Abstract
Research on Blockchain implementation in the Pharmaceutical Supply Chains (PSC) is lacking despite its strong potential to overcome conventional supply chain challenges. Thus, this study aims to provide critical insight into the nexus between Blockchain and PSC and further build a conceptual framework for implementation within the pharmaceutical industry. Following a systematic literature review and text mining approach, 65 interdisciplinary articles published between 2010 and 2021 were studied to capture the decade long developments. Descriptive and thematic analysis showcases nascent developments of Blockchain in PSC. The drivers and barriers to adoption, implementation stages, and applications identified through the thematic analysis guide in setting the agenda for future research, primarily focussing on the use of Blockchain for drug counterfeiting, recall issues, along with other sector-specific challenges such as patient privacy, regulations and clinical trials. Research on Blockchain for PSC has been slow compared to other sectors, but has accelerated since the Covid-19 pandemic. Identified influential factors, implementation process and apparent applications are expected to influence researchers and practitioners in developing a roadmap for adopting Blockchain in the pharmaceutical industry. The proposed conceptual framework is novel and provides valuable directions to producers, regulators and governments to implement Blockchain in the pharmaceutical industry.
1. Introduction
Pharmaceutical supply chains (PSC) deliver medicines to multiple stakeholders, including biotechnology companies, health regulatory agencies, pharmacies, hospitals, and patients. With the ongoing Covid-19 pandemic and geopolitical situations, the shortage of medicines has placed PSC at the top of several governments’ priorities. According to the World Health Organization (WHO) estimate, nearly 10 percent of drugs in circulation are substandard, and almost 50 percent of drugs sold online are counterfeit (WHO Citation2017). These drugs containing toxic, dangerous ingredients are on the rise in the pharmaceutical industry (Sylim et al. Citation2018; Ahmadi et al. Citation2020; Hosseini-Motlagh, Nematollahi, and Nami Citation2021), posing increased risks to public health (Chang and Chen Citation2020).
In the pharmaceutical supply chains (PSC), the Food and Drug Agency (FDA), USA, is a regulatory body responsible for monitoring the safety, quality and effectiveness of drugs (Azzi, Chamoun, and Sokhn Citation2019). The Drug Supply Chain Security Act, first signed in 2013, urged drug/medicine supply chain participants to collaborate and generate an electronic and interoperable system to trace prescribed drugs through their physical movement by 2023 (FDA Citation2021). However, despite strict policies by regulatory bodies and other collaborative initiatives by the pharmaceutical industry, drug breach incidents are still on the rise, putting millions at risk and are expected to cost billions in healthcare fraud.
The low efficiency of PSCs is due to a lack of coordination among supply chain members and stakeholders (Moktadir et al. Citation2018). Lack of transparency and visibility of drug supply within PSC are fundamental reasons for the increased disease progression, drug resistance, and death (Buckley and Gostin Citation2013). Fortunately, global sourcing and online markets make it easier to procure and supply medicines in different countries; however, they pose an increased health risk without the ability to trace the origin. Even with stringent standards and the latest technologies (e.g. holograms, barcodes, RFID tags) to maintain the integrity of pharmaceutical products, counterfeiting remains a major challenge for global PSC. Recently, Interpol observed a significant rise in fake pharmaceutical products during Covid-19 and seized around 4.4 million units of illicit pharmaceuticals worldwide (Interpol Citation2020).
Blockchain, a shared digital ledger, is used by the peer-to-peer network to achieve immutable recording and self-executive transactions using cryptography principles (Saberi et al. Citation2019; Pournader et al. Citation2020); Wang, Han, and Beynon-Davies Citation2019). With increasing focus and successful implementation attempts in other sectors such as finance, energy, food/agriculture, there is a strong potential for Blockchain’s implementation in the pharmaceutical sector due to its distinct intrinsic attributes such as decentralisation, immutability and auditability (Karamchandani et al. Citation2021). Yaqoob et al. (Citation2019) pointed out that decentralisation could be used to upend the healthcare hierarchy by developing new systems to help patients manage their data. Blockchain technology could maintain patients’ sovereignty and control over their personal data, providing the most accurate data for precision medicine (Panwar et al. Citation2022). Furthermore, high data transparency of the Blockchain can improve trust in drug delivery, conditions, documentation, and end-to-end visibility, especially for the cold chain management. Several studies have identified Blockchain’s strong potential for pharmaceutical supply chains (e.g. Hussien et al. Citation2019; Jangir et al. Citation2019; Fraga-Lamas and Fernandez-Carames Citation2020; Chang, Iakovou, and Shi Citation2020; Bamakan, Moghaddam, and Manshadi Citation2021). However, a comprehensive or systematic literature review discussing Blockchain interfacing the PSC is still missing.
Most importantly, a framework for possible Blockchain implementation in PSC is non-existent in the extant literature. Thus, this study aims to conduct a holistic review of Blockchain adoption in PSC and develop a conceptual framework. A systematic literature review combined with a text mining approach followed by Ghadge, Dani, and Kalawsky (Citation2012) and Galati and Bigliardi (Citation2019) is adopted to investigate the advancements in Blockchain implementation in the PSC. The thematic analysis builds the agenda for future research and the development of an implementation framework. This study provides practitioners and researchers with a holistic understanding of the nexus between Blockchain and PSC.
The rest of the paper is structured as follows: Section 2 presents the research methodology adopted for conducting a systematic literature review. Section 3 and Section 4 provide the descriptive and thematic analysis, respectively. Section 5 develops a conceptual framework of Blockchain implementation in the PSC, along with the identification of future research avenues. Finally, section 6 concludes this study with discussion, contribution to theory and practice, and limitations.
2. Research methodology
A systematic literature review (SLR) provides a comprehensive, replicable and transparent overview of the study (Tranfield, Denyer, and Smart Citation2003). Furthermore, predefined inclusion and exclusion criteria during data identification help make the SLR objective compared to the narrative literature reviews (Durach, Kembro, and Wieland Citation2017; Thomé, Scavarda, and Scavarda Citation2016). To make the SLR process unbiased and robust in its approach, text mining has been effectively used for analysing literature reviews (e.g. Ghadge, Dani, and Kalawsky Citation2012; Khan, Akhtar, and Merali Citation2018; Galati and Bigliardi Citation2019; Ghadge, Weiß et al. Citation2020). Besides, the text mining approach supports the SLR process by cutting a few manual, iterative processes such as identifying search strings and data synthesis (Daneshvar Kakhki and Gargeya Citation2019).
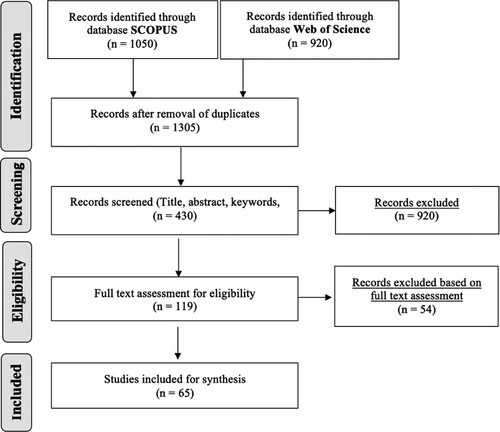
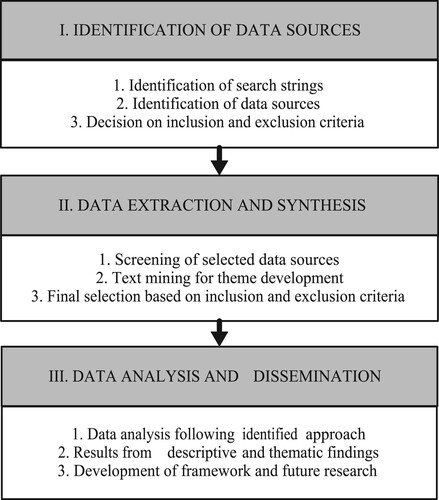
A structured process adopted from Tranfield, Denyer, and Smart (Citation2003) and Ghadge, Dani, and Kalawsky (Citation2012) for conducting a systematic literature review supported with text mining is presented in Figure . The process can be broadly divided into three stages – Identification of data sources, Data extraction & synthesis and Data analysis & Dissemination. A brief description of the activities involved in each stage of SLR is presented below.
Figure 1. Systematic literature review process (adapted from Tranfield, Denyer, and Smart Citation2003, Ghadge, Dani, and Kalawsky Citation2012).
2.1. Identification of data sources
Data identification is a critical stage in the SLR, attempting to clearly define the scope and quality of the study (Swanson et al. Citation2018). Here the keywords are identified for searching relevant information from multiple data sources. The pilot search results for apparent keywords: ‘Blockchain’ and ‘Pharmaceutical supply chain*’ received only 20 and 86 hits from Web of Science and EBSCO databases, respectively. Scanning through these limited papers thoroughly, other keywords were identified. In the Blockchain review articles (Wang et al. Citation2018; Chang and Chen Citation2020), other synonyms or technical names such as ‘distributed ledger’, ’shared ledger’ and ‘Smart contracts’ were found to be commonly used.
Similarly, the pharmaceutical and healthcare industries closely collaborate and associate with each other (Narayana, Kumar Pati, and Vrat Citation2014). In the pharmaceutical sector, the words – medicine’, ‘pharmaceutical’ and ‘drug’ are often used interchangeably (Taylor Citation2015). Two commonly used online databases, namely Scopus and Web of Science, were used to identify peer-reviewed, high quality (based on ranking, citations, impact factor, etc.) papers for conducting the SLR. Both databases are commonly used to screen data sources and are proven to provide reasonably comprehensive results (Harzing and Alakangas Citation2016). The choice of two different databases was to capture the inter-disciplinary work comprehensively. The Boolean operations connected different terms with ‘OR’ and ‘AND’. The detailed choice of search strings used for this study is presented in Table .
Table 1. Search strings used for SLR.
Predefined inclusion criteria comprised papers published between 2010–2021 to capture a decade long horizon. Although the first Blockchain-related paper was published in 2014, a more significant time span was selected to capture the broader growth of the PSC. Only peer-reviewed academic journals were included, while conference proceedings, books, book chapters and other grey literature (e.g. newspapers, white papers, HTML links, etc.) were excluded. This exclusion of the ‘grey literature’ in SLR studies helps to focus on the quality publications (Seuring and Müller, Citation2008; Ghadge, Dani, and Kalawsky Citation2012). Given the interdisciplinary nature of the study capturing the nexus between Blockchain and the pharmaceutical industry, papers published in wider journals (beyond operations and supply chain discipline) were considered to ensure the study is holistic and unbiased.
2.2. Data extraction & synthesis
The data extraction & synthesis stage helps to filter highly relevant papers. After removing the duplicates and excluding literature from the two selected databases; each paper was carefully synthesised by considering the title, keywords, and abstract to select 119 papers that were found to be relevant to the study. Following an iterative process, a full-text assessment of the selected paper identified 65 papers for final assessment. Figure shows the flow diagram followed for screening the dataset, adapting a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) approach, a systematic method to visualise the study selection process (Siddaway, Wood, and Hedges Citation2019). Text mining is used in this study for the data synthesis activity. Text mining of selected papers was conducted to validate the search strings identified in the previous stage and provide further support for the data analysis (Ghadge, Wurtmann, and Seuring Citation2020). QDA Miner©, a qualitative data analysis software developed by Provalis Research, was used as a text mining platform.
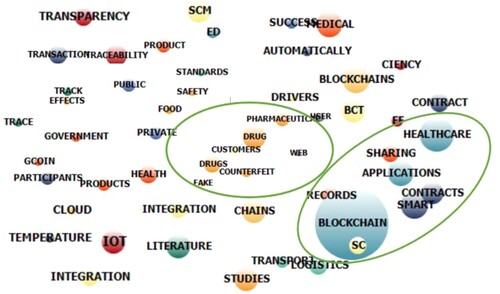
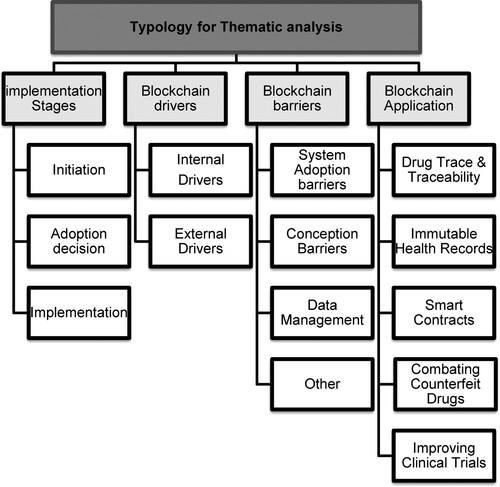
Table presents the most important words or phrases identified in the selected database by their term frequency and inverse document frequency (TF-IDF). TF-IDF is a measure of significance and provides information regarding the number of occurrences of a given word or phrase in the selected dataset (Aizawa Citation2003). It is noticeable that Blockchain, healthcare, supply chains, smart contracts appear as the most frequent words or phrases through text mining/analysis. Furthermore, most of the identified words and phrases (refer to Table ) strongly correlate with identified search strings for data identification. This retrospective validation of the pre-identified search strings provides confidence in the reliability of the process followed for data identification and analysis. Furthermore, data sources were (text) mined for extracting patterns to develop suitable themes using the clustering and correlation approach. Finally, the emerging themes were used for developing a typology for conducting thematic analysis, as shown in Figure .
Table 2. Keywords and phrases captured by text mining.
2.3. Data analysis & dissemination
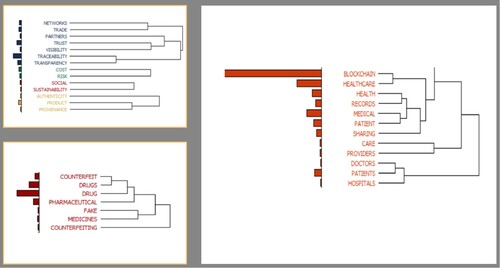
The data analysis and dissemination stage consist of descriptive and thematic analysis (Tranfield, Denyer, and Smart Citation2003). The former analysis aims to provide a brief overview capturing the depth and spread of the subject under study. In contrast, the thematic analysis attempts to provide comprehensive insights based on the developed themes. Following text mining, common themes were identified and formulated on different ‘clusters’ shaped in dendrograms and cluster diagrams (examples shown in Figures and ). Below identified ‘cluster-based’ themes were extensively discussed between authors to finalise a comprehensive typology for the thematic analysis and are shown in Figure .
Based on implementation stages: This theme attempts to capture developments through three stages adopted for implementing Blockchain: initiation, adoption decision, and implementation.
Based on drivers for Blockchain implementation: This theme attempts to identify enablers or drivers for adopting Blockchain technology in pharmaceutical supply chains.
Based on barriers to Blockchain implementation: This theme summarises the barriers/challenges to implementing Blockchain in PSC.
Based on Blockchain applications: This theme captures the broader applications of Blockchain within the pharmaceutical sector.
3. Descriptive analysis
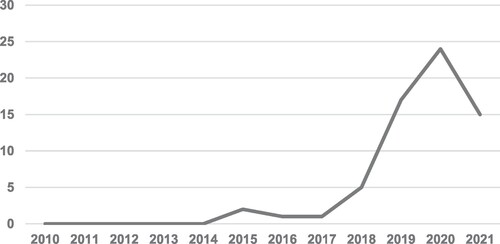
The descriptive analysis captures an overview of the development of Blockchain technology within PSC. Academic papers published over the last decade show that research on Blockchain within PSC has grown significantly over the last six years (2015-2021). Although Blockchain has been explored by researchers in other sectors (such as the financial, energy, food and construction), it is observed that the growth of research on Blockchain for pharmaceutical supply chains is slow compared to other sectors. Figure shows an exponential growth of research since 2018. Clearly, there is a growing interest in Blockchain implementation in PSC; however, it is believed that the research is still in the nascent stage, demanding several SC managers, decision-makers and researchers to explore the feasibility of this technology further.
In terms of the geographical location of these selected studies, the USA and the UK were the leading countries for exploring Blockchain in the pharmaceutical sector. Other countries such as China, India, UAE, Germany and Brazil also contribute to the growth of this research field. Developed countries have more mature use cases or ‘pilots’ on Blockchain for the medicine/drug supply chain, while the development of Blockchain in the pharmaceutical sector in developing countries is restricted by poor healthcare systems and networks (Kassab et al. Citation2019). This could be a possible reason for most studies undertaken in developed countries. Significant growth in the pharmaceutical sector in India and China has been observed since the pandemic and could be a reason for the growth in the research interest.
Most studies linking Blockchain with PSC are qualitative in nature, with only a small number of quantitative studies. It was noticed that the number of literature reviews on the broad topic of Blockchain implementation within various logistics and supply chain areas accounts for the majority (38%) of studies. Conceptual (15%) and pilot/case studies (15%) were also leading in the selected papers. Conceptual research involves interpreting the Blockchain’s potential or utilising existing operations/SCM theories to generate new insights. In contrast, case studies are an effective way to develop hypotheses and theories (Levy Citation2008). Few conceptual models attempted to demonstrate how Blockchain can be adopted in the supply chain for logistics monitoring, information sharing and integration with supply chain networks. These models are often combined with other disruptive technologies from Industry 4.0, such as IoT, Automation and Big Data Analytics (BDA), to achieve higher efficiency and reduce the adoption barriers (Ghadge, Kara et al. Citation2020). For example, IoT combined with Blockchain can help to record SC activities for network-wide security. Similarly, Blockchain coupled with BDA can support informed decision-making.
In contrast, proof of concept studies attempts to demonstrate Blockchain implementation’s functionality in SCs and verify the concepts following pilot programme(s). Business cases and surveys by ‘early adopters’ and Blockchain experts are observed to primarily study the feasibility of Blockchain adoption and its drivers and barriers within supply chains. Research attempting to conduct empirical/experimental studies to build the link between Blockchain and PSC is missing. A mixed methodology approach combining surveys and modelling may be helpful for the nature of the study. Operation and supply chain management theories have been broadly explored, namely- theory of constraints, system theory, game theory and transaction costs theory. Furthermore, only one-fourth of the selected studies contain some form of framework or model development approach following the use of existing theories (Table ). This further evidence that the theoretical perspectives on the Blockchain are inadequate and entail more research following existing or new theoretical lenses.
Table 3. Use of conceptual models/theories.
4. Thematic analysis
The thematic analysis provides comprehensive and interpretative research findings following a mixed- inductive and deductive approach (Castleberry and Nolen Citation2018; Nowell et al. Citation2017). The thematic analysis follows the typology-based assessment model shown in Figure . Blockchain implementation stages, drivers and barriers to adoption and applications within the PSC context are explored in this section.
4.1. Blockchain implementation stages for PSC
IT innovation adoption follows user acceptance and technology acquisition model stages: pre-adoption, adoption, and post-adoption (Hameed and Arachchilage Citation2016; Pichlak Citation2015). With the process of the adoption phase, many impacting factors are influencing the project’s success, such as organisational and environmental factors and user acceptance. It is observed that the implementation process and associated activities for integrating new technology within SC/Business models remain consistent (Hameed, Counsell, and Swift Citation2012; Pichlak Citation2015). Based on this generic principle and research development by authors (Refer to Vu, Ghadge, and Bourlakis Citation2021a, Citation2021b), the Blockchain implementation in PSC can be broadly classified into three stages: (1) Initiation – the organisation explores various aspects of the innovation in consideration, (2) Adoption decision – the organisation decides whether and how to implement the innovation, and (3) Implementation – the organisation deploys large scale implementation and integrates the innovation into its structure.
4.1.1. Initiation stage
Blockchain’s initiation stage captures the understanding of Blockchain and its potential benefits, including the rationale for adoption (Pichlak Citation2015). The external environment and technology initiatives drive the need for Blockchain adoption. Majority of conceptual studies in the pharmaceutical sector claim that the immutable and decentralised distributed ledger could stimulate a transparent, efficient, falsified-free pharmaceutical supply chain network. However, without common technical standards and other related regulations in the Blockchain-based solution for the PSC, stakeholders will not be willing to take risks to support the adoption. Tseng et al. (Citation2018) suggest an open government surveillance net by risk benchmark on medication movement in the PSC. The government and other participants in this supply chain could require inspecting the transaction information and protect the drugs’ authenticity. The initiation stage supports conceptualising a healthy Blockchain ecosystem for the PSC and further helps in enhancing the user acceptance of this technology.
4.1.2. Adoption decision stage
Moving from initiation to the adoption decision stage requires strategic, financial and technological evaluation for organisations (Pichlak Citation2015). This stage evaluates the inter-organisation resources and allocates them to support technology adoption. In other technology implementation cases such as RFID and IoT, top management commitment in the adoption decision stage is critical (Zelbst et al. Citation2019; Reyes, Visich, and Jaska Citation2020) as this is a strategic level decision, which is likely to change the structure and resource requirements of the organisation. The stakeholders’ support is beneficial in this stage to develop a Blockchain-based culture within the organisations and acquire related knowledge on Blockchain. The cost model is needed for the stakeholders to explain how Blockchain benefit the whole pharmaceutical supply chain from the financial perspective (Kshetri Citation2018; Chukwu and Garg Citation2020; Khatoon Citation2020). The long-term benefits of Blockchain, such as improved supply chain efficiency, high data security and combating counterfeited drugs, could persuade large enterprises to join the pilot project for implementation. Furthermore, the technical issues need to be tackled with the help of Blockchain platform companies (Hölbl et al. Citation2018; Chang and Chen Citation2020). Rather than integrating different healthcare information systems and pharmaceutical producers’ ERP systems, Blockchain provides interoperable interfaces with different systems and shares drug movement.
4.1.3. Implementation stage
The implementation stage refers to the practical use of Blockchain at the firm or supply chain level. It is reasonable to start a pilot project with few supply chain participants and test Blockchain’s feasibility in the PSC. van Hoek (Citation2019) emphasised that meaningful and valuable lessons can be obtained through pilot projects on a small scale. MediLedger is the famous FDA pilot project, including the collaboration of 25 leading pharmaceutical companies and other pharmaceutical supply chain participants, aiming to track drugs at a package level. This pilot project is appealing to more stakeholders and is likely to convince small businesses to join the Blockchain-based ecosystem of the pharmaceutical industry. With the success of pilot projects, scaling up those projects at a supply chain level is the next step. However, as identified earlier, scalability, especially the transaction speed and interoperability for multiple nodes, are the barriers that need to be overcome (Monfared Citation2016; Hughes et al. Citation2019; Wang et al. Citation2019; Singh, Dwivedi, and Srivastava Citation2020). If successful, Blockchain can be commonly accepted by users, with fewer technical issues and implementation costs. Bocek et al. (Citation2017) predict that the more mature the Blockchain market, the lesser the Blockchain adoption costs. Interestingly, the pharmaceutical market is projected to achieve at least 1.6 billion dollars in 2025, contributing to Blockchain implementation.
4.2. Drivers of blockchain implementation in PSC
Blockchain has a strong potential to mitigate pharmaceutical supply chain issues (Clauson et al. Citation2018; Bamakan, Moghaddam, and Manshadi Citation2021; Babu et al. Citation2022), and several practitioners such as IBM have adopted pilot projects with pharmaceutical manufacturers, distributors, and hospitals to prove the feasibility of Blockchain-based solutions in the PSC. Internal and external drivers facilitate this cooperation of multi-participants in PSC to implement Blockchain successfully.
4.2.1. Internal drivers
Thematic analysis into internal drivers identified three internal drivers for successfully implementing Blockchain in PSC. The need for improved transparency and visibility, enhanced data security and improved operational efficiency within the pharmaceutical supply chain were identified as key drivers.
Improved transparency and visibility
Sensitive medical records of patients are scattered across hospitals, manufacturers and distributors primarily for the demand planning of medicines/drugs. However, patients do not have access to the drug provenance (Jamil et al. Citation2019; Abbas et al. Citation2020; Musamih et al. Citation2021). Therefore, achieving total transparency and visibility in PSC is a challenge unlike any other supply chain but can help to increase collaboration, trust and efficiency. Thus, the immutable, tamper-proof, interoperable characteristic of Blockchain is a driving force in overcoming the above challenges (van Hoek Citation2019; Hastig and Sodhi Citation2020; Helo and Hao Citation2019; Reyes, Visich, and Jaska Citation2020). Due to these attributes, the use of Blockchain to influence personal health records while adhering to data protection regulations is highly attractive (Fang et al. Citation2021). In doing this, PSC stakeholders will have access to the transactional data without exposing it to third parties (Bocek et al. Citation2017; Wang et al. Citation2018; Sharma, Kaur, and Singh Citation2021). In addition, it can help with optimised data accuracy, contributing to the reduction in information delays. Recently, BlockRx, a pharma ecosystem, collaborated with pharma manufacturers, retailers, and pharmacies to integrate their existing information systems, break the information silos, and provide transparency within the supply chain (Farouk et al. Citation2020).
Enhanced data security
Central cloud storage of electronic medical records has been proved as a standard way of dealing with dispersed data, while it is vulnerable and easy to be damaged by cyber-attacks (Hussien et al. Citation2019). By adopting Blockchain, each transaction record will be grouped into time-stamping and immutable blocks and can be shared through the decentralised distributed ledger (McGhin et al. Citation2019; Vu, Ghadge, and Bourlakis Citation2021a). Thus, Blockchain protects healthcare and medicine/drug data from being attacked by hackers or cybercriminals.
Interestingly, medical data management has received substantial interest in Blockchain for PSC (e.g. Radanović and Likić Citation2018; Wang et al. Citation2018; Khezr et al. Citation2019; De Aguiar et al. Citation2020). Some pharmaceutical products, such as vaccines, require delicate temperature monitoring and humidity during transportation. Utilising IoT devices (e.g. GPS, temperature sensors) during shipment can quickly capture any unqualified environmental conditions for drugs and generate an alert to the authorised parties on the Blockchain platform (Zhao, Li, and Yao Citation2019; Leal et al. Citation2021). Patients can also secure the transportation conditions of vaccines throughout the supply chain (Singh, Dwivedi, and Srivastava Citation2020).
Smart contracts in this process play a vital role in verifying the authenticity of products and contingency of pre-set protocols and rules, as well as the termination of contracts and refunds (van Hoek Citation2019). In addition, several Blockchain companies recommend Blockchain-based projects such as ‘SmartHub’ to track and trace pharmaceutical product characteristics- such as location, ingredients, temperature settings and product images while dealing with international trade (Helo and Hao Citation2019). Another use case, ‘LedgerDomain’, utilises BRUINchain and bar-coding for patients to trace and track the drugs (Palas and Bunduchi Citation2021). In short, the need for traceability and security of drugs drives the Blockchain-based implementation in the PSC.
Improved efficiency
Adopting Blockchain helps to improve supply chain efficiency (Hald and Kinra Citation2019; Wong et al. Citation2020). Blockchain is expected to positively impact quick response to emergencies, accelerating the payment process and forecasting in the pharmaceutical industry (Ding Citation2018; Schmidt and Wagner Citation2019; Reyes, Visich, and Jaska Citation2020). In previous studies, the PSC’s notification process and drug recall were time-consuming due to the manual paperwork and fragmented, complex systems, leading to long response times and dissatisfied healthcare services. With the increased trust and information transparency among the supply chain parties, Blockchain helps speed up the processing time and warn supply chain parties quickly regarding drug recalls (Hosseini-Motlagh, Nematollahi, and Nami Citation2021). Besides, smart contracts can eliminate other intermediates and reduce labour costs, where the validation process is completed within the Blockchain network. The self-executed agreement will be valid when each party’s pre-set conditions, rights, and obligations are satisfied. Furthermore, Blockchain increases the extent of information sharing, contributing to identifying bottlenecks, avoiding overstocking, and eventually achieving higher efficiency in the pharmaceutical supply chain.
4.2.2. External drivers
Apart from internal drivers, the external market environment, such as the macroeconomics, regulations, and pressure from the competitors, also stimulates the adoption of new technology (Ciliberti, Carraresi, and Bröring Citation2016; Vu, Ghadge, and Bourlakis Citation2021b). In addition, external drivers are motivated by external environments such as suppliers, pharmaceutical regulatory bodies, governments and patients.
Enforcement by regulatory bodies
The pharmaceutical industry is strictly supervised by government departments such as the FDA and FMD (Falsified Medication Directive) and other non-profit organisations like WHO. Organisations are required to follow the regulations and combat counterfeited products. Developed countries are taking more practical actions in detecting falsified drugs than developing countries. The pilot projects on Blockchain, such as MediLedger and PharmaLedger, are designed to collaborate with global pharmaceutical manufacturers, distributors, hospitals, and pharmacies to make transactions on the distributed ledger. Similarly, the European Federation of Pharmaceutical Industries and Associations (EFPIA) is also focused on detecting counterfeited products in the PSC and are trying to eliminate unqualified drugs. The NHS (National Health Service, United Kingdom) follows the FMD directives and brings pharmacies together to supervise this medication movement. Developing countries like India and Brazil also promote Blockchain projects to improve their healthcare service level (Dhagarra et al. Citation2019).
Stakeholder’s pressure
Patients are increasingly interested in the provenance and ingredients of the medicines they use for checking side effects or adhering to sustainability and quality certificates (Yang et al. Citation2017). Furthermore, during the COVID-19 pandemic, consumers were restricted in using local pharmacies, leading to an increasing trend toward purchasing drugs online. Thus, governments demanded strict supervision of online pharmacies, especially their legitimacies and authenticity to reduce the chances of purchasing falsified drugs. My Net Doctor, a registered online pharmacy, recently signed up to join MediConnect’s Blockchain platform in the UK. This collaboration enhances the PSC traceability of online drugs, especially prescription drugs. MediConnect’s private, permissioned distributed ledger will store confidential customer prescription data and provenance data for medicines, preventing the misuse and overprescribing of prescription drugs.
4.3. Barriers to blockchain implementation in PSC
Previous studies emphasise Blockchain’s challenges in three aspects: system-related adoption barrier, conception and regulation barrier and data security barrier.
4.3.1. System adoption
Regarding technology adoption, technical and operational issues are the most common issues faced during implementation (Haseeb et al. Citation2019; Aceto, Persico, and Pescapé Citation2020). In the multidiscipline studies, scalability, smart contract design, and high adoption costs of Blockchain are ranked as the highest barriers to adoption.
Scalability: New technology adoption undergoes hundreds of market tests to prove its plausibility and feasibility. Blockchain application in the pharmaceutical industry is still in the early stage with limited successful pilot projects. According to the previous studies, scaling the Blockchain to a supply chain network level will need to meet thousands of transactions per second and manage large data capacity (Kassab et al. Citation2019). Healthcare and pharmaceutical industry transactions could lead to 10 billion drugs based on Taiwan’s healthcare insurance system (Tseng et al. Citation2018). While the current Gcoin Blockchain system, a Blockchain-based solution for drug governance, can only process a million transactions per day thus, time latency for verifying some of the transaction data will be evident. Moreover, complex and large medical supplies and decentralised distributed ledger mean multiple nodes are required to process each transaction. Therefore, scalability is a generic issue for wider Blockchain adoption and stands true in the case of PSC.
Smart contract design: Besides, self-executive smart contract design and verification between parties would also make mistakes if there were several permissioned parties. For example, the process of translating the PSC operations and regulations to smart contracts is complicated due to the balance of conflicts and benefits among different stakeholders (Wang, Han, and Beynon-Davies Citation2019). The ambiguous clause generated in the interpretation could cause an unsuccessful or wrong transaction. Besides, the initial smart contracts can only carry out simple processes like payment transactions, while laws and regulations are not included in the verification clauses (Kumar, Liu, and Shan Citation2020). An increase in transaction volumes and contractual agreements may make smart contracts inefficient in detecting counterfeit products. To overcome this, IBM has developed a ‘crypto-anchor’ platform to ensure receiving correct products through user authentication/certification (Prada-Delgado et al. Citation2021).
Operational cost: Blockchain adoption is likely to be delayed by the high system adoption (mining) cost and related costs like system maintenance and specialist consultant costs (Cole, Stevenson, and Aitken Citation2019). Practitioners from the pharmaceutical company are currently finding it hard to see whether Blockchain benefits in terms of improved efficiencies (Bocek et al. Citation2017) and cost reduction (Kshetri Citation2018) could cover its high technology implementation costs. Furthermore, the current ‘immature’ Blockchain technology market (van Hoek Citation2019) may lead to high system adoption costs and technical issues during the adoption. It is expected that large enterprises may be ‘early adopters’ of this technology, but medium and small enterprises may avoid taking this leap due to associated financial risks. Some scholars also recommend the combination of Blockchain with other technologies like Cloud, IoT and RFID for best results (Park and Park Citation2017; Aryal et al. Citation2020; Abbas et al. Citation2020; Singh, Dwivedi, and Srivastava Citation2020). Thus, the cost of integrating different systems would also be high. In addition, technical training for employees and cost of consultants for implementation would be high for Blockchain adoption at the supply chain level.
4.3.2. Conception and regulation
The support from the top management plays a vital role in the implementation success (Cole, Stevenson, and Aitken Citation2019; van Hoek Citation2019). In the literature study, leadership commitment and engagement are the success factors of Blockchain adoption in the pharmaceutical industry. However, pharmaceutical SC stakeholders have different interests in Blockchain projects due to a complex supply network. Thus, decisions based on a few successful pilot projects are challenging to justify an agreement for large-scale investment by the senior management. On the other hand, the lack of a defined industry governance framework associated with the Blockchain network and standards for information exchange (like GSI) is another conception barrier (Wang et al. Citation2019). Although some government sectors regulate counterfeited drugs, Blockchain-related laws and regulations are still insufficient, which poses more risks and pressure for managers to adopt Blockchain in healthcare as well as the pharmaceutical industry. Clearly, the industrial architecture and managerial support hinder the Blockchain adoption in PSC.
4.3.3. Data security-cyber risks
Digital supply chains will have an increased threat from cyber risks in the near future (Ghadge, Wurtmann, and Seuring Citation2020). Aptly, another challenge in the pharmaceutical industry is cyber security issues associated with data management. The cryptographic mechanism for protecting a patient’s medical records and medicine transaction information from cyber risks is still in the testing phase (Hussien et al. Citation2019). Patients have concerned about their privacy leakage and are unwilling to provide helpful information for the Blockchain-based solution. Past incidences, such as privacy leakage cases like AMCA (American Medical Collection Agency) data breach in 2018, undermine the patient’s confidence in data security. Moreover, the loss of core keys could damage the whole Blockchain operation since the verification process is ceased without private keys, causing millions of transaction records to be postponed (Dwivedi, Amin, and Vollala Citation2020).
Most importantly, the irreversible characteristic of Blockchain could be a barrier to data accuracy (Tseng et al. Citation2018; Wang et al. Citation2018; Chukwu and Garg Citation2020). Although incorrect data in Blockchain can be corrected in the sense that a second transaction corrects the first transaction. However, this makes the search in a Blockchain system more complex and can be time-consuming. Such inaccurate input data in the Blockchain can be concerning when emergent cases like drug recalls happen. Besides, the process of verifying and deciphering codes to text in the Blockchain may cause mistakes due to the misunderstanding of the coding languages (Dhagarra et al. Citation2019).
4.4. Applications of blockchain within PSC
Based on the selected studies, Blockchain applications can be broadly summarised in five parts, namely- (1) traceability of drugs, (2) combating counterfeited drugs, (3) improving clinical trials, (4) immutable data sharing, and (5) smart contracting.
The pilot projects and use cases captured in the review are shown in Table - the use cases like Medichain and BlockRx Pharma track drug status from raw ingredients to end patients. The real-time tracking of transaction records on the distributed ledger reduces the chance of counterfeited drugs in the pharmaceutical industry. For example, pharmaceutical company Pfizer has taken the Blockchain pilot project to ascertain the authenticity of their drugs. Besides, Blockchain platforms such as MeDshare proposed in Wang et al. (Citation2018) protect patients from cyber-attacks and ensure data security. Furthermore, smart contracting in the Blockchain assures financial transactions, reducing intermediaries’ costs.
Table 4. Pilot Blockchain applications.
5. Framework and future directions
It is observed from the selected past studies that Blockchain adoption in the PSC is still in its developmental stage. Due to the lack of empirical research, large-scale successful Blockchain-based platforms for the PSC are challenging. Besides, practitioners have difficulty defining the steps to implementing Blockchain technology in the pharmaceutical sector, as research demonstrating a framework for implementation in such a specific sector is non-existent within the supply chain management context. Therefore, following the insights generated from the thematic analysis, a conceptual framework of Blockchain implementation in the PSC is attempted. The proposed framework is likely to benefit the successful implementation of Blockchain in PSC. Although conceptual, it can be retrospectively validated on Blockchain pilot studies within PSC or tested on the new initiatives in PSC attempting to implement Blockchain. In the end, unique research gaps are identified, paving the way for future research directions in the selected area of study.
5.1. Development of a conceptual framework
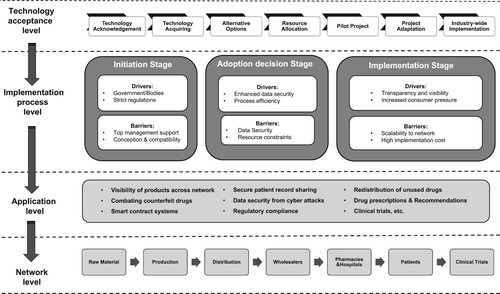
The developed conceptual framework integrates multiple layers to capture the Blockchain implementation within PSC holistically. As shown in Figure , the proposed framework has four layers: technology acceptance/integration layer, implementation process layer, application layer and network layer. Understanding of the first layer and coordination of the second layer is crucial for the successful implementation of Blockchain in PSC and for achieving benefits observed in the application layer. Under the established three stages of Blockchain implementation - initiation, adoption decision, and implementation, a set of influencing factors (drivers and barriers) are captured and presented in the implementation process layer.
With the development of Blockchain, technology user acceptance is expected to increase and ultimately promote several applications in the PSC. The user acceptance model is a ‘sequent model’ based on the degree of Blockchain technology acceptance. First, businesses realise the need to introduce technology (in this case, Blockchain) to address sector-specific supply chain issues. After obtaining the knowledge and deciding whether adopting Blockchain is suitable for the organisation, the resource requirements (like human labour, and technical support) are identified in the next stage. In the pre-final stage, small-scale pilot projects with few participants are proposed. The last phase of Blockchain implementation is scaling up those successful projects in a wider environment with more participants nodes and transactions being processed.
In the implementation process layer, several influential factors are identified for initiation, adoption, and implementation stages, as shown in Figure . Drivers and barriers are grouped following their appropriateness to highlighted stages. Multiple Blockchain applications benefiting the whole pharmaceutical supply chain network are captured in the application layer. The most common applications are tracking and tracing drugs throughout the PSC and combating counterfeited drugs. Besides, smart contracting rules among different participants assure safe payment and accelerate supply chain operation, enhancing supply chain efficiency. Early adoption work has seen first cases of safe electronic health records, redistribution of unused drugs, anti-counterfeiting and reliable clinical trials.
As part of the network later, Blockchain technology connects with seven key stakeholders of PSC and plays a vital role in balancing the conflicts and benefits among multiple supply chain nodes. For suppliers and producers, the quality of the raw ingredients of the medicine is guaranteed due to the visibility of data provenance. With the verification of ingredients, unqualified raw materials will be refused to trade in the PSC, and the pre-set rules in smart contracts can also intrigue payment termination. Furthermore, the identification code and serial numbers used on drug packages are helpful for distributors tracking and tracing these drugs throughout the whole PSC.
Downstream PSC network- patients, hospitals, and clinical trials can benefit from Blockchain in securing healthcare records and personal sensitive healthcare/medicine data. This proposed ‘layered’ framework captures multiple aspects by exploring the nexus between Blockchain and PSC. It also enables PSC participants to decide whether Blockchain is a suitable solution for their organisation based on the identification of influencing factors for each stage of implementation and wider applications across PSC.
5.2. Research gaps and avenues for future research
Potential to enhance sustainability and circular economy in PSC
Blockchain adoption in the PSC is strongly linked with environmental and social dimensions of sustainability (Wang, Lin, and Choi Citation2020). Personal information of patients and medical data can be kept confidential without compromising accessibility to data for demand planning and other supply chain activities through Blockchain implementation. On the other hand, Shipchain utilises smart contracts to achieve 100% digital transactions reducing the amount of paper wasted. Future researchers can explore more sustainable and circular economy practices with the use of Blockchain in the pharmaceutical industry, like patient-centred drug supply and sustainable reuse and disposal of the drugs (Ding Citation2018).
Enhancing drug recall management
Supply chain practitioners are still seeking proactive, efficient drug recall management systems, and with the help of Blockchain, it is possible to assure drug authenticity and bring transparency and visibility across drug supply chain networks (Hosseini-Motlagh, Jazinaninejad, and Nami Citation2020; Wu and Lin Citation2019). The possibility of Blockchain technology use for reverse pharmaceutical logistics for expired or recalled drugs is another exciting research direction.
Governance of Blockchain standards and regulations
The Blockchain ecosystem in the PSC is still in the initial stage due to inadequate successful practices and clear regulations for this digital domain (De Aguiar et al. Citation2020). The review clearly identified and highlighted this concern while discussing implementation drivers and barriers. Pharmaceutical supply chain practitioners share concerns related to limited regulations like protection privacy rules to sensitive data that could hinder Blockchain adoption on a large scale. Besides, Zwitter and Hazenberg (Citation2020) suggested that the governance model is likely to be updated and changed by implementing a Blockchain consortium. Clearly, standardising Blockchain practices and well-defined PSC regulations is lacking and needs further extensive research.
Scalability and need for a solid transaction mechanism
The scalability of the Blockchain platform will be a technical barrier when millions of healthcare/pharmaceutical transactions are required to be processed simultaneously (Khezr et al. Citation2019). Practitioners can consider designing a more secure and robust mechanism and practices for smart contract rules to meet high throughput requirements.
Improving the interoperability and compatibility of Blockchain
Blockchain interoperability may provide an opportunity for collaboration among different Blockchain systems. However, there are concerns that Blockchain interoperability in the healthcare/pharmaceutical system may be challenging. Besides, integrating Blockchain with other support technologies such as IoT and RFID is beneficial for PSC in improving supply chain traceability (Fernández-Caramés and Fraga-Lamas Citation2018; Botcha, Chakravarthy, and Anurag Citation2019; Bamakan, Moghaddam, and Manshadi Citation2021); but this opportunity has not been fully explored. A Blockchain-based IoT system, designed by Ahmadi et al. (Citation2020), assures drug provenance through its life cycle. To better manage larger data, ERP-integrated Blockchain systems should be established. Practitioners can explore further possibilities of enhancing Blockchain interoperability and compatibility with other disruptive technologies in the future.
Building performance measurement of Blockchain
Liu et al. (Citation2019) suggested that the performance optimisation system of Blockchain is beneficial for organisations to evaluate its success and failures and decide whether Blockchain is genuinely needed in this industry. As the Blockchain pilot projects in the PSC increase, there is a need to build a performance measurement system to measure the efficiency of the implemented Blockchain system. Kuhi, Kaare, and Koppel (Citation2018) pointed out that performance measurement could facilitate organisations to follow the benchmarking standards to evaluate their performance at each implementation stage, ultimately improving the whole supply chain operations. Research attempting to conduct empirical/experimental studies to build the link between Blockchain and PSC is also found to be necessary.
6. Conclusion
6.1. Discussion
This study aimed to holistically explore the current state of Blockchain implementation in the PSC and build a conceptual framework following a systematic literature review. First, screening academic studies in the PSC provided an overview and potential of Blockchain as a disruptive technology. The descriptive analysis provided insights into the generic trend from a geographical and publication perspective, along with adapted theories for conceptualisation. The thematic analysis explored influential factors (drivers and barriers) to integration, implementation stages and applications for PSC. Finally, the insights generated following thematic analysis identified a conceptual framework and research gaps.
In conclusion, it is observed that the growth of research in Blockchain for pharmaceutical supply chains is slow compared to other sectors. Blockchain development in the PSC is still at the initiation stage and is likely to grow significantly in the future. The requirement for enhanced transparency, efficiency, traceability, and data security drives Blockchain adoption in the pharmaceutical supply chain. In addition, external pressure, such as patients, competitors, and regulators, drives SC managers to adopt this technology. Based on the current Blockchain pilot projects and use cases in the PSC, combating falsified drugs, recall of medicines, and data security, compliance to pharmaceutical standards, and reliability of clinical trials are the most promising Blockchain applications. While PSC system and technology integration-related identified barriers could hinder the industry-wide agreement to use Blockchain.
6.2. Contribution to theory and practice
This review delivers several contributions to theory as well as practice. Firstly, the SLR provides a broad overview of challenges faced within PSC and captures the potential of Blockchain to overcome them. To the best of the authors knowledge, it is the first review attempting to explore the potential of Blockchain in pharmaceutical supply chains. The systematic review provides SC managers and academics insights into implementation stages, adoption drivers and barriers, along with the latest applications of Blockchain. The conceptual framework based on Blockchain adoption stages provides directives for successful implementing Blockchain in PSC. Farouk et al. (Citation2020) stressed that Blockchain regulations enforcement could facilitate more small and medium organisations to join the Blockchain ecosystem. Identified multiple research opportunities provide a good starting point for exploring the nexus between Blockchain and PSC. For the government and other policymakers, findings can benefit in designing new regulatory standards and rules for Blockchain-enabled PSCs.
6.3. Limitations of the study
There are some limitations to this study. Only 65 interdisciplinary papers were selected for the study. The selection of keywords and screening process can be subjective and biased; however, an attempt was made to overcome this limitation through the use of text mining to validate the choice of keywords. Besides, the study mainly focuses on the pharmaceutical sector, and thus, studies not strongly interlinking with the healthcare sector may be neglected. The technical aspects related to Blockchain such as compatibility, interoperability and large data sharing during and after the implementation, along with legal & regulatory challenges of PSC are not considered in detail, needing future focus. Following the empirical study, future research will test the proposed conceptual framework for feasibility in a practical setting.
Acknowledgement
The authors are grateful to AE and the anonymous reviewers for their constructive comments and suggestions to improve the contents of this paper. The authors acknowledge the support of MSc student Ms Lu Zhu from Cranfield University for preliminary data collection and synthesis of the literature.
Data availability statement
The data that support the findings of this study is available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors

Abhijeet Ghadge
Abhijeet Ghadge is an Associate professor of Supply Chain Management at Cranfield School of Management, UK. He holds PhD in Operations and Supply Chain Management from Loughborough University, UK, and MTech in Industrial Engineering and Management from Indian Institute of Technology, India. He has several years of industrial, academic and consulting experience working with a wide range of UK, European and Asian organisations. Dr Ghadge has published widely in leading operations, logistics and supply chain management journals. He follows a practice-driven approach to problems across the broad domains of supply chain resilience, and supply chain 4.0.

Michael Bourlakis
Michael Bourlakis is the Director of Research for Cranfield School of Management and the Head of the Logistics, Procurement and Supply Chain Management Group. Michael is an internationally renowned and established authority in logistics and supply chain management. He has been very successful in winning substantial external funding. Specifically, he has won 35 research and consulting projects totalling more than £33.5 million (total allocated project funding) from various bodies and private companies. Professor Michael Bourlakis has generated 85 journal papers and 5 edited books. Professor Bourlakis has been a Chair and Keynote Speaker for leading logistics academic and industry conferences worldwide and his multidisciplinary research work and impactful thought leadership has received considerable attention by the media (BBC News, ITV News, BBC Radio 4 Today, The Guardian, Wall Street Journal etc.). Michael joined the Research Excellence Framework (REF2021) Panel for Business and Management Studies (UoA 17) being involved with the evaluation of all UK Business Schools in relation to logistics, supply chain and operations management outputs.

Sachin Kamble
Sachin Kamble is a Professor of Strategy at EDHEC Business School, France. He has over 20 years of academic experience and is associated with leading manufacturing organisations in India, as a consultant and trainer. His research interest is inclined towards understanding the impact of emerging technologies such as Blockchain, Industry 4.0, and Big Data Analytics on sustainable supply chain performance. His work has been published in high-impact journals such as the International Journal of Production Economics, International Journal of Production Research, Computers in Industry, and Production Planning and Control.

Stefan Seuring
Stefan Seuring is full professor of Supply Chain Management at the University of Kassel, Germany. Stefan is one of the globally leading authors on sustainable supply chain management, but also covers topics on supply chain strategy, digitisation and circular economy. Stefan has collaborated interdisciplinary in research project with colleagues from agriculture, engineering and political sciences. The Web of Science and Clarivate Analytics listed him as a highly cited researcher, in 2018 for Cross-field in 2019 and 2020 for Economics and Business.
References
- Abbas, K., M. Afaq, T. Ahmed Khan, and W. C. Song. 2020. “A Blockchain and Machine Learning-Based Drug Supply Chain Management and Recommendation System for Smart Pharmaceutical Industry.” Electronics 9 (5): 852.
- Aceto, G., V. Persico, and A. Pescapé. 2020. “Industry 4.0 and Health: Internet of Things, Big Data, and Cloud Computing for Healthcare 4.0.” Journal of Industrial Information Integration 18: 100129.
- Ahmadi, V., S. Benjelloun, M. El Kik, T. Sharma, H. Chi, and W. Zhou. 2020. “Drug Governance: Iot-Based Blockchain Implementation in the Pharmaceutical Supply Chain.” 2020 6th international conference on mobile and secure services, MOBISECSERV 2020, Institute of Electrical and Electronics Engineers Inc.
- Aizawa, A. 2003. “An Information-Theoretic Perspective of Tf-Idf Measures.” Information Processing and Management 39: 45–65.
- Aryal, A., Y. Liao, P. Nattuthurai, and B. Li. 2020. “The Emerging big Data Analytics and IoT in Supply Chain Management: A Systematic Review.” Supply Chain Management: An International Journal 25 (2): 141–156.
- Azzi, R., R. K. Chamoun, and M. Sokhn. 2019. “The Power of a Blockchain-Based Supply Chain.” Computers & Industrial Engineering. Elsevier Ltd 135: 582–592.
- Babu, E. S., I. Kavati, S. R. Nayak, U. Ghosh, and W. Al Numay. 2022. “Secure and Transparent Pharmaceutical Supply Chain Using Permissioned Blockchain Network.” International Journal of Logistics Research and Applications, 1–28. doi:10.1080/13675567.2022.2045578.
- Bamakan, S. M. H., S. G. Moghaddam, and S. D. Manshadi. 2021. “Blockchain-enabled Pharmaceutical Cold Chain: Applications, key Challenges, and Future Trends.” Journal of Cleaner Production 127021.
- Bocek, T., B. B. Rodrigues, T. Strasser, and B. Stiller. 2017. “Blockchains Everywhere - A use-Case of Blockchains in the Pharma Supply-Chain.” Proceedings of the IM 2017 - 2017 IFIP/IEEE international symposium on integrated network and service management, Institute of Electrical and Electronics Engineers Inc., pp. 772–777.
- Botcha, K. M., V. V. Chakravarthy, and A. Anurag. 2019. “Enhancing Traceability in Pharmaceutical Supply Chain Using Internet of Things (IoT) and Blockchain.” Proceedings - 2019 IEEE international conference on intelligent systems and green technology, ICISGT 2019, Institute of Electrical and Electronics Engineers Inc, pp. 45–48.
- Buckley, G. J., and L. O. Gostin. 2013. “Causes of Falsified and Substandard Drugs.” In Countering the Problem of Falsified and Substandard Drugs. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK202531/ (Accessed: 19 September 2022).
- Castleberry, A., and A. Nolen. 2018. “Thematic Analysis of Qualitative Research Data: Is it as Easy as it Sounds?” Currents in Pharmacy Teaching and Learning 10: 807–815.
- Chang, S. E., and Y. Chen. 2020. “When Blockchain Meets Supply Chain: A Systematic Literature Review on Current Development and Potential Applications.” IEEE Access. Institute of Electrical and Electronics Engineers Inc 8: 62478–62494.
- Chang, Y., E. Iakovou, and W. Shi. 2020. “Blockchain in Global Supply Chains and Cross Border Trade: A Critical Synthesis of the State-of-the-art, Challenges and Opportunities.” International Journal of Production Research 58 (7): 2082–2099.
- Choudhury, O., I. Sylla, N. Fairoza, and A. Das. 2019. “A Blockchain Framework for Ensuring Data Quality in Multi-Organisational Clinical Trials.” 2019 IEEE international conference on healthcare informatics, ICHI 2019, Institute of Electrical and Electronics Engineers Inc.
- Chukwu, E., and L. Garg. 2020. “A Systematic Review of Blockchain in Healthcare: Frameworks, Prototypes, and Implementations.” IEEE Access. Institute of Electrical and Electronics Engineers Inc 8: 21196–21214.
- Ciliberti, S., L. Carraresi, and S. Bröring. 2016. “Drivers of Innovation in Italy: Food Versus Pharmaceutical Industry.” British Food Journal. Emerald Group Publishing Ltd. 118 (6): 1292–1316.
- Clauson, K. A., E. A. Breeden, C. Davidson, and T. K. Mackey. 2018. “Leveraging Blockchain Technology to Enhance Supply Chain Management in Healthcare: An Exploration of Challenges and Opportunities in the Health Supply Chain.” Blockchain in Healthcare Today 1 (3): 1–12.
- Cole, R., M. Stevenson, and J. Aitken. 2019. “Blockchain Technology: Implications for Operations and Supply Chain Management.” Supply Chain Management: An International Journal. Emerald Group Publishing Ltd. 24 (4): 469–483.
- Daneshvar Kakhki, M., and V. B. Gargeya. 2019. “Information Systems for Supply Chain Management: A Systematic Literature Analysis.” International Journal of Production Research 57 (15-16): 5318–5339.
- De Aguiar, E. J., B. S. Faiçal, B. Krishnamachari, and J. Ueyama. 2020. “A Survey of Blockchain-Based Strategies for Healthcare.” ACM Computing Surveys. Association for Computing Machinery (ACM) 53 (2): 1–27.
- Dhagarra, D., M. Goswami, P. R. S. Sarma, and A. Choudhury. 2019. “Big Data and Blockchain Supported Conceptual Model for Enhanced Healthcare Coverage: The Indian Context.” Business Process Management Journal. Emerald Group Publishing Ltd. 25 (7): 1612–1632.
- Ding, B. 2018. “Pharma Industry 4.0: Literature Review and Research Opportunities in Sustainable Pharmaceutical Supply chains.” Process Safety and Environmental Protection. Institution of Chemical Engineers 119: 115–130.
- Durach, C. F., J. Kembro, and A. Wieland. 2017. “A New Paradigm for Systematic Literature Reviews in Supply Chain Management.” Journal of Supply Chain Management 53 (4): 67–85.
- Dwivedi, S. K., R. Amin, and S. Vollala. 2020. “Blockchain Based Secured Information Sharing Protocol in Supply Chain Management System with key Distribution Mechanism.” Journal of Information Security and Applications. Elsevier Ltd 54: 102554.
- Fang, H. S. A., T. H. Tan, Y. F. C. Tan, and C. J. M. Tan. 2021. “Blockchain Personal Health Records: Systematic Review.” Journal of Medical Internet Research 23 (4): e25094.
- Farouk, A., A. Alahmadi, S. Ghose, and A. Mashatan. 2020. “Blockchain Platform for Industrial Healthcare: Vision and Future opportunities.” Computer Communications 154: 223–235.
- FDA. 2021. “FDA in Brief: FDA Provides New Guidance to Further Enhance the Security of Prescription Drugs in the U.S. Supply Chain.” Accessed September 19 2022. https://www.fda.gov/news-events/press-announcements/fda-brief-fda-provides-new-guidance-further-enhancesecurity-prescription-drugs-us-supply-chain.
- Fernández-Caramés, T. M., and P. Fraga-Lamas. 2018. “A Review on the Use of Blockchain for the Internet of Things.” IEEE Access 6: 32979–33001.
- Fraga-Lamas, P., and T. M. Fernandez-Carames. 2020. “Fake News, Disinformation, and Deepfakes: Leveraging Distributed Ledger Technologies and Blockchain to Combat Digital Deception and Counterfeit Reality.” IT Professiona. IEEE Computer Societyl 22 (2): 53–59.
- Galati, F., and B. Bigliardi. 2019. “Industry 4.0: Emerging Themes and Future Research Avenues Using a Text Mining Approach.” Computers in Industry 109: 100–113.
- Ghadge, A., S. Dani, and R. Kalawsky. 2012. “Supply Chain Risk Management: Present and Future Scope.” The International Journal of Logistics Management 23: 313–339.
- Ghadge, A., M. E. Kara, H. Moradlou, and M. Goswami. 2020. “The Impact of Industry 4.0 Implementation on Supply Chains.” Journal of Manufacturing Technology Management 31 (4): 669–686.
- Ghadge, A., M. Weiß, N. D. Caldwell, and R. Wilding. 2020. “Managing Cyber Risk in Supply Chains: A Review and Research Agenda.” Supply Chain Management: An International Journal 25 (2): 223–240.
- Ghadge, A., H. Wurtmann, and S. Seuring. 2020. “Managing Climate Change Risks in Global Supply Chains: A Review and Research Agenda.” International Journal of Production Research 58 (1): 44–64.
- Hald, K. S., and A. Kinra. 2019. “How the Blockchain Enables and Constrains Supply Chain Performance.” International Journal of Physical Distribution & Logistics Management 49 (4): 376–397.
- Hameed, M. A., and N. A. G. Arachchilage. 2016. A Model for the Adoption Process of Information System Security Innovations in Organisations: A Theoretical Perspective. arXiv preprint arXiv:1609.07911. doi:10.48550/arXiv.1609.07911.
- Hameed, M. A., S. Counsell, and S. Swift. 2012. “A Conceptual Model for the Process of IT Innovation Adoption in organisations.” Journal of Engineering and Technology Management - JET-M. Elsevier 29 (3): 358–390.
- Harzing, A. W. 2016. “Google Scholar, Scopus and the Web of Science: A Longitudinal and Cross-Disciplinary Comparison.” Scientometrics 106 (2): 787–804.
- Haseeb, M., H. I. Hussain, B. Ślusarczyk, and K. Jermsittiparsert. 2019. “Industry 4.0: A Solution Towards Technology Challenges of Sustainable Business Performance.” Social Sciences. MDPI AG 8 (5): 154.
- Hastig, G. M., and M. S. Sodhi. 2020. “Blockchain for Supply Chain Traceability: Business Requirements and Critical Success Factors.” Production and Operations Management. Wiley-Blackwell 29 (4): 935–954.
- Helo, P., and Y. Hao. 2019. “Blockchains in Operations and Supply Chains: A Model and Reference Implementation.” Computers & Industrial Engineering. Elsevier Ltd 136: 242–251.
- Hölbl, M., M. Kompara, A. Kamišalić, and L. Nemec Zlatolas. 2018. “A Systematic Review of the Use of Blockchain in Healthcare.” Symmetry. MDPI AG 10 (10): 470.
- Hosseini-Motlagh, S. M., M. Jazinaninejad, and N. Nami. 2020. “Recall Management in Pharmaceutical Industry through Supply Chain Coordination.” Annals of Operations Research 1–39. doi:10.1007/s10479-020-03720-7.
- Hosseini-Motlagh, S.-M., M. Nematollahi, and N. Nami. 2021. “Drug Recall Management and Channel Coordination Under Stochastic Product Defect Severity: A Game-Theoretic Analytical Study.” International Journal of Production Research 59 (6): 1649–1675.
- Hughes, L., Y. K. Dwivedi, S. K. Misra, N. P. Rana, V. Raghavan, and V. Akella. 2019. “Blockchain Research, Practice and Policy: Applications, Benefits, Limitations, Emerging Research Themes and Research Agenda.” International Journal of Information Management. Elsevier Ltd 49: 114–129.
- Hussien, H. M., S. M. Yasin, S. N. I. Udzir, A. A. Zaidan, and B. B. Zaidan. 2019. “A Systematic Review for Enabling of Develop a Blockchain Technology in Healthcare Application: Taxonomy, Substantially Analysis, Motivations, Challenges, Recommendations and Future Direction.” Journal of Medical Systems. Springer New York LLC 43 (10): 1–35.
- Interpol. 2020. Global operation sees a rise in fake medical products related to COVID-19, https://www.interpol.int/en/News-and-Events/News/2020/Global-operation-sees-a-rise-in-fake-medical-products-related-to-COVID-19.
- Jamil, F., L. Hang, K. Kim, and D. Kim. 2019. “A Novel Medical Blockchain Model for Drug Supply Chain Integrity Management in a Smart Hospital.” Electronics. MDPI AG 8 (5): 505.
- Jangir, S., A. Muzumdar, A. Jaiswal, C. N. Modi, S. Chandel, and C. Vyjayanthi. 2019. “A Novel Framework for Pharmaceutical Supply Chain Management Using Distributed Ledger and Smart Contracts.” 2019 10th international conference on computing, communication and networking technologies, ICCCNT 2019, Institute of Electrical and Electronics Engineers Inc.
- Karamchandani, A., S. K. Srivastava, S. Kumar, and A. Srivastava. 2021. “Analysing Perceived Role of Blockchain Technology in SCM Context for the Manufacturing Industry.” International Journal of Production Research 59 (11): 3398–3429.
- Kassab, M., J. DeFranco, T. Malas, P. Laplante, G. Destefanis, and V. V. G. Neto. 2019. “Exploring Research in Blockchain for Healthcare and a Roadmap for the Future.” IEEE Transactions on Emerging Topics in Computing 9 (4): 1835–1852.
- Khan, M. N., P. Akhtar, and Y. Merali. 2018. Industrial Management & Data Systems. Taylor & Francis Group.
- Khatoon, A. 2020. “A Blockchain-Based Smart Contract System for Healthcare Management.” Electronics. MDPI AG 9 (1): 94.
- Khezr, S., M. Moniruzzaman, A. Yassine, and R. Benlamri. 2019. “Blockchain Technology in Healthcare: A Comprehensive Review and Directions for Future Research.” Applied Sciences (Switzerland) 9 (9): 1–28.
- Kshetri, N. 2018. “1 Blockchain’s Roles in Meeting key Supply Chain Management objectives.” International Journal of Information Management 39: 80–89.
- Kuhi, K., K. Kaare, and O. Koppel. 2018. “Ensuring Performance Measurement Integrity in Logistics Using Blockchain.” Proceedings of the 2018 IEEE international conference on service operations and logistics, and informatics, SOLI 2018, Institute of Electrical and Electronics Engineers Inc., pp. 256–261.
- Kumar, A., R. Liu, and Z. Shan. 2020. “Is Blockchain a Silver Bullet for Supply Chain Management? Technical Challenges and Research Opportunities.” Decision Sciences. Blackwell Publishing Ltd 51 (1): 8–37.
- Leal, F., A. E. Chis, S. Caton, H. González–Vélez, J. M. García–Gómez, M. Durá, and M. Mier. 2021. “Smart Pharmaceutical Manufacturing: Ensuring end-to-end Traceability and Data Integrity in Medicine Production.” Big Data Research 24: 100172.
- Ledger Insights. 2020. “Bahrain to Use Blockchain for Food, Pharma Traceability, Customs Amid COVID-19.” Accessed September 19 2022. https://www.ledgerinsights.com/bahrain-to-use-blockchain-for-food-pharma-traceability-customs-amid-covid-19/.
- Levy, J. S. 2008. “Case Studies: Types, Designs, and Logics of Inference.” Conflict Management and Peace Science 25 (1): 1–18.
- Liu, M., F. R. Yu, Y. Teng, V. C. Leung, and M. Song. 2019. “Performance Optimization for Blockchain-Enabled Industrial Internet of Things (IIoT) Systems: A Deep Reinforcement Learning Approach.” IEEE Transactions on Industrial Informatics. IEEE Computer Society 15 (6): 3559–3570.
- López, D., and B. Farooq. 2020. “A Multi-Layered Blockchain Framework for Smart Mobility Data-markets.” Transportation Research Part C: Emerging Technologies. Elsevier Ltd 111: 588–615.
- McGhin, T., K. K. R. Choo, C. Z. Liu, and D. He. 2019. “Blockchain in Healthcare Applications: Research Challenges and opportunities.” Journal of Network and Computer Applications 135 (c): 62–75.
- Moktadir, M. A., S. M. Ali, S. K. Mangla, T. A. Sharmy, S. Luthra, N. Mishra, and J. A. Garza-Reyes. 2018. “Decision Modeling of Risks in Pharmaceutical Supply Chains.” Industrial Management and Data Systems 118 (7): 1388–1412.
- Monfared, R. 2016. “Blockchain ready manufacturing supply chain using distributed ledger, Accepted to the International Journal of Research in Engineering and Technology-IJRET.” Accessed June 12 2020. http://esatjournals.net/ijret/2016v05/i09/IJRET20160509001.pdfMetadataRecord:https://dspace.lboro.ac.uk/2134/22625.
- Musamih, A., K. Salah, R. Jayaraman, J. Arshad, M. Debe, Y. Al-Hammadi, and S. Ellahham. 2021. “A Blockchain-Based Approach for Drug Traceability in Healthcare Supply Chain.” IEEE Access 9: 9728–9743.
- Narayana, S. A., R. Kumar Pati, and P. Vrat. 2014. “Managerial Research on the Pharmaceutical Supply Chain - A Critical Review and Some Insights for Future directions.” Journal of Purchasing and Supply Management. Pergamon 20 (1): 18–40.
- Nowell, L. S., J. M. Norris, D. E. White, and N. J. Moules. 2017. “Thematic Analysis.” International Journal of Qualitative Methods. SAGE Publications Inc. 16 (1): 160940691773384.
- Palas, M. J. U., and R. Bunduchi. 2021. “Exploring Interpretations of Blockchain’s Value in Healthcare: A Multi-Stakeholder Approach.” Information Technology and People 34 (2): 453–495.
- Panwar, A., V. Bhatnagar, M. Khari, A. W. Salehi, and G. Gupta. 2022. “A Blockchain Framework to Secure Personal Health Record (PHR) in IBM Cloud-Based Data Lake.” Computational Intelligence and Neuroscience. doi:10.1155/2022/3045107.
- Park, Jin, and Jong Park. 2017. “Blockchain Security in Cloud Computing: Use Cases, Challenges, and Solutions.” Symmetry. MDPI AG 9 (8): 164.
- Pham, H. L., T. H. Tran, and Y. Nakashima. 2019. “Practical Anti-Counterfeit Medicine Management System Based on Blockchain Technology.” TIMES-iCON 2019 - 2019 4th technology innovation management and engineering science international conference, Institute of Electrical and Electronics Engineers Inc.
- Pichlak, M. 2015. “The Innovation Adoption Process: A Multidimensional Approach.” Journal of Management and Organization. Cambridge University Press, 476–494.
- Pournader, M., Y. Shi, S. Seuring, and S. L. Koh. 2020. “Blockchain Applications in Supply Chains, Transport and Logistics: A Systematic Review of the Literature.” International Journal of Production Research. Taylor and Francis Ltd. 58 (7): 2063–2081.
- Prada-Delgado, M. A., G. Dittmann, I. Circiumaru, and J. Jelitto. 2021. “A Blockchain-Based Crypto-Anchor Platform for Interoperable Product Authentication.” IEEE international symposium on circuits and systems (ISCAS), pp. 1–5.
- Queiroz, M. M., R. Telles, and S. H. Bonilla. 2020. “Blockchain and Supply Chain Management Integration: A Systematic Review of the Literature.” Supply Chain Management: An International Journal 25 (2): 241–254.
- Radanović, I., and R. Likić. 2018. “Opportunities for Use of Blockchain Technology in Medicine.” Applied Health Economics and Health Policy. Springer International Publishing 16 (5): 583–590.
- Reyes, P. M., J. K. Visich, and P. Jaska. 2020. “Managing the Dynamics of New Technologies in the Global Supply Chain.” IEEE Engineering Management Review. Institute of Electrical and Electronics Engineers Inc. 48 (1): 156–162.
- Saberi, S., M. Kouhizadeh, J. Sarkis, and L. Shen. 2019. “Blockchain Technology and Its Relationships to Sustainable Supply Chain Management.” International Journal of Production Research 57 (7): 2117–2135.
- Schmidt, C. G., and S. M. Wagner. 2019. “Blockchain and Supply Chain Relations: A Transaction Cost Theory Perspective.” Journal of Purchasing and Supply Management. Elsevier Ltd 25 (4): 100552.
- Seuring, S., and M. Müller. 2008. “From a Literature Review to a Conceptual Framework for Sustainable Supply Chain Management.” Journal of Cleaner Production 16 (15): 1699–1710.
- Sharma, A., S. Kaur, and M. Singh. 2021. “A Comprehensive Review on Blockchain and Internet of Things in Healthcare.” Transactions on Emerging Telecommunications Technologies 32 (10): e4333.
- Siddaway, A. P., A. M. Wood, and L. V. Hedges. 2019. “How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses.” Annual Review of Psychology 70: 747–770.
- Singh, R., A. D. Dwivedi, and G. Srivastava. 2020. “Internet of Things Based Blockchain for Temperature Monitoring and Counterfeit Pharmaceutical Prevention.” Sensors 20 (14): 3951–3923.
- Swanson, D., L. Goel, K. Francisco, and J. Stock. 2018. “An Analysis of Supply Chain Management Research by Topic.” Supply Chain Management: An International Journal 23 (2): 100–116.
- Sylim, P., F. Liu, A. Marcelo, and P. Fontelo. 2018. “Blockchain Technology for Detecting Falsified and Substandard Drugs in Distribution: Pharmaceutical Supply Chain Intervention.” Journal of Medical Internet Research 20 (9): e10163.
- Taylor, D. 2015. “The Pharmaceutical Industry and the Future of Drug Development.” In Pharmaceuticals in the Environment, 1–33. doi:10.1039/9781782622345-00001.
- Thomé, A. M. T., L. F. Scavarda, and A. J. Scavarda. 2016. “Conducting Systematic Literature Review in Operations Management.” Production Planning & Control 27 (5): 408–420.
- Tranfield, D., D. Denyer, and P. Smart. 2003. “Towards a Methodology for Developing Evidence-Informed Management Knowledge by Means of Systematic Review.” British Journal of Management 14 (3): 207–222.
- Tseng, J. H., Y. C. Liao, B. Chong, and S. W. Liao. 2018. “Governance on the Drug Supply Chain via Gcoin Blockchain.” International Journal of Environmental Research and Public Health. MDPI AG 15 (6): 1055.
- van Hoek, R. 2019. “Developing a Framework for Considering Blockchain Pilots in the Supply Chain – Lessons from Early Industry adopters.” Supply Chain Management: An International Journal. Emerald Group Publishing Ltd. 25 (1): 115–121.
- van Hoek, R. 2019. “Exploring Blockchain Implementation in the Supply Chain.” International Journal of Operations & Production Management. Emerald Group Publishing Ltd. 39: 829–859.
- Vu, N., A. Ghadge, and M. Bourlakis. 2021a. “Blockchain Adoption in Food Supply Chains: A Review and Implementation Framework.” Production Planning & Control, 1–18. doi:10.1080/09537287.2021.1939902.
- Vu, N., A. Ghadge, and M. Bourlakis. 2021b. “Evidence-Driven Model for Implementing Blockchain in Food Supply Chains.” International Journal of Logistics research and Applications, 1–21. doi:10.1080/13675567.2022.2115987.
- Wang, Y., J. H. Han, and P. Beynon-Davies. 2019. “Understanding Blockchain Technology for Future Supply Chains: A Systematic Literature Review and Research Agenda.” Supply Chain Management: An International Journal 24 (1): 62–84.
- Wang, Y., J. Lin, and T. M. Choi. 2020. “Gray Market and Counterfeiting in Supply Chains: A Review of the Operations Literature and Implications to Luxury industries.” Transportation Research Part E: Logistics and Transportation Review. Elsevier Ltd 133: 101823.
- Wang, S., L. Ouyang, Y. Yuan, X. Ni, X. Han, and F. Y. Wang. 2019. “Blockchain-Enabled Smart Contracts: Architecture, Applications, and Future Trends.” IEEE Transactions on Systems, Man, and Cybernetics: Systems. Institute of Electrical and Electronics Engineers Inc. 49 (11): 2266–2277.
- Wang, S., L. Ouyang, Y. Yuan, X. Ni, X. Han, and F. Y. Wang. 2019. “Blockchain-Enabled Smart Contracts: Architecture, Applications, and Future Trends.” IEEE Transactions on Systems, Man, and Cybernetics: Systems 49 (11): 2266–2277.
- Wang, Y., M. Singgih, J. Wang, and M. Rit. 2019. “Making Sense of Blockchain Technology: How Will it Transform Supply Chains?” International Journal of Production Economics. Elsevier B.V. 211: 221–236.
- Wang, S., J. Wang, X. Wang, T. Qiu, Y. Yuan, L. Ouyang, Y. Guo, and F. Y. Wang. 2018. “Blockchain-Powered Parallel Healthcare Systems Based on the ACP Approach.” IEEE Transactions on Computational Social Systems. Institute of Electrical and Electronics Engineers Inc. 5 (4): 942–950.
- WHO News. 2017. https://www.who.int/news/item/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified.
- Wong, L. W., G. W. H. Tan, V. H. Lee, K. B. Ooi, and A. Sohal. 2020. “Unearthing the Determinants of Blockchain Adoption in Supply Chain Management.” International Journal of Production Research 58 (7): 2100–2123.
- Wu, X., and Y. Lin. 2019. “Blockchain Recall Management in Pharmaceutical Industry.” Procedia CIRP 83: 590–595.
- Yang, K., H. Shen, D. Forte, S. Bhunia, and M. Tehranipoor. 2017. “Hardware-Enabled Pharmaceutical Supply Chain Security.” ACM Trans. Des. Autom. Electron. Syst 23 (23): 1–26.
- Yaqoob, S., M. M. Khan, R. Talib, A. D. Butt, S. Saleem, F. Arif, and A. Nadeem. 2019. “Use of Blockchain in Healthcare: A Systematic Literature Review.” International Journal of Advanced Computer Science and Applications 10 (5). doi:10.14569/IJACSA.2019.0100581.
- Zelbst, P. J., K. W. Green, V. E. Sower, and P. L. Bond. 2019. “The Impact of RFID, IIoT, and Blockchain Technologies on Supply Chain Transparency.” Journal of Manufacturing Technology Management. Emerald Group Publishing Ltd. 31 (3): 441–457.
- Zhao, S., S. Li, and Y. Yao. 2019. “Blockchain Enabled Industrial Internet of Things Technology.” IEEE Transactions on Computational Social Systems. Institute of Electrical and Electronics Engineers Inc. 6 (6): 1442–1453.
- Zwitter, A., and J. Hazenberg. 2020. “The DAO Controversy: The Case for a New Species of Corporate Governance?” Frontiers in Blockchain. Frontiers Media SA 3: 25.