Abstract
Air-borne foliar diseases as well as soil-borne diseases can cause substantial losses in agricultural production systems. One of the strategies to overcome production losses caused by plant diseases is the targeted use of disease defence mechanisms that are inherent to plants. In this paper, the potential to enhance the plant's health status either by inducing resistance through optimized soil management techniques or by foliar application of inducers of resistance is explored on the basis of a literature review and results from laboratory and field experiments. In our studies, the focus was on recent research about the use of dl-β-aminobutyric acid (BABA) and an aqueous extract of Penicillium chrysogenum (Pen) as elicitors. We conclude that BABA as well as Pen can contribute to disease control strategies. The use of soil fertility management techniques to reduce diseases was explored in recent research about the impact of short- and long-term management practices on soil suppressiveness to air-borne and soil-borne diseases, with the aim to elucidate the influence of soil properties and to quantify the relative importance of site-specific vs cultivation-mediated soil properties. The results indicate that site-specific factors, which cannot be influenced by agronomic practices have a greater impact than cultivation-specific effects within the same site. Nevertheless, short- and long-term management strategies were shown to have the potential for influencing soil suppressiveness to certain diseases such as Rhizoctonia solani.
1 Introduction
In agriculture, infection of crops by pathogens like fungi, bacteria and viruses can cause high yield losses. To prevent damage from diseases, strategies have been developed that include the use of high-quality propagation material, sanitation measures (e.g., removal of overwintering sources of inoculum or infected volunteer plants), avoidance techniques, crop rotation, soil management, plant nutrition, and resistant varieties [Citation1]. In addition, pesticides or antagonists are widely applied. However, especially in organic agriculture, for whose products the demand has increased highly in the last decades [Citation2], it is imperative to substitute the use of plant protection chemicals such as copper and sulphur by improved biological methods [Citation3].
One of the strategies to overcome production problems caused by plant diseases is the targeted use of disease defence mechanisms that are inherent to plants [Citation1,Citation4,Citation5]. Besides preformed barriers and constitutively expressed antimicrobials, plants possess inducible defence mechanisms that are activated upon contact with pathogenic or non-pathogenic micro-organisms, extracts of micro-organisms or chemicals, thus providing protection against a broad spectrum of pathogens. Chemicals known to induce disease resistance in some plants include salicylic acid (SA) [Citation6], isonicotinic acid (INA) [Citation7], jasmonic acid [Citation8], acibenzolar-S-methyl (BTH) (commercially known as Bion®) [Citation9,Citation10], probenazole [Citation11], and dl-β-aminobutyric acid (BABA) [Citation12].
Furthermore, it has been shown that plants can recognize general structures associated with micro-organisms, so-called elicitors or PAMPs (Pathogen Associated Molecular Patterns) [Citation13], such as flagellin [Citation14] and harpin from bacteria [Citation15,Citation16], chitin [Citation17], ergosterol [Citation18] and several cell-wall glucans [Citation19,Citation20] from fungi and lamarins from algae [Citation21]. After bonding with a specific receptor of the plant, elicitors trigger a signalling cascade, eventually resulting in biochemical and mechanical defence mechanisms such as production of phytoalexins [Citation22], translation of specific proteins with putative antimicrobial activities [Citation23,Citation24] and mechanical strengthening of the cell walls [Citation25–Citation27]. It has been shown that depending on the stimulus, specific signal transduction pathways involving one or several of these key regulators are activated, leading to resistance against specific sets of pathogens. Besides these well-defined, pure molecules, various crude extracts from micro-organism or plants activating plant defence mechanisms have been described, including an extract from the giant knotweed (Reynoutria sachaliensis) (sold under the commercial name Milsana) [Citation3,Citation28,Citation29], or an aqueous extract from the ascomycete Penicillium chrysogenum (Pen) studied by Thürig et al. [Citation30,Citation31].
Extracts or chemical compounds inducing resistance are often referred to as ‘plant activators’, ‘inducers’ or, if derived from micro-organisms, ‘elicitors’. Classical inducers do not have a direct impact on pathogens, which clearly distinguishes them from fungicides [Citation32]. Inducers to be used in commercial agriculture have to be available in sufficient quantities, be of constant quality and be effective under field conditions. Moreover, to be acceptable in organic agriculture the compounds have to occur in nature and must not derive from genetically modified organisms [Citation33,Citation34].
In this paper, based on a literature review, on previously published research, and on some so far unpublished experiments, we explore the potential of enhancing the plant's health status either by inducing resistance via optimized soil management techniques or by foliar application of inducers of resistance.
2 Evaluation of elicitors and inducers of resistance
2.1 Plasmopara viticola in grapevine
Most studies on induced resistance have been performed on annual plants; much less is known about the effect of inducers on woody perennial plants under field conditions. Over the last decade, several substances or complex commercial products have been reported that are active against important plant diseases such as Plasmopara viticola in grapevine. Yet, so far, BABA and Pen appeared the only compounds with a proven efficacy against P. viticola in the field. In our experiments we have evaluated, under controlled conditions, several compounds including Agromos, BABA, Bion, ISR2000, Messenger, Milsana, Pen, salicylic acid and Stimulase against P. viticola. Next, the compounds with proven activity were further evaluated under field conditions. Methods used for the evaluation are described in detail in [Citation30]. In brief, seedlings of the grapevine cultivar Chasselas (kindly provided by Syngenta AG, Stein, Switzerland) were grown in the greenhouse until 3–4 fully expanded leaves had been formed. Then, the test substances were applied in an atomatized-spray cabinet where the plants were incubated at 100% relative humidity (RH) for 5 days before being inoculated with two drops (10 μl) per leaf of a sporangia suspension (50,000 sporangia per ml). Subsequently, the plants were incubated for 24 h at 100% RH, followed by 6 days at 60–80% RH before being returned to 100% RH, 12 h prior to measuring lesion diameters. Selected substances were tested in 2003 on the grapevine cvs. Riesling × Sylvaner and Chasselas (both on rootstock 5BB) in a field experiment in the institute's experimental vineyard in Frick, Switzerland (47°31′N, 8°01′E, at 376 m a.s.l.). The experiment was of the complete randomized block design with 12 treatments, 4 replications (6 plants per plot). The test products were applied taking into account weather conditions, plant growth, and the risk of infection by P. viticola over 5–10 day intervals, as determined by the model Vitimeteo [Citation35]. The substances were applied using air-assisted spraying equipment, which ensured coverage of lower and upper sides of the leaves and efficiently prevented drift. Disease incidence and severity were assessed 3–5 times per season, depending on disease progress. Efficacy was calculated according to [Citation36] as 100 × (1 − a × b−1) where a = lesion diameter on the treated leaves and b = lesion diameter on the control leaves. Data were analysed by ANOVA followed by a Tukey test at α = 0.05 for multiple comparisons.
Our experiments showed that under controlled conditions, Agromos, BABA, Bion, Pen, and salicylic acid reduced disease incidence significantly at least at one of the evaluated application dosages, whereas Stimulase, ISR2000, Messenger, and Milsana showed no significant activity at any of the tested concentrations (). Under field conditions, BABA and PEN significantly reduced disease incidence, while Bion and Stimulase were not effective (). These results suggest that it is difficult to control P. viticola by means of inducers of resistance. For further details about the interpretation of the variables assessed see [Citation30].
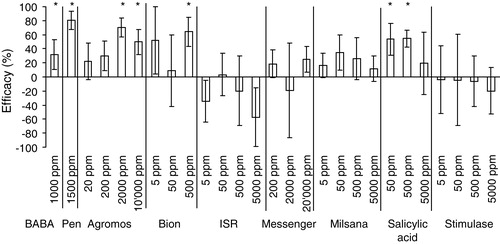
Table 1 Efficacy of elicitors against P. viticola in grapevine cv. Riesling × Sylvaner under field conditions in 2003.
2.2 Bremia lactucae in lettuce
Besides P. viticola, also other oomycete pathogens such as Bremia lactucae in lettuce and Phytophthora infestans in tomato are notoriously difficult to control and may cause substantial losses in organic vegetable production systems. Breeding for varietal resistance is very costly and novel varieties (especially those of lettuce) are introduced at high rates. However, the varietal resistance is often overcome within a very short period of time due to highly adaptive pathogen populations. Thus, induced resistance might be a promising alternative to both conventional fungicides and to breeding of resistant cultivars.
In a recent study, Cohen et al. [Citation37] evaluated the efficacy of dl-β-aminobutyric acid (BABA) in controlling downy mildew (B. lactucae) in lettuce with a focus on disease control under field conditions. dl-3-amino-n-butanoic acid (dl-β-aminobutyric acid, BABA) is a non-protein amino acid that has shown to induce resistance against about 50 plant pathogens in a large number of annual and perennial crops [Citation38,Citation39]. Oomycetes suppressed in their respective host tissues by BABA include Aphanomyces euteiches in pea [Citation40], Peronospora tabacina in tobacco [Citation41], Peronospora parasitica in Arabidopsis [Citation42] and cauliflower [Citation43], P. infestans in tomato and potato [Citation38], Phytophthora capsici in pepper (Capsicum sp.) [Citation44], P. viticola in vinegrape [Citation45,Citation46], Plasmopara halstedii in sunflower [Citation47], Sclerospora graminicola in sorghum [Citation48] and Pseudoperonospora cubensis in cucumber [Citation49]. Ascomycetes/Fungi Imperfecti controlled by BABA are Fusarium oxysporum f. sp. solani in tomato [Citation38], Botrytis cinerea and Plectosphaerella cucumerina in Arabidopsis [Citation50,Citation51], Monosporascus cannonballus in melon [Citation38], Alternaria alternata in apple [Citation52], Alternania brassicicola in Arabidopsis [Citation50] and Penicillium digitatum in grapefruit [Citation53].
Our studies have demonstrated that BABA was effective in controlling downy mildew in lettuce [Citation37]. In potted plants, a foliar spray with 250 mg BABA per litre, or a soil drench with 1.25 mg BABA per pot was sufficient to reduce the disease by ≥90%. The Systemic Acquired Resistance (SAR)-inducing compound sodium salicylate (NaSA) and its functional analogue benzodiothiazol-S-methyl ester (BTH) (Bion) were ineffective compared with BABA. In other pathosystems, these SAR compounds operate via the salicylic acid (SA) pathway by inducing pathogenesis related (PR-) proteins [Citation54–Citation56]. Their failure to protect lettuce against downy mildew might suggest that BABA may operate via a different, SA-independent pathway. Indeed, BABA was shown to protect tobacco against P. tabacina via an SA-independent pathway [Citation41]. In Arabidopsis, SA-dependent as well as SA-independent pathways have been reported [Citation57,Citation58]. Our results corroborate with those of Pajot et al. [Citation59], who tested BABA against B. lactucae in 7-days-old lettuce plants, showing that a 10 mM (1000 mg per litre) foliar spray reduced the disease index by 98%. No experiments, however, were conducted with lower concentrations, and neither was BABA tested by soil application.
Furthermore, a major finding of our study [Citation37] was that BABA was efficient in controlling downy mildew in lettuce not only under controlled conditions in growth chambers but also under field conditions. Foliar sprays with 201 and 1039 mg per litre resulted in 50 and 90% control of the disease, respectively. This may encourage the introduction of BABA to agriculture as a SAR compound against lettuce downy mildew. Due to the fact that BABA occurs naturally in tomato plants (Y. Cohen, unpublished data) it might also be considered for registration in organic farming. Only a limited number of studies were conducted with BABA in the field. Our own studies showed efficacy against downy mildew in grapevine [Citation60], late blight in potato and tomato [Citation38], rust in sunflower [Citation61] and moldy core in apple [Citation52]. Shailasree et al. [Citation48] showed that soaking the seeds of pearl millet in 50 mM BABA for 6 h induced durable resistance against downy mildew.
Still, the mode of action of BABA is not fully understood. BABA did not affect spore germination in vitro of B. lactucae, or germination and appressoria formation in planta [Citation37]. Post infection applications of BABA were highly effective in inhibiting the disease, indicating that penetration of the pathogen into the host was not affected. Our finding that BABA was effective even when applied after inoculation suggests no adverse effect on establishment of the pathogen in the host tissue but rather on tissue colonization by the fungus. In other pathosystems, BABA was shown to potentiate in the host enhanced callose and/or lignin depositions [Citation42], reactive oxygen species accumulation [Citation62], increased peroxidase activity [Citation63], enhanced synthesis of PR-proteins or their transcripts and elevated levels of transcripts of jasmonic acid or abscicic acid related transcripts [Citation39,Citation50,Citation57]. PR-protein analysis by Pajot et al. [Citation59] showed that BABA induced a weak accumulation of acidic PR-2 (β-1,3-glucanase) at 3–7 days after treatment, but not PR-1, PR-5 or PR-9. β-1,3-glucanase activity increased with time in BABA-treated plants.
In conclusion, BABA was shown to effectively control downy mildew development in lettuce in growth chambers and in the field. It was also effective when applied to the foliage or the root system. It exhibited pre (protective) and post infection (curative) efficacy and provided durable resistance against the disease in the field.
2.3 Various diseases in crop plants
Another example of an inducer of resistance that has recently been studied is Pen, an aqueous extract of the mycelium of the ascomycete P. chrysogenum [Citation30,Citation31]. The mycelium of this fungus is obtained as a by-product from penicillin production and is thus relatively cheap and available in sufficient quantities, both prerequisites for a potential use in practice. The objectives of our study were (1) to examine the effect of Pen on several plant × pathogen interactions under greenhouse and field conditions with a special focus on the systems grapevine – P. viticola and tomato – P. infestans, (2) to assess the quality of the raw material for the production of the aqueous extract, and (3) to evaluate potential side-effects of Pen.
It was demonstrated that Pen protects many crop plant species against several pathogens under greenhouse and field conditions [Citation30]. Pen-mediated resistance was effective under field conditions against powdery (Uncinula necator) and downy mildew (P. viticola) in grapevine, against scab (Venturia inaequalis) in apple, downy mildew (Peronospora destructor) in onion, and late blight (P. infestans) in tomato under greenhouse conditions. Furthermore, Pen was even effective under very high disease pressure, as described for P. viticola in 1997, U. necator in 1998 and P. destructor in 2000. Efficacy of Pen in grapevine and apple in the field was comparable with the effect of standard fungicides such as copper and sulphur. Furthermore, if compared with other well-known inducers such as BABA and Bion, efficacy of Pen was equal or superior in most plant–pathogen systems. The only exception was cucumber, where Bion performed much better against the two tested pathogens Colletotrichum lagenarium and P. cubensis.
The replacement of copper by other, more environmental friendly products has been a major research focus in organic agriculture in the last few years [Citation64]. However, no real alternative products that conform to the guidelines of organic agriculture have been found yet. Pesticides to be applied in organic agriculture have to fulfil several criteria [Citation65–Citation67]. One criterion is the way of production. Only natural products or products identical to natural products may be used. Furthermore, natural products may not be obtained from genetically modified organisms. The Pen extract complies with the guidelines, in contrast to inducers such as Bion (containing the synthetic active compound BTH) and Messenger® (containing the bacterial protein harpin obtained from genetically modified bacteria). In addition, the raw material for the production of the extract is relatively cheap and available in large quantities of constant quality, prerequisites for its application in practice. However, phytotoxic side-effects have been observed related to the Pen extract.
In conclusion, we have shown that Pen, the aqueous extract from the mycelium of P. chrysogenum, induces resistance against a broad range of pathogens in several crop plants under both greenhouse and field conditions. Particularly its effect against downy mildews in grapevine and onion is promising. However, potential phytotoxic side-effects are undesirable. Yet, our data (results not shown) suggest that phytotoxicity can be reduced by appropriate techniques, which have still to be improved.
In conclusion, inducers of resistance may enhance the inherent plant defence status under field conditions and thus reduce the dependence on foliar-applied fungicides. The control of oomycete pathogens is notoriously difficult. However, BABA as well as Pen has the potential to become commercially available alternatives with proven efficacy.
3 Optimized soil fertility management strategies
3.1 Introduction
Agricultural practices not only have an obvious impact on crops by affecting soil parameters such as erosion stability, nutrient availability and water holding capacity, they also affect soil organisms and their activities [Citation68–Citation70]. An active and abundant soil flora and fauna improves soil fertility and soil quality parameters [Citation71]. Soil (micro-)organisms have been shown to be a key factor in the suppression of soil-borne diseases [Citation72–Citation76]. Mechanisms involved in the suppression of soil-borne diseases by soil micro-organisms have been studied extensively and include competition for nutrients and space, antibiosis, hyperparasitism and the induction of plant disease resistance [Citation77–Citation79]. Some studies have demonstrated that soil micro-organisms may also reduce disease development of air-borne, foliar diseases [Citation80]. Here, beneficial micro-organisms and plant pathogens are physically separated, and induced systemic resistance (ISR) has been identified as the main underlying mechanism [Citation81]. The occurrence of ISR against air-borne diseases has been demonstrated mainly under controlled conditions, while little is known about the occurrence and relevance of this phenomenon under field conditions [Citation82].
Several studies suggest that soil type is a key determinant for soil microbial activity and community structure [Citation83]. Yet, organic material amendments (e.g., manure, compost, plant residues) to the soil have also been shown to affect soil microbial populations and soil suppressiveness by promoting beneficial micro-organisms native to the soils and/or by introducing new beneficial micro-organisms [Citation70,Citation84–Citation88]. Furthermore, long-term experiments have shown that organic farming systems using regular organic material amendments have a higher soil microbial biomass activity and diversity compared with conventional farming systems using inorganic fertilizers only [Citation89]. Fertilizer inputs to soils are an important means to improve plant production in agricultural systems. While most conventional or integrated farming systems are based on regular inorganic N, P and K fertilizer inputs (water soluble and immediately and easily available to the crop from the soil solution), fertilizers used in organic farming systems are based on organic inputs (e.g., green and animal manures, compost) the nutrients of which only become available to the crop after unlocking them from the solid phase through weathering and mineralization of the resulting organic matter. Mineralization of organic matter by soil micro-organisms is crucial for nutrient delivery to the crops in organic agriculture. Rapid mineralization requires an active and abundant soil flora and fauna, and their activity in turn depends on soil temperature, soil moisture and the chemical composition of the fertilizer input.
3.2 Impact of management strategies on soil parameters
Fließbach et al. [Citation90] and Tamm et al. [Citation91] evaluated the long- and short-term effects of organic fertilizer inputs on physical, chemical and biological soil properties as part of the long-term DOK trial in Therwil, Switzerland, from short-term fertilizer input experiments with lettuce in Bonn, Germany, and with onions in Yorkshire (UK) (). The analyses of the DOK trial confirmed that long-term soil management strategies changed soil properties, depending on amount and quality of fertilizer inputs. The trials at Bonn (BON), Tadcaster (TAD) and Stocksbridge (STC) comprised one single fertilizer application per treatment followed by the growing of one crop (). Soil samples taken after the amendment of organic or inorganic fertilizers and after growing onions (trial sites STC and TAD) or lettuce (trial site BON) (AFI) differed from the corresponding before-fertilizer input (BFI) soil samples in terms of biological and chemical parameters. At the trial site BON, the AFI soil samples had lower microbial biomass and microbial activities than the BFI samples, whereas at the trial sites TAD and STC, many biological parameters were higher in the AFI than in the BFI soil samples. The type of amendment had only a small effect on soil parameters. Differences were mainly found between soils fertilized with farmyard manure (FYM) and soils fertilized with inorganic (MIN) fertilizers at the BON site. This finding was in accordance with the results from the DOK trial, where the application of FYM as opposed to inorganic fertilizer was identified as a key determinant for the differences in some soil parameters [Citation92].
Table 2 Details of the four fertilizer experiments used for evaluating suppressiveness to soil-borne and air-borne diseases in 2004 [Citation91].
3.3 Impact of soil management strategies on suppressiveness
Soils from the DOK long-term trial and from the three short-term fertilizer input trials were also evaluated for differences in suppressiveness to soil- and air-borne diseases using the bioassay systems basil (Ocimum basilicum) – Rhizoctonia solani, cress (Lepidium sativum) – Pythium ultimum, Arabidopsis thaliana – Hyaloperonospora parasitica and tomato (Solanum lycopersicum) – P. infestans [Citation91]. We found that soil type is a key determinant for suppressiveness to diseases. Soil from the STC site showed the highest level of suppressiveness to all tested diseases, a result that was confirmed by soil samples taken in the subsequent year and evaluated in two bioassays [Citation91,Citation93]. The causal mechanisms for the high suppressiveness of the STC soil were not identified. However, earlier studies comparing the suppressiveness of soils to P. ultimum also detected levels of suppressiveness in sandy soils that were higher than in clay soils, but the underlying mechanisms were not determined [Citation72].
Furthermore, we have shown that site-specific suppressiveness can be modulated by long-term soil management, and, to a lesser extent, by short-term fertilizer inputs [Citation91]. For instance, within the DOK trial, A. thaliana plants growing on the least suppressive soil (CONMIN) showed around 30% more disease incidence than plants growing on the most suppressive soil (BIODYN). Furthermore, there was a significant correlation between suppressiveness and soil microbial biomass (; unpublished data). Similarly, weight reductions of basil caused by R. solani varied between 30% and 46% among the long-term treatments. So far only few studies have shown that soil amendments not only affect suppressiveness to soil-borne diseases, but also the resistance of host plants to air-borne diseases. For instance, Vallad et al. [Citation94] showed that composted paper-mill residues amended to field soils reduced air-borne diseases caused by Pseudomonas syringae pv. tomato in A. thaliana and tomato.

As for soil parameters, short-term fertilizer input treatments had little effect on the suppressiveness of soils to the three pathogens included in the study [Citation91]. Exceptions were (1) a significant reduction of disease caused by H. parasitica in A. thaliana grown in soils amended with composted FYM when compared with chicken manure in soil samples from the TAD site, and (2) a significant weight reduction in O. basilicum infected with R. solani in soils amended with FYM compared with soils amended with inorganic fertilizer in soil samples from BON.
In conclusion, site-specific factors, which cannot be influenced by agronomic practices, were found to have a greater impact than cultivation-specific effects within the same site. Nevertheless, short-term, but in particular long-term management strategies have been shown to have the potential to influence suppressiveness of soils to certain diseases.
4 Conclusions
In this paper we explored the potential to enhance the plant's health status either by inducing resistance via optimized soil management techniques or by foliar application of inducers of resistance. The foliar application of inducers of resistance may enhance the inherent plant defence status under field conditions and thus reduce the dependence on foliar fungicide sprays against several key pathogens, although the control of oomycete pathogens is notoriously difficult. However, BABA as well as Pen has the potential to become commercially available alternatives with proven efficacy. The systematic use of soil fertility management techniques to reduce diseases is an intriguing concept in theory, but is not yet widely used in practice, partly because of lack of understanding the underlying principles. We demonstrated that site-specific factors, which cannot be influenced by agronomic practices, have a greater impact than cultivation-specific effects within the same site. Nevertheless, short-term, but particularly long-term management strategies have been shown to have the potential to influence the suppressiveness of soils to certain diseases. Within limits, a better understanding of the processes will help to adapt management practices in order to reduce crop losses due to noxious organisms.
Acknowledgements
The authors gratefully acknowledge funding from the European Community financial participation under the Sixth Framework Programme for Research, Technological Development and Demonstration Activities for the Integrated Project QUALITYLOWINPUTFOOD, FP6-FOOD-CT-2003-506358, and the Swiss National Science Foundation (NCCR plant survival).
References
- G.N.AgriosPlant Pathology4th ed.1997Academic PressSan Diego
- L.TammOrganic agriculture: development and state of the artJ. Environ. Monit.320019296
- S.SchneiderW.R.UllrichDifferential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic and biotic inducersPhysiol. Mol. Plant Pathol.451994291304
- R.HammerschmidtInduced Resistance to Disease in Plants1995Kluwer Academic PublishersDordrecht
- J.KucConcepts and direction of induced systemic resistance in plants and its applicationEur. J. Plant Pathol.1072001712
- R.F.WhiteAcetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobaccoVirology991979410412
- E.WardS.J.UknesS.C.WilliamsS.S.DincherD.L.WiederholdD.C.AlexanderP.Ahl GoyJ.P.MétrauxJ.RyalsCoordinate gene activity in response to agents that induce systemic acquired resistancePlant Cell3199110851094
- Y.CohenU.GisiT.NidermanLocal and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid and jasmonic methyl esterPhytopathology83199310541062
- L.FriedrichK.LawtonW.RuessP.MasnerN.SpeckerM.Gut RellaB.MeierS.DincherT.StaubS.UknesJ.-P.MétrauxH.KessmannJ.RyalsA benzothiadiazole derivative induces systemic acquired resistance in tobaccoPlant J.1019966170
- J.GorlachS.VolrathG.Knauf-BeiterG.HengyU.BeckhoveK.H.KogelM.OostendorpT.StaubE.WardH.KessmannJ.RyalsBenzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheatPlant Cell81996629643
- YSekizawaS.MaseMode of controlling action of probenazole against rice blast disease with reference to the induced resistance mechanism in rice plantJ. Pesticide Sci.619819194
- Y.CohenT.NidermanE.MösingerR.Fluhrβ-Aminobutyric acid induces the accumulation of pathogenesis-related proteins in tomato (Lycopersicon esculentum L.) plants and resistance to late blight infection caused by Phytophthora infestansPlant Physiol.10419945966
- T.NürnbergerF.BrunnerInnate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patternsCurr. Opin. Plant Biol.52002318324
- G.FelixJ.D.DuranS.VolkoT.BollerPlants have a sensitive perception system for the most conserved domain of bacterial flagellinPlant J.181999265276
- H.DongT.P.DelaneyD.W.BauerS.V.BeerHarpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 genePlant J.201999207215
- N.E.StrobelC.JiS.GopalanJ.A.KucS.Y.HeInduction of systemic acquired resistance in cucumber by Pseudomonas syringae pv syringae 61 HrpZPss proteinPlant J.91996431439
- G.FelixM.RegenassT.BollerSpecific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory statePlant J.41993307316
- JGranadoG.FelixT.BollerPerception of fungal sterols in plantsPlant Physiol.1071995485490
- J.K.SharpB.ValentP.AlbersheimPurification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybeanJ. Biol. Chem.25919841131211320
- T.YamaguchiA.YamadaN.HongT.OgawaT.IshiiN.ShibuyaDifferences in the recognition of glucan elicitor signals between rice and soybean: β-glucan fragments from the rice blast disease fungus Pyricularia oryzae that elicit phytoalexin biosynthesis in suspension-cultured rice cellsPlant Cell122000817826
- A.AzizB.PoinssotX.DaireM.AdrianA.BézierB.LambertJ.-M.JoubertA.PuginLaminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticolaMol. Plant Microbe Interact.16200311181128
- J.W.MansfieldAntimicrobial compounds and resistance. The role of phytoalexins and phytoanticipinsA.SlusarenkoR.S.S.FraserL.C.Van LoonMechanisms of Resistance to Plant Diseases2000Kluwer Academic PublishersDordrecht325370
- H.J.M.LinthorstPathogenesis-related proteins of plantsCrit. Rev. Plant Sci.101991123150
- L.C.van LoonOccurrence and properties of plant pathogenesis-related proteinsS.K.DattaS.MuthukrishnanPathogenesis-related Proteins in Plants1999CRC PressBoca Raton119
- N.BenhamouJ.W.KloepperA.Quadt-HallmanS.TuzunInduction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteriaPlant Physiol.1121996919929
- U.MaternB.GrimmigR.E.KneuselPlant cell wall reinforcement in the disease-resistance response: Molecular composition and regulationCan. J. Bot.731995511517
- B.Mauch-ManiA.SlusarenkoProduction of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasiticaPlant Cell81996203212
- F.DaayfA.SchmittR.R.BélangerEvidence of phytoalexins in cucumber leaves infected with powdery mildew following treatment with leaf extracts of Reynoutria sachalinensisPlant Physiol.1131997719727
- G.HergerF.KlingaufD.MangoldE.H.PommerM.SchererEfficacy of extracts of Reynoutria sachalinensis (F. Schmidt) Nakai (Polygonaceae), against fungal diseases, especially powdery mildewsNachrichtenblatt des Deutschen Pflanzenschutzdienstes4019885660
- BThuerigA.BinderT.BollerU.GuyerS.JiménezC.RentschL.TammAn aqueous extract of the dry mycelium of Penicillium chrysogenum induces resistance in several crops under controlled and field conditionsEur. J. Plant Pathol.1142006185197
- B.ThuerigG.FelixA.BinderT.BollerL.TammAn extract of Penicillium chrysogenum elicits early defense-related responses and induces resistance in Arabidopsis thaliana independently of known signalling pathwaysPhysiol. Mol. Plant Pathol.672006180193
- J.KucInduced systemic resistance in plants to diseases caused by fungi and bacteriaJ.A.BaileyB.J.DeverallThe Dynamics of Host Defense1983Academic PressNew York191221
- Codex Alimentarius Commission. Guidelines for the production, processing, labelling and marketing of organically produced foods, CAC/GL 32-1999, Rev. 1-2001, 1999.
- B.SpeiserL.TammV.MaurerA.BernerM.WalkenhorstHilfsstoffliste: Zugelassene und empfohlene Hilfsstoffe für den biologischen Landbau2004Research Institute of Organic Agriculture (FiBL)Frick
- O.ViretB.BloeschA.L.FabreW.SiegfriedG.BleyerB.HuberH.H.KassemeyerV.SteinmetzVitimeteo: a new model for forecasting grapevine mildewRevue Suisse de Viticulture, Arboriculture et Horticulture3720056568
- W.S.AbbottA method for computing the effectiveness of an insecticideJ. Econ. Entomol.181925265267
- Y.CohenA.BaiderD.GotliebA.RubinControl of Bremia lactucae in field-grown lettuce by dl-3-amino-n-butanoic acid (BABA)U.NiggliC.LeifertT.AlföldiL.LuckH.WillerImproving sustainability in organic and low input food production systems. 3rd Intn. Cong. European integrated project ‘Quality Low Input Food’ (QLIF)University of Hohenheim, Germany(2007) 172–176.
- Y.Cohenβ-Aminobutyric acid-induced resistance against plant pathogensPlant Dis.862002448457
- G.JakabJ.TonV.FlorsL.ZimmerliJ.P.MétrauxB.Mauch-ManiEnhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responsesPlant Physiol.1392005267274
- G.C.PapavizasC.B.DaveyEffect of amino compounds and related substances lacking sulfur on Aphanomyces root rot of peasPhytopathology531963116122
- Y.Cohen3-Aminobutyric acid induces systemic resistance against Peronospora tabacinaPhysiol. Mol. Plant Pathol.441994273288
- L.ZimmerliG.JakabJ.-P.MétrauxB.Mauch-ManiPotentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acidProc. Nat. Acad. Sci. U.S.A.9720001292012925
- D.SilueE.PajotY.CohenInduction of resistance to downy mildew (Peronospora parasitica) in cauliflower by dl-β-amino-n-butanoic acid (BABA)Plant Pathol.51200297102
- B.K.HwangJ.Y.SunwooY.J.KimB.S.KimAccumulation of β-1,3-glucanase and chitinase isoforms, and salicylic acid in the dl-beta-amino-n-butyric acid-induced resistance response of pepper stems to Phytophthora capsiciPhysiol. Mol. Plant Pathol.511997305322
- Y.CohenM.ReuveniA.BaiderLocal and systemic activity of BABA (dl-3-aminobutyric acid) against Plasmopara viticola in grapevinesEur. J. Plant Pathol.1051999351361
- M.M.HamiduzzamanG.JakabL.BarnavonJ.-M.NeuhausB.Mauch-Maniβ-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signalingMol. Plant Microbe Interact.182005819829
- L.TosiR.LuigettiA.ZazzeriniInduced resistance against Plasmopara helianthi in sunflower plants by dl-β-amino-n-butyric acidJ. Phytopathol.1461998295299
- S.ShailasreeB.R.SaroshN.S.VasanthiH.S.ShettySeed treatment with β-aminobutyric acid protects Pennisetum glaucum systemically from Sclerospora graminicolaPest Manage. Sci.572001721728
- R.BitonA.BaiderA.OvadiaY.CohenInduced resistance against Fusarium oxysporum f. sp. melonis in melon plants in response to β-aminobutyric acidI.J.PorterProc. 2nd Aust. Soilborne Dis. Symp.20017172
- J.TonB.Mauch-Maniβ-Amino-butyric acid-induced resistance against necrotrophic pathogensis based on ABA-dependent priming for callosePlant J.382004119130
- L.ZimmerliJ.-P.MétrauxB.Mauch-Maniβ-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinereaPlant Physiol.1262001517523
- M.ReuveniD.SheglovY.CohenControl of moldy-core decay in apple fruits by β-aminobutyric acids and potassium phosphitesPlant Dis.872003933936
- R.PoratV.VinokurB.WeissL.CohenA.DausE.E.GoldschmidtS.DrobyInduction of resistance to Penicillium digitatum in grapefruit by β-aminobutyric acidEur. J. Plant Pathol.1092003901907
- L.SticherB.Mauch-ManiJ.-P.MétrauxSystemic acquired resistanceAnn. Rev. Phytopathol.351997235270
- L.C.van LoonInduced resistance in plants and the role of pathogenesis-related proteinsEur. J. Plant Pathol.1031997753765
- D.WaltersD.WalshA.NewtonG.LyonInduced resistance for plant disease control: Maximizing the efficacy of resistance elicitorsPhytopathology95200513681373
- U.ConrathG.J.M.BeckersV.FlorsP.Garcia-AgustinG.JakabF.MauchM.A.NewmanC.M.J.PieterseB.PoinssotM.J.PozoA.PuginU.SchaffrathJ.TonD.WendehenneL.ZimmerliB.Mauch-ManiPriming: getting ready for the battleMol. Plant Microbe Interact.19200610621071
- J.TonV.ToquinV.FlorsA.IavicoliM.N.MaederJ.P.MétrauxB.Mauch-ManiDissecting the β-aminobutyric acid-induced priming phenomenon in ArabidopsisPlant Cell172005987999
- E.PajotD.Le CorreD.SiluePhytogard(R) and dl-beta-amino butyric acid (BABA) induce resistance to downy mildew (Bremia lactucae) in lettuce (Lactuca sativa L.)Eur. J. Plant Pathol.1072001861869
- M.ReuveniT.ZahaviY.CohenControlling downy mildew (Plasmopara viticola) in field-grown grapevine with β-aminobutyric acid (BABA)Phytoparasitica292001125133
- E.AmzalekY.CohenComparative efficacy of SAR compounds against rust infection in sunflower plantsPhytopathology972007179186
- J.SiegristM.OroberH.Buchenauerβ-Aminobutyric acid-mediated enhancement of resistance in tobacco to tobacco mosaic virus depends on the accumulation of salicylic acidPhysiol. Mol. Plant Pathol.56200095106
- O.BaysalY.Ziya GursoyH.OrnekA.DuruInduction of oxidants in tomato leaves with dl-β-aminobutyric acid (BABA) and infected with Clavibacter michiganensis subp. michiganensisEur. J. Plant Pathol.1122005361369
- B.SpeiserA.BernerA.HäseliL.TammControl of downy mildew of grapevine with potassium phosphonate: effectivity and phosphonate residues in wineBiol. Agric. Hort.172000305312
- Eu, Council Regulation (EEC) No. 2092/91 of 24 June 1991 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs as amended, 1991.
- Eu, Council Regulation (EEC) No. 1488/97 of 29 July 1997 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs as amended, 1997.
- IfOAM, Basic Standards for Organic Agriculture. D-Tholey-Theley, 2000.
- B.GovaertsM.MezzalamaY.UnnoD.D.SayreM.Luna-GuidoK.VanherckL.DendoovenJ.DeckersInfluence of tillage, residue management, and crop rotation on soil microbial biomass and catabolic diversityAppl. Soil Ecol.3720071830
- D.RotenbergR.JoshiM.-S.BenitezL.G.ChapinA.CampC.ZumpettaA.OsborneW.A.DickB.B.M.GardenerFarm management effects on rhizosphere colonization by native populations of 2,4-diacetylphloroglucinol-producing Pseudomonas spp. and their contributions to crop healthPhytopathology972007756766
- J.D.Van ElsasP.GarbevaJ.SallesEffects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogensBiodegradation1320022940
- J.W.DoranM.SarrantonioM.A.LiebergSoil health and sustainabilityAdv. Agron.561996154
- I.M.B.KnudsenK.M.LarsenD.F.JensenJ.HockenhullPotential suppressiveness of different field soils to Pythium damping-off of sugar beatAppl. Soil Ecol.212002119129
- J.G.MenziesOccurrence and transfer of a biological factor in soil that suppresses potato scabPhytopathology491959648652
- P.J.ShiptonR.J.CookJ.W.SittonOccurrence and transfer of a biological factor in soil that suppresses take-all of wheat in Eastern WashingtonPhytopathology631973511517
- E.W.StutzG.DéfagoH.KernNaturally occurring fluorescent Pseudomonads involved in suppression of black root rot of tobaccoPhytopathology761986181185
- B.M.WisemanS.M.NeateK.O.KellerS.E.SmithSuppression of Rhizoctonia solani anastomosis group 8 in Australia and its biological natureSoil Biol. Biochem.281996727732
- D.HaasG.DéfagoBiological control of soil-borne pathogens by fluorescent pseudomonadsNat. Rev. Microbiol.32005307319
- H.A.J.HoitinkD.M.J.Van DorenA.F.SchmitthennerSuppression of Phytophthora cinnamomi in a composted hardwood bark potting mediumPhytopathology671977561565
- M.TheodoreJ.A.ToribioSuppression of Pythium aphanidermatum in composts prepared from sugarcane factory residuesPlant Soil1771995219223
- L.C.van LoonP.A.H.M.BakkerC.M.J.PieterseSystemic resistance induced by rhizosphere bacteriaAnn. Rev. Phytopathol.361998453483
- L.C.van LoonA.H.M.BakkerInduced systemic resistance as a mechanism of disease suppression by rhizobacteriaZ.A.SiddiquiPGPR: Biocontrol and Biofertilization2005SpringerDordrecht3966
- J.E.KloepperR.Rodriguez-UbanaG.W.ZehnderJ.F.MurphyE.SikoraC.FernandezPlant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseasesAust. Plant Pathol.2819992126
- M.S.GirvanJ.BullimoreJ.N.PrettyA.M.OsbornA.S.BallSoil type is the primary determinant of the composition of the total and active bacterial communities in arable soilsAppl. Environ. Microbiol.69200318001809
- H.ChuX.LinT.FujiiS.MorimotoK.YagiJ.HuJ.ZhangSoil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer managementSoil Biol. Biochem.39200729712976
- E.InbarS.J.GreenY.HadarD.MinzCompeting factors of compost concentration and proximity to root affect the distribution of streptomycetesMicrob. Ecol.5020057381
- G.InnerebnerB.KnappT.VasaraM.RomantschukH.InsamTraceability of ammonia-oxidizing bacteria in compost-treated soilsSoil Biol. Biochem.38200610921100
- A.Pérez-PiqueresV.Edel-HermannC.AlabouvetteC.SteinbergResponse of soil microbial communities to compost amendmentsSoil Biol. Biochem.382006460470
- C.Serra-WittlingS.HouotC.AlabouvetteIncreased soil suppressiveness to Fusarium wilt of flax after addition of municipal solid waste compostSoil Biol. Biochem.28199612071214
- P.MäderA.FließbachD.DuboisL.GunstP.FriedU.NiggliSoil fertility and biodiversity in organic farmingScience296200216941697
- A.FließbachC.SchmidtC.BrunsM.PalmerB.NietlispachC.LeifertL.TammSoil biological quality in short- and long-term field trials with conventional and organic fertility input typesAnnual QLIF Conference2007
- L.TammB.ThuerigC.BrunsJ.G.FuchsU.KöpkeC.LeifertN.MahlbergC.SchmidtA.FliessbachSoil type, management history, and soil amendments influence development of soil-borne (Rhizoctonia solani, Pythium ultimum) and air-borne (Phytophthora infestans, Hyaloperonospora parasitica) diseasesEur. J. Plant Pathol.1272010465481
- F.WidmerA.FliessbachE.LaczkoJ.Schulze-AurichJ.ZeyerAssessing soil biological characteristics: a comparison of bulk soil community DNA-, PLFA-, and Biolog™-analysesSoil Biol. Biochem.33200110291036
- B.ThuerigA.FliessbachN.BergerJ.FuchsN.KrausN.MahlbergB.NietlispachL.TammRe-establishment of suppressiveness to soil- and air-borne diseases by re-inoculation of soil microbial communitiesSoil Biol. Biochem.41201021532161
- G.E.ValladL.CooperbandR.M.GoodmanPlant foliar disease suppression mediated by composted forms of paper mill residuals exhibits molecular features of induced resistancePhysiol. Mol. Plant Pathol.6320036577