Abstract
Phenotypic plasticity in plants is a naturally occurring phenomenon that plants have evolved to survive environmental change. In agriculture, environmental stress such as insect infestation can lead to reduction in yield components. Although insect resistance can be bred into crops, insect genetic variation can cause variability in resistance leading to yield reduction. However, the extent to which insect resistance is plastic may depend on crop genotype. A genotype × genotype matrix was designed to study the effect of within-species genetic variation on wheat × aphid interactions. We found that wheat yield components and aphid population growth were significantly influenced by both wheat genotype and aphid genotype. Furthermore, plasticity in wheat yield components depended on the wheat–aphid combination. The results indicate that wheat plasticity not only has a genetic basis, but that it is also influenced by the biotic environment. The consequences of plasticity in resistance to aphid genotypes found in our study in relation to crop breeding for insect resistance are discussed.
1 Introduction
Phenotypic plasticity is a plant's ability to adapt to and survive environmental change [Citation1,Citation2]. In agriculture, plasticity in yield components like grain set and grain mass can be caused by abiotic (climatic) or biotic (e.g., insect infestation) stress factors, and can be problematic. For example, when plants are infested with insect pests, they may direct their resources towards defence away from reproduction [Citation2], leading to reduced yield. In the case of major pests and diseases, crop varieties are bred with resistance so that yield reduction is minimized, which is a crucial strategy for organic and low-input agriculture where the use of pesticides is prohibited or restricted.
The ability to breed plants for resistance against insect pests relies on naturally occurring genetic variation in a plant's response to insect attack [Citation3]. Similarly, insects exhibit naturally occurring genetic variation in their attack on plants and responses to plant resistance [Citation4,Citation5]. These two characteristics have led to plant genotype × insect genotype interactions that have been demonstrated in both experimental (e.g., [Citation6,Citation7]) and agricultural plant–insect systems [Citation4,Citation5,Citation8]. Genotype × genotype interactions expose the plasticity of plant × insect interactions, and are a problem in breeding strategies that focus on breeding resistance against a single insect genotype. For example, the extent to which crop yield is affected by an insect species can be influenced by the insect genotype attacking the crop, as well as by the crop genotype that is being attacked.
Phenotypic plasticity affecting fitness-related traits (e.g., crop yield) is genetically based, and has been mapped to specific genomic regions [Citation9,Citation10]. Such studies indicate the possibility to breed crops with reduced plasticity. However, this would require a complete understanding of the pathways that influence plasticity in characteristics like yield traits in variable environments. Plasticity in crop yield caused by aphid attack may be linked to the different types of resistance (i.e., antixenosis, antibiosis, tolerance) [Citation11], which vary in their effect on different aphid species in wheat [Citation12].
In this study we used a wheat–aphid (Triticum aestivum – Sitobion avenae) model system to examine the effect of wheat and insect genetic variation on wheat resistance and grain yield. Clonal variation in aphids occurs naturally, and genetically distinct clones of S. avenae can coexist on cereal crops in an agricultural environment [Citation13–Citation15]. The objectives of this study were to test (1) contrasting winter wheat genotypes (T. aestivum) for their resistance to the cereal aphid S. avenae, and (2) whether intraspecific genetic variation in aphids would alter wheat resistance to the cereal aphid and cause plasticity in crop yield.
2 Materials and methods
2.1 Experimental design
A genotype × genotype matrix was designed using six winter wheat (T. aestivum) genotypes and two cereal aphid (S. avenae) genotypes plus a control (no aphids), yielding 18 treatments. Each treatment was replicated eight times, giving a total of 144 plants. The experiment was of the randomized block design.
2.2 Plant growth
The experiment was carried out in a glasshouse at the Close House Field Station (Newcastle University) in the period April–September 2007. The six contrasting winter wheat genotypes were obtained from different suppliers, and consisted of four short-straw varieties: Battalion, Gladiator, Zebedee (supplied by Mastock), and Malacca (by Horizon Seeds) and two long-straw varieties: Pollux (by Gilchester Organics) and Deben (by Horizon Seeds). Seedlings were raised in the glasshouse in trays containing John Innes Compost No. 2. After seven days the trays were placed in a coldroom (2–4 °C) for 6 weeks for vernalization. The seedlings were then transplanted into 10-cm pots containing the same type of compost. They were watered twice daily via the saucers to ensure that all plants received the same amount of water. Eleven days after transplanting, two adult aphids were placed onto each plant as appropriate. Fine-mesh bags were secured on top of each plant. At four weeks after infestation the aphids were manually removed. Wheat yield measurements were taken at the end of the plant's life cycle, and included the mass of 10 grains, total grain weight, and the number of ears. Average grain mass was calculated from the mass of 10 grains. The number of grains per plant was estimated from the total grain weight divided by the average grain mass. We also harvested the shoot and leaves, and dried them for three days at 80 °C for dry biomass assessment.
2.3 Aphid measurements
Three aphid treatments were used, consisting of two genotypes (H1 and HF92a) and a no-aphids control. The aphid genotypes were originally collected from UK field sites and genotypes were identified using microsatellite markers (Rothamsted Insect Survey, Rothamsted Research, Harpenden). Aphid population size was recorded 2 and 4 weeks after the plants had been infested.
2.4 Statistical analysis
We analysed the two-way interaction (wheat × aphid) using a two-way ANOVA in the statistical software package Minitab 15. Both, wheat genotype and aphid treatment were treated as random factors. Phenotypic plasticity was calculated using the character state approach [Citation16]. In this method, plasticity is the difference in a population mean trait between two environments.
3 Results
3.1 Influence of wheat and aphid genotype
The five grain yield traits (mass of 10 grains, total grain mass, number of ears, average mass per grain, and grain set) and dry shoot biomass were significantly influenced by wheat genotype (). In addition, three of these grain yield traits (mass of 10 grains, total grain mass, average grain mass) and dry shoot biomass were also significantly influenced by aphid genotype. No statistically significant interaction between wheat genotype and aphid treatment was found: the aphid treatment tended to have the same effect on all wheat genotypes. Both aphid genotypes (H1 and HF92a) reduced all wheat performance traits compared with the control (no aphids). The extent to which wheat performance was reduced differed between the aphid genotypes but the difference was not statistically significant. The largest difference was observed between the control (no aphids) and both aphid genotypes ().
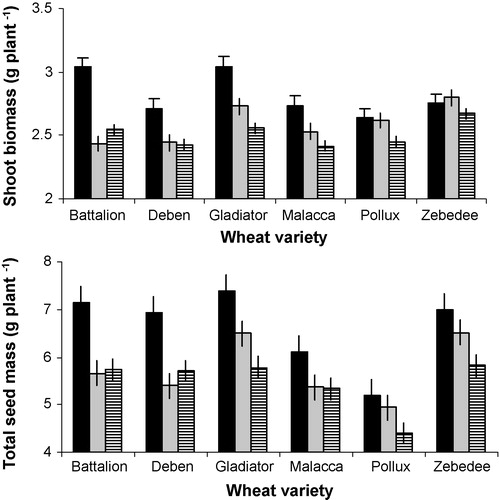
Table 1 ANOVA results for wheat grain yield and biomass traits.
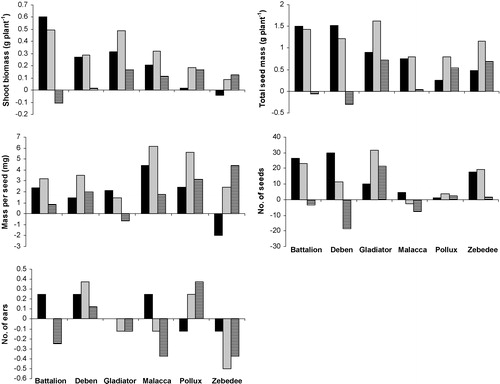
3.2 Variation in phenotypic plasticity across wheat genotypes
Phenotypic plasticity of a wheat genotype was defined as the difference in mean performance between the Hi and HF92a aphid treatments. The three aphid treatments (no aphids (control), genotype H1 and genotype HF92a) implied three paired environments to calculate phenotypic plasticity: control–H1, control–HF92a, and H1–HF92a. illustrates the phenotypic plasticity calculated for each of the six wheat performance traits. Within each wheat genotype the magnitude and polarity of phenotypic plasticity was influenced by aphid treatments. For example in ‘seed set’ for wheat genotype Pollux, control–H1 showed a relatively low positive phenotypic plasticity (i.e., seed set was greater without aphids than with H1 aphids), but control–HF92a showed a relatively high negative phenotypic plasticity (i.e., seed set was greater with H1 aphids than without aphids). Within each pair of aphid treatments, plasticity in wheat performance varied with wheat genotype. For example, in the trait ‘mass of 10 grains’, Battalion control–H1 gave a relatively small negative phenotypic plasticity. However, control–HF92a resulted in a higher and positive phenotypic plasticity, indicating that the effect of HF92a had a larger effect on grain mass than H1 compared with the control, and also that HF92a reduced grain mass, whereas H1 slightly increased it. The difference H1–HF92a resulted in a larger and positive phenotypic plasticity, illustrating the difference in effect between these two aphid genotypes on grain mass. Looking across wheat genotypes, the difference H1–HF92a can lead to any combination of relatively large or small and positive or negative phenotypic plasticity.
3.3 Influence of aphid and wheat genotype on aphid performance
Aphid population size was recorded two and four weeks after infesting the plants with two adult aphids. At both moments, aphid performance was significantly influenced by both aphid genotype and wheat genotype (). No interaction between aphid genotype and wheat genotype was found. On both moments, the aphid population on all wheat genotypes was larger for aphid genotype HF92a than for aphid genotype H1 (). The differences in population size between the two aphid genotypes were smaller on some wheat genotypes (e.g., Pollux) than on other ones (e.g., Malacca, Gladiator), indicating that Pollux may have less plasticity in resistance to these aphid genotypes than Malacca and Gladiator.
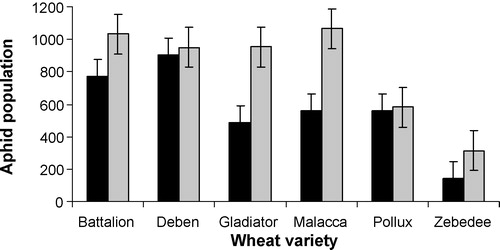
Table 2 ANOVA results for aphid performance two and four weeks after infestation.
Aphid plasticity across wheat genotypes was influenced by aphid genotype. For example, for aphid genotype H1 population size was similar on Gladiator, Malacca and Pollux, but was significantly larger on Battalion and Deben and lower on Zebedee. For aphid genotype HF92a, however, population size was similar on Battalion, Deben, Gladiator and Malaccca, but significantly lower on Zebedee and Pollux.
4 Discussion
4.1 The occurrence of genotype × genotype interactions
A wheat genotype × aphid genotype matrix was designed to investigate the effect of wheat and aphid genetic variation on both species’ performance. Both wheat and aphid genetic variation significantly influenced numerous wheat yield and growth components and aphid performance. The influence of genetic variation in plants and insects on their reciprocal interaction can cause unpredictability in crop breeding for resistance to insect pests. Genotype × genotype interactions are a naturally occurring phenomenon and may be the result of variation in the types of response in plants that can be elicited by insect infestation. Plants can be resistant in terms of antixenosis (preference), antibiosis (reduced lifespan), or tolerance (plants performs well despite insect attack) [Citation11]. These different modes of resistance may be controlled by different plant pathways, and be influenced by genetic variation. Other components of resistance are known to be influenced by genetic variation, such as hydroxamic acids [Citation6], and by sap composition [Citation17]. Although we were concerned with how wheat genetics influences the wheat × aphid interaction, it is worth noting how aphid traits involved in their interaction with wheat are genetically variable and can be the source of unpredictability in breeding for crop resistance. For example, differences in aphid feeding behaviour can determine whether aphids are sensitive or insensitive to hydroxamic acids in wheat [Citation6].
4.2 Implications of genetically based phenotypic plasticity
Phenotypic plasticity in wheat yield across different aphid genotypes could cause problems in breeding for resistance against insect attack. We demonstrated that some winter wheat genotypes were more plastic to the two aphid genotypes than other wheat genotypes. For example, the difference in population size between the two aphid genotypes was less on Pollux than on Malacca, and Gladiator plants. This indicates that Pollux may be less plastic in its resistance to these aphid genotypes than Malacca and Gibraltar. This is corroborated by the lower effect of aphid attack on shoot biomass of Pollux compared with the other wheat genotypes. However, it is also possible that Pollux is a poor host (i.e., lower sap nutrient content [Citation17]) for aphids, considering that Pollux had a lower shoot biomass (and total seed mass) than the other genotypes even in the no-aphids environment. Understanding which genotypes elicit which type of resistance and the effect of the mode of resistance on plasticity of yield may aid breeding strategies that aim to produce new varieties with resistance to insects while maintaining robust crop yield.
4.3 The prevalence of genetic variability in aphids
In this study we highlighted the potential problem of genetic variability in aphids and its possible consequences for wheat resistance and yield. The potential for this to occur in a crop under field conditions is made more realistic if we consider the prevalence of intraspecific genetic variation in natural ecosystems. For our aphid species (S. avenae) by itself, studies already reported the natural existence of intraspecific genetic variation [Citation13–Citation15], and numerous aphid genotypes have been documented within the same geographic location [Citation4,Citation18]. Coexisting genotypes of S. avenae have been shown to have host specialization with regards to the species of grass or cereal crop [Citation15,Citation18], suggesting a transition to speciation. Phenotypic plasticity among aphid genotypes as seen in this study, can alter aphid fitness and could be the starting point for their evolution via speciation [Citation19]. Thus the phenomenon of phenotypic plasticity is not unusual and has strong implications for breeding for insect resistance.
5 Conclusions
The results from this study show that breeding for resistance against aphids (and possibly insects in general) could benefit from taking into account variation in resistance due to genetic variability in the insect species. Crops bred with low plasticity in resistance against different genotypes of insect species could have more robust yields. Therefore, understanding the genetic basis of phenotypic plasticity of resistance, and its relation to yield, could be essential for breeding new varieties for low input and organic agriculture [Citation20].
Acknowledgments
The authors wish to thank Robert Hodgson and Alan Craig at Close House Field Station for their assistance during the glasshouse trial. We also thank Peter Shotton at Nafferton Farm for his help in identifying suitable wheat varieties and seed suppliers. This study was financed by European Community financial participation under the Sixth Framework Programme for Research, Technological Development and Demonstration Activities, for the Integrated Project QUALITYLOWINPUTFOOD, FP6-FOOD-CT-2003-506358.
References
- A.D.BradshawEvolutionary significance of phenotypic plasticity in plantsAdv. Genet.131965115155
- D.L.MarshallD.A.LevinN.L.FowlerPlasticity of yield components in response to stress in Sesbania macrocarpa and Sesbania vesicaria (Leguminosae)Am. Nat.1271986508521
- A.M.CastroA.VasicekC.EllerbrookD.O.GimenezE.TochoM.S.TacalitiA.CluaJ.W.SnapeMapping quantitative trait loci in wheat for resistance against greenbug and Russian wheat aphidPlant Breed.1232004361365
- M.C.CaillaudC.A.DedryverJ.P.DipietroJ.C.SimonF.FimaB.ChaubetClonal variability in the response of Sitobion avenae (Homoptera: Aphididae) tot resistant and susceptible wheatBull. Entomol. Res.851995189195
- E.GianoliC.M.CaillaudB.ChaubetJ.P.DiPietroH.M.NiemeyerVariability in grain aphid (Homoptera: Aphididae) performance and aphid-induced phytochemical responses in wheatEnviron. Entomol.261997638641
- A.GivovichH.M.NiemeyerComparison of the effect of hydroxamic acids from wheat on 5 species of cereal aphidsEntomol. Exp. Appl.741995115119
- C.Tétard-JonesM.A.KerteszP.GalloisR.F.PreziosiGenotype-by-environment interactions modified by a third species in a plant–insect systemAm. Nat.1702007492499
- S.M.MiguiR.J.LambSources of variation in the interaction between three cereal aphids (Hemiptera: Aphididae) and wheat (Poaceae)Bull. Entomol. Res.962006235241
- E.W.GuttelingJ.A.G.RiksenJ.BakkerJ.E.KammengaMapping phenotipc plasticity and genotype–environment interactions affecting life-history traits in Caenorhabditis elegansHeredity9820072837
- X.LacazeP.M.HayesA.B.KorolGenetics of phenotypic plasticity: QTL analysis in barley, Hordeum vulgareHeredity1022009163173
- G.PowellJ.HardieHost-selection behaviour by genetically identical aphids with different plant preferencesPhysiol. Entomol.2520005462
- A.M.CastroA.VasicekS.RamosA.WorlandE.SuarezM.MunozD.GimenezA.A.CluaDifferent types of resistance against green bug, Schizaphis graminum Rond, and the Russian wheat aphid, Diuraphis noxia Mordvilko, in wheatPlant Breed.1181999131137
- K.S.LlewellynH.D.LoxdaleR.HarringtonC.P.BrookesS.J.ClarkP.SunnucksMigration and genetic structure of grain aphid (Sitobion avenae) in Britain related to climate and clonal fluctuation as revealed using microsatellitesMol. Ecol.1220032134
- G.LushaiH.D.LoxdaleC.P.BrookesN.VonMendeR.HarringtonJ.HardieGenotypic variation among different phenotypes within aphid clonesProc. R. Soc. Lond. Ser. B-Biol. Sci.2641997725730
- P.SunnucksP.J.DeBarroG.LushaiN.MacleanD.HalesGenetic structure of an aphid studied using microsatellites: cyclic parthenogenesis, differentiated lineages and host specializationMol. Ecol.6199710591073
- S.ViaR.GomulkiewiczG.DejongS.M.ScheinerC.D.SchlichtingP.H.VantienderenAdaptive phenotypic plasticity – consensus and controversyTrends Ecol. Evol.101995212217
- S.GoundoudakiJ.A.TsitsipisJ.T.MargaritopoulosK.D.ZarpasS.DivanidisPerformance of the tobacco aphid Myzus persicae (Hemiptera: Aphididae) on oriental and Virginia tobacco varietiesAgric. Forest Entomol.52003285291
- G.LushaiO.MarkovitchH.D.LoxdaleHost-based genotype variation in insects revisitedBull. Entomol. Res.922002159164
- G.GorurC.LomonacoA.MackenziePhenotypic plasticity in host–plant specialisation in Aphis fabaeEcol. Entomol.302005657664
- G.BackesH.OstergardMolecular markers to exploit genotype–environment interactions of relevance in organic growing systemsEuphytica1632008523531