Biesemeier A, Schweizer A, Röllinghoff M, et al. Induction of SAP7 correlates with virulence in an intravenous infection model of candidiasis but not in a vaginal infection model in mice. Infect Immun 2005; 73:7061 - 3; http://dx.doi.org/10.1128/IAI.73.10.7061-7063.2005; PMID: 16177393
- Albrecht A, Felk A, Pichova I, Naglik JR, Schaller M, de Groot P, et al. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J Biol Chem 2006; 281:688 - 94; http://dx.doi.org/10.1074/jbc.M509297200; PMID: 16269404
- Schaller M, Korting HC, Schäfer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol 1999; 34:169 - 80; http://dx.doi.org/10.1046/j.1365-2958.1999.01590.x; PMID: 10540295
- Hube B, Sanglard D, Odds FC, Hess D, Monod M, Schäfer W, et al. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun 1997; 65:3529 - 38; PMID: 9284116
- Lermann U, Morschhäuser J. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans.. Microbiology 2008; 154:3281 - 95; http://dx.doi.org/10.1099/mic.0.2008/022525-0; PMID: 18957582
- Correia A, Lermann U, Teixeira L, Cerca F, Botelho S, da Costa RM, et al. Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect Immun 2010; 78:4839 - 49; http://dx.doi.org/10.1128/IAI.00248-10; PMID: 20679440
- Moran GP, Coleman DC, Sullivan DJ. Candida albicans versus Candida dubliniensis: Why Is C. albicans More Pathogenic?. Int J Microbiol 2012; 2012:205921; http://dx.doi.org/10.1155/2012/205921; PMID: 21904553
- Niewerth M, Korting HC. Phospholipases of Candida albicans.. Mycoses 2001; 44:361 - 7; http://dx.doi.org/10.1046/j.1439-0507.2001.00685.x; PMID: 11766099
- Mavor AL, Thewes S, Hube B. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets 2005; 6:863 - 74; http://dx.doi.org/10.2174/138945005774912735; PMID: 16375670
- Leidich SD, Ibrahim AS, Fu Y, Koul A, Jessup C, Vitullo J, et al. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans.. J Biol Chem 1998; 273:26078 - 86; http://dx.doi.org/10.1074/jbc.273.40.26078; PMID: 9748287
- Theiss S, Ishdorj G, Brenot A, Kretschmar M, Lan CY, Nichterlein T, et al. Inactivation of the phospholipase B gene PLB5 in wild-type Candida albicans reduces cell-associated phospholipase A2 activity and attenuates virulence. Int J Med Microbiol 2006; 296:405 - 20; http://dx.doi.org/10.1016/j.ijmm.2006.03.003; PMID: 16759910
- Fu Y, Ibrahim AS, Fonzi W, Zhou X, Ramos CF, Ghannoum MA. Cloning and characterization of a gene (LIP1) which encodes a lipase from the pathogenic yeast Candida albicans.. Microbiology 1997; 143:331 - 40; http://dx.doi.org/10.1099/00221287-143-2-331; PMID: 9043110
- Hube B, Stehr F, Bossenz M, Mazur A, Kretschmar M, Schäfer W. Secreted lipases of Candida albicans: cloning, characterisation and expression analysis of a new gene family with at least ten members. Arch Microbiol 2000; 174:362 - 74; http://dx.doi.org/10.1007/s002030000218; PMID: 11131027
- Gácser A, Stehr F, Kröger C, Kredics L, Schäfer W, Nosanchuk JD. Lipase 8 affects the pathogenesis of Candida albicans.. Infect Immun 2007; 75:4710 - 8; http://dx.doi.org/10.1128/IAI.00372-07; PMID: 17646357
- Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol 2009; 12:365 - 70; http://dx.doi.org/10.1016/j.mib.2009.05.006; PMID: 19632143
- Fonzi WA. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of beta-1,3- and beta-1,6-glucans. J Bacteriol 1999; 181:7070 - 9; PMID: 10559174
- Mühlschlegel FA, Fonzi WA. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol 1997; 17:5960 - 7; PMID: 9315654
- De Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans.. Infect Immun 1998; 66:3317 - 25; PMID: 9632601
- Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, Hube B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol 2007; 63:1606 - 28; http://dx.doi.org/10.1111/j.1365-2958.2007.05614.x; PMID: 17367383
- Mitchell BM, Wu TG, Jackson BE, Wilhelmus KR. Candida albicans strain-dependent virulence and Rim13p-mediated filamentation in experimental keratomycosis. Invest Ophthalmol Vis Sci 2007; 48:774 - 80; http://dx.doi.org/10.1167/iovs.06-0793; PMID: 17251477
- Yuan X, Mitchell BM, Hua X, Davis DA, Wilhelmus KR. The RIM101 signal transduction pathway regulates Candida albicans virulence during experimental keratomycosis. Invest Ophthalmol Vis Sci 2010; 51:4668 - 76; http://dx.doi.org/10.1167/iovs.09-4726; PMID: 20375342
- Davis D, Edwards JE Jr., Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun 2000; 68:5953 - 9; http://dx.doi.org/10.1128/IAI.68.10.5953-5959.2000; PMID: 10992507
- Nobile CJ, Solis N, Myers CL, Fay AJ, Deneault JS, Nantel A, et al. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol 2008; 10:2180 - 96; http://dx.doi.org/10.1111/j.1462-5822.2008.01198.x; PMID: 18627379
- Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio 2011; 2:e00055 - 11; http://dx.doi.org/10.1128/mBio.00055-11; PMID: 21586647
- Mayer FL, Wilson D, Jacobsen ID, Miramón P, Große K, Hube B. The novel Candida albicans transporter Dur31 Is a multi-stage pathogenicity factor. PLoS Pathog 2012; 8:e1002592; http://dx.doi.org/10.1371/journal.ppat.1002592; PMID: 22438810
- Brown AJP, Haynes K, Gow NAR, Quinn J. Stress Responses in Candida. In: Calderone RA, Clancy, C.J., ed. Candida and Candidiasis: ASM Press, Washington, DC, pp. 225-242., 2012.
- Brock M. Fungal metabolism in host niches. Curr Opin Microbiol 2009; 12:371 - 6; http://dx.doi.org/10.1016/j.mib.2009.05.004; PMID: 19535285
- Fleck CB, Schöbel F, Brock M. Nutrient acquisition by pathogenic fungi: nutrient availability, pathway regulation, and differences in substrate utilization. Int J Med Microbiol 2011; 301:400 - 7; http://dx.doi.org/10.1016/j.ijmm.2011.04.007; PMID: 21550848
- Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol 2009; 71:240 - 52; http://dx.doi.org/10.1111/j.1365-2958.2008.06528.x; PMID: 19019164
- Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 2004; 3:1076 - 87; http://dx.doi.org/10.1128/EC.3.5.1076-1087.2004; PMID: 15470236
- Ghosh S, Navarathna DH, Roberts DD, Cooper JT, Atkin AL, Petro TM, et al. Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infect Immun 2009; 77:1596 - 605; http://dx.doi.org/10.1128/IAI.01452-08; PMID: 19188358
- Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, et al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 2012; 14:1319 - 35; http://dx.doi.org/10.1111/j.1462-5822.2012.01813.x; PMID: 22587014
- Lorenz MC, Fink GR. The glyoxylate cycle is required for fungal virulence. Nature 2001; 412:83 - 6; http://dx.doi.org/10.1038/35083594; PMID: 11452311
- Wysong DR, Christin L, Sugar AM, Robbins PW, Diamond RD. Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect Immun 1998; 66:1953 - 61; PMID: 9573075
- Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, Kang SO. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 2002; 148:3705 - 13; PMID: 12427960
- Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell 2004; 15:456 - 67; http://dx.doi.org/10.1091/mbc.E03-03-0179; PMID: 14617819
- Hromatka BS, Noble SM, Johnson AD. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol Biol Cell 2005; 16:4814 - 26; http://dx.doi.org/10.1091/mbc.E05-05-0435; PMID: 16030247
- Monge RA, Román E, Nombela C, Pla J. The MAP kinase signal transduction network in Candida albicans.. Microbiology 2006; 152:905 - 12; http://dx.doi.org/10.1099/mic.0.28616-0; PMID: 16549655
- Mayer FL, Wilson D, Jacobsen ID, Miramón P, Slesiona S, Bohovych IM, et al. Small but crucial: the novel small heat shock protein Hsp21 mediates stress adaptation and virulence in Candida albicans. PLoS One 2012; 7:e38584; http://dx.doi.org/10.1371/journal.pone.0038584; PMID: 22685587
- Diez-Orejas R, Molero G, Navarro-García F, Pla J, Nombela C, Sanchez-Pérez M. Reduced virulence of Candida albicans MKC1 mutants: a role for mitogen-activated protein kinase in pathogenesis. Infect Immun 1997; 65:833 - 7; PMID: 9009353
- Alonso-Monge R, Navarro-García F, Molero G, Diez-Orejas R, Gustin M, Pla J, et al. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans.. J Bacteriol 1999; 181:3058 - 68; PMID: 10322006
- Csank C, Schröppel K, Leberer E, Harcus D, Mohamed O, Meloche S, et al. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun 1998; 66:2713 - 21; PMID: 9596738
- Lindquist S. Heat-shock proteins and stress tolerance in microorganisms. Curr Opin Genet Dev 1992; 2:748 - 55; http://dx.doi.org/10.1016/S0959-437X(05)80135-2; PMID: 1458023
- Lindquist S. The heat-shock response. Annu Rev Biochem 1986; 55:1151 - 91; http://dx.doi.org/10.1146/annurev.bi.55.070186.005443; PMID: 2427013
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 2010; 40:253 - 66; http://dx.doi.org/10.1016/j.molcel.2010.10.006; PMID: 20965420
- Fiori A, Kucharíková S, Govaert G, Cammue BP, Thevissen K, Van Dijck P. The heat-induced molecular disaggregase Hsp104 of Candida albicans plays a role in biofilm formation and pathogenicity in a worm infection model. Eukaryot Cell 2012; 11:1012 - 20; http://dx.doi.org/10.1128/EC.00147-12; PMID: 22635920
- Cowen LE, Singh SD, Köhler JR, Collins C, Zaas AK, Schell WA, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 2009; 106:2818 - 23; http://dx.doi.org/10.1073/pnas.0813394106; PMID: 19196973
- LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AA, et al. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 2010; 6:e1001069; http://dx.doi.org/10.1371/journal.ppat.1001069; PMID: 20865172
- Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, et al. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 2009; 19:621 - 9; http://dx.doi.org/10.1016/j.cub.2009.03.017; PMID: 19327993
- Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 2009; 5:e1000532; http://dx.doi.org/10.1371/journal.ppat.1000532; PMID: 19649312
- Li XS, Reddy MS, Baev D, Edgerton M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem 2003; 278:28553 - 61; http://dx.doi.org/10.1074/jbc.M300680200; PMID: 12761219
- Li XS, Sun JN, Okamoto-Shibayama K, Edgerton M. Candida albicans cell wall ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J Biol Chem 2006; 281:22453 - 63; http://dx.doi.org/10.1074/jbc.M604064200; PMID: 16720580
- Sun JN, Li W, Jang WS, Nayyar N, Sutton MD, Edgerton M. Uptake of the antifungal cationic peptide Histatin 5 by Candida albicans Ssa2p requires binding to non-conventional sites within the ATPase domain. Mol Microbiol 2008; 70:1246 - 60; http://dx.doi.org/10.1111/j.1365-2958.2008.06480.x; PMID: 19006817
- Leach MD, Stead DA, Argo E, Brown AJ. Identification of sumoylation targets, combined with inactivation of SMT3, reveals the impact of sumoylation upon growth, morphology, and stress resistance in the pathogen Candida albicans.. Mol Biol Cell 2011; 22:687 - 702; http://dx.doi.org/10.1091/mbc.E10-07-0632; PMID: 21209325
- Sorger PK, Pelham HR. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J 1987; 6:3035 - 41; PMID: 3319580
- Sorger PK, Pelham HR. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 1988; 54:855 - 64; http://dx.doi.org/10.1016/S0092-8674(88)91219-6; PMID: 3044613
- Inglis DO, Arnaud MB, Binkley J, Shah P, Skrzypek MS, Wymore F, et al. The Candida genome database incorporates multiple Candida species: multispecies search and analysis tools with curated gene and protein information for Candida albicans and Candida glabrata.. Nucleic Acids Res 2012; 40:Database issue D667 - 74; http://dx.doi.org/10.1093/nar/gkr945; PMID: 22064862
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev 2002; 66:64 - 93; http://dx.doi.org/10.1128/MMBR.66.1.64-93.2002; PMID: 11875128
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, et al. Hsp26: a temperature-regulated chaperone. EMBO J 1999; 18:6744 - 51; http://dx.doi.org/10.1093/emboj/18.23.6744; PMID: 10581247
- Eyles SJ, Gierasch LM. Nature’s molecular sponges: small heat shock proteins grow into their chaperone roles. Proc Natl Acad Sci U S A 2010; 107:2727 - 8; http://dx.doi.org/10.1073/pnas.0915160107; PMID: 20133678
- Cashikar AG, Duennwald M, Lindquist SL. A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J Biol Chem 2005; 280:23869 - 75; http://dx.doi.org/10.1074/jbc.M502854200; PMID: 15845535
- Fu MS, De Sordi L, Mühlschlegel FA. Functional characterization of the small heat shock protein Hsp12p from Candida albicans.. PLoS One 2012; 7:e42894; http://dx.doi.org/10.1371/journal.pone.0042894; PMID: 22880130
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 2012; 10:525 - 37; http://dx.doi.org/10.1038/nrmicro2836; PMID: 22796883
- Almeida RS, Wilson D, Hube B. Candida albicans iron acquisition within the host. FEMS Yeast Res 2009; 9:1000 - 12; http://dx.doi.org/10.1111/j.1567-1364.2009.00570.x; PMID: 19788558
- Cleary IA, Reinhard SM, Miller CL, Murdoch C, Thornhill MH, Lazzell AL, et al. Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology 2011; 157:1806 - 15; http://dx.doi.org/10.1099/mic.0.046326-0; PMID: 21436220
- Heymann P, Gerads M, Schaller M, Dromer F, Winkelmann G, Ernst JF. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect Immun 2002; 70:5246 - 55; http://dx.doi.org/10.1128/IAI.70.9.5246-5255.2002; PMID: 12183576
- Weissman Z, Kornitzer D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol 2004; 53:1209 - 20; http://dx.doi.org/10.1111/j.1365-2958.2004.04199.x; PMID: 15306022
- Braun BR, Head WS, Wang MX, Johnson AD. Identification and characterization of TUP1-regulated genes in Candida albicans.. Genetics 2000; 156:31 - 44; PMID: 10978273
- Citiulo F, Jacobsen ID, Miramón P, Schild L, Brunke S, Zipfel P, et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 2012; 8:e1002777; http://dx.doi.org/10.1371/journal.ppat.1002777; PMID: 22761575
- Soloviev DA, Jawhara S, Fonzi WA. Regulation of innate immune response to Candida albicans infections by αMβ2-Pra1p interaction. Infect Immun 2011; 79:1546 - 58; http://dx.doi.org/10.1128/IAI.00650-10; PMID: 21245270
- Marvin ME, Williams PH, Cashmore AM. The Candida albicans CTR1 gene encodes a functional copper transporter. Microbiology 2003; 149:1461 - 74; http://dx.doi.org/10.1099/mic.0.26172-0; PMID: 12777486
- Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today 2009; 14:214 - 22; http://dx.doi.org/10.1016/j.drudis.2008.11.013; PMID: 19152839
