Abstract
Globally, the human respiratory syncytial virus (hRSV) is the major cause of lower respiratory tract infections (LRTIs) in infants and children younger than 2 years old. Furthermore, the number of hospitalizations due to LRTIs has shown a sustained increase every year due to the lack of effective vaccines against hRSV. Thus, this virus remains as a major public health and economic burden worldwide. The lung pathology developed in hRSV-infected humans is characterized by an exacerbated inflammatory and Th2 immune response. In order to rationally design new vaccines and therapies against this virus, several studies have focused in elucidating the interactions between hRSV virulence factors and the host immune system. Here, we discuss the main features of hRSV biology, the processes involved in virus recognition by the immune system and the most relevant mechanisms used by this pathogen to avoid the antiviral host response.
Introduction
HRSV is the leading cause of lower respiratory tract infections (LRTIs) in infants and young children worldwide.Citation1 Epidemiological data show that more than 70% of children under 1 y old and the 100% children at age 2 have been infected by hRSV.Citation2-Citation4 HRSV can also infect the elderly and immunocompromised individuals, however the most severe disease manifestations (i.e., being more virulent) occur in infants younger than 6 mo.Citation5,Citation6
HRSV pathology includes a broad spectrum of disease manifestations, ranging from milder upper respiratory tract infection to severe bronchiolitis, alveolitis, and pneumonia.Citation7,Citation8 Generally, hRSV infections are not lethal and the virus is eliminated from the airways during disease resolution. Nerveless, global epidemiological studies have estimated that hRSV causes over 34 million LRTIs and more than 200 000 deaths every year.Citation9 HRSV spread occurs through contact with large-particle aerosol droplets or direct contact with infected patients. The infection begins in the nasopharynx of the host and progress with the spreading of the virus to the lower respiratory tract.Citation10-Citation12 At these sites the main target of hRSV are epithelial cells present in the airways, but it also may infect lung resident myeloid cells, as evidenced by the detection of infected mononuclear cells in circulation.Citation13,Citation14
Molecular Composition of hRSV
HRSV is an enveloped virus classified in the order Mononegavirales, the Paramyxoviridae family, and the Pneumovirus genus.Citation15 HRSV contains a non-segmented, negative-sensed and single-stranded RNA genome of 15.2 kb of length. The hRSV genome has 10 genes in the order 3′-NS1-NS2-N-P-M-SH-F-G-M2-L-5′, which are transcribed in 10 different monocistronic mRNAs. The hRSV genome encodes nine structural proteins and two non-structural proteins. The structural proteins include three envelope glycoproteins (F, G, and SH), the nucleocapsid proteins (N, P, and L), the nucleocapsid-associated proteins (M2–1 and M2–2), the matrix protein (M), and the non-structural proteins NS1 and NS2.Citation16 Different from other viral mRNAs, the M2 mRNA is translated into two different proteins, namely M2–1 and M2–2, through a process of ribosomal termination-dependent re-initiation mechanism ().Citation17 The M2 gene products, M2–1 and M2–2, are pivotal regulatory proteins that modulate the replication cycle of hRSV. More specifically, M2–1 integrates the ribonucleoprotein complex that mediates transcription of viral mRNAs, whereas the M2–2 protein regulates the switch from transcription to replication process.Citation18,Citation19
Figure 1. Schematic representation of the hRSV genome indicating the known function for each encoded protein. The figure shows the order of the 10 genes of hRSV and the known function of the 11 encoded proteins.
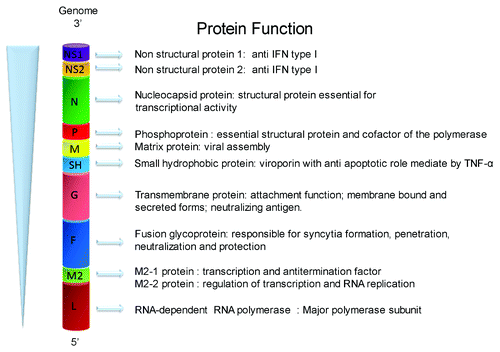
Once hRSV reaches the host target cell, the infection cycle begins with the attachment and entry process, which is mediated by the G and F glycoproteins, respectively.Citation16 It has been reported that G and F glycoproteins can interact with the cell surface receptors CX3CR1 and TLR4,Citation20,Citation21 respectively, as well as glycosaminoglycan (GAGs)Citation22 and C-type lectinsCitation23 to promote viral infection. Further, nucleolin was recently described as the functional host receptor that interacts with hRSV F glycoprotein.Citation24 After fusion of the viral envelope and the cell plasma membrane, the viral nucleocapsid is released into the cytosol of infected cells where transcription of viral mRNAs and replication of the viral genome are initiated.Citation1,Citation25 HRSV replication requires the synthesis of a complementary, polycistronics ssRNA (+) antigenome, which is used as a template for the synthesis of new full-length ssRNA(-) genomes.Citation26 Both, genomes and antigenomes are independently wrapped by the N protein, forming stable nucleocapsids.Citation27
HRSV virions are pleomorphic, spherical structures with a diameter ranging from 100–350 nm to 100–100 nm, which are assembled in cholesterol-enriched domains at the host cell membrane.Citation28 Recent studies suggest that the majority of hRSV strains form long filamentous projections at sites of virus assembly and budding.Citation29 Furthermore, assembly and maturation of hRSV filaments on the surface of infected cells depend on the proper destination of M protein to cholesterol rich domains of the plasma membrane.Citation30 Therefore, during genome packaging and virus assembly the hRSV particle acquires a lipid envelope of host origin.Citation31 Because glycoproteins located at the viral envelope participate in host cell recognition, attachment and infection, this structure is pivotal for the infectivity of hRSV.Citation32
Modulation of Host Cell Biology by hRSV Replication
Upon infection, hRSV modulates several biological processes of the infected cells to enhance their replication. Studies performed in A549 and primary human epithelial cells (PHBE cells) have shown that hRSV infection induces the production of TGFβ1 and the decrease of the p53, which results in G1/S and a G2/M cell-cycle arrest and a subsequent enhancement of hRSV replication.Citation33 HRSV infection also promotes the formation of host cytoplasmic stress granules (SG) in epithelial cells.Citation34 Although the formation of SG has been associated with increased viral replication,Citation34 these structures are also recognized by the cytoplasmic RLR receptor MDA5. This protein is activated by viral dsRNA or by 5′-triphosphorylated un-capped viral RNA,Citation35which activates the type I interferon (IFN-α/β) response.Citation36 Recently, it was described that MDA5 specifically recognizes dsRNA replication intermediates in viral infected cells.Citation34,Citation37,Citation38 SGs increase in size during the course of infection and contain the N, P, M2–1, L, and M proteins.Citation37 In addition, hRSV induces changes in the expression of neurotropic factors and receptors, which are involved in airway inflammation and hyperreactivity. Recent reports have shown that hRSV induces the upregulation of the nerve growth factor (NGF) and their receptor tropomyosin-related kinase A (TrkA), with concomitant downregulation of the low-affinity pan-neurotrophin p75NTR receptor.Citation39 The NGF–TrkA axis prevents apoptosis by increasing the expression of anti-apoptotic Bcl-2 family members, whereas p75NTR signaling promotes apoptosis via JNK. These mechanisms keep the infected cells alive and promote viral replication.Citation39 The same study suggested that upregulation of the NGF–TrKA axis induced by hRSV infection in human bronchial epithelial cell takes place through silencing of miR-221 expression and also induces the downregulation of other 24 miRNA.Citation39 MiRNAs are small ssRNA molecules that modulate the gene expression at the post-transcriptional level.Citation40 The role of these miRNA in the modulation of both innate and adaptive immune response has been broadly studied.Citation41 Indeed, miRNAs participate in the maintenance of the airway epithelial barrier and in the modulation of the antiviral defense of epithelial cells.Citation40 Infection of A549 cell line with hRSV increases the production of miRNA let-7f expression, probably due to the signaling triggered by the G glycoprotein.Citation8
Let-7 miRNAs regulate several key host genes induced during hRSV infection that also controls virus replication.Citation42 Furthermore, let-7f regulates cell-cycle genes (CCND1, DYRK2, and ELF4),Citation42 the gene encoding the CCL7 chemokine and the gene encoding the suppressor of cytokine signaling 3 (SOCS3).Citation17,Citation42 Also, the regulation of ELF4 by let-7f modulates the expression of IL-8, which plays an important role in the pathogenesis of hRSV. Also, has been described that G hRSV glycoprotein can modulate the expression of IL-8 thought let-7f miRNA.Citation43,Citation44
HRSV Recognition by PRRs Receptors
Innate immunity is the first line of defense against virus infection, before induction of the adaptive immune response.Citation45 It is well established that innate immunity is critical to restrain virus spreading and infection, resulting in diminished disease burden. After hRSV infection, the virus infects epithelial cells, alveolar macrophages and dendritic cells, which triggers an innate antiviral response thought pattern recognition receptors (PRRs), including toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) and nucleotide-biding oligomerization domain (NOD)-like receptors (NLRs). All these proteins recognize pathogen-associated molecular patterns (PAMPs) from the virus or damage-associated molecular patterns (DAMPs) derived from host cells after virus infection.Citation43,Citation46
TLRs play an important role in the recognition of hRSV. The TLR-2 and TLR-6 form the cell-surface heterodimer TLR4/TLR6 on immune cells. After activation by hRSV, this complex triggers a signaling cascade in leukocytes that activates innate immunity by promoting the production of tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), CCL2, and CCL5.Citation47 Also, TLR-4 associates with CD14 and the complex recognizes LPS from gram-negative bacteria and the hRSV-F glycoprotein. Activation of TLR4/CD14 complex requires binding of MD2, leading to NFκB activation. The final result of this activation pathway is the secretion of IL-8, IL-10, and IL-6, and also the upregulation of TLR4 on epithelial cells.Citation48
HRSV infection is also sensed by the intracellular receptor TLR-3. This receptor localizes on the surface of endosomes and recognizes double-stranded RNA (dsRNA) produced in the cytoplasm of the infected cell during viral replication.Citation45 The induction of TLR3 activates the innate immune response through the TRIF-mediated pathway that promotes the production of CCL-5, IFN-α, and IFN-β.Citation49 TLR7 is also involved in the recognition of hRSV, which binds to the viral ssRNA genome in endosomes during the fusion process. When TLR7 is activated after hRSV infection it signals via the MyD88 pathway.Citation50
Similar to MDA5, RIG-I is another receptor belonging to the RLRs family and is activated by viral dsRNA or 5′-triphosphorylated un-capped viral RNA in the cytoplasm.Citation51 Specifically, RIG-I recognizes ssRNA viral genomes bearing 5′-triphosphates, whereas MDA5 recognize long dsRNA molecules.Citation52 The induction either RIG-I or MDA5 leads to the activation of downstream NFκB and IRF3 pathways by interacting with the mitochondrial antiviral-signaling protein (MAVS; IFN-β promoter stimulator 1 [IPS-1]). RIG-1 detects hRSV during replication and subsequently activates IFN regulatory factors 3 and 7 (IRF-3 and IRF-7), which are transcription factors for IFN-α and IFN-β.Citation45 Finally, the nucleotide-binding oligomerization domain 2 (NOD2) detects the ssRNA genome and triggers innate immune activation by binding with the adaptor MAVS. NOD2 is required for IRF3 activation and IFN-β production in hRSV infection in vitro.Citation45
Airway Immune Response against hRSV
The epithelial cells from the airway (tracheal, bronchial, and bronchiolar cells) actively contribute to activate the immune response after hRSV infection, through the secretion of immunomodulatory molecules with innate antimicrobial activity, as well as secretion of cytokines and chemokines upon infection to recruit immune cells.Citation53 HRSV infection induces the secretion of surfactant proteins A and D (SP-A and SP-D). Both SP-A and SP-D are polypeptides of the collectin family that bind pathogens and play an important role in host defense and regulation of inflammatory processes in the lung.Citation54 SPs can act as opsonins, but also stimulate macrophages (Mφ) activation, increasing chemotaxis, phagocytosis and modulating cytokine secretion.Citation55,Citation56 In vitro infections of human peripheral blood monocytes and HEp-2 cells with hRSV show that SP-A interacts with G and F glycoproteins and favors the binding and uptake of the virus.Citation57,Citation58 Also, SP-A modulates the activity of Mφ, including activation of the NFκB signaling pathway and the upregulation of cytokine synthesis, mediated by TLR4 complex.Citation59 SP-A and SP-D bind to CD14,Citation60 whereas SP-A also binds to TLR2, but does not activate downstream NFκB signaling.Citation61 Surfactant protein D also binds the RSV G protein and inhibits hRSV infection in vitro and in vivo.Citation58
Recent reports have also shown that infection of airway epithelial cells by hRSV induces the secretion of thymic stromal lymphopoietin (TSLP), an epithelium derived cytokine that plays an important role in the development of allergic asthma via activation of RIG-1 antiviral pathway.Citation62 Additional evidence supports the notion that TSLP induces myeloid dendritic cells (mDCs) to express the OX40 ligand (OX40L), which is a member of the TNF superfamily that has been involved in the B cell–T cell interaction, the DC–T cell interaction, and the initiation of Th2 cell responses through OX40L expressed on these DCs.Citation63-Citation65 DCs are professional antigen presenting cells (APCs) with superior capacity to activate antigen-inexperienced T cells, thus being an APC subset linking the innate and adaptive immunity.Citation66 To achieve their function, DCs that are infected by pathogens or that have up-taken antigens at mucosal tissues of the body need to undergo a phenotype change process known as maturation, which has been demonstrated to be a critical response to establish CD8+ T cell memory to infections.Citation67 Along these lines, previous studies have shown that TSLP promotes mDCs maturation, evidenced by the upregulation of major histocompatibility molecules (MHC) class I and II and costimulatory molecules (CD40, CD80, CD83, and CD86).Citation43,Citation64 In addition, other reports have shown that TSLP can act either in an autocrine or paracrine manner on epithelial cells. Thus, airways cells contribute to the TSLP response and drives the production of the Th2 chemokine CCL17, allowing epithelial cells to induce a Th2-type response due to hRSV infection.Citation62 Another study performed in primary rat airway epithelial cells (PRAECs) shows that hRSV induced the production of both TSLP mRNA and protein at 18 h post-infection.Citation64 In this work, it was shown that hRSV-treated PRAECs induce the maturation of mDCs, which have enhanced levels of OX40L and CCL17 mRNAs.Citation64 Is has been also described that the presence of TSLP in mixed lymphocyte reactions increases the expression of MHC-II and CD86 and promotes enhanced T-cell proliferation.Citation64 CCL17 have a key role in the Th2 response, because it binds to their chemokine receptor CCR4, which is expressed in almost 100% on Th2 cells that produce IL-4, IL-5, and IL-10.Citation64,Citation68 This chemokine also participates in the recruitment of Th2 cells and eosinophils to the lungs.Citation68
Role of hRSV Proteins in Immune System Evasion
During host-virus co-evolution, several strategies has been developed by viruses to interfere with critical functions of the immune system, including antigen presentation, T-cell activation, and the development of the host humoral response.Citation69 Among the viral components modulating the host immunity, several hRSV proteins have been attributed with the capacity to modulate the function of either innate or adaptive immune cells (summarized in ). In the next sections, we review the virulence determinants used by hRSV to negatively modulate the antiviral immunity, from the early innate responses to the highly specific T-cell responses required to clear the infection.
Table 1. HRSV interaction with host innate and adaptive immune response
The Nonstructural NS1 and NS2 Proteins
NS1 and NS2 are two small proteins (139 and 124 amino acids respectively), which are not included as structural elements in the viral particle.Citation70 NS1 and NS2 are encoded by the first two genes of the hRSV genome and their mRNAs are the most abundant during the infective cycle.Citation70 Accordingly, NS1 has been shown to be the most abundantly produced protein in hRSV-infected cells, suggesting that these proteins are involved in the modulation of the innate immune response during early stages of the virus replication cycle.Citation71 In agreement with this notion, both proteins modulate the host immune responses by impairing the induction/signaling of interferons, dendritic cells (DCs) maturation and T lymphocyte activation.Citation72-Citation74 Also, NS1 and NS2 have been associated with the apoptosis inhibition, thus prolonging the life of the infected cell and increasing viral yield.Citation75,Citation76
Inhibition of the Type I Interferon Response by NS Proteins
The first evidence supporting that NS proteins inhibit the type I interferon pathway showed that virus lacking both NS1 and NS2 failed at preventing the activation of the interferon regulatory factor 3 (IRF-3) and its nuclear translocation.Citation72,Citation76,Citation77 Further studies demonstrated that NS1 inhibits the phosphorylation of IRF-3, thereby interrupting the binding of this protein to the interferon gene promoter. On the other hand, NS2 causes the degradation of STAT2, an important signaling component of the JAK/STAT cascade that is triggered by interferon receptors. Additionally, NS2 interacts with the RIG-I, preventing the the activation of IRF-3 and the interferon stimulated genes involved in the innate antiviral response.Citation76-Citation78 Moreover, NS1 and NS2 activate the phosphoinositide 3-kinase (PI3K) pathway, which both promote survival of infected epithelial cells and mediates virus maturation and budding.Citation79-Citation81 In agreement with this notion, suppression of NS1 and/or NS2 expression by either small interfering RNAs (siRNAs) or by viral gene deletion suppressed the activation of the PI3K pathway, which resulted in accelerated apoptosis of hRSV-infected cells and a reduction in virus yield.Citation78 By activating the PI3K pathway, NS1 and NS2 increased the survival time of the infected cell and increased the yield of viral progeny.Citation82
Effects of NS Proteins in the Maturation of Dendritic Cells
It has been demonstrated that hRSV mutants lacking either NS1 or NS1/NS2 have an increased capacity to induce DC maturation as compared with wild-type viruses, as evidenced by an increased expression of maturation markers and secretion of pro-inflammatory cytokines, both of which are known changes associated with DCs maturation.Citation73 However, NS1 appears to exert most of the modulatory effect over DCs maturation, as evidenced by a non-significant modulation of NS2 knockout hRSV. The upregulation described was inhibited by pretreatment with a blocking antibody against the type I IFN receptor, suggesting that suppression of DCs maturation by NS1/NS2 is associated with antagonism of the type I IFN pathway by these proteins.Citation73 Furthermore, suppression of DCs maturation negatively affected antigen presentation and T-lymphocyte activation, suggesting that reduced immune responses against hRSV are at least in part due to the effects of NS proteins over DCs maturation.Citation73
Negative Modulation of T-Cell Responses by NS Proteins
The hRSV NS proteins can modulate the activation and proliferation of T cells. As described by Munir and coworkers, deletion of NS1, but not NS2, produced an increased activation and proliferation of CD8+ T cells expressing the tissue homing integrin CD103. Because this integrin leads CD8+ T-cell recruitment into the respiratory tract mucosa, which favors its cytolytic activity in infected airways, it is though that NS1 negatively modulates cytotoxicity in vivo.Citation74 Also, NS1 knockout mutants display increased activation and proliferation of Th17 cells within the lungs, which have anti-viral effects and also indirectly attract neutrophils; and decreased activation of IL-4-producing CD4+ T cells and reduced proliferation of total CD4+ T cells.Citation74 Except for total CD4+ T-cell proliferation, none of the T-cell effects appeared to be due to increased type I IFN signaling. Data from a previous study show that in infected DCs, deletion of the NS1 and NS2 genes strongly upregulated the expression of cytokines and other molecules involved in DCs maturation.Citation73 This was partly IFN-I-independent, and thus might account for the T-cell effects. Taken together, these reports demonstrate that the NS1 protein suppresses proliferation and activation of two protective T-cell populations (CD103+ CD8+ T cells and Th17 cells), and promotes proliferation and activation of deleterious Th2 cells that may enhance lung hRSV immunopathology.Citation74
The Small Hydrophobic (SH) Glycoprotein
The SH glycoprotein gene encodes 64 or 65 amino acids depending of the hRSV serotype (A or B),Citation83-Citation85 which is highly conserved among all hRSV A subtypes.Citation84 In cells infected with hRSV strain A2, the SH glycoprotein can adopt several forms, such as SH 0, SH g, and SH p,Citation84,Citation85 while in cells infected with B1 strain similar glycosylated and non-glycosylated forms are found.Citation85 Depending of the glycosylation pattern of the SH glycoprotein, three forms have been described, which are: 7.5 kDa non-glycosylated form (SH0), a 13–15 kDa N-linked glycosylated form (SHg), and a polylactosaminoglycan-modified form of the protein (SHp), which varies between 21 and 30 kDa.Citation84 The SH glycoprotein is found anchored at the cellular membrane by the N-terminus and the C-terminal amino acids are extracellular.Citation84 Several reports indicate that SH glycoprotein can form pentamers and when expressed in Escherichia coli, it changes the membrane permeability of the bacteria, allowing the entry of low-molecular-weight compounds.Citation86-Citation88 Structural modeling analyses have demonstrated that this glycoprotein is an ion channel-forming viroporin.Citation84,Citation85,Citation88,Citation89 Viroporins belong to a group of small/highly hydrophobic virus proteins that can oligomerize forming pores in the cell membrane, causing varying effects in the physiology of infected cells.Citation86
Currently, it is know that SH glycoprotein is not important for the viral replication but hRSV lacking SH glycoprotein was attenuated in mouse and chimpanzee models, which indicates that SH glycoprotein is important for hRSV pathogenesis.Citation82-Citation84,Citation88 Mutant viruses generated by reverse genetics, in which the SH gene has been deleted, suggest that it is dispensable for virus growth, virus entry into host cells or syncytium formation, but may be necessary for the evasion of the host immune system.Citation84,Citation85 To evaluate the participation of the SH glycoprotein as a virulence factor, experiments has been performed comparing the hRSV SH protein with another member of the Paramixoviridae family, Parainfluenza virus 5 (PIV5), because hRSVΔSH shows a phenotype similar to rPIV5ΔSH: a normal growth in vitro but attenuated growth in vivo.Citation85 Also, studies of rPIV5ΔSH have shown the role of this protein in the inhibition of apoptosis mediate by TNF-α.Citation85 In the absence of SH glycoprotein during PIV5 infection, there is an increase of production of TNF-α and activation of NFκB, due to the translocation of the p65 subunit of NFκB into the nucleus of PIV5ΔSH-infected L929 cells.Citation85 A similarly ability to inhibit the activation of NFκB by TNF-α in the L929 cells has been observed for hRSVΔSH, independent of the strain used.Citation85 Other characteristic of hRSVΔSH infection is the high cytophatic effect and the high rate of the apoptosis produced in infected cells, compared with the wild-type hRSV.Citation85 This observation suggest that hRSV SH glycoprotein play an important role in the inhibition of apoptosis during the infection, to favor the viral replication.Citation85
The hRSV infection induces the secretion of IL-1β in the respiratory tract in mouse and humans and its secretion is relevant for the anti-viral immune response to clear virus.Citation90 Recently, the activation of the nucleotide binding oligomerization domain like receptor (NLR) inflammasome, principally NOD-like receptor family, pryin domain containing 3 (NLRP3) has been described during the hRSV infection, resulting in the pro-IL1β cleavage and secretion of the processed cytokine.Citation90 Given that the triggering of NLRP3 inflammasome requires the permeability of cellular membrane, the participation of the SH glycoprotein was evaluate.Citation87 In this study it was observed that a mutant strain of hRSV lacking SH glycoprotein fails at triggering inflammasome activation.Citation87 This result suggests that SH glycoprotein is required for the trigger of signal 2 (), due to the formation of a pore or channel on the plasma membrane.Citation87 More studies are required to understand the role of SH glycoprotein as a hRSV virulence factor.
The Attachment (G) Glycoprotein
The G hRSV glycoprotein contains 298 amino acid and besides of its role in the attachment process, it seems to have additional features, unrelated with attachment proteins from other Paramyxoviridae family members.Citation91 The G glycoprotein has a trans-membrane domain near the N-terminus and the major part of the molecule, including the C-terminus, is external. However, the G glycoprotein exists also in a secreted form lacking this trans-membrane domain.Citation91 The G glycoprotein has a central conserved cysteine region that contains a CX3C chemokine motif at amino acid positions 182–186.Citation92 This CX3C motif interacts with the CX3CR1, whose ligand CX3CL1 (also known as fractalkine). Therefore, the G glycoprotein establishes a competitive inhibition with CX3CL1 for the binding to CX3CR1, facilitating infection and impairing CX3CL1-mediated responses.Citation92
CX3CL1 has several functions related to leukocyte biology, including adhesion, chemoattraction and immunomodulation of T cells.Citation93 The membrane-anchored form of CX3CL1 promotes cell adhesion with CX3CR1 expressed primarily on cytotoxic cells, e.g., T cells, natural killer NK cells, and monocytes/macrophages, and the soluble form acts as a chemoattractant for CX3CR1+ cells.Citation94 Studies of the interaction between CX3CL1-CX3CR1, using either blocking antibodies for CX3CL1/CX3CR1 or knockout mice for CX3CR1, have shown a high inhibition of leukocyte migration and chemotaxis after hRSV infection.Citation20,Citation93-Citation95 Also, it has been suggested that G glycoprotein is important for the development of enhanced pulmonary disease in the vaccination model of formalin-inactivated hRSV,Citation95 and also increases the expression of the pro-inflammatory tachykinin sustance P during hRSV infection.Citation96 Indeed, antibodies that block hRSV G glycoprotein CX3C–CX3CR1 interaction prevent many of the immunomodulatory effects associated with RSV G glycoprotein,Citation97 supporting the idea that the interaction of CX3 mimetic domain in the G glycoprotein with CX3CR1 receptor have an important role in the hRSV infection and disease pathogenesis.Citation98
Modulatory Effects on the Host Innate Immune Response by G Glycoprotein
HRSV G glycoprotein has the ability to modify the immune response at different levels, affecting the function of chemokines, cytokines and leukocytes. Competition with the CX3C chemokine is one of the most described effects of G glycoprotein attributed to the central conserved region, which contains a CX3C motif. Also the G glycoprotein presents a structural homology with the fourth subdomain of the tumor necrosis factor receptor (TNFr).Citation89 TNF-α and TNF-β are important cytokines of the inflammatory response, and the structural homology with the TNFr of the G glycoprotein suggest that this protein could bind to TNF-α and TNF-β, affecting the antiviral response against hRSV.Citation89
Experimental approaches using mutant hRSV virus lacking the G gene showed a increases in the recruitment of natural killer cells into the lungs, as well as increases in the production of IFN-γ and TNF-α, supporting the involvement of the G glycoprotein in the inhibition of NK cells infiltration and proinflammatory cytokine secretion.Citation99 The G glycoprotein has been associated with the induction of a Th2 response and the increased eosinophils recruitment into the lungs after hRSV infection. The secreted form of G glycoprotein increases the IL-5 levels, producing a more severe immunopathology due the activation and migration of eosinophils.Citation100 Other studies have also shown that G glycoprotein decreases the expression of macrophage inflammatory protein (MIP-1a), MIP-1b and MIP-2 and monocyte chemoattractant protein (MCP-1), which have attracting function over NK cells into the lungs.Citation98
Modulation of the Host Adaptive Immune Response by the G Glycoprotein
Several pieces of evidence support the notion that hRSV G glycoprotein has important immune modulatory effects. For instance, it has been shown that during hRSV infection the G glycoprotein promotes a Th2 immune response in pulmonary CD3+ T cells (high expression of IL-4 and IL-5) by negatively modulating Th1 cytokines, including IFN-γ and IL-2. It is possible that this phenotype is due to alterations in DCs recruitment/activation and effects in signaling important in T-cell activation, such as substance P.Citation99 Moreover, recent studies have suggested a possible mechanism by which the G glycoprotein may be interfering with the cytotoxic T-cell response (which is essential for viral clearance and the control of virus replication) by antagonizing the activities of the chemokine CX3CL1 over the CX3CR1+ cells, characterized by a Th1 response.Citation94 Several studies performed in the murine model suggest that hRSV suppresses the effector activity of CD8+ T cells and the development of pulmonary CD8+ T-cell memory,Citation101 which can be recovered by exogenous IL-2 treatment.Citation102 These findings are consistent with hRSV G glycoprotein-associated reduction of Th1-type cytokine responses.Citation97 Because CX3CR1 plays an important role as a chemotactic and adhesion receptor for CX3CL1+ cells, it is though that the hRSV G glycoprotein through its CX3C motif may be involved in the negative regulation of T-cell function observed in vivo. Indeed, the G glycoprotein differentially affects the trafficking of CD8+CX3CR1+ T cells into the lungs and the mediastinal lymph nodes (MLN) of hRSV infected mice. Furthermore, additional evidence suggest that the hRSV G protein may affect the antiviral response thought the modulation of perforin and granzyme B expression in cytotoxic CX3CR1+ cellsCitation94 and also expression of the G glycoprotein during hRSV infection induces an exacerbated Th2 type cytokine expression.Citation98
The Fusion (F) Glycoprotein
The F hRSV glycoprotein is a type I integral membrane protein of 574 amino acid similar to the fusion proteins of other Paramyxoviridae family members, which participates in both the fusion of the viral envelope with the host cell membrane during viral entry and the formation of syncytia.Citation103 The F glycoprotein is highly conserved among hRSV genogroups, displaying amino acid sequence identities of 90% or higher between serogroups A and B.Citation104 The F glycoprotein is synthesized as an inactive F0 precursor; three F0 monomers assemble into a trimer, which is further modified and activated in the Golgi apparatus by the host furin-like protease.Citation82,Citation83 This protease cleaves at amino acid positions 109 and 136, therefore forming three polypeptides. Finally, the N-terminal and C-terminal polypeptides (named F2 and F1 subunits) are linked by two disulfide bonds.Citation83 During the replication of hRSV, the F mRNA is produced in the cytosol and the functional F protein exists as a trimer located in the virion membrane as a metastable pre-fusion form, which upon binding to its relevant ligand; the nucleolin, undergoes a refolding process into a post-fusion conformation.Citation105 This conformational change promotes the fusion of the virus and cell membranes, allowing virus entry and the initiation of the hRSV replication cycle (reviewed inCitation103). Because of this, the F glycoprotein is essential for the infective cycle, as evidenced by studies showing complete loss of virus infectivity both in vivo and in vitro in hRSV strains lacking the F glycoprotein (RSVΔF).Citation106
As the major protein mediating infection of target cells, the F protein has been attributed as a major target for the pattern recognition receptors (PRRs) of the innate immune system. Indeed, through binding to the TLR4/CD14 complex expressed on the surface of monocytes, the F protein triggers the NFκB pathway and the secretion of pro-inflammatory cytokines, including IL-6, IL-1β, and IL-8 in vitro.Citation107 Because these cytokines act as chemoattractants, they promote the recruitment of neutrophils and macrophage cells into injured tissues.Citation108 Furthermore, the TLR4 pathway appears to be essential for the efficient elimination of hRSV from the infected lungs, as evidenced by studies showing severe impairment of viral clearance in TLR4 null mice. Although the mechanism underlying increase susceptibility to hRSV in TLR4 null mice is not fully understood, studies with these mice show an overall impairment of the innate antiviral immunity, including IL-12 expression and diminished numbers of NK cells and CD14+cells in hRSV-infected lungs. Also, the infiltrating NK cells have a significantly diminished cytotoxicity.Citation108
Besides being a major hRSV signature for the innate immune system, the hRSV fusion protein has been recognized as a major CD8+ T-cell antigen, as evidenced by the identification of several F-derived antigenic peptides both in humans and mice.Citation83,Citation109,Citation110
CTL responses in mice
In agreement with this notion, it has been shown that approximately 4.8% of the pulmonary CD8+ T cells activating the adaptive antiviral response during the infection peak (day 8) are indeed F85–93-specific.Citation83 Nevertheless, the F-specific T-cell repertory generated during the experimental infection with hRSV display a substantially reduced cytotoxic capacity when compared with the F repertory induced through vaccination with a recombinant influenza or adenovirus vaccine,Citation111 suggesting that hRSV negatively modulates the expansion and generation of CD8+-specific T cells. Furthermore, F-specific CD8+ T cells isolated from hRSV infected mice exhibited lower IFN-γ synthesis and impaired cytolytic activity ex vivo.Citation83,Citation112 Although the mechanisms accounting for inefficient cytolytic activity of CD8+ T cells are not fully understood, it has been shown that the heterologous expression of the hRSV F protein in epithelial cells reduced their sensitivity to CD8+ cytotoxicity in vitro.
Recently, studies reported the interaction of F glycoprotein with the intracellular adhesion molecule (ICAM)-1 expressed on the cell surface, suggesting a possible role of this molecule in the viral fusion. Indeed, hRSV infection increases expression of ICAM-1 on epithelial cells and the ICAM-1 cross-linking of human epithelial cells induced the expression of IL-1β, which is also induced by hRSV infection, suggesting the participation of ICAM-1 in this process.Citation113 Because the high conservation of the F protein and its pivotal role in the infection of target cells and the modulation of the host immune response, make the pre-fusion form of the F glycoprotein an ideal target for neutralizing antibodies and the development of antiviral therapies.Citation104
The Nucleoprotein (N) as a Novel Modulator of T-Cell Activation
The hRSV N protein is a non-glycosylated, 43 kDa protein with essential roles in virus transcription and replication, acting as a scaffold for the assembly of the hRSV ribonucleoprotein complex. Although its major role in infected cells has been attributed to the protection of viral RNA species, including the wrapping of genomes and polycistronic antigenomes,Citation114,Citation115 a recent work of our group has proposed that N protein could be expressed in the surface of infected cells at early stages of the viral replication cycle (Céspedes et al., manuscript accepted). Although the mechanism of surface N protein destination is still elusive, it may occur through interactions with the M and P proteins.Citation116 M protein has a highly positive face able to interact directly with the inner leaflet of the plasma membrane,Citation117 and the P protein serving as chaperone of N protein that prevents its interaction with RNA.Citation118 Furthermore, surface expression of N was shown to interfere with the assembly of the T-cell activating immunological synapse (IS) when expressed in the surface of infected APCs, mainly by interfering with the molecular interaction between the T-cell receptor (TCR) and antigenic pMHCs. This observation was in agreement with our previous work showing impairment of IS assembly between hRSV-infected DCs and naïve T cells, which was characterized as a contact-dependent mechanismCitation119 as shows the .
Figure 2. HRSV infection blockade of IS assembly between infected DCs and T cells as a novel RSV virulence mechanism. A novel mechanism to avoid the host immune response elicit by hRSV is the impairment of immunological synapse (IS) assembly between hRSV-infected DCs and naïve T cells that impairs the T cells activation. During IS assembly DCs provide three different signals to promote the T-cell activation: Signal 1 (antigenic presentation in p-MHC), signal 2 (Co-stimulation) and signal 3 (cytokines) represented in the left part of cartoon. In hRSV infected DCs, as show the right part of cartoon, hRSV effectors are expressed in the surface membrane and interfering with the molecular interaction between the T-cell receptor (TCR) and antigenic pMHCs impairing the T-cell activation. This phenomenon is evidenced by a reduction in the Golgi apparatus polarization toward the DC and decreases in IL-2 secretion in T cells.
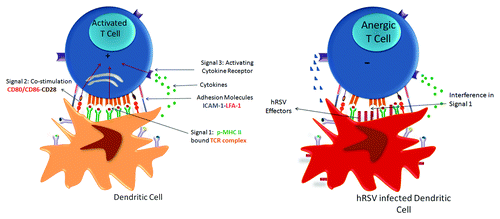
Because the priming of T cells largely depends on the assembly of the IS with DCs (and in a lesser extent with other APCs), these observations suggest that the major mechanism explaining the broad impairment of CD4+ and CD8+ T-cell activation by hRSV is due to expression of hRSV proteins over APCs ().
Concluding Remarks
HRSV has been recognized as the major viral agent causing severe acute respiratory infections in children. However, and despite considerable efforts made to understand the molecular mechanisms explaining the immunopathology and the evasion of the host-immune response, there is still no appropriate vaccine for hRSV prophylaxis. It is known that hRSV pathology is due to an excessive inflammation of the respiratory tract, characterized initially as a Th2, allergic-like immune response. Because the hRSV-elicited immune response is non-optimal for virus clearance (due to broad impairment with T-cell functions), infected individuals can eliminate the virus at the expense of an exaggerated inflammatory response in the lungs. Furthermore, the aberrant immune response observed in the airways of hRSV-infected animals have been associated with several viral proteins, each of them with unique complementary functions in the modulation of the innate antiviral response of the host, thus facilitating viral propagation. Indeed, hRSV use multiple mechanisms to avoid the host immune response including interference with type I IFN responses (mediated by hRSV NS1 and NS2 proteins) and the antagonism of CX3CL1 chemokine mediated by the mimetic domain of the hRSV G glycoprotein. The pieces of evidence suggesting immune regulatory roles for hRSV F and SH glycoproteins is not conclusive yet. However, our knowledge is still insufficient to understand the complete picture of hRSV infection necessary to design an effective and safe vaccine available for the population most affected by this pathogen.
Considering the accumulated knowledge of hRSV proteins that act as negative modulators of T-cell physiology, we propose that during host-virus co-evolution the hRSV has selected/evolved virulence determinants with complementary functions at inhibiting T-cell activation and effector functions. Indeed, hRSV proteins impair several critical steps occurring during the development of T-cell responses; from naïve T-cell priming (by hRSV N protein), the acquisition of proper Th1 anti-viral CD4+ responses (by hRSV NS1 protein), the proper recruitment of CTLs and helper T cells within infected lungs (by hRSV G glycoprotein) and the execution of antigen-specific CD8+ cytotoxicity (by hRSV F glycoprotein). Accordingly, new, rational vaccine design strategies should consider the development of T-cell responses with the capacity to circumvent the mechanisms imposed by hRSV to restraint the host adaptive immunity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Millennium Institute on Immunology and Immunotherapy from Chile (P09/016-F for AMK and SB), La Région Pays de la Loire through the “Chaire d’excellence program” for AMK and Grant “Nouvelles Equipes-nouvelles thématiques”(to AMK and SMB), the ECOS France-Chile grant, FONDECYT no 1070352, FONDECYT no 1050979, FONDECYT no 1040349, FONDECYT no 1100926, FONDECYT no 1110397, FONDECYT no 1131012, FONDECYT no 1140010, FONDECYT no 3140455 and the Biomedical Research Consortium CTU06. JAE and KB are CONICYT-Chile fellows.
References
- Bueno SM, González PA, Pacheco R, Leiva ED, Cautivo KM, Tobar HE, Mora JE, Prado CE, Zúñiga JP, Jiménez J, et al. Host immunity during RSV pathogenesis. Int Immunopharmacol 2008; 8:1320 - 9; http://dx.doi.org/10.1016/j.intimp.2008.03.012; PMID: 18687294
- Bueno SM, González PA, Riedel CA, Carreño LJ, Vásquez AE, Kalergis AM. Local cytokine response upon respiratory syncytial virus infection. Immunol Lett 2011; 136:122 - 9; http://dx.doi.org/10.1016/j.imlet.2010.12.003; PMID: 21195729
- Hacking D, Hull J. Respiratory syncytial virus--viral biology and the host response. J Infect 2002; 45:18 - 24; http://dx.doi.org/10.1053/jinf.2002.1015; PMID: 12217726
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543 - 6; PMID: 3706232
- El Saleeby CM, Devincenzo JP. Respiratory syncytial virus load and disease severity in the community. J Med Virol 2011; 83:904 - 5; http://dx.doi.org/10.1002/jmv.22039; PMID: 21412798
- El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011; 204:996 - 1002; http://dx.doi.org/10.1093/infdis/jir494; PMID: 21881113
- van Drunen Littel-van den Hurk S, Watkiss ER. Pathogenesis of respiratory syncytial virus. Curr Opin Virol 2012; 2:300 - 5; http://dx.doi.org/10.1016/j.coviro.2012.01.008; PMID: 22709517
- Thornburg NJ, Hayward SL, Crowe JE Jr.. Respiratory syncytial virus regulates human microRNAs by using mechanisms involving beta interferon and NF-κB. MBio 2012; 3:e00220-12; http://dx.doi.org/10.1128/mBio.00220-12; PMID: 23249809
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917 - 30; http://dx.doi.org/10.1016/S0140-6736(11)61051-9; PMID: 22078723
- Gralton J, Tovey ER, McLaws ML, Rawlinson WD. Respiratory virus RNA is detectable in airborne and droplet particles. J Med Virol 2013; 85:2151 - 9; PMID: 23959825
- Hall CB, Douglas RG Jr., Schnabel KC, Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun 1981; 33:779 - 83; PMID: 7287181
- Hall CB, Douglas RG Jr.. Modes of transmission of respiratory syncytial virus. J Pediatr 1981; 99:100 - 3; http://dx.doi.org/10.1016/S0022-3476(81)80969-9; PMID: 7252646
- Domurat F, Roberts NJ Jr., Walsh EE, Dagan R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J Infect Dis 1985; 152:895 - 902; http://dx.doi.org/10.1093/infdis/152.5.895; PMID: 2931491
- Yui I, Hoshi A, Shigeta Y, Takami T, Nakayama T. Detection of human respiratory syncytial virus sequences in peripheral blood mononuclear cells. J Med Virol 2003; 70:481 - 9; http://dx.doi.org/10.1002/jmv.10421; PMID: 12767015
- Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res 2011; 162:80 - 99; http://dx.doi.org/10.1016/j.virusres.2011.09.020; PMID: 21963675
- Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 1999; 12:298 - 309; PMID: 10194461
- Ahmadian G, Randhawa JS, Easton AJ. Expression of the ORF-2 protein of the human respiratory syncytial virus M2 gene is initiated by a ribosomal termination-dependent reinitiation mechanism. EMBO J 2000; 19:2681 - 9; http://dx.doi.org/10.1093/emboj/19.11.2681; PMID: 10835365
- Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A 1999; 96:11259 - 64; http://dx.doi.org/10.1073/pnas.96.20.11259; PMID: 10500164
- Cheng X, Park H, Zhou H, Jin H. Overexpression of the M2-2 protein of respiratory syncytial virus inhibits viral replication. J Virol 2005; 79:13943 - 52; http://dx.doi.org/10.1128/JVI.79.22.13943-13952.2005; PMID: 16254330
- Johnson CH, Miao C, Blanchard EG, Caidi H, Radu GU, Harcourt JL, Haynes LM. Effect of chemokine receptor CX3CR1 deficiency in a murine model of respiratory syncytial virus infection. Comp Med 2012; 62:14 - 20; PMID: 22330646
- Marr N, Turvey SE. Role of human TLR4 in respiratory syncytial virus-induced NF-κB activation, viral entry and replication. Innate Immun 2012; 18:856 - 65; http://dx.doi.org/10.1177/1753425912444479; PMID: 22535679
- Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 2000; 74:10508 - 13; http://dx.doi.org/10.1128/JVI.74.22.10508-10513.2000; PMID: 11044095
- Johnson TR, McLellan JS, Graham BS. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J Virol 2012; 86:1339 - 47; http://dx.doi.org/10.1128/JVI.06096-11; PMID: 22090124
- Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med 2011; 17:1132 - 5; http://dx.doi.org/10.1038/nm.2444; PMID: 21841784
- González PA, Carreño LJ, Bueno SM, Riedel CA, Kalergis AM. Understanding respiratory syncytial virus infection to improve treatment and immunity. Curr Mol Med 2013; 13:1122 - 39; http://dx.doi.org/10.2174/1566524011313070007; PMID: 23157678
- Noton SL, Deflubé LR, Tremaglio CZ, Fearns R. The respiratory syncytial virus polymerase has multiple RNA synthesis activities at the promoter. PLoS Pathog 2012; 8:e1002980; http://dx.doi.org/10.1371/journal.ppat.1002980; PMID: 23093940
- Collins PL, Murphy BR. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc 2005; 2:166 - 73; http://dx.doi.org/10.1513/pats.200501-011AW; PMID: 16113487
- Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc Natl Acad Sci U S A 2013; 110:11133 - 8; http://dx.doi.org/10.1073/pnas.1309070110; PMID: 23776214
- Rodriguez R, Ramilo O. Respiratory syncytial virus: how, why and what to do. J Infect 2014; 68:Suppl 1 S115 - 8; http://dx.doi.org/10.1016/j.jinf.2013.09.021; PMID: 24171820
- Mitra R, Baviskar P, Duncan-Decocq RR, Patel D, Oomens AG. The human respiratory syncytial virus matrix protein is required for maturation of viral filaments. J Virol 2012; 86:4432 - 43; http://dx.doi.org/10.1128/JVI.06744-11; PMID: 22318136
- Batonick M, Wertz GW. Requirements for Human Respiratory Syncytial Virus Glycoproteins in Assembly and Egress from Infected Cells. Adv Virol 2011; 2011; http://dx.doi.org/10.1155/2011/343408; PMID: 21931576
- Feldman SA, Audet S, Beeler JA. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol 2000; 74:6442 - 7; http://dx.doi.org/10.1128/JVI.74.14.6442-6447.2000; PMID: 10864656
- Gibbs JD, Ornoff DM, Igo HA, Zeng JY, Imani F. Cell cycle arrest by transforming growth factor beta1 enhances replication of respiratory syncytial virus in lung epithelial cells. J Virol 2009; 83:12424 - 31; http://dx.doi.org/10.1128/JVI.00806-09; PMID: 19759128
- Lindquist ME, Lifland AW, Utley TJ, Santangelo PJ, Crowe JE Jr.. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J Virol 2010; 84:12274 - 84; http://dx.doi.org/10.1128/JVI.00260-10; PMID: 20844027
- Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem 2007; 282:15315 - 8; http://dx.doi.org/10.1074/jbc.R700007200; PMID: 17395582
- Onomoto K, Jogi M, Yoo JS, Narita R, Morimoto S, Takemura A, Sambhara S, Kawaguchi A, Osari S, Nagata K, et al. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS One 2012; 7:e43031; http://dx.doi.org/10.1371/journal.pone.0043031; PMID: 22912779
- Lindquist ME, Mainou BA, Dermody TS, Crowe JE Jr.. Activation of protein kinase R is required for induction of stress granules by respiratory syncytial virus but dispensable for viral replication. Virology 2011; 413:103 - 10; http://dx.doi.org/10.1016/j.virol.2011.02.009; PMID: 21377708
- Langereis MA, Feng Q, van Kuppeveld FJ. MDA5 localizes to stress granules, but this localization is not required for the induction of type I interferon. J Virol 2013; 87:6314 - 25; http://dx.doi.org/10.1128/JVI.03213-12; PMID: 23536668
- Othumpangat S, Walton C, Piedimonte G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLoS One 2012; 7:e30030; http://dx.doi.org/10.1371/journal.pone.0030030; PMID: 22272270
- Głobińska A, Pawełczyk M, Kowalski ML. MicroRNAs and the immune response to respiratory virus infections. Expert Rev Clin Immunol 2014; 10:963 - 71; http://dx.doi.org/10.1586/1744666X.2014.913482; PMID: 24784476
- Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev 2013; 253:112 - 28; http://dx.doi.org/10.1111/imr.12060; PMID: 23550642
- Bakre A, Mitchell P, Coleman JK, Jones LP, Saavedra G, Teng M, Tompkins SM, Tripp RA. Respiratory syncytial virus modifies microRNAs regulating host genes that affect virus replication. J Gen Virol 2012; 93:2346 - 56; http://dx.doi.org/10.1099/vir.0.044255-0; PMID: 22894925
- Qin L, Hu CP, Feng JT, Xia Q. Activation of lymphocytes induced by bronchial epithelial cells with prolonged RSV infection. PLoS One 2011; 6:e27113; http://dx.doi.org/10.1371/journal.pone.0027113; PMID: 22216085
- Tripp RA. Respiratory Syncytial Virus (RSV) Modulation at the Virus-Host Interface Affects Immune Outcome and Disease Pathogenesis. Immune Netw 2013; 13:163 - 7; http://dx.doi.org/10.4110/in.2013.13.5.163; PMID: 24198740
- Kim TH, Lee HK. Innate immune recognition of respiratory syncytial virus infection. BMB Rep 2014; 47:184 - 91; http://dx.doi.org/10.5483/BMBRep.2014.47.4.050; PMID: 24568879
- Zeng R, Cui Y, Hai Y, Liu Y. Pattern recognition receptors for respiratory syncytial virus infection and design of vaccines. Virus Res 2012; 167:138 - 45; http://dx.doi.org/10.1016/j.virusres.2012.06.003; PMID: 22698878
- Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol 2009; 83:1492 - 500; http://dx.doi.org/10.1128/JVI.00671-08; PMID: 19019963
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 2000; 1:398 - 401; http://dx.doi.org/10.1038/80833; PMID: 11062499
- Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol 2006; 176:1733 - 40; http://dx.doi.org/10.4049/jimmunol.176.3.1733; PMID: 16424203
- Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol 2010; 185:2231 - 9; http://dx.doi.org/10.4049/jimmunol.1000733; PMID: 20624950
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005; 6:981 - 8; http://dx.doi.org/10.1038/ni1243; PMID: 16127453
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006; 441:101 - 5; http://dx.doi.org/10.1038/nature04734; PMID: 16625202
- Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 2004; 23:327 - 33; http://dx.doi.org/10.1183/09031936.03.00098803; PMID: 14979512
- LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001; 167:5868 - 73; http://dx.doi.org/10.4049/jimmunol.167.10.5868; PMID: 11698462
- Wright JR. Immunomodulatory functions of surfactant. Physiol Rev 1997; 77:931 - 62; PMID: 9354809
- Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol 2001; 63:521 - 54; http://dx.doi.org/10.1146/annurev.physiol.63.1.521; PMID: 11181966
- Barr FE, Pedigo H, Johnson TR, Shepherd VL. Surfactant protein-A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. Am J Respir Cell Mol Biol 2000; 23:586 - 92; http://dx.doi.org/10.1165/ajrcmb.23.5.3771; PMID: 11062136
- Hickling TP, Bright H, Wing K, Gower D, Martin SL, Sim RB, Malhotra R. A recombinant trimeric surfactant protein D carbohydrate recognition domain inhibits respiratory syncytial virus infection in vitro and in vivo. Eur J Immunol 1999; 29:3478 - 84; http://dx.doi.org/10.1002/(SICI)1521-4141(199911)29:11<3478::AID-IMMU3478>3.0.CO;2-W; PMID: 10556802
- Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 2002; 168:5989 - 92; http://dx.doi.org/10.4049/jimmunol.168.12.5989; PMID: 12055204
- Sano H, Chiba H, Iwaki D, Sohma H, Voelker DR, Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem 2000; 275:22442 - 51; http://dx.doi.org/10.1074/jbc.M001107200; PMID: 10801802
- Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem 2002; 277:6830 - 7; http://dx.doi.org/10.1074/jbc.M106671200; PMID: 11724772
- Miazgowicz MM, Elliott MS, Debley JS, Ziegler SF. Respiratory syncytial virus induces functional thymic stromal lymphopoietin receptor in airway epithelial cells. J Inflamm Res 2013; 6:53 - 61; PMID: 23576878
- Han J, Dakhama A, Jia Y, Wang M, Zeng W, Takeda K, Shiraishi Y, Okamoto M, Ziegler SF, Gelfand EW. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J Allergy Clin Immunol 2012; 130:1175 - 86, e9; http://dx.doi.org/10.1016/j.jaci.2012.08.033; PMID: 23036746
- Qiao J, Li A, Jin X. TSLP from RSV-stimulated rat airway epithelial cells activates myeloid dendritic cells. Immunol Cell Biol 2011; 89:231 - 8; http://dx.doi.org/10.1038/icb.2010.85; PMID: 20603637
- Lay MK, González PA, León MA, Céspedes PF, Bueno SM, Riedel CA, Kalergis AM. Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect 2013; 15:230 - 42; http://dx.doi.org/10.1016/j.micinf.2012.11.012; PMID: 23246463
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med 1974; 139:380 - 97; http://dx.doi.org/10.1084/jem.139.2.380; PMID: 4589990
- Zammit DJ, Cauley LS, Pham QM, Lefrançois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 2005; 22:561 - 70; http://dx.doi.org/10.1016/j.immuni.2005.03.005; PMID: 15894274
- Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW. Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol 2007; 179:1648 - 58; http://dx.doi.org/10.4049/jimmunol.179.3.1648; PMID: 17641031
- Iannello A, Debbeche O, Martin E, Attalah LH, Samarani S, Ahmad A. Viral strategies for evading antiviral cellular immune responses of the host. J Leukoc Biol 2006; 79:16 - 35; http://dx.doi.org/10.1189/jlb.0705397; PMID: 16204622
- Pretel E, Camporeale G, de Prat-Gay G. The non-structural NS1 protein unique to respiratory syncytial virus: a two-state folding monomer in quasi-equilibrium with a stable spherical oligomer. PLoS One 2013; 8:e74338; http://dx.doi.org/10.1371/journal.pone.0074338; PMID: 24058549
- Ling Z, Tran KC, Teng MN. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol 2009; 83:3734 - 42; http://dx.doi.org/10.1128/JVI.02434-08; PMID: 19193793
- Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol 2005; 79:5353 - 62; http://dx.doi.org/10.1128/JVI.79.9.5353-5362.2005; PMID: 15827150
- Munir S, Le Nouen C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J Virol 2008; 82:8780 - 96; http://dx.doi.org/10.1128/JVI.00630-08; PMID: 18562519
- Munir S, Hillyer P, Le Nouën C, Buchholz UJ, Rabin RL, Collins PL, Bukreyev A. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog 2011; 7:e1001336; http://dx.doi.org/10.1371/journal.ppat.1001336; PMID: 21533073
- Bitko V, Shulyayeva O, Mazumder B, Musiyenko A, Ramaswamy M, Look DC, Barik S. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth. J Virol 2007; 81:1786 - 95; http://dx.doi.org/10.1128/JVI.01420-06; PMID: 17151097
- Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, Buick R, Stevenson NJ, Touzelet O, Gadina M, et al. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol 2007; 81:3428 - 36; http://dx.doi.org/10.1128/JVI.02303-06; PMID: 17251292
- Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O’Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, et al. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis 2006; 193:573 - 81; http://dx.doi.org/10.1086/499600; PMID: 16425137
- Wu W, Tran KC, Teng MN, Heesom KJ, Matthews DA, Barr JN, Hiscox JA. The interactome of the human respiratory syncytial virus NS1 protein highlights multiple effects on host cell biology. J Virol 2012; 86:7777 - 89; http://dx.doi.org/10.1128/JVI.00460-12; PMID: 22593156
- Jeffree CE, Brown G, Aitken J, Su-Yin DY, Tan BH, Sugrue RJ. Ultrastructural analysis of the interaction between F-actin and respiratory syncytial virus during virus assembly. Virology 2007; 369:309 - 23; http://dx.doi.org/10.1016/j.virol.2007.08.007; PMID: 17825340
- Groskreutz DJ, Monick MM, Yarovinsky TO, Powers LS, Quelle DE, Varga SM, Look DC, Hunninghake GW. Respiratory syncytial virus decreases p53 protein to prolong survival of airway epithelial cells. J Immunol 2007; 179:2741 - 7; http://dx.doi.org/10.4049/jimmunol.179.5.2741; PMID: 17709487
- Lindemans CA, Coffer PJ, Schellens IM, de Graaff PM, Kimpen JL, Koenderman L. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J Immunol 2006; 176:5529 - 37; http://dx.doi.org/10.4049/jimmunol.176.9.5529; PMID: 16622022
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 2008; 82:2040 - 55; http://dx.doi.org/10.1128/JVI.01625-07; PMID: 17928346
- Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review. Virus Genes 2006; 33:235 - 52; PMID: 16972040
- Rixon HW, Brown G, Aitken J, McDonald T, Graham S, Sugrue RJ. The small hydrophobic (SH) protein accumulates within lipid-raft structures of the Golgi complex during respiratory syncytial virus infection. J Gen Virol 2004; 85:1153 - 65; http://dx.doi.org/10.1099/vir.0.19769-0; PMID: 15105532
- Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol 2007; 81:8361 - 6; http://dx.doi.org/10.1128/JVI.02717-06; PMID: 17494063
- Gan SW, Ng L, Lin X, Gong X, Torres J. Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci 2008; 17:813 - 20; http://dx.doi.org/10.1110/ps.073366208; PMID: 18369195
- Triantafilou K, Kar S, Vakakis E, Kotecha S, Triantafilou M. Human respiratory syncytial virus viroporin SH: a viral recognition pathway used by the host to signal inflammasome activation. Thorax 2013; 68:66 - 75; http://dx.doi.org/10.1136/thoraxjnl-2012-202182; PMID: 23229815
- Gan SW, Tan E, Lin X, Yu D, Wang J, Tan GM, Vararattanavech A, Yeo CY, Soon CH, Soong TW, et al. The small hydrophobic protein of the human respiratory syncytial virus forms pentameric ion channels. J Biol Chem 2012; 287:24671 - 89; http://dx.doi.org/10.1074/jbc.M111.332791; PMID: 22621926
- Langedijk JP, de Groot BL, Berendsen HJ, van Oirschot JT. Structural homology of the central conserved region of the attachment protein G of respiratory syncytial virus with the fourth subdomain of 55-kDa tumor necrosis factor receptor. Virology 1998; 243:293 - 302; http://dx.doi.org/10.1006/viro.1998.9066; PMID: 9568029
- Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 2012; 7:e29695; http://dx.doi.org/10.1371/journal.pone.0029695; PMID: 22295065
- Melero JA, García-Barreno B, Martínez I, Pringle CR, Cane PA. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol 1997; 78:2411 - 8; PMID: 9349459
- Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol 2013; 87:13466 - 79; http://dx.doi.org/10.1128/JVI.01741-13; PMID: 24089561
- Harcourt JL, Karron RA, Tripp RA. Anti-G protein antibody responses to respiratory syncytial virus infection or vaccination are associated with inhibition of G protein CX3C-CX3CR1 binding and leukocyte chemotaxis. J Infect Dis 2004; 190:1936 - 40; http://dx.doi.org/10.1086/425516; PMID: 15529257
- Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 2006; 176:1600 - 8; http://dx.doi.org/10.4049/jimmunol.176.3.1600; PMID: 16424189
- Johnson TR, Teng MN, Collins PL, Graham BS. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J Virol 2004; 78:6024 - 32; http://dx.doi.org/10.1128/JVI.78.11.6024-6032.2004; PMID: 15141000
- Tripp RA, Dakhama A, Jones LP, Barskey A, Gelfand EW, Anderson LJ. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J Virol 2003; 77:6580 - 4; http://dx.doi.org/10.1128/JVI.77.11.6580-6584.2003; PMID: 12743318
- Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab’)2 components mediates reduced pulmonary inflammation in mice. J Gen Virol 2009; 90:1119 - 23; http://dx.doi.org/10.1099/vir.0.009308-0; PMID: 19264600
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2001; 2:732 - 8; http://dx.doi.org/10.1038/90675; PMID: 11477410
- Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol 1999; 73:7099 - 107; PMID: 10438795
- Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol 1998; 72:2871 - 80; PMID: 9525607
- Stevens WW, Sun J, Castillo JP, Braciale TJ. Pulmonary eosinophilia is attenuated by early responding CD8(+) memory T cells in a murine model of RSV vaccine-enhanced disease. Viral Immunol 2009; 22:243 - 51; http://dx.doi.org/10.1089/vim.2009.0016; PMID: 19594395
- Chang J, Choi SY, Jin HT, Sung YC, Braciale TJ. Improved effector activity and memory CD8 T cell development by IL-2 expression during experimental respiratory syncytial virus infection. J Immunol 2004; 172:503 - 8; http://dx.doi.org/10.4049/jimmunol.172.1.503; PMID: 14688360
- Mastrangelo P, Hegele RG. RSV fusion: time for a new model. Viruses 2013; 5:873 - 85; http://dx.doi.org/10.3390/v5030873; PMID: 23518574
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340:1113 - 7; http://dx.doi.org/10.1126/science.1234914; PMID: 23618766
- Ternette N, Tippler B, Uberla K, Grunwald T. Immunogenicity and efficacy of codon optimized DNA vaccines encoding the F-protein of respiratory syncytial virus. Vaccine 2007; 25:7271 - 9; http://dx.doi.org/10.1016/j.vaccine.2007.07.025; PMID: 17825960
- Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev 2005; 18:541 - 55; http://dx.doi.org/10.1128/CMR.18.3.541-555.2005; PMID: 16020689
- Takeuchi R, Tsutsumi H, Osaki M, Sone S, Imai S, Chiba S. Respiratory syncytial virus infection of neonatal monocytes stimulates synthesis of interferon regulatory factor 1 and interleukin-1beta (IL-1beta)-converting enzyme and secretion of IL-1beta. J Virol 1998; 72:837 - 40; PMID: 9420296
- Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol 2001; 75:10730 - 7; http://dx.doi.org/10.1128/JVI.75.22.10730-10737.2001; PMID: 11602714
- McDermott DS, Knudson CJ, Varga SM. Determining the breadth of the respiratory syncytial virus-specific T cell response. J Virol 2014; 88:3135 - 43; http://dx.doi.org/10.1128/JVI.02139-13; PMID: 24371055
- Rock MT, Crowe JE Jr.. Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology 2003; 108:474 - 80; http://dx.doi.org/10.1046/j.1365-2567.2003.01619.x; PMID: 12667209
- Fu Y, He J, Zheng X, Wu Q, Zhang M, Wang X, Wang Y, Xie C, Tang Q, Wei W, et al. Intranasal immunization with a replication-deficient adenoviral vector expressing the fusion glycoprotein of respiratory syncytial virus elicits protective immunity in BALB/c mice. Biochem Biophys Res Commun 2009; 381:528 - 32; http://dx.doi.org/10.1016/j.bbrc.2009.02.075; PMID: 19233131
- De Baets S, Schepens B, Sedeyn K, Schotsaert M, Roose K, Bogaert P, Fiers W, Saelens X. Recombinant influenza virus carrying the respiratory syncytial virus (RSV) F85-93 CTL epitope reduces RSV replication in mice. J Virol 2013; 87:3314 - 23; http://dx.doi.org/10.1128/JVI.03019-12; PMID: 23302879
- Behera AK, Matsuse H, Kumar M, Kong X, Lockey RF, Mohapatra SS. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem Biophys Res Commun 2001; 280:188 - 95; http://dx.doi.org/10.1006/bbrc.2000.4093; PMID: 11162498
- Ruigrok RW, Crépin T. Nucleoproteins of negative strand RNA viruses; RNA binding, oligomerisation and binding to polymerase co-factor. Viruses 2010; 2:27 - 32; http://dx.doi.org/10.3390/v2010027; PMID: 21994598
- Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagné N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 2009; 326:1279 - 83; http://dx.doi.org/10.1126/science.1177634; PMID: 19965480
- Oliveira AP, Simabuco FM, Tamura RE, Guerrero MC, Ribeiro PG, Libermann TA, Zerbini LF, Ventura AM. Human respiratory syncytial virus N, P and M protein interactions in HEK-293T cells. Virus Res 2013; 177:108 - 12; http://dx.doi.org/10.1016/j.virusres.2013.07.010; PMID: 23892143
- Money VA, McPhee HK, Mosely JA, Sanderson JM, Yeo RP. Surface features of a Mononegavirales matrix protein indicate sites of membrane interaction. Proc Natl Acad Sci U S A 2009; 106:4441 - 6; http://dx.doi.org/10.1073/pnas.0805740106; PMID: 19251668
- Galloux M, Tarus B, Blazevic I, Fix J, Duquerroy S, Eléouët JF. Characterization of a viral phosphoprotein binding site on the surface of the respiratory syncytial nucleoprotein. J Virol 2012; 86:8375 - 87; http://dx.doi.org/10.1128/JVI.00058-12; PMID: 22623798
- González PA, Prado CE, Leiva ED, Carreño LJ, Bueno SM, Riedel CA, Kalergis AM. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A 2008; 105:14999 - 5004; http://dx.doi.org/10.1073/pnas.0802555105; PMID: 18818306

