Abstract
Recent and historic gold mines and mine wastes in the South Island of New Zealand have been exposed under a wide range of climatic conditions for up to 100 yr. The studied sites are in rain shadow areas east of the Southern Alps, and in wet areas west of the mountains. Arsenic concentrations in mine wastes are up to 20 wt% locally. The arsenic is primarily derived from arsenopyrite in gold-bearing ore, although roasting and oxidation of ore occurred at some mines. Arsenolite was a byproduct of roasting at two sites, and this was also discharged into mine wastes. Five secondary As minerals, scorodite, kankite, pharmacosiderite, zykaite, and bukovskyite, have formed in different mines and mine wastes during surficial oxidation and remobilisation. The minerals formed under different chemical and climatic conditions. Ferrihydrite with combined arsenate (up to 20 wt% As) occurs at all localities. Scorodite is the most common secondary As mineral, replacing arsenopyrite and/or arsenolite, and forming cement in mine wastes. Dissolution and precipitation of scorodite, which is strongly pH dependent, controls maximum dissolved As concentrations on a regional scale. Kankite has formed instead of scorodite in a persistently moist microclimate east of the mountains. Pharmacosiderite formed instead of scorodite on the 50 yr time-scale in mine tunnels at pH 7–8. Pharmacosiderite is distinctly less soluble than scorodite in this groundwater setting at pH 7–8, and apparently restricts dissolved As to <25 mg/L locally. Bukovskyite and zykaite form where dissolved sulfate concentrations are elevated and the host mine wastes are perennially damp.
Introduction
Gold mines are notorious for the high concentrations of As associated with them, because of the almost ubiquitous natural close geochemical relationship between gold and As (Bowell Citation1992; Roussel et al. Citation1998; Lottermoser Citation2003). This association is so close that the gold commonly occurs encapsulated in As-bearing sulfide mineral grains at the micrometre or millimetre scale (Cathelineau et al. Citation1989; La Brooy et al. Citation1994; Petrie et al. Citation2005). Whereas gold is commonly mined at grades <10 mg/kg, As concentrations in the same ore can be 100 or 1000 times higher (Bowell Citation1992; Petrie et al. Citation2005; Salzsauler et al. Citation2005). Hence, gold mines are commonly considered by environmental regulators to be highly contaminated with As, although since the As is entirely natural, the word ‘contaminated’ is inappropriate in this setting. High As concentrations in mine waters arise because of enhanced oxidation of As-bearing sulfide minerals in the disturbed rock mass (Bowell Citation1992; Lottermoser Citation2003; Salzsauler et al. Citation2005). This issue is exacerbated in abandoned historic mine sites, where no attempts were made to limit this oxidation process, and some of these historic sites continue to discharge highly As-rich (tens of mg/L) waters (Ashley et al. Citation2003; Hewlett et al. Citation2005).
Because of the very large amounts of As available for dissolution at many mine sites, the maximum dissolved As concentrations are defined by the solubility of secondary As minerals, and the kinetics of precipitation of these secondary minerals (Krause & Ettel Citation1988; Vink Citation1996; Magalhães Citation2002; Salzsauler et al. Citation2005; Langmuir et al. Citation2006). Despite the potential environmental significance of these secondary As minerals, there is limited information on the environmental stability of them. This is partly because of the many practical difficulties, dominated by kinetics and crystallinity, associated with conducting laboratory experiments with these minerals (Chukhlantsev Citation1956; Nishimura & Tozawa Citation1978; Krause & Ettel Citation1988; Vircikova et al. Citation1995; Langmuir et al. Citation2006). There are also problems in translating experimental results to the natural environment, which is far more complex than a laboratory.
One way to get around these laboratory-related problems is to compile direct observations of the environmental settings of precipitation of different secondary As minerals, and thereby deduce environmental stability of the minerals in the natural environment. This empirical approach has its limitations because of the large number of variables involved in a range of sites. Nevertheless, the empirical approach provides a useful starting point for making predictions of maximum dissolved As concentrations at As-rich mine sites. In this paper, we present some empirical observations from southern New Zealand on secondary As minerals () formed at mine sites with some very high arsenic concentrations in both solids and waters, under a range of climatic and geochemical conditions. We use these observations to provide indications of the relative stabilities and solubilities of five secondary As minerals.
Table 1 List of arsenic minerals in this study, and their formulas
Background
The South Island of New Zealand has a well-defined mountain backbone, the Southern Alps, which separates the main geological terranes and the main climate zones (). The Southern Alps form a barrier to the prevailing westerly winds, and this barrier results in large climate contrast across the South Island. A zone of high orographic rainfall occurs on the western side of the mountains, and a rain shadow prevails on the eastern side. Hence, while the whole island has a generally temperate climate, there is a steep rainfall gradient across the island (). The rain shadow zone receives frequent strong dry winds to exposed areas in all seasons, but has well-defined hot summers and cold winters. The high relief generates numerous microclimates within the contrasting climatic zones ().
Fig. 1 Digital elevation model of the South Island of New Zealand (downloaded, with permission, from www.geographx.co.nz), showing the topography of the Southern Alps that form the island backbone and induce differing climatic regimes as indicated. Localities mentioned in the text are shown, with the secondary As minerals () indicated. Localities are described in .
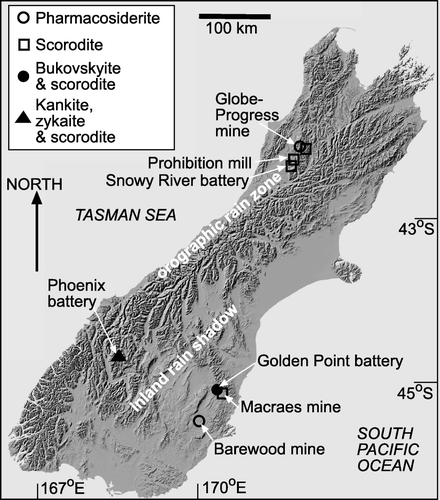
Table 2 Localities mentioned in the text (see ), and their environmental settings
West of the mountains, the rocks are predominantly Paleozoic, and include a suite of Ordovician metasedimentary rocks, the Greenland Group, that host gold-bearing fault systems (Christie & Brathwaite Citation2003). Mesozoic metasedimentary rocks dominate to the east of the mountains, and these are locally mineralised with gold-bearing fault systems as well. The host rocks in both terranes are broadly similar, consisting of metagreywackes with greenschist facies mineralogy: quartz, albite, muscovite, chlorite, calcite, with minor epidote, titanite, and pyrite. The mineralised structures are also broadly similar in both geological terranes, and contain variable amounts of hydrothermal quartz, arsenopyrite, and pyrite. Gold is closely associated with, and generally encapsulated in, the sulfide minerals. There has been historic mining in many mineralised zones on both sides of the mountains (; ). Historic mines were primarily underground tunnels (adits), some of which were accessed by vertical shafts. In 1990 a large, modern, open cut gold mine opened, at Macraes () and recently (2007) the Globe-Progress mine reopened as an open cut () and its ore concentrate is being transported across the mountains to Macraes for gold extraction.
Gold was extracted from the various historic mines from crushed ore slurries by mercury amalgamation or cyanidation. Most historic mines focused on extraction of coarse-grained free gold, rather than refractory gold, which is encapsulated in sulfide minerals. However, more sophisticated processing plants produced sulfide mineral concentrates, from which gold was extracted after finer crushing and/or roasting. Arsenic-rich tailings from these plants were disposed of immediately outside the processing plants, and some of these wastes are the subject of this study.
Methods
All the localities mentioned in this study have been examined in detail as part of a long-term research programme on mine wastes associated with orogenic gold deposits, and most of this research has been published as studies of particular aspects distinctive to the individual sites (Craw et al. Citation1999 , Citation2000 , Citation2002 , Citation2007; Hewlett et al. Citation2005; Mains & Craw Citation2005; Haffert & Craw Citation2008). This study uses some of this published research, combined with new mineral identifications, to provide a more integrated view of secondary mineral occurrences spanning all of the sites studied in detail.
Mineral identifications were done primarily with powder X-ray diffraction (XRD) on pressed-powdered discs of mineral samples selected in the field for their relative purity. Diffractograms were obtained using a PANalytical X'Pert-Pro MPD PW3040/60 at the Geology Department, University of Otago, with Cu Kα radiation at a scanning rate of 0.05°/s. Results were interpreted with the support of the PANalytical High Score software package. Mineral identifications were further facilitated with X-ray fluorescence analyses of bulk material. Well-cemented samples of minerals were made into polished thin sections and examined under a semi-automated JEOL JXA-8600 electron microprobe analyser (EPMA) at the Geology Department, University of Otago. EPMA methods include scanning electron microscopy (SEM), and energy dispersive (EDS) and wavelength dispersive (WDS) analysis. Quantitative EPMA analysis was hindered by the high porosity material and admixed impurities, so most results were semiquantitative only. Brown iron oxyhydroxide (ferrihydrite) is an especially common accompanying and admixed mineral at many localities, and this material influenced the colours of some secondary arsenic mineral specimens ().
Water analyses for dissolved As for this study were compiled from the more detailed studies mentioned above and from available databases constructed by the mining industry (Craw & Pacheco Citation2002; Haffert et al. Citation2006). In particular, the Database for Assessment of Mine Environment (DAME) contains a wide variety of mine water compositional data for the Westland region (Haffert et al. Citation2006; Pope et al. Citation2006). Most of these analyses were done by atomic absorption spectrophotometry. Detection limits varied for the many different sources, but were between 0.001 and 0.05 mg/L, significantly lower than the As concentrations that are of direct interest in this study. Major ion analyses for east Otago and Reefton waters have been published previously by Craw & Nelson (Citation2000) and Hewlett et al. (Citation2005), and additional major ion data have been compiled in the DAME database.
The pH of mine wastes was determined in situ on associated waters, where present, using a field pH meter calibrated in the field with buffer solutions. Paste pH of dry mine wastes was determined with the same pH meter, using the technique outlined by Sobek et al. (Citation1978).
Arsenic minerals
The arsenic minerals that are discussed in this paper are listed in . These minerals are all well-crystallised phases with well-defined X-ray diffraction peaks that allow unequivocal identification. Arsenopyrite is the principal primary arsenic mineral, although minor As occurs in solid solution in some hydrothermal pyrite (e.g., Petrie et al. Citation2005). Arsenopyrite grains are typically 0.1–2 mm across, and are disseminated through vein quartz and adjacent host rock at all the studied gold mines. Waste rock and some tailings from modern and historic mines typically contain abundant arsenopyrite. Arsenolite is a product of the ore roasting process undertaken at some mines to release encapsulated gold from arsenopyrite and pyrite. Arsenolite is an As(III) compound which forms when incomplete arsenic oxidation has occurred in the roasting process, and this material was discarded with tailings.
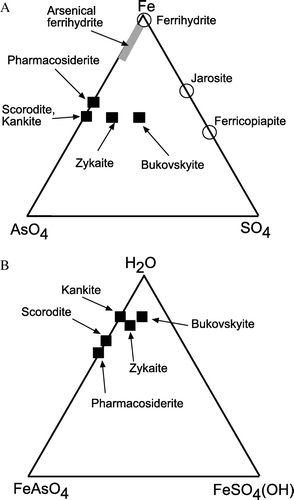
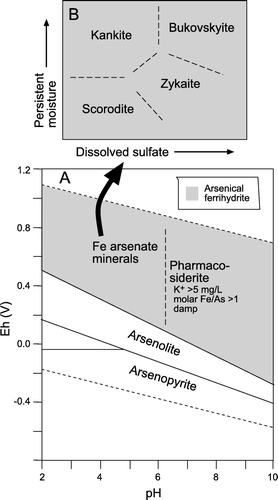
Secondary arsenic minerals that formed during post-mining weathering are all ferric iron bearing As(V) compounds that precipitated during complete oxidation of arsenopyrite and/or arsenolite (). The compositional relationships of these arsenate minerals, in terms of sulfate content and degree of hydration, are indicated in A, B, along with iron sulfate minerals commonly found in the same deposits. The structures of all these minerals are different, but some solid solution between minerals may occur.
Fig. 2 Ternary diagrams, showing the relative compositions of ideal secondary arsenic minerals in this study (black squares). Broadly related common secondary iron minerals in mine wastes are indicated with open circles. (A) Diagram showing iron, arsenate and sulfate variations. (B) Diagram showing iron arsenate, iron sulfate, and hydration variations.
Most localities described below also have varying amounts of poorly crystalline brown iron-rich material, as well as the minerals listed in . Some of this brown material has two broad diffuse X-ray diffraction peaks that are typical of two-line ferrihydrite (Jambor & Dutrizac Citation1998). Most of the brown material also contains some arsenic, locally up to 20 wt%. This brown material, commonly called arsenical ferrihydrite, generally includes a mixture of amorphous ferric arsenate and ferrihydrite (Paktunc et al. Citation2008), as well as some arsenate adsorbed on to the ferrihydrite surfaces (Waychunas et al. Citation1996; Roddick-Lanzilotta et al. Citation2002).
Secondary arsenic mineral localities
The localities described below are diverse in geographical setting, microclimate, mine type, mine processing procedures, and water chemistry. These differences led to general differences in the secondary arsenic minerals that have formed. The following descriptions, summarised in , provide the observational background to this set of generalisations. The generalisations are then developed into empirical phase diagrams that provide speculations on relative stability relationships among the secondary As minerals and their effects on dissolved As concentrations at mine sites. All the discharge waters from the sites mentioned in this study are subject to dilution by streams and groundwater, generally within a few hundred metres of a mine site. These diluting waters commonly have naturally elevated dissolved As concentrations from unmined mineralised rocks, and this natural dissolved As flux dominates over mine site discharges (Hewlett et al. Citation2005).
Localities east of the Southern Alps
Some abandoned mine adits intersect the local water table and form channels for groundwater. Flow rates are generally low (<0.01 L/s) because of the low rainfall of the area and low permeability of the host rocks. Abundant metamorphic calcite in the host rocks ensures that groundwater pH is generally between 7 and 7.5 despite sulfide mineral oxidation in exposed veins (Craw et al. Citation2000). This seeping groundwater leaves widespread precipitates on damp adit walls, and these precipitates are dominated by amorphous or poorly crystalline two-line ferrihydrite, commonly with adsorbed arsenic (Craw et al. Citation2000). Locally, precipitates of pharmacosiderite have formed on and near mineralised veins in these adits, particularly at Barewood mining area (Craw et al. Citation2000; ; ). These precipitates have formed over c. 100 yr since the adits were abandoned. Pharmacosiderite precipitates are 1–3 mm thick and form encrustations on quartz vein protuberances in the damp adits. The grain size of the pharmacosiderite is micrometre scale. The shallow groundwater that pervades the schist basement and precipitates pharmacosiderite in mine adits has low dissolved potassium (1–14 mg/L; ), and low dissolved sulfate (<10 mg/L) (Craw & Nelson Citation2000). The groundwater obtains its dissolved constituents primarily from dissolution of schist minerals, including muscovite (K), albite (Na), and pyrite (S) (Craw & Nelson Citation2000).
Fig. 3 The concentrations of sodium and potassium in groundwaters and mine waters in the Reefton and Macraes areas, South Island. Mine water compositions are affected by mining and processing activity.
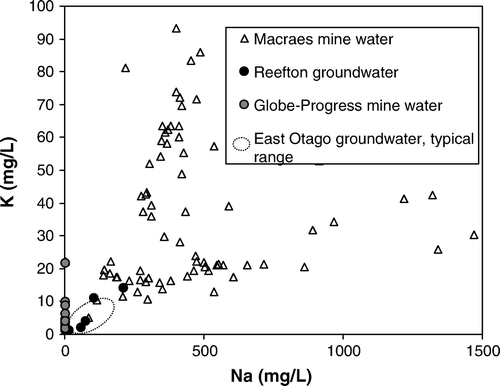
The active open cut Macraes mine () is being developed in a historic underground mining area. Outcrops and historic tunnels of sulfide-rich ore (pyrite and arsenopyrite) commonly have joints coated in scorodite and/or ferrihydrite, and rainwater running off these surfaces has pH between 4 and 7. The modern mine routinely produces a sulfide concentrate rich in arsenopyrite, and this material passes through the cyanidation plant at pH 10–11. When the mine opened in 1990, the sulfide concentrate tailings were stored in a dedicated impoundment. Oxidation and regular evaporative drying of exposed surfaces of this concentrate caused acidification (down to pH 1) and precipitation of scorodite (Craw et al. Citation1999 , Citation2002). Scorodite redissolves under alkaline conditions (pH 7–8; Craw et al. Citation2002). The tailings water from which scorodite precipitated contained elevated dissolved potassium and sodium compared to natural groundwaters () because of the processing activity (Craw & Nelson Citation2000). Dissolved sulfate concentrations of 1000–2000 mg/L prevailed (Craw & Nelson Citation2000).
The Macraes concentrate is now roasted and oxidised before cyanidation, producing mainly poorly crystalline As-bearing ferrihydrite material, with minor scorodite (Craw Citation2003). Minor arsenolite forms during the roasting process, but this is essentially all oxidised to arsenate compounds before discharge (Craw Citation2003). The concentrate tailings are mixed with the flotation tailings and disposed of in a large impoundment. Water from the large impoundment precipitates poorly crystalline ferrihydrite with abundant adsorbed arsenic (Roddick-Lanzilotta et al. Citation2002).
A small historic processing plant, the Golden Point battery (), processed the same type of ore as the Macraes mine. A sulfide concentrate of this ore was roasted before mercury amalgamation, and the tailings were deposited outside the battery in a pile <1 m thick (Mains & Craw Citation2005). Roasting was inefficient and some relict pyrite remains, oxidation of which causes acidification (pH 2–3) of the tailings. These tailings have undergone some chemical remobilisation in situ, and they are now cemented with ferrihydrite and secondary arsenic minerals. Scorodite occurs as a cement in the surficial portion of the tailings pile, and bukovskyite forms cement and veinlets in the interior of the pile (Mains & Craw Citation2005).
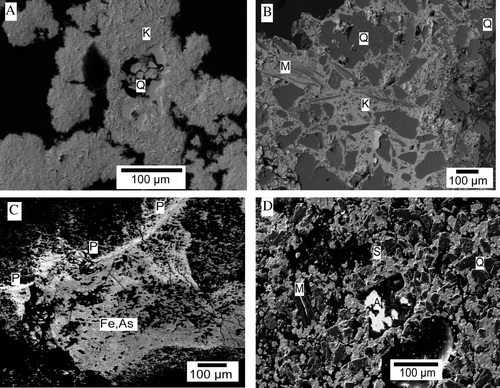
The Phoenix battery was associated with the Bullendale mining area in the southeastern part of the Southern Alps (MacKenzie et al. Citation2007) (; ). The battery site is in a steep-sided canyon beneath 1800 m high mountains. Ore rich in arsenopyrite and pyrite was processed and discarded outside the battery. The tailings pile has since been partially eroded by the steep mountain river that passes the site. The surface of the tailings pile has been cemented by As-rich ferrihydrite from oxidised pyrite. The eroded remnants of the once arsenopyrite-rich tailings beneath this cap have abundant kankite encrustations, cement, and cavity-filling crystal masses (A, B). Zykaite is a relatively uncommon accompanying mineral, with textures similar to the kankite. Scorodite was also identified as inclusions in the cemented cap and in some portions of the tailing. Paste pH of these materials are as low as 3–4, whereas nearby surface waters are alkaline (8.0– 8.5). More detailed descriptions of this site are presented by Haffert & Craw (Citation2010).
Fig. 4 Electron backscatter images of some secondary arsenic minerals and textures in South Island mine wastes. (A) Crystalline kankite (K) encrustations with scattered quartz (Q) grains, Phoenix battery. (B) Massive fine-grained kankite (K) cementing mine waste grains of quartz (Q) and muscovite (M). (C) Precipitated material from the Globe-Progress mine lower tunnel apron (). Amorphous ferrihydrite (black) and amorphous ferrihydrite with abundant admixed arsenate (Fe,As; middle grey) dominate this material, which is cut by a veinlet of secondary pharmacosiderite (P; pale grey). (D) Scorodite (S; middle grey) forms a cement in mine wastes dominated by dark grey quartz (Q) and muscovite (M). A relict arsenolite grain (white, A) has been partially plucked during specimen preparation, leaving a black hole.
Localities west of the Southern Alps
Numerous historic adits remain at the Globe-Progress mine, and these are discharging groundwater that has chemically interacted with the host rocks, in particular, oxidising sulfides (pyrite and arsenopyrite). Discharging waters are precipitating arsenical ferrihydrite at pH between 7 and 7.5, commonly forming an apron of brown precipitate with adsorbed As (Hewlett et al. Citation2005). The topographically lowest adit at the Globe-Progress mine has developed the largest ferrihydrite apron, which is up to 4 m thick. The ferrihydrite typically contains between 10 and 20 wt% As, laminated on the 100 micrometre scale (Hewlett et al. Citation2005; Craw et al. Citation2007). Local remobilisation of this material has resulted in precipitation of veinlets of pharmacosiderite (C) that are up to 5 mm thick. The ferrihydrite apron and included pharmacosiderite have formed over the past c. 50 yr since the underground mines were abandoned. The modern Globe-Progress open cut mine has exposed partially oxidised rocks that contain ferrihydrite and/or scorodite on joint surfaces. These minerals have formed over geological time before the open cut was excavated.
Prohibition Mill and Snowy River Battery (; ) processed ore at different times from the same major quartz vein, and this ore was rich in arsenopyrite with only minor pyrite. The ore was roasted to release enclosed gold, and the processing plants were set up to condense volatilised arsenic and save this as a byproduct. The condensate was dominated by arsenolite; and residues of this material, with associated hematite, were left lying around both Prohibition and Snowy River sites when the mines closed (Haffert & Craw Citation2008). These mine wastes have low sulfur contents because most sulfur was volatilised during roasting. Waters discharging from these sites have dissolved sulfate concentrations <15 mg/L. Exposed arsenolite has partially dissolved in the wet climate, and As has reprecipitated as scorodite cement encapsulating silicate wastes (D), thereby forming a hard crust (up to 10 cm thick) as the site substrate. Oxidation of As in this remobilisation process causes acidification of the substrate down to pH 3 (Haffert & Craw Citation2008).
Dissolved arsenic maxima
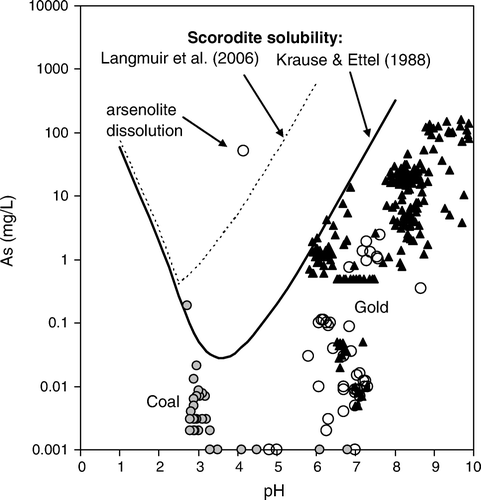
The As content of waters associated with the mine wastes described above are highly variable because of dilution associated with rain events and the mixing of surface and groundwaters (). Maximum values of dissolved As are the most significant for this study, and we focus on these here. Adit waters in the Barewood and Macraes mining areas have dissolved As up to 5 mg/L, and Macraes mine processing and tailings waters range up to 200 mg/L dissolved As () (Craw et al. Citation2000; Craw & Pacheco Citation2002). Globe-Progress mine adits have dissolved As up to 60 mg/L, and the lower adit discharge waters forming the thick apron of precipitates (above) had up to 23 mg/L dissolved As (Hewlett et al. Citation2005). Water runoff from arsenolite-bearing wastes at the Prohibition mine has up to 52 mg/L dissolved As, and at least some of this dissolved As is As(III) () (Haffert & Craw Citation2008). The set of As analyses near pH 3 in is from acid mine waters in Westland coal mines (DAME database), and is presented here for comparison to the generally circumneutral gold mine data.
Fig. 5 Compositions of South Island mine waters on a dissolved As versus solution pH diagram. Black triangles are for Otago (east of mountains) (Craw & Pacheco Citation2002). Circles are for mines west of the mountains (DAME database); open circles=gold mines; grey circles=coal mines. Scorodite solubility curves are from Krause & Ettel (Citation1988) and Langmuir et al. (Citation2006).
Discussion
Scorodite solubility
The solubility of scorodite has been widely investigated because of the significance of scorodite and related ferric arsenate and arsenical ferrihydrite for storage of As-bearing waste material (Krause & Ettel Citation1988; Waychunas et al. Citation1996; Langmuir et al. Citation2006; Paktunc et al. Citation2008). The stability of scorodite under acid conditions is well established () (Krause & Ettel Citation1988; Langmuir et al. Citation2006). However, thermodynamic predictions for circumneutral solutions (Langmuir et al. Citation2006) suggest scorodite solubility that is more than an order of magnitude higher than laboratory experiments indicate (Krause & Ettel Citation1988) (). The lower experimental scorodite solubility arises because the high dissolved As and sulfate concentrations kinetically inhibit precipitation of associated ferrihydrite, thus maintaining high dissolved ferric ion concentrations (Langmuir et al. Citation2006).
Because of the above described kinetic effect, the Krause & Ettel (Citation1988) solubility curve is the best predictor of environmental dissolved As concentrations in association with scorodite (). The Krause & Ettel (Citation1988) scorodite solubility curve apparently defines the upper limit for dissolved As concentrations at many of the sites examined in this study (). In particular, the As concentrations of Macraes mine waters are apparently limited by formation of scorodite (), which is abundant at the site (Craw & Pacheco Citation2002; Craw et al. Citation2002). Scorodite precipitation apparently limits the maximum environmental concentrations of As at a wide range of water pH values (). An exception to this arises where the site chemical system is dominated by more less-oxidised As in arsenolite, as at the Prohibition Mill site. Dissolved As can rise to >50 mg/L in moderately acid waters () because of the high solubility of arsenolite as As(III) (Haffert & Craw Citation2008). Scorodite precipitates almost immediately from solution, and locally directly replaces arsenolite, where this As(III) becomes oxidised by atmospheric oxygen (Haffert & Craw Citation2008). Scorodite precipitation is commonly presumed to control the dissolved As contents of mine waters elsewhere in the world (Krause & Ettel Citation1988; Vink Citation1996; Ashley & Lottermoser Citation1999; Williams Citation2001; Smedley & Kinniburgh Citation2002; Borba et al. Citation2003; Garcia-Sanchez & Alvarez-Ayuso Citation2003).
Scorodite crystallinity has an effect on dissolved As concentrations, and poorly crystalline scorodite may precipitate at higher dissolved As concentrations than indicated for crystalline scorodite in (Krause & Ettel Citation1988; Vircikova et al. Citation1995). However, the abundance of crystalline scorodite at the studied New Zealand sites, and the apparently good relationship between experimental scorodite solubility and maximum observed dissolved As concentrations (), suggest that poorly crystalline scorodite is not a significant phase at these sites.
Arsenical ferrihydrite and pharmacosiderite
Chemical systems with high molar Fe/As ratios (>2) favour precipitation of arsenical ferrihydrite, with associated dissolved As 1–2 orders of magnitude lower than that accompanying scorodite precipitation (Langmuir et al. Citation2006). High Fe/As ratios arise where arsenopyrite is accompanied by abundant pyrite and the two sulfide minerals oxidise at the same time. These conditions occur in historic mine adits, and the precipitates in and near these adits are dominated by arsenical ferrihydrite without scorodite (; C). Roddick-Lanzilotta et al. (Citation2002) and Craw et al. (Citation2004) show that ferrihydrite efficiently scavenges dissolved As from solution with a distribution coefficient of 105–106 L/kg at some of these mine sites, and this adsorption contributes to formation of arsenical ferrihydrite with up to 20 wt% As (above). Arsenical ferrihydrite also occurs as direct pseudomorphous replacement of some arsenopyrite grains in mine wastes (Craw et al. Citation2000 , Citation2002; Hewlett et al. Citation2005).
Pharmacosiderite, not scorodite, is the crystalline iron arsenate mineral that forms on aging (≥50 yr), remobilisation, and crystallisation of arsenical ferrihydrite precipitates (; C). These adit waters have up to 23 mg/L dissolved As (above), which is at least an order of magnitude lower than scorodite solubility () (Krause & Ettel Citation1988). Our observations suggest that pharmacosiderite is less soluble than scorodite under neutral to alkaline conditions, and pharmacosiderite will form instead of scorodite on the 50 yr time-scale under the perennial damp conditions of mine adits. Pharmacosiderite has slightly higher molar Fe/As ratio than scorodite (A, B), and this may facilitate formation of pharmacosiderite in these adits. Although pharmacosiderite formation also requires some dissolved K, the low dissolved K concentrations of groundwater in the adits (<20 mg/L) are apparently sufficient (). However, the Macraes mine waters contain much higher K concentrations (up to 90 mg/L; ) than natural groundwater, yet scorodite is the mineral that precipitates in that setting in the short term (). We suggest that pharmacosiderite forms, or will ultimately form instead of scorodite, under damp neutral to alkaline conditions with low dissolved K, where molar Fe/As is >1 (A).
Fig. 6 Relative stabilities of As minerals discussed in this study, as deduced from empirical observations of South Island mine sites. (A) Eh-pH diagram, after Vink (Citation1996) and Craw et al. (Citation2003), for total dissolved As of c. 10-4 moles/L, Fe/As ≥1, and dissolved K+ of c. 5 mg/L. Secondary As minerals are commonly accompanied by arsenical ferrihydrite (grey ornament). The boundary between pharmacosiderite and scorodite-like minerals has not been quantified thermodynamically. (B) Schematic phase diagram showing qualitative controls on iron arsenate mineral formation. The boundaries separating the different minerals in B have not been quantified thermodynamically, but reflect differences in mineral formulae () and observed occurrences (). The ‘persistent moisture’ axis reflects microclimates as outlined in the text.
Other secondary As minerals
Kankite is essentially hydrated scorodite, so precipitation and dissolution of kankite probably result in similar dissolved As concentrations to those associated with scorodite (). Bukovskyite and zykaite are more hydrated than scorodite as well (; B). Kankite and zykaite occur in Phoenix battery mine wastes that are kept constantly damp in a shady valley for several months of each year (). This persistent dampness may explain why kankite is more common than scorodite at this locality (B). Likewise, relatively hydrated bukovskyite (B, B) formed in the centre of a mine waste pile at Golden Point battery () where consistent dampness may have prevailed, whereas scorodite formed on the exposed evaporatively dry surface of the same pile.
Precipitation of sulfate-bearing arsenates zykaite or bukovskyite apparently occur in preference to, or as well as, scorodite or kankite formation in moderately acid gold mine wastes (). This implies that zykaite and bukovskyite are less soluble than scorodite under these conditions, provided there is sufficient available dissolved sulfate. Pyrite is the dominant source of dissolved sulfate in oxidising gold mine wastes, so zykaite or bukovskyite should be expected on oxidation of pyrite-rich arsenopyrite wastes. These secondary minerals are conspicuously absent from the acidic pyrite-poor wastes at Prohibition Mill and Snowy River battery () where dissolved sulfate concentrations are low (<15 mg/L; Haffert & Craw Citation2008). Formation of bukovskyite rather than zykaite, or vice versa, is probably dependent on aqueous sulfate concentration and the presence or absence of persistent moisture (microclimate) of the site (; B). In contrast, under the alkaline conditions prevailing at the Macraes mine site, scorodite is the dominant secondary arsenate despite the very high dissolved sulfate in the mine waters (up to 2000 mg/L; Craw & Nelson Citation2000).
Conclusions
Secondary As minerals have formed in As-rich gold mine wastes in contrasting climatic regimes in the South Island of New Zealand, ranging from high rainfall areas to low rainfall areas with high evaporation. Microclimates in high relief areas appear to exert some influence on minerals formed. Secondary arsenic minerals form as direct replacement of primary arsenopyrite grains, as recrystallised arsenical ferrihydrite, and as precipitates that cement the wastes. Scorodite is the most common secondary As mineral, and this mineral occurs locally in all of the areas examined. Scorodite is highly soluble under alkaline conditions (up to 100s of mg/L dissolved As). Scorodite will form in the short-term (months) via evaporative concentration of mine waters, and also redissolves readily on wetting. Pharmacosiderite is less soluble than scorodite at pH 7–8, and forms instead of scorodite from historic mine adit waters on the 50 yr time-scale from groundwaters with low dissolved potassium concentrations (<10 mg/L) and Fe/As >1. Pharmacosiderite restricts dissolved As to c. 25 mg/L at pH 7–8. Kankite, a more hydrated form of scorodite, forms instead of scorodite in a locality that has a relatively dry regional climate but a microclimate in which the site is damp for several months at a time during winter. Bukovskyite and zykaite are apparently less soluble than scorodite under acidic conditions, and form in mine wastes with pH near 3–4 where there is elevated dissolved sulfate and persistent moisture. In the absence of other ferric arsenate minerals, scorodite dissolution and precipitation control dissolved As concentrations in discharge waters, and this dissolved As is strongly affected by pH.
Acknowledgements
This paper is an outgrowth of many individual site studies, and we are grateful to the following for their input to the databases and the ideas behind this paper: Amanda Black, Debra Chappell, Debbie Clarke, Lucy Hewlett, Dusk Mains, Craig McIntosh, Leonita Pacheco, and Simone Vellekoop. The research was funded by University of Otago, NZ Foundation for Research, Science and Technology, and CRL Energy. The Department of Conservation permitted access to some of the studied sites, and Oceana Gold provided data and logistical support. Technical assistance was ably provided by Loraine Patterson and Damian Walls. Helpful reviews by D. Kinniburgh and an anonymous referee improved the clarity of the manuscript.
References
- Ashley , PM and Lottermoser , BG . 1999 . Arsenic contamination at the Mole River mine, northern New South Wales . Australian Journal of Earth Science , 46 : 861 – 874 .
- Ashley , PM , Craw , D , Graham , BP and Chappell , DA . 2003 . Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand . Journal of Geochemical Exploration , 77 : 1 – 14 .
- Borba , RP , Figueiredo , BR and Matschullat , J . 2003 . Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from Iron Quadrangle, Brazil . Environmental Geology , 44 : 39 – 52 .
- Bowell , RJ . 1992 . Supergene gold mineralogy at the Ashanti mine, Ghana: implications for the supergene behaviour of gold . Mineralogical Magazine , 56 : 545 – 560 .
- Cathelineau , M , Boiron , M-C , Holliger , P , Marion , P and Denis , M . 1989 . Gold in arsenopyrites: crystal chemistry, location, and state, physical and chemical conditions of deposition . Economic Geology Monograph , 6 : 328 – 341 .
- Christie , AB and Brathwaite , RL . 2003 . Hydrothermal alteration in metasedimentary rock-hosted orogenic gold deposits, Reefton goldfield, South Island, New Zealand . Mineralium Deposita , 38 : 87 – 107 .
- Chukhlantsev , VG . 1956 . The solubility products of a number of arsenates . Journal of Analytical Chemistry (USSR) , 11 : 565 – 571 .
- Craw , D , Chappell , D , Nelson , M and Walrond , M . 1999 . Consolidation and incipient oxidation of alkaline arsenopyrite-bearing mine tailings, Macraes Mine, New Zealand . Applied Geochemistry , 14 : 485 – 498 .
- Craw , D , Chappell , D , Reay , A and Walls , D . 2000 . Mobilisation and attenuation of arsenic around gold mines, east Otago, New Zealand . New Zealand Journal of Geology and Geophysics , 43 : 373 – 383 .
- Craw , D and Nelson , M . 2000 . Geochemical signatures of discharge waters, Macraes mine flotation tailings, east Otago, New Zealand . New Zealand Journal of Marine and Freshwater Research , 34 : 597 – 613 .
- Craw , D , Koons , PO and Chappell , DA . 2002 . Arsenic distribution during formation and capping of an oxidized sulphidic minesoil, Macraes mine, New Zealand . Journal of Geochemical Exploration , 76 : 13 – 29 .
- Craw , D and Pacheco , L . 2002 . Mobilisation and bioavailability of arsenic around mesothermal gold deposits in a semiarid environment, Otago, New Zealand . The Scientific World , 2 : 308 – 319 .
- Craw , D . 2003 . Geochemical changes in mine tailings during a transition to pressure–oxidation process discharge, Macraes Mine, New Zealand . Journal of Geochemical Exploration , 80 : 81 – 94 .
- Craw , D , Falconer , D and Youngson , JH . 2003 . Environmental arsenopyrite stability and dissolution: theory, experiment, and field observations . Chemical Geology , 199 : 71 – 82 .
- Craw , D , Wilson , N and Ashley , PM . 2004 . Geochemical controls on the environmental mobility of Sb and As in mesothermal antimony and gold deposits . Transactions of the Institute of Mining & Metallurgy, Applied Earth Science , 113 : B3 – B10 .
- Craw , D , Rufaut , CG , Haffert , L and Paterson , LA . 2007 . Plant colonization and arsenic uptake on high arsenic mine wastes, New Zealand . Water, Air and Soil Pollution , 179 : 351 – 364 .
- Garcia-Sanchez , A and Alvarez-Ayuso , E . 2003 . Arsenic in soils and waters and its relation to geology and mining activities (Salamanca Province, Spain) . Journal of Geochemical Exploration , 80 : 69 – 79 .
- Haffert L , Craw D , Pope J 2006 . Quantification and controls on As distribution in Westland, NZ . Australasian Institute of Mining and Metallurgy 39th New Zealand Branch Annual Conference, August 2006 , Waihi . 115 126
- Haffert , L and Craw , D . 2008 . Mineralogical controls on environmental mobility of arsenic from historic mine processing residues, New Zealand . Applied Geochemistry , 23 : 1467 – 1483 .
- Haffert , L and Craw , D . 2010 . Geochemical processes influencing arsenic mobility at Bullendale historic gold mine, Otago, New Zealand . New Zealand Journal of Geology and Geophysics , 53 : 129 – 142 .
- Hewlett , L , Craw , D and Black , A . 2005 . Comparison of trace element contents of discharges from adjacent coal and gold mines, Reefton, New Zealand . Marine and Freshwater Research , 56 : 983 – 995 .
- Jambor , JL and Dutrizac , JE . 1998 . Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide . Chemistry Reviews , 98 : 2549 – 2585 .
- Krause , E and Ettel , VA . 1988 . Solubility and stability of scorodite, FeAsO4.2H2O: new data and further discussion . American Mineralogist , 73 : 850 – 854 .
- La Brooy , SR , Linget , HG and Walker , GS . 1994 . Review of gold extraction from gold ores . Minerals Engineering , 7 : 1213 – 1241 .
- Langmuir , D , Mahoney , J and Rowson , J . 2006 . Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4.2H2O) and their application to arsenic behaviour in buried mine tailings . Geochimica et Cosmochimica Acta , 70 : 2942 – 2956 .
- Lottermoser BG 2003 . Mine wastes: characterization, treatment and environmental impacts . Springer , Berlin
- MacKenzie , DJ , Craw , D and Begbie , M . 2007 . Mineralogy, geochemistry, and structural controls of a disseminated gold-bearing alteration halo around the schist-hosted Bullendale orogenic gold deposit, New Zealand . Journal of Geochemical Exploration , 93 : 160 – 176 .
- Magalhães , MCF . 2002 . Arsenic. An environmental problem limited by solubility . Pure and Applied Chemistry , 74 : 1843 – 1850 .
- Mains , D and Craw , D . 2005 . Composition and mineralogy of historic gold processing residues, east Otago, New Zealand . New Zealand Journal of Geology and Geophysics , 48 : 641 – 647 .
- Nishimura , T and Tozawa , K . 1978 . On the solubility products of ferric, calcium and magnesium arsenates . Bulletin of the Institute of Mineral Dressing and Metallurgy , 34 : 20 – 26 .
- Paktunc , D , Dutrizac , J and Gertsman , V . 2008 . Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: implications for arsenic mobility . Geochimica et Cosmochimica Acta , 72 : 2649 – 2672 .
- Petrie , BS , Craw , D and Ryan , CG . 2005 . Geological controls on refractory ore in an orogenic gold deposit, Macraes mine, New Zealand . Mineralium Deposita , 40 : 45 – 58 .
- Pope J , Newman N , Craw D 2006 . Coal mine drainage geochemistry, West Coast, South Island: a preliminary water quality hazard model . Australasian Institute of Mining and Metallurgy 39th New Zealand Branch Annual Conference, August 2006 , Waihi . 1 12
- Roddick-Lanzilotta , AJ , McQuillan , AJ and Craw , D . 2002 . Infrared spectroscopic characterisation of arsenate(V) ion adsorption from mine waters, Macraes Mine, New Zealand . Applied Geochemistry , 17 : 445 – 454 .
- Roussel , C , Bril , H and Fernandez , A . 1998 . Hydrogeochemical survey and mobility of As and heavy metals on the site of a former gold mine (Saint-Yrieix mining district, France) . Hydrogeology , 1 : 3 – 12 .
- Salzsauler , KA , Sidenko , NV and Sheriff , BL . 2005 . Arsenic mobility in alteration products of sulfide-rich, arsenopyrite-bearing mine wastes, Snow Lake, Manitoba, Canada . Applied Geochemistry , 20 : 2303 – 2314 .
- Sobek A , Schuller WA , Freeman JR , Smith RM 1978 . Field and laboratory methods applicable to overburden and minesoils Environmental Protection Agency Washington, DC EPA-600/2-78-054
- Smedley , PL and Kinniburgh , DG . 2002 . A review of the source, behaviour and distribution of arsenic in natural waters . Applied Geochemistry , 17 : 517 – 568 .
- Vink , B . 1996 . Stability relations of antimony and arsenic compounds in the light of revised and extended Eh-pH diagrams . Chemical Geology , 130 : 21 – 30 .
- Vircikova , E , Molnar , J , Lech , P and Reitznerova , E . 1995 . Solubilities of amorphous Fe-As precipitates . Hydrometallurgy , 38 : 111 – 123 .
- Waychunas , GA , Fuller , CC , Rea , BA and Davis , JA . 1996 . Wide-angle X-ray scattering (WAXS) study of ‘two-line’ ferrihydrite structure: effect of arsenate sorption and counterion variation and comparison with EXAFS results . Geochimica et Cosmochimica Acta , 60 : 1765 – 1781 .
- Williams , M . 2001 . Arsenic in mine waters: an international study . Environmental Geology , 40 : 267 – 278 .