Abstract
Porcine Circovirus type 2 (PCV2) infections and associated diseases have been rarely studied in Africa. There is no report of PCV2 infection-associated morbidity and the level of awareness of stakeholders has never been investigated in Uganda. This cross sectional survey investigated the occurrence of Porcine Circovirus type 2 – systemic disease (PCV2-SD) among pigs and the associated level of awareness of stakeholders in Central Uganda. Data were collected using questionnaires, Focus Group Discussions (FGDs), key informant interviews and laboratory investigations. All respondents (n = 131) and farmers attending FGDs (n = 31) had never heard of PCV2-SD and only 16.7% (n = 2) of the interviewed animal health workers (n = 12) knew about the disease. Among the farms, 20 piglets presenting with a chronic wasting and a persistent diarrhea were detected and sampled for laboratory investigations. Severe lymphoid depletion with histiocytic and macrophage infiltration in lymphoid organs (n = 8), shortening of intestinal villi (n = 9), abscesses in various organs (n = 15) and granulomatous pneumonia (n = 2) were the major histopathological lesions described. Immunohistochemistry and PCR assays on organs with implicating lesions confirmed PCV2 infection in 25% (n = 5) of the 20 pigs. The study confirmed the occurrence of PCV2 infections among piglets with persistent diarrhea on pig farms in central Uganda and revealed a low level of associated knowledge among farmers and veterinary practitioners. The study arouses the need for systematic studies on prevalence of PCV2 infections and sensitization of stakeholders on occurrence of PCV2 infections in Uganda.
Introduction
Porcine Circovirus associated diseases (PCVADs) are emerging epizootic diseases of economic significance in swine [Citation1]. The occurrence of PCVADs is associated with Porcine Circovirus type 2 (PCV2) which is a single-stranded DNA virus that was first detected and described in Western Canada [Citation1,Citation2]. PCV2 infections have been reported across Europe, the Americas, Africa and Asia [Citation3–Citation6]. By far, Porcine Circovirus type 2 – systemic disease (PCV2-SD) previously called Postweaning Multisystemic Wasting Syndrome (PMWS) and PCV2 sub clinical infections are considered the most economically important PCVADs [Citation7]. Although, infections occur early in life and during fetal development [Citation8], PCV2-SD typically presents in piglets of 4–15 weeks of age [Citation9]. However, older pigs towards finishing stage are also susceptible [Citation9]. Morbidity often varies from 5 to 30% among cohorts in the nursery or finishing stages while case fatality rate may be above 50% [Citation9]. However, most infections remain unapparent and onset of PCV2-SD is triggered by several environmental stressors and immunosuppression of the host [Citation1].
Characteristically, PCV2-SD clinically presents with growth retardation, emaciation, laboured breathing, anaemia, persistent diarrhoea and sometimes jaundice among affected piglets [Citation10]. The most common necropsy findings include generalized lymphadenopathy and mottling of the lungs [Citation10]. In addition to PCV2-SD; PCV2 has been linked to several other PCVADs, such as granulomatous enteritis, porcine dermatitis and nephropathy syndrome (PDNS), porcine respiratory disease complex and occasional reproductive disorders [Citation11–Citation13].
Although no report is available on the economic importance of PCV2-SD to the pig industry, losses to individual farms accrue from high mortality rates, retarded growth, low carcass weights and increased management costs arising from treatment of PCV2-associated immunosuppressive conditions [Citation14]. The associated immunosuppression [Citation14] increases the prevalence and shedding of food-borne pathogens such as Campylobacter jejuni, Salmonella typhimurium, and Yersinia enterocolitica at slaughter [Citation15]. This increases the incidence of carcass contamination at slaughter and presents a serious public health challenge. Furthermore, repeated inadvertent use of antibiotics in PCV2-SD cases contributes to antimicrobial resistance and antibiotic residues in pork and pork products.
Studies on the occurrence of PCV2 infections are very limited in Africa [Citation16]. The occurrence of PCV2 infections was recently reported in Uganda [Citation6,Citation17]. Serological surveys have reported point prevalences ranging of up to 50.8% among domestic pigs [Citation17] while a molecular study on pig lymph nodes from an abattoir detected three cases out of the 35 sampled (8.6%) [Citation6]. However, there is no report on occurrence of PCV2 infections and Porcine Circovirus associated diseases (PCVADs) on pig farms in Central Uganda. Given the limited reports on PCVADs in Uganda, it remains unclear if animal health workers (AHWs) and farmers are aware of the occurrence of PCV2 infections. This study was therefore aimed at detecting PCV2-SD on commercial pig farms and assessment of stakeholders’ associated knowledge in Central Uganda.
Materials and methods
.1 Ethical approval
The study was approved by the higher degrees committee of the School of Veterinary Medicine and Animal Resources, Makerere University (HD/Min/3.1/11/AUG/2011). Written informed consent was obtained from farm owners and key informants to participate in the study.
.2 Study area, study population, study design and sampling
A cross sectional and descriptive study was conducted from four districts of central Uganda (Kampala, Mpigi, Wakiso and Mukono; ) from March to November 2013. These districts have the highest population of pigs in Uganda [Citation18]. Only pig farms which contacted the ambulatory clinic of the School of Veterinary Medicine and Animal Resources, Makerere University for veterinary services and those identified through district animal health workers (AHWs) were included in the study. Key informants included area AHWs and veterinary drug sellers. Only piglets that looked unthrifty and had a history of refractory diarrhea (unresponsive to antibiotic therapy) and dyspnoea were selected from study farms for detailed laboratory investigations.
.3 Collection of data on farm management practices and stakeholders’ knowledge on PCV2-SD
After obtaining consent to participate in the study, pre-tested semi-structured questionnaires were administered to collect data from the farmers or their representatives. On farm level, data were collected on pig farm management practices and occurrence of PCV2-SD implicating clinical signs. In-depth interviews were conducted using interview guides to establish key informants’ (n = 12) knowledge and awareness on PCV2-SD. Key informants included actively practicing veterinarians from the study districts (n = 6), clinicians from the Veterinary School, Makerere University (n = 2) and veterinary practitioners working in major veterinary drug outlets in each of the study districts (n = 4). Three FGDs were conducted in each of the districts of Mpigi, Mukono and Wakiso to validate data collected using questionnaires and get an in depth insight into the level of pig farmers knowledge on PCV2-SD. The FGDs were constituted by 9 to 12 farmers each (total of 31 participants) and lasted up to 50 min. The discussions focused on: knowledge/awareness about PCV2-SD and treatment/prevention of chronic diarrhea. Proceedings were documented in writing by two independent repertoires.
.4 Necropsy and collection of tissue samples for laboratory investigations
Pigs on farms were examined to identify piglets showing clinical signs suggestive of PCV2-SD (weaned piglets presenting with emaciation and persistent diarrhea). Necropsies were performed on the sampled piglets using a standard autopsy technique as described by King et al. [Citation19]. The organ samples (lungs, spleen, kidneys, lymph nodes, tonsils, intestines, liver and heart) were taken for further laboratory analysis. During autopsy, gross lesions were described. Tissue samples were immediately fixed in 10% buffered formalin and then submitted to Central Diagnostic Laboratory (CDL), Makerere University for histopathological, immunohistochemical (IHC) and polymerase chain reaction (PCR) analyses.
.5 Histopathology and Immunohistochemistry
After fixation (maximum of 14 days), approximately 5 mm thick tissue sections were cut for processing in an automated histokinete (SLEE, Germany) into paraffin blocks. Tissue processing was done in accordance with standard histopathology procedures described by Edna et al. [Citation20]. After processing, tissues were then embedded in molten paraffin using individual base moulds attached to plastic cassettes before cooling on a cold surface. The excess paraffin was trimmed off before sections of 5 µM thickness were cut with a microtome for Haematoxylin and Eosin (H&E) and IHC staining using standard methods (Mayer’s H&E and PAP for IHC, respectively) [Citation20].
Paired sections were made and stained as described in the IHC protocol provided by the manufacturer (DAKO, Tokyo, Japan). Tissue sections for IHC (spleen, intestines and lymph nodes) were held on silanised slides (DAKO, Tokyo, Japan). Briefly, the tissue sections were deparaffinized in xylene and decreasing concentrations of ethanol before washing in distilled water and Phosphate Buffered Saline (PBS). Endogenous peroxidase was blocked by immersion of the deparaffinized tissues in 3% hydrogen peroxide mixed with 0.5% methanol for 30 min before rising with distilled water and PBS. For antigen retrieval, slides were incubated in freshly prepared trypsin (0.25%) at 37 °C for 10 min and then washing with PBS for three times each five minutes. The sections were then incubated at 4 °C in PCV2 specific primary antibody (Ingenasa, Madrid, Spain) overnight before rinsing in PBS. This was followed by incubation at room temperature in a secondary antibody (rabbit anti-mouse IgG) conjugated to Horseradish peroxidase enzyme labeled polymer (Sigma, Aldrich, Germany). Development was effected with DAB (3,3′-diaminobenzidine tetrachloride) and hydrogen peroxide in PBS for 3 min before rinsing with PBS followed by distilled water. Mayer’s Hematoxylin was used as a counterstaining for 3 min. Eventually the sections were dehydrated in graded alcohol (70%X2 for 3 min each, 100%X2 for 20 min each), cleared with xylene for 20 min before mounting with DPX.
The processed slides were viewed under a light microscope at various magnifications and findings were described. Major findings were also documented by microphotography. Examination of H&E slides was for identification of established PCV2 infection implicating lesions while IHC was used for localization of antigens in tissues positive on PCR or those with PCV2-like lesions identified by H&E technique. Tissue samples of known PCV2 status (negative and positive controls; gracefully provided by Prof. Dr. Manfred Reinacher, University of Giessen, Germany) were processed alongside the samples to validate the results.
.6 Diagnostic PCR for PCV2
For PCR, 10 sections (only spleen and lymph nodes) of 10 µm thick for each pig were cut and placed in 1.5 mL eppendorf® tubes containing 1 mL of xylene for deparaffinization. In between samples, microtome blades were cleaned with xylene to prevent cross contamination of samples. Extraction of DNA was done using QIAamp® DNA Formalin Fixed Paraffin Embedded (FFPE) Tissue Kit (QIAGEN GmbH, D-40724 Hilden, Germany) as per the manufacturer’s protocol. Negative and positive controls from the University of Giessen, Germany were also processed alongside the samples to validate the findings.
Amplification of target DNA was done using QIAGEN HotStar HiFidelity PCR Kit as per the manufacturer’s protocol. Briefly, 5 µL of sample DNA template was added to 10 µL of Master mix in a PCR tube and run in a thermocycler (PTC-100™, MJ Research, Inc. USA). The components for master mix (included 2.5 µL of 10xPCR buffer, 5 µL of 5xSoln Q, 8.75 µL of ddH2O, 1 µL of 25 mM MgCl2, 0.5 µL of dNTPs 10 mM, 1 µL of ORF2-PCV2-Forward, 1 µL of ORF2-PCV2-Reverse primer and 0.25 µL HotStar HiFidelity DNA polymerase. PCV2 specific primer pair described by Ouardani et al., 1999: ORF2.PCV2.S4-Forward (CACGGATATTGTAGTCCTGGT) and ORF2.PCV2.AS4-Reverse (CCGCACCTTCGGATATACTGTC) from HVD Biotech Vertriebs Ges.mbH, Austria with an expected product of 480 bp were used.
PCR conditions included initial activation step of heating at 94 °C for 5 mins, denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C and the cycle from denaturation to extension was repeated for 40 cycles, then final extension at 72 °C for 5 min and end of PCR cycling at 4 °C. 5 µL of the amplified products were analyzed by electrophoresis (120v for 1 h) on 1.5% (w/v) agarose gels and visualized under UV light after staining with ethidium bromide and photographs taken.
.7 Statistical analysis
Data obtained during the field survey were entered in Microsoft Excel sheets (Microsoft 2007) before exportation to SPSS 11.5 program for statistical analysis. Summarized data were presented in tables as frequencies and percentages. Chi-square test and binomial test were used to establish the significance of differences between proportions and P values of <.05 were considered significant. For histopathology, IHC and PCR, the findings were described and documented by microphotography. Results of laboratory findings were also summarized as percentages. Qualitative data from the FGDs and key informant interviews were summarized according to themes and presented as summary statements with some sample quotations presented in the results.
Results
.1 Socio-demographic background of farms
In total, 131 pig farms were sampled for the study. shows the demographic characteristics of the respondents.
able 1 Socio-demographic characteristics of the respondents.
.2 Managemental practices on the pig farms and occurrence of PCV2-SD-like conditions
All the farmers interviewed in this study reported death of weaned piglets in the past six months. Majority of the farmers (57.3%; P = .058) had ever observed emaciation among weaned piglets before death. The farmers reported emaciated piglets practiced semi intensive (n = 11) and intensive management systems (n = 64), P = .27. Diarrhea refractory to treatments was reported among few pig farms (n = 37; P < .0001). and highlight the husbandry practices and extent of occurrence of PCV2-SD-like clinical signs on the study farms, respectively. None of the farmers screened pigs for major infectious diseases before introduction into their farms.
able 2 Management practices of farms (n = 131).
able 3 Occurrence of PCV2-SD implicating clinical signs and their management.
.3 Farmer knowledge on PCV2-SD
All the farmers or their representatives (n = 131; 100%) had never heard of PCV2-SD as a disease of pigs. Neither use of any vaccine against PCV2-SD nor diagnosis of PCV2-SD was reported among the pigs on any of the farms visited. In the three focus group discussions (FGDs), although all the respondents reported ever seeing weaned piglets getting emaciated, none knew about PCV2-SD and as such, there were no local names for the disease. In one of the FGDs, one participant (male, Wakiso) reported: “I have never heard about such a disease”. “[……] not even the doctors who treat my pigs have ever told me that my pigs are suffering from the disease you mentioned”. Generally, farmers attributed the emaciation of pigs to poor nutrition, helminthiasis or other long standing diseases. When the implicating clinical picture was presented to the farmers, farmers reported the occurrence of such morbidity among grower pigs but no technical personnel had ever talked to them about the disease. One of the farmers (male) with over 600 pigs in Wakiso said, ‘When you find any positive result, you let us know such that we improve our management’. Antibiotic therapy especially sulphonamides administration (S-Dime®, Norodine®) and deworming were the common treatments administered to the affected piglets. Regular deworming and proper feeding were reported as the main control practices for progressive weight loss.
.4 Responses of animal health workers regarding PCV2-SD
The actual records of pig farmers in the districts visited were not available although veterinarians had personal contacts with farmers and had some information on approximate number of farmers in their respective areas of operation. Of the 12 veterinarians interviewed only 2 (all from the university) had ever heard about PCV2-SD. However, all the field practitioners reported seeing progressive loss of weight in weaned piglets. Such morbidities were attributed to weaning stress, helminthiasis, poor nutrition and other enteropathies such as corona virus and rotavirus infections. Two veterinarians in Wakiso reported experiencing progressive weight loss coupled with intermittent diarrhea which was refractory to prolonged antibacterial therapy (S-Dime®, Enrosol®, Oxytetracycline, Norodine®) and disinfectants in water. The drug sellers reported selling anti-diarrheals and antibiotics to pig farmers to manage chronic diarrhoea. The drug dealers attributed the increased use of antibiotic among pig farmers to outbreaks of African Swine Fever (ASF) and various bacterial diseases. No drug company reported importation of vaccines specifically for pigs. Moreover, dispensing personnel did not know that the commercial vaccines for PCV2 were available.
.5 Occurrence of chronic wasting among piglets on farms and their clinical presentations
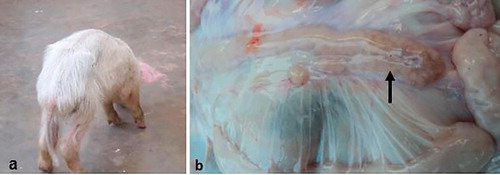
Among the 131 farms sampled, twenty chronically emaciated piglets were detected. Of these; twelve (60%) were from Wakiso, Five (25%) from Mukono, two (10%) from Mpigi and one (5%) from Kampala. All the affected pigs were on intensive management with over 100 pigs in a herd. Weaning of piglets on these farms was done between 5 and 6 weeks of age and all farms were mixing different litters. On the respective farms, there were reports of piglets that had clinical signs of chronic wasting () soon after weaning with intermittent diarrhoea. One farm from Wakiso also reported reproductive problems ranging from increased number of stillbirths of different sizes to unthrifty piglets born with tremors. The same farm also reported incidences of skin lesions that appeared as necrotic patches on ventral abdomen and flanks among weaners and fatteners. On this particular farm, none of the cases was thoroughly investigated but two of the sampled piglets were positive to PCV2 on PCR and IHC. Other than cachexia and persistent diarrhea, these two moribund piglets also presented with breathing difficulties and they were reportedly non responsive to treatment with Enrosol® (Enrofloxacin 10%).
.6 Gross and histopathological findings
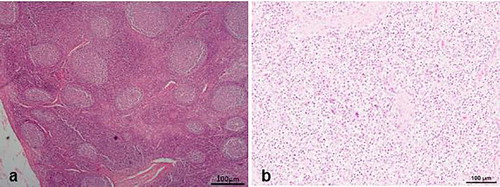
From the twenty piglets with cachexia from farms, 8 had enlarged inguinal and mesenteric lymph nodes (), all of which had necrotic cut surfaces. Six piglets were found to have ulcerative gastroenteritis and these had persistent diarrhoea. Moribund cases (n = 11) had an average of 109 cfu/g of β-haemolytic E. coli colonies in the duodenal contents. In all these cases, enterotoxigenic E. coli (ETEC) was identified by demonstrating fimbriae antigens K88, K99 987P and F41. Five of these cases were positive on PCR and Immunohistochemistry for PCV2 infections. Some pigs (n = 15) also had non-collapsing lungs which appeared grayish and pneumonic. Multiple abscesses were detected in lungs, lymph nodes and abdominal viscera in the piglets with lymphadenopathy.
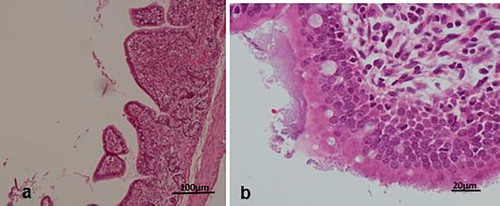
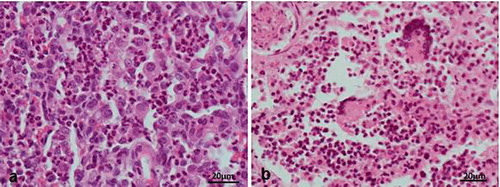
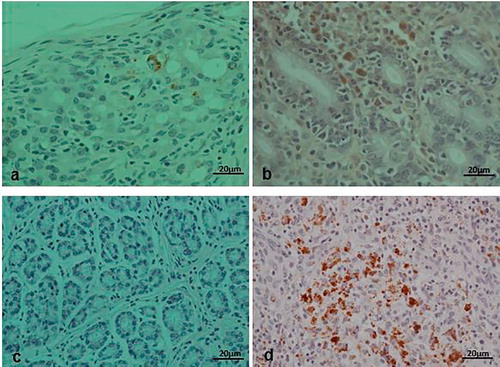
Histologically, the diseased pigs which presented with enlarged lymph nodes (n = 8) had lymphoid necrosis or severe lymphoid depletion with total loss of follicular architecture () coupled with extensive histiocytic and macrophages infiltrations. The intestinal villi were shortened and in some cases bacteria were attached to the mucosal surfaces (n = 6) (). Granulomatous inflammation with large numbers of macrophages and giant cells were visible in the lungs of two pigs from Wakiso ().
.7 PCR result
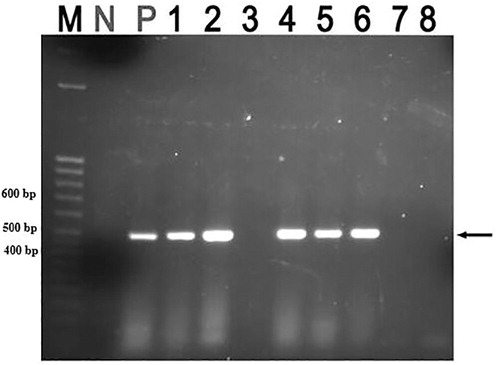
A total of five samples from emaciated pigs on farms were found to be positive for PCV2 (), three of which were from the same farm. The other two cases came from two other different farms and all positive cases came from Wakiso district ().
.8 Immunohistochemistry results
The positive lymph node samples (5 in number) to PCV2 on PCR and those with highly suspicious on H&E were processed using immunohistochemical techniques. In total, lymph nodes from 5 animals were positive to PCV2 antigens on IHC and these were the same positive animals on PCR analysis. Positive result was shown by brown deposits of the substrate chromogen in the affected cells ().
Discussion
PCV2-SD is a recently characterized disease of pigs [Citation1]. The confirmation of PCV2 infections is by demonstration of viral antigens or DNA through IHC or in situ hybridisation [Citation21,Citation22] and PCR [Citation23]. These two diagnostic techniques require well established laboratory facilities and trained personnel. Since Uganda currently lacks these facilities at district level; confirmatory diagnosis, surveillance and subsequent information dissemination to pig farmers (mainly small holders) may not be possible. This may partly explain the limited level of awareness of animal health practitioners and the farmers.
Lack of appropriate information on PCV2 infections and associated diseases is likely to contribute to the propagation of these infections. Also, appropriate management aimed at minimizing the risk factors, may be overlooked by farmers. For example, 99.2% (P < .001) of the farmers mixed weaned piglets from different sows thus age groups and different sources. Also, early weaning (below five weeks) was being practiced by over 30% of the farmers. The later contributes to early withdrawal of maternal antibodies before development of the immune system. The risk of occurrence and aggravation of morbidity due to PCV2 infections is known to be compounded by the above factors [Citation1,Citation24–Citation26]. Moreover, most pig farms in Uganda hardly implement proper bio-security measures [Citation17]. Although effective commercial vaccines against PCV2 infections have been developed [Citation27,Citation28], their use to control PCVADs has never been explored in Uganda. Occurrence of PCV2 infections on some farms as revealed by this study indicates failure to control triggers for onset of PCV2-SD which presents a big risk for occurrence of epidemic outbreaks.
PCV2 infections are immunosuppressive and frequently occur with persistent secondary bacterial infections. Limited knowledge of pig farmers and field animal health workers (AHWs) on the existence of PCV2-SD presents clinical diagnostic errors which may inadvertently contribute to unwarranted use of antibiotics and other chemotherapeutic agents targeting diseases with similar manifestations. Indeed, 87% of the farmers reported persistent diarrhea in this study continued treating refractory cases when the pigs did not improve after the first treatment regimen. Such practices may result in contamination of pork and pork products with antibiotic and other drug residues.
Immunosuppression, a major feature of infected pigs, may also contribute to pig products contamination with food borne pathogens like Salmonella typhimurium, Campylobacter jejuni and Yersinia enterocolitica [Citation14]. Also, persistent use of antibiotics among pigs with PCV2-SD may contribute to the emergence of multi-antibiotic resistant bacteria. The above health hazards may discourage consumers from the consumption of pig products. This may retard the growth of the pig industry, one of the most rapidly growing animal enterprises in Uganda [Citation18].
Only five (25%) of the 20 chronically wasted pigs were positive for PCV2. This emphasizes the importance of confirmatory diagnosis in disease management. Prolonged fixation of tissues in formalin may affect the sensitivity of IHC and PCR. In this study, samples were processed within two weeks to minimize the effects of prolonged fixation of tissues in formalin. Despite the confirmation of PCV2 infections from one district (Wakiso), the extent of the problem may be widespread since there is a limited control in the movement of breeding stock amidst poor diagnosis, subclinical states and limited knowledge. Actually, the study sampled only pigs presenting with chronic diarrhea for laboratory detection of PCV2 in tissues hence subclinical infections which are highly prevalent in other regions of Uganda [Citation6,Citation17] were not investigated. Chronic wasting among pigs that were negative for PCV2 could not be fully characterized as their causes were not investigated. Such may include malnutrition and viral enteropathies such as rotavirus, parvovirus and corona virus infections among others. Future studies are recommended to investigate the extent of PCVADs and other enteropathies and their contribution to the onset of PCV2-SD.
Conclusions
This study demonstrated the occurrence of PCV2 infections associated with chronic wasting and persistent diarrhea in pigs on farms in Central Uganda and low levels of knowledge on PCV2-SD among farmers and AHWs. It is therefore imperative to sensitize farmers and veterinary practitioners about the existence of PCV2 infections and PCVADs with their possible effects on the Ugandan pig industry, public health and pig welfare. Further studies on the prevalence and mapping of PCV2 infections and PCVADs are required to establish the extent of the infection to guide interventions. Vaccination against PCVADs should also be encouraged to control the disease on pig farms.
Competing interests
Authors have no conflict of interest to declare.
Acknowledgement
We acknowledge the support of pig farmers and animal health workers in the study districts. Positive and negative control blocks for IHC and PCR were gracefully provided by Prof. Dr. Manfred of Reinacher, University of Giessen, Germany. This work was part of Wilfred Eneku’s MSc. Dissertation.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- M.K.GhebremariamE.GruysPostweaning Multisystemic Wasting Syndrome (PMWS) in pigs with particular emphasis to causative agent, the mode of transmission, the diagnostic tools and the control measures: a reviewVet Q105–1162005
- J.C.S.HardingE.G.ClarkJ.H.StrokappeP.I.WilsonJ.A.EllisPost-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentationJ Swine Health Prod61998249254 [Accessed on 30 April 2018]
- C.ChaePostweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathologyVet J16820044149 10.1016/j.tvjl.2003.09.018
- P.WallgrenF.HasslungG.BergströmA.LinderK.BelakC.Hård af SegerstadPostweaning multisystemic wasting syndrome–PMWS: the first year with the disease in SwedenVet Q262004170187
- O.GarkavenkoR.ElliottM.CroxsonIdentification of pig circovirus type 2 in New Zealand pigsTransplant Proc2005372018506509 10.1016/j.transproceed.2004.12.301 [Accessed on 13 August]
- L.OjokJ.OkuniC.HohlochW.HechtM.ReinacherDetection and characterisation of porcine circovirus 2 from Ugandan pigsIndian J Vet Pathol3720137780 http://www.indianjournals.com/ijor.aspx?target=ijor:ijvp&volume=37&issue=1&article=019; [Accessed on 30 April 2018]
- A.R.ResendesJ.SegalesCharacterisation of vascular lesions in pigs affected by porcine circovirus type – systemic diseaseVet Pathol522015497504 https://doi.org/10.1177/0300985814540542https://doi.org/http://journals.sagepub.com/doi/abs/10.1177/0300985814540542?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed [Accessed on 13 August 2018]
- L.Dieste-PerezA.Van NesK.Van MaanenT.DuinhofT.TobiasThe prevalence of PCV2 viremia in newborn piglets on four endemically infected Dutch sow farms is very lowPrev Vet Med15320184246 10.1016/j.prevetmed.2018.03.001
- G.M.AllanJ.A.EllisPorcine circoviruses: a reviewJ Vet Diagn Invest122000314http://journals.sagepub.com/doi/abs/10.1177/104063870001200102?url_ver=Z39.88-2003%26rfr_id=ori:rid:crossref.org%26rfr_dat=cr_pub%3dpubmed
- E.AlbinaC.TruongE.HutetP.BlanchardR.L.CarioletR.HospitalierAn experimental model for postweaning multisystemic wasting syndrome in growing pigletsJ Comp Pathol1252001292303
- C.ChaeA review of porcine circovirus 2-associated syndromes and diseasesVet J1692005326336 10.1016/j.tvjl.2004.01.012
- B.MeehanF.McNeillyI.McNairI.WalkerJ.EllisS.KrakowkaIsolation and characterisation of porcine circovirus 2 from cases of sow abortion and porcine dermatitis and nephropathy syndromeArch Virol1462001835842
- J.SegalesC.RosellM.DomingoPathological findings associated with naturally acquired porcine circovirus type 2 associated diseaseVet Microbiol982004137149 10.1016/j.vetmic.2003.10.006
- J.SegalésM.DomingoF.ChianiniN.MajóJ.DomínguezL.DarwichImmunosuppression in postweaning multisystemic wasting syndrome affected pigsVet Microbiol982004151158 10.1016/j.vetmic.2003.10.007
- A.J.C.CookS.R.PascoeA.C.J.WilesmithA case-control study of post-weaning multi-systemic wasting syndrome (PMWS) and porcine dermatitis nephropathy syndrome (PDNS)Pig J4820015360
- K.AfolabiB.IwerieborA.OkohL.ObiGlobal Status of Porcine circovirus Type 2 and Its Associated Diseases in Sub-Saharan AfricaAdv Virol2017 6807964 10.1155/2017/6807964 [Accessed on 13 August 2018]
- M.DioneC.MasembeJ.AkolW.AmiaJ.KunguB.WielandThe importance of on-farm biosecurity: sero-prevalence and risk factors of bacterial and viral pathogens in smallholder pig systems in UgandaActa Trop2018 10.1016/j.actatropica.2018.06.025 [Accessed on 13 September 2018]
- A.TatwangireUganda Smallholder Pigs Value Chain Development: Situation Analysis and Trends2014International Livestock Research Institute (ILRI)Nairobi, Kenya
- J.KingL.Roth-JohnsonD.DoddM.NewsonThe Necropsy Book. 6245 Formoor Lane2005Charles Louis Davis, D.V.M. FoundationGurnee, Illinois, 60031-4757, USA
- B.EdnaM.BobB.JacquelynH.LeslieLaboratory Methods in Histotechnology1992American Registry of PathologyWashington, D.C
- F.ChianiniN.MajóJ.SegalésJ.DomínguezM.DomingoImmunohistochemical characterisation of PCV2 associate lesions in lymphoid and non-lymphoid tissues of pigs with natural postweaning multisystemic wasting syndrome (PMWS)Vet Immunol Immunopathol9420036375 10.1016/S0165-2427(03)00079-5
- F.McNeillyS.KennedyD.MoffettB.MeehanJ.FosterE.ClarkeA comparison of in situ hybridisation and immunohistochemistry for the detection of a new porcine circovirus in formalin fixed tissues from pigs with postweaning multisystemic wasting syndrome (PMWS)J Virol Methods801999123128 10.1016/S0166-0934(99)00043-9
- A.CaprioliF.Mc NeillyI.McNairP.Lagan-TregaskisJ.EllisS.KrakowkaPCR detection of Porcine Circovirus type 2 (PCV2) DNA in blood, tonsillar and faecal swabs from experimentally infected pigsRes Vet Sci812006287292 10.1016/j.rvsc.2006.01.001
- J.SegalésPorcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosisVirus Res16420121019 10.1016/j.virusres.2011.10.007
- N.RoseG.LarourG.Le DiguerherE.EvenoJ.JollyP.BlanchardRisk factors for porcine postweaning multisystemic wasting syndrome in 149 French farrow-to-finish herdsPrev Vet Med612003209225 10.1016/j.prevetmed.2003.07.003
- S.López-SoriaJ.SegalésN.RoseM.ViñasP.BlanchardF.MadecAn exploratory study on risk factors for postweaning multisystemic wasting syndrome (PMWS) in SpainPrev Vet Med69200597107 10.1016/j.prevetmed.2004.11.015
- J.JeongC.ParkS.KimS.J.ParkI.KangK.H.ParkEvaluation of the efficacy of a novel porcine circovirus type 2 synthetic peptide vaccineCan J Vet Res822018146153
- X.LiuT.OuyangT.MaH.OuyangD.PangL.RenImmunogenicity evaluation of inactivated virus and purified proteins of porcine circovirus type 2 in miceBMC Vet Res142018137 10.1186/s12917-018-1461-9