Abstract
Coal mine drainage chemistry on the West Coast of the South Island is highly variable; pH ranges from about 2–8, and chemical concentrations vary by several orders of magnitude. Factors that influence mine drainage chemistry on the West Coast include regional geology, mine type, hydrogeology, and local geology. At a regional scale, mine drainage chemistry is bimodal and relates to geological formations. Mines within the Paparoa Coal Measures have neutral mine drainage, whereas those within the Brunner Coal Measures have acid mine drainage. This is related to the availability of SO4 during coal measures deposition and, in combination with Fe and organic material, the subsequent formation of pyrite during burial. Paparoa Coal Measures were deposited in alluvial to lucustrine environments where SO4 was relatively unavailable, whereas Brunner Coal Measures were deposited in alluvial to estuarine to marginal marine settings where marine SO4 was abundant. Brunner Coal Measures acid mine drainage chemistry is influenced by mine type; open cast mines have Al- and trace element-rich acid mine drainage compared to underground mines. Acid mine drainage forming reactions that release trace elements and Al proceed more rapidly and completely at open cast mines where mine waste has a higher reactive surface area compared to waste rock at underground mines. Brunner coal mine drainage chemistry is also influenced by hydrogeology where flooded underground mines release less acid than free-draining mines because there is less oxygen available to react with pyrite. In addition, local geology overprints mine drainage chemistry where differences in acid mine drainage chemistry arise from changes in contributing lithologies either within a single mine or between different coalfields. Identification of factors that control mine drainage chemistry enables prediction of mine drainage chemistry. These predictions have application to the mining industry for managing, mitigating, monitoring, and remediation of mine drainages that would otherwise cause negative environmental impact.
Introduction
Under the current regulatory regime in New Zealand, environmental impact is an important factor that determines profitability of operating mines and the success or failure of permit applications for new mines. At some mine sites water quality downstream of the mine is the most challenging environmental factor to manage during mine operations and after mine closure. There are several ways that mining can have a negative impact on downstream water quality including; increase in suspended sediment, release of environmentally significant trace elements without acid (neutral mine drainage [NMD] or contaminated neutral drainage [CND]), or release of low pH and acidic mine effluent with or without environmentally significant trace elements (acid mine drainage [AMD]). The consequences of poor environmental management on water quality downstream of mines can be seen at many locations on the West Coast of the South Island where historically coal mines were operated with little concern for the downstream environment. This paper presents chemical data from immediately downstream of historic and current coal mines and identifies geological, hydro-geological, and mine operational factors that influence mine drainage chemistry. Recognition of the influence of these factors can be used to improve rehabilitation at historic mines and management strategies at current and future mines (Trumm et al. Citation2008).
At coal mines on the West Coast of the South Island, the most serious potential environmental impact is AMD (Alicorn Leon & Anstiss Citation2002; de Joux Citation2003; Hewlett Citation2003; Hewlett & Craw Citation2003; James Citation2003; Lindsay et al. Citation2003; Black et al. Citation2005; de Joux & Moore Citation2005; Pope et al. Citation2005; Pope et al. Citation2006; Davies et al. Citation2008; Trumm et al. Citation2008). AMD can form where sulfide minerals are exposed by mining to humid air or oxygenated water (Plumlee & Logsdon Citation1999). Acid rock drainage (ARD) is caused by identical sulfide oxidation processes but not necessarily related to mining.
Background
AMD chemistry
There are three important chemical steps in the formation of AMD, oxidation of reduced S to (Eq. Equation1), oxidation of Fe(II) to Fe(III) (Eq. Equation2 —this reaction is acid consuming), and hydrolysis of Fe(III) to precipitate ferrihydrite, Fe(OH)3 (Eq. Equation3). In detail, these reactions are often chemically or biologically catalysed, include partially oxidised S species as well as transient complex Fe ions, and involve a number of secondary minerals (Evangelou Citation1998). Despite these complexities, the ratio of pyrite consumed to acid formed is stoichiometric and remains the same for the complete oxidation/hydrolysis process (Eq. Equation4).
Secondary Fe minerals such as melanterite (FeSO4·7H2O), jarosite (KFe3(SO4)2(OH)6), schwertmannite (Fe8O8OH4.5(SO4)1.75), or ferrihydrite (Fe(OH)3) are common in AMD formation zones and catchments. Acidity is also contributed by elevated Al3+ that is released when AMD or ARD reacts with alumino-silicates. For example, the breakdown of feldspar during reaction with AMD releases acid, alkali or alkaline earth metals, and Al3+, and can be accompanied by precipitation of jarosite (Eq. Equation5). When substantial Al is dissolved in AMD, it can buffer pH in an acidic range controlled by hydrolysis of Al3+ through precipitation and dissolution of Al(OH)3 (Eq. Equation6).
Sulfide mineral chemistry, reactions between AMD and other rocks as well as secondary minerals that form in AMD environments are many and complex, so AMD chemistry is highly variable. Reactions with some minerals, such as carbonates, can have an acid-neutralising effect that mitigates AMD and therefore the chemistry depends on the relative abundances and reactivity of acid-forming minerals and acid-neutralising minerals (Pope et al. Citation2010) under site-specific geological, hydrological, and environmental conditions. The abundance of acid-forming and neutralising minerals can be quantified by standardised rock analyses (Kleinmann Citation2000; Smart et al. Citation2002). The variability of coal mine drainage chemistry is demonstrated by a large range in physiochemical properties and concentrations identified in this study and others (Cravotta III Citation2008aIII 2008b). The impact of regional and site specific factors on mine drainage chemistry is outlined in this paper for mines in the Brunner and Paparoa Coal Measures.
Downstream of the mine environment, mine drainage chemistry changes are caused by normal stream processes such as dilution, neutralisation, buffering, oxidation, hydrolysis, and precipitation. Often this leads to a predictable series of chemical changes (Espana et al. Citation2005). Although AMD impacts can be completely attenuated by natural stream processes, in severe cases, AMD impact can contaminate large river systems from headwaters to river mouth. Our research examines the chemistry of coal mine drainages at source, before the onset of downstream processes. The objective is to determine the variability of AMD and identify factors that control AMD chemistry before release into the receiving stream and river environment. Improved prediction of mine drainage chemistry will assist optimal management and remediation strategies (Ellis Citation2008; Trumm et al. Citation2008; McCauley et al. in press).
Geology
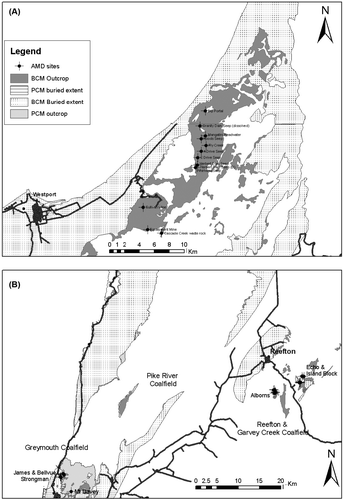
Current and historic coal mining on the West Coast of the South Island is mostly hosted in the Paparoa and Brunner Coal Measures (). Paparoa Coal Measures are Cretaceous–Paleocene (Raine Citation1984) and are deposited onto earlier Cretaceous terrestrial sediments of the Pororari Group, Cambrian–Ordovician Greenland Group metasediments, and granitic basement rocks. Paparoa Coal Measures were deposited in fluvial to lacustrine environments without marine influence (Nathan Citation1986; Newman & Newman Citation1992). The Paparoa sequence (up to 750 m thick) was deposited rapidly into fault-controlled basins with complex interplay of fluvial systems, lakes, and coal forming mires. Outcrop of Paparoa Coal Measures is mostly restricted to the Greymouth, Pike River and Punakaiki Coalfields, and the subsurface extent of these coal measures mostly coincides with a depositional structure known as the Paparoa Trough (Nathan et al. Citation2002).
Fig. 1 Outcrop and subsurface extent of Brunner Coal Measures (BCM) and Paparoa Coal Measures (PCM) or co-relative rocks on the West Coast of the South Island.
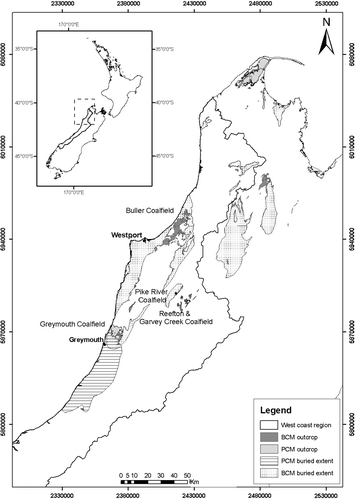
Brunner Coal Measures (Paleocene–Oligocene but mostly Eocene) were deposited in a fluvial to estuarine environment in response to a gradual and tectonically controlled transgression. The Brunner Coal Measures paraconformably lie on Paparoa Coal Measures and disconformably upon an eroded and often weathered surface of granitoids and Greenland Group rocks (Suggate Citation1959; Nathan Citation1986; Nathan et al. Citation2002). Sediments of the Brunner Coal Measures are commonly quartzose and represent slow accumulation of sediment. In contrast to the restricted and fault-bounded Paparoa Coal Measures, the Brunner Coal Measures are extensive but comparatively thin, usually<30 m. Exceptions occur locally in sub-basins controlled by subsidence where several hundred metres of Brunner Coal Measures can occur, such as within the Garvey Creek Coalfield and parts of the Buller Coalfield. (Nathan Citation1986; Titheridge Citation1992; Leask Citation1993; Nathan Citation1996; Nathan et al. Citation2002). Brunner Coal Measures are conformably overlain by marine rocks of the Kaiata Formation, typically a shallow water marine mudstone (Kaiata Mudstone) but locally a facies equivalent shallow marine sandstone (Island Sandstone).
Methods
Sampling
During 2005 and 2006 a suite of mine drainage samples was collected to examine coal mine drainage chemistry from Paparoa and Brunner hosted mines (). All samples were collected from mine drainage seeps or mine adits as close to source as possible to minimise the overprint of downstream geochemical processes on mine drainage chemistry. Many sample locations focused on catchments that had previously been identified as mining impacted (Hewlett & Craw Citation2003; James Citation2003; Bell unpubl. data 2005). However, samples were also collected from mine drainage at sites where mining-related water quality impacts had not previously been reported. Analyses of mine drainage chemistry collected for this study have been collated into the Database for Assessment of Mine Environment (DAME) (Pope et al. Citation2005; Pope et al. Citation2006) that also includes other published information, data from regional councils, mining companies, and university theses.
Column leach sample
ARD was produced in the laboratory by leaching a Brunner Coal Measures rock with oxygenated water to provide an example of worst case drainage from acid forming rocks on the West Coast. The leaching procedure followed a free draining method (Pope Citation2007) and concentrations of trace elements and metals and major components were analysed in the leachate.
Analyses
At each AMD sample location, field measurements of pH, electrical conductivity (ec), dissolved oxygen (DO) and temperature were completed and water samples were collected for major metal and trace element analyses. At all sites, samples were filtered through a 0.45 µm disposable syringe filter to remove suspended particulate material then acidified (nitric acid to pH <2). At a selection of sites, unfiltered, acidified water samples were collected to determine the amount of trace elements transported by suspended particulate material. Metal and trace element analyses were conducted at an accredited commercial laboratory by inductively coupled plasma mass spectrometry (ICP-MS).
Results
Mine drainage chemistry from coal mines on the West Coast of the South Island ( and ) is variable and ranges from NMD without elevated concentrations of environmentally significant trace elements, to highly acidic AMD with enriched concentrations of environmentally significant trace elements. The pH range for data presented here is from 1.8 to 7.5, the range in DO is from <10% saturated to oversaturated, and electrical conductivity (ec) values are between 100 µS/cm to about 12,000 µS/cm. Dissolved concentrations of trace elements are variable in AMD and ARD but concentrations can be enriched by several orders of magnitude compared to baseline samples ().
Fig. 3 Enrichment of AMD, natural ARD, and synthetic ARD (Leach), compared to background water from the West Coast. Where analyses were below detection, detection limits were used to calculate enrichment factors.
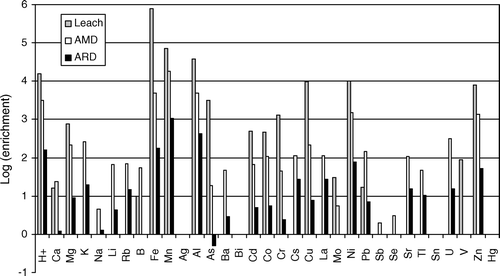
Table 1 Mine drainage sample site descriptions and physiochemical parameters, ec=electrical conductivity
Table 2 Metal and trace element analyses (mg/L) of mine drainage chemistry from coal mines on the West Coast of New Zealand (samples filtered to 0.45 µm and acidified)
In general, the maximum concentration of trace elements in AMD is about an order of magnitude higher than concentrations within a typical sample of naturally occurring ARD. The ARD sample () comes from a small creek draining unmined Brunner Coal Measures in the Garvey Creek Coalfield. Laboratory generated ARD leachate contains concentrations of elements about an order of magnitude higher than AMD samples reported here. However recently, small seeps have been found with concentrations of trace elements and metals that approach those in the laboratory leachate (McCauley et al. Citation2010). Extensive studies of naturally occurring ARD or laboratory produced ARD have not been published and it is possible that examples of more acidic and trace element enriched samples could be found or produced.
At sample sites where both filtered and unfiltered samples were collected, the difference in concentrations between samples is almost always <10%, indicating that trace elements are mostly transported in dissolved form. In samples from the Paparoa Coal Measures total concentrations of Fe exceeded dissolved concentrations of Fe by more than 10%, indicating the presence of colloidal Fe(III) oxyhydroxide minerals in suspension. These colloids did not substantially impact trace element concentrations.
Discussion
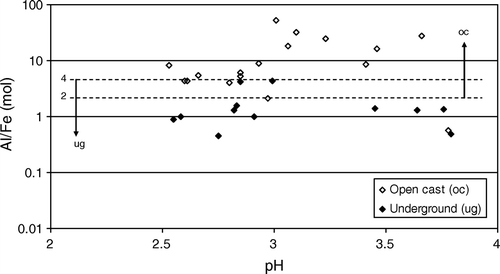
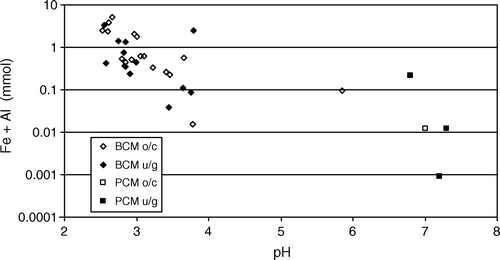
Mine drainage chemistry from West Coast coal mines has bimodal frequency distributions for pH and concentrations of dissolved components (). Mines in the Paparoa Coal Measures produce neutral mine drainage (NMD) pH 6.5–7.5 with relatively low Fe, Al, and trace element concentrations. In comparison, mines in the Brunner Coal Measures almost always produce AMD that is variably enriched in Fe, Al, and trace elements. Several factors have been identified that influence mine drainage chemistry on the West Coast, including regional geology and paleo-environmental setting, mine type, hydrogeology, and local rock type variations.
Fig. 4 Bimodal mine drainage chemistry from West Coast coal mines, Paparoa Coal Measures (PCM), Brunner Coal Measures (BCM). oc=open cast; ug=under ground.
Regional geology
Bimodal results in mine drainage chemistry for Paparoa and Brunner Coal Measures reflect control of AMD chemistry by regional geology. In general, Brunner Coal Measures are more acid producing than Paparoa Coal Measures because they formed in a fluvial-estuarine environment where marine SO4-rich water can interact with coal measures during burial. The average sulphate concentration of seawater is 2712 mg/L (Stumm & Morgan Citation1996) compared to about 7 mg/L in average surface water (Berner & Berner Citation1987). In the Brunner Coal Measures, marine SO4 and terrestrial Fe combine with reducing conditions caused by breakdown of organic material to form FeS2. In contrast, the Paparoa Coal Measures formed in alluvial to lucustrine environments where SO4 is unavailable at the time of deposition or immediately after burial. Pyrite is present in the Paparoa Coal Measures, especially near the top of the sequence where marine rocks overlie unconformably, but it is much less common than in Brunner Coal Measures (Suggate Citation1959; Pope et al. Citation2006).
Regional geological influences on mine drainage chemistry, related to depositional control of the abundance of pyrite and carbonate minerals, have been identified in North America and Australia (Brady et al. Citation2000). There are standard geochemical methods to predict the acid producing characteristics of rocks (Kleinmann Citation2000; Smart et al. Citation2002) and rock chemistry results from New Zealand coal measures (Hughes et al. Citation2004; Pope et al. Citation2006; Craw et al. Citation2008) have been published and reviewed (Pope et al. Citation2010).
All West Coast AMD sampled during this study are from mines in the Brunner Coal Measures. The acidity of Brunner Coal Measures AMD is high, in some cases pH <2.5 with >10 mmol/L dissolved Fe+Al. The chemistry of Brunner Coal Measures AMD is variable and the relationship between major acid components (Fe3+, Al3+ and H+) is complex despite similarities in the composition of the source rocks.
Mine type
AMD from open cast mines hosted in the Brunner Coal Measures typically has a higher Al:Fe ratio than AMD from underground mines (). There are two explanations for the high Al:Fe ratio in open cast AMD, firstly at underground mines high concentrations of Fe(II) are more likely, and secondly reactions that release Al are more likely at open cast mines. The most likely source of Al in Brunner Coal Measures AMD is reaction between H2SO4 produced by pyrite oxidation and alumino-silicate minerals such as clays and feldspars (Eq. Equation5) in the coal measures. This reaction and other similar reactions can release aluminium and consume Fe and therefore have a compounded effect on the Al:Fe ratio. In general, these reactions can proceed more rapidly and to a greater extent at open cast mines because coal measure sediments are more disturbed and have a higher reactive surface area in mine waste rock dumps compared to underground mines. At underground mines, there is less reaction between the H2SO4 from pyrite oxidation and coal measure sediment and therefore relatively higher Fe compared to Al.
Hydrogeology
At underground mines on the West Coast hydrogeology influences the chemistry of AMD. Many mines on the West Coast are free-draining because the terrain and dip of the seams make monitor (water jet) style coal cutting and gravity assisted fluming of coal an attractive mode of operation. However, three of the mines that were sampled are flooded and do not freely drain surrounding coal measure sequences. Samples collected from the three flooded mines with flowing adits had lower acidity and higher pH than samples from underground mines that freely drain updip workings and overlying coal measures (). Flooding of underground mines is commonly used to minimise the amount of oxygen ingress into abandoned mines and mitigate or minimise AMD chemistry and volumes (Skousen et al. Citation1998).
Local rock type variations
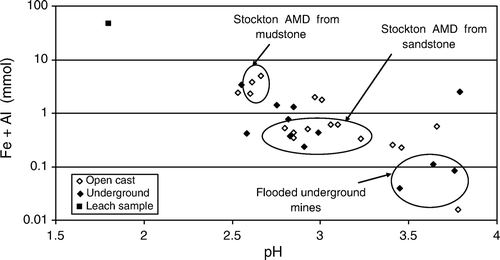
Local variations in coal measures rock types are present throughout the West Coast coalfields. In the Buller Coalfield, the distribution of different rock types is attributed to controls on depositional environment by differential compaction and syn-depositional fault displacement (Titheridge Citation1992). Several samples have been collected at Stockton open cast mine where variations in rock type have been identified in previous work (Titheridge Citation1992; Alicorn Leon & Anstiss 2002). Samples collected in areas where mine waste is mudstone rich have higher total acidity than samples where mine waste is sandstone rich (). It is possible that the high total acidity relates to fine grained, framboidal, reactive sulfide minerals in the mudstone compared to coarse, euhedral, less reactive sulfides in the sandstone rocks (Caruccio Citation1970). Research relating to variations in acid production from different rock types and relationships with pyrite morphology and other factors is in progress at Stockton Mine (Weisener & Weber Citation2010).
Trace element geochemistry of Brunner Coal Measures AMD
The concentration of trace elements in drainages from mines in the Brunner Coal Measures is enriched compared to background (), especially Mn, Zn, Ni, Cu, As, Cr, Co, and Cd. Typically, mine drainage from open cast mines has higher concentrations of these trace elements than mine drainage from underground mines (). However there is little difference between the total acidity and pH values in drainage from open cast mines compared to underground mines (). Although the concentrations of Fe and Al are inversely correlated with pH, concentrations of trace element and pH are poorly correlated ().
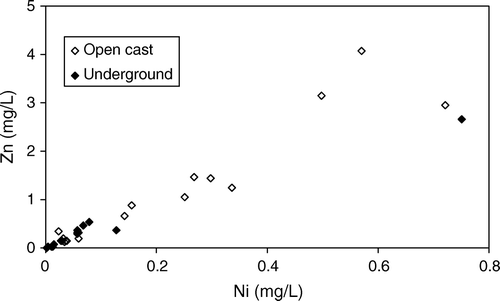
Fig. 7 Concentration of Ni compared to concentration of Fe in Brunner Coal Measures AMD. Ni concentrations are higher in AMD from open cast mines. Most other trace elements have a similar relationship to Fe.
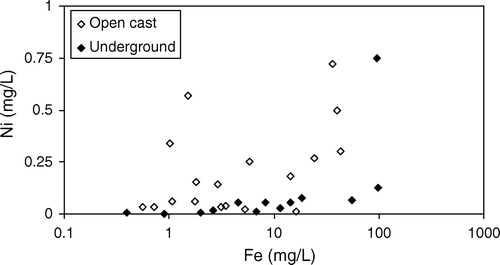
Fig. 8 Brunner Coal Measures AMD. (A) No relationship between pH and Zn; (B) poor relationship between pH and Cu. Other trace elements have similarly poor relationships to pH and other acidity parameters.
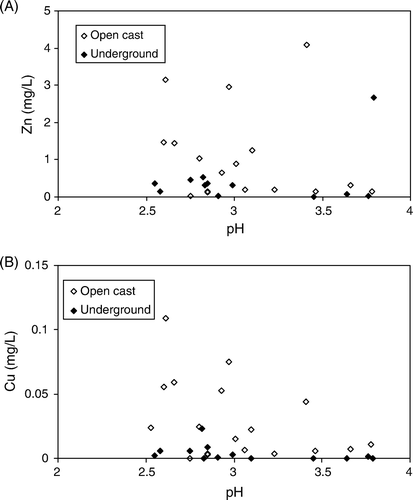
In the coal mine drainages, pH and concentrations of Fe and Al are regulated by secondary minerals (Eq. Equation5, Equation6) whereas trace elements accumulate in Brunner Coal Measures AMD as reactions continue. Concentrations of trace elements coal mine drainages can be limited by solubility or alternatively adsorption processes even at low pH (Cravotta III Citation2008b). However, in the samples examined to date, trace element concentrations in Brunner Coal Measures AMD appear to be below levels required for precipitation of trace element rich minerals, and the low pH of samples from mines in this study are unfavourable for adsorption of most cations. Therefore, the trace element concentrations in our mine drainage samples reflect the degree of dissolution of their source minerals. At open cast mines AMD forming reactions that release trace elements proceed further than at underground mines because of the increased surface areas as well as greater ingress of O2 and therefore trace elements can accumulate to higher concentrations ().
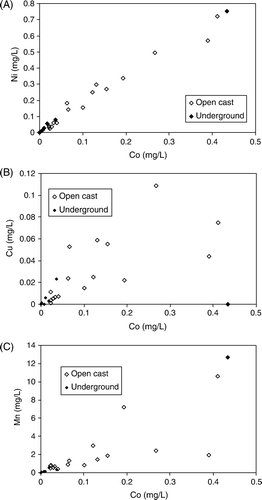
There are relationships between the concentrations of different trace elements in Brunner Coal Measures AMD. For example, some of the most enriched trace elements, Zn, Ni and Co, are strongly related to each other (, ) and more weakly to other trace elements such as Cu and Mn (, ). This indicates that the source of these trace elements is similar and that the concentrations of Zn, Ni and Co are conservative (they remain dissolved rather than partition into secondary minerals) in the AMD forming environment. It is possible that other trace elements such as Cu and Pb have the same source but are partially removed by adsorption because this can take place at low pH for Cu and Pb (Cravotta III Citation2008b).
Fig. 10 Relationship between Co and: (A) Nickel (Ni); (B) Copper (Cu); and (C) Manganese (Mn) for all samples of Brunner Coal Measures AMD. Note strong relationship between Co and Ni.
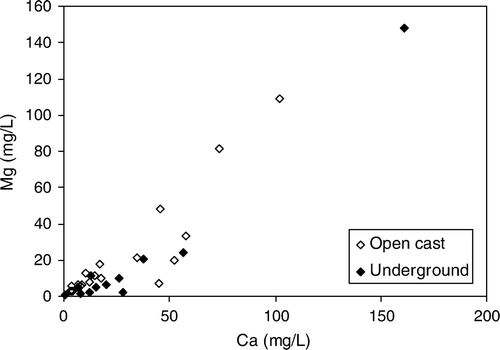
Concentrations of Ca and Mg in AMD from the Brunner Coal Measures are correlated (). However, other base cations are not correlated with Mg and Ca probably because of precipitation of secondary minerals (e.g. Jarosite, Eq. Equation5). Relationships between trace elements and alkaline earth metals (Ca and Mg) highlight variations in the geochemistry of coal measures in different coalfields. The ratio of trace elements such as Zn and Ni to Ca and Mg is bimodal (). Most samples from the Garvey Creek Coalfield have a lower Zn to Mg ratio than samples from the Buller and Greymouth Coalfields. It is possible these different ratios relate to impurities in dolomite (Henshaw & Back Citation1979) identified in Garvey Creek Coalfield (Newman Citation1988) but unreported in the Buller Coalfield.
Fig. 12 Bimodal ratio between Zn and Mg strongly related to coalfield in Brunner Coal Measures AMD. Garvey Creek coalfield is has a lower Zn:Mg ratio than Greymouth and Buller Coalfield.
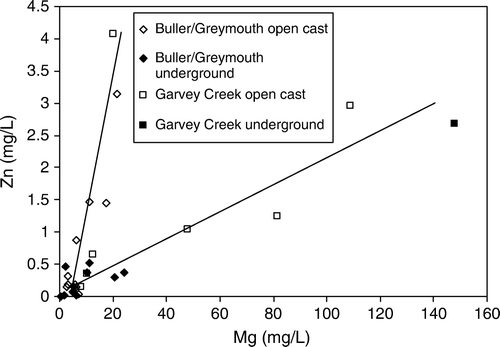
One sample plots as an outlier in Fig and represents a sample from an underground seep or spring with chemistry more similar to samples from open cast mines. This sample comes from a seep that is interpreted to be a collapsed adit directly down gradient of an open cast mine that reworked old underground operations. At this site it is likely that open cast AMD seeps into underground workings and flows out the collapsed adit or spring. This result indicates the complexity of mine drainage seeps and highlights that trace element chemistry can be used to identify the contribution of AMD from different sources where this is complex such as areas with extensive historic mining activity.
Relationships between trace elements and local geological factors require combined analyses of AMD and sulfide mineral composition at specific sites, and studies of this type have been recently completed (Hewlett Citation2003; de Joux & Moore Citation2005; Hewlett et al. Citation2005; Weber et al. Citation2006; Weisener & Weber Citation2010) and are in progress. In general, these studies have found that sulphide minerals are enriched in trace elements that correlate with those present in related AMD. However, no comprehensive studies have been completed that examine the trace element concentrations of silicate minerals as well as sulphides and therefore contributions to trace element chemistry by silicates in coal measures can not be ruled out.
Implications and limitations
Several factors that influence mine drainage chemistry have been identified including regional geology and paleoenvironmental setting, mine type (underground or open cast), hydrogeology, and local geology. Interpretation of these factors has implications for mine regulation, operation, management, and remediation on the West Coast. Prediction of mine drainage chemistry assists mine industry regulators to identify areas that have high and low AMD risk so that requirements for data collection and monitoring can be optimised. Mine operators are able to identify factors that impact mine drainage chemistry prior to mining, budget accordingly, and optimise management and remediation strategies.
Mine drainage chemistry evolves with time (Perry & Rauch Citation2006) and this paper does not explore the influence of time between mine closure and sample collection on mine drainage chemistry. In order to quantify these effects in a meaningful manner, repeated sampling is required at appropriate time intervals and under measured flow conditions. To date there are insufficient data available to evaluate on the evolution of mine drainage chemistry.
Sampling and analysis of appropriate rock geochemistry such as acid base accounting data would improve regional and local geological interpretations of mine drainage chemistry. Publications including these types of analyses are available (Hughes et al. Citation2004; Pope et al. Citation2006; Craw et al. Citation2008) and there are substantial datasets held by mining companies. Interpretations of acid base accounting data from previous studies are summarised in (Pope et al. Citation2010).
Summary and conclusion
Coal mine drainage chemistry on the West Coast of the South Island is highly variable. The pH is between 2 and 8, chemical concentrations vary by several orders of magnitude, and ratios of major components also vary by several orders of magnitude. Factors that influence mine drainage on the West Coast of the South Island include regional geology and paleoenvironemental setting, mine type, hydrogeology, and local geology. West Coast coal mine drainage chemistry is bimodal and controlled by regional geology. Mines in Paparoa Coal Measures have neutral mine drainage whereas those in Brunner Coal Measures have acid mine drainage. This is primarily related to the depositional environment of the coal measures, however, additional analysis of rock geochemistry is required to improve and refine these interpretations.
Brunner Coal Measures AMD chemistry is influenced by mine type. Open cast mines have Al and trace element rich AMD compared to underground mines. This relates to reactions between AMD and silicate minerals which release Al and consume Fe. In addition, AMD forming reactions release trace elements and the concentration of many of these components is not limited by secondary mineral formation, instead they are conservative and accumulate in the AMD. Brunner Coal Measures mine drainage chemistry is also influenced by hydrogeology; flooded underground mines release less acid than free-draining mines because the surface area available for oxygen to react with pyrite is lower. In addition, local geology overprints mine drainage chemistry, for example AMD from mudstone rich mine waste is more acidic than AMD from sandstone waste at Stockton Mine. Local geology also influences the ratios of trace elements (Zn, Co, Ni) to alkaline earth metals (Ca and Mg) so that AMD Garvey Creek Coalfield can be distinguished from Buller Coalfield much of the time. It is possible these ratios relate to dolomite identified in Garvey Creek Coalfield but unreported in Buller Coalfield.
Coal mine drainage chemistry on the West Coast of the South Island is highly variable but can be predicted based on several geological factors. These predictions have application to the mining industry for managing, mitigating, monitoring, and remediation of mine drainages that would otherwise cause negative environmental impact.
Acknowledgements
This research is funded by the Foundation for Research, Science and Technology under contract FRST CRLX401—Delivering pathways to mineral wealth. Important support for this programme is provided by several organisations including, the West Coast Regional Council, Environment Southland, DOC, Solid Energy New Zealand, Oceana Gold, New Zealand Coal and Carbon Ltd, New Zealand Coal Association, and Minerals West Coast. Thanks to Paul Weber, Charles Cravotta and an anonymous reviewer for constructive comments.
References
- Alicorn Leon , E and Anstiss , RG . 2002 . Selected trace elements in Stockton, New Zealand waters . New Zealand Journal for Marine and Freshwater Science , 36 : 81 – 87 .
- Berner , EK and Berner , RA . 1987 . Global water cycle geochemistry and environment , Englewood Cliffs, NJ : Prentice Hall .
- Black , A , Trumm , D and Lindsay , P . 2005 . “ Impacts of Coal mining on water quality and metal mobilisation: case studies from West Coast and Otago ” . In Metal contaminants in New Zealand , Edited by: Moore , TA , Black , A , Centeno , JA , Harding , JS and Trumm , DA . 247 – 260 . Resolutionz Press .
- Brady , K , Hornberger , R , Chisholm , W and Sames , G . 2000 . “ How geology affects mine drainage prediction ” . In Prediction of water quality at surface coal mines , Edited by: Kleinmann , RLP . 9 – 35 . Morgantown, West Virginia, , USA : The National Mine Land Reclamation Centre .
- Caruccio FT 1970 . The quantification ofreactive pyrite by grain size . Proceedings 2nd symposium on coal mine drainage research. Pittsburgh, National Coal Association – Bituminous Coal Research Inc . 123 131 .
- Cravotta , CA III . 2008a . Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania. USA. Part 1: Constituent quantities and correlations . Applied Geochemistry , 23 : 166 – 202 .
- Cravotta , CA III . 2008b . Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania. USA. Part 2: Geochemical controls on consituent concentrations . Applied Geochemistry , 23 : 203 – 226 .
- Craw , D , Mulliner , T , Haffert , L , Paulsen , HK , Peake , B and Pope , J . 2008 . Stratigraphic controls on water quality at coal mines in southern New Zealand . New Zealand Journal of Geology and Geophysics , 51 : 59 – 72 .
- Davies HG , Craw D , Peake BM , Weber PA , Lindsay P 2008 . Geochemical changes following pH remediation within Mangatini Stream, Stockton Mine, West Coast, New Zealand . New Zealand Branch Conference , 31st August–3 Sept 2008, AusIMM, Wellington . 129 140 .
- de Joux A 2003 . Geochemical investigations and computer modelling of acid mine drainage, Sullivan Mine, Denniston Plateau, West Coast . MSc thesis, University of Canterbury, Christchurch
- de Joux , A and Moore , TA . 2005 . “ Geological controls on the source of nickel in Rapid Stream, South Island ” . In Metal Contaminants in New Zealand , Edited by: Moore , TA , Black , A , Centeno , JA , Harding , JS and Trumm , DA . 261 – 276 . Christchurch : Resolutionz Press .
- Ellis MJ 2008 . Stockton Mine water treatment plant – the removal of black water from the Mangatini Stream . New Zealand Branch Conference, AusIMM , Wellington . 145 155 .
- Espana , JS , Pamo , EL , Santofimia , E , Aduvire , O , Reyes , J and Barettino , D . 2005 . Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications . Applied Geochemistry , 20 : 1320 – 1356 .
- Evangelou , VP . 1998 . Environmental soil and water chemistry , New York : John Wiley and Sons .
- Henshaw , BB and Back , W . 1979 . Major geochemical processes in the evolution of carbonate-aquifer systems . Journal of Hydrology , 43 : 287 – 312 .
- Hewlett L 2003 . Environmental geology of gold and coal mines, Reefton, New Zealand . BSc Hons thesis, University of Otago , Dunedin .
- Hewlett L , D Craw 2003 . Comparison ofenvironmental discharges from historic coal and gold mines, Reefton, New Zealand . Opportunities for the New Zealand Mining and Minerals Industry, AusIMM, 3 Sept–5 Sept 2003, Greymouth . 8 .
- Hewlett , L , Craw , D and Black , A . 2005 . Comparison of arsenic and trace metal contents of discharges from adjacent coal and gold mines, Reefton, New Zealand . Marine and Freshwater Research , 56 : 983 – 995 .
- Hughes J , Lindsay P , Peake B , Craw D 2004 . An environmental evaluation ofthe geochemical properties ofKiata Mudstone and Brunner Caol Measures, Cypres and Stockton Mines, West Coast, NZ . Looking Forward, Looking Back, AusIMM, 29 August–1 Sept 2004, Nelson . 41 51 .
- James T 2003 . Water quality of streams draining various coal measures in the north central West Coast . Opportunities for the New Zealand Mining and Minerals Industry. Crown Minerals, MED, 3 Sept–5 sept 2003, Greymouth, not paginated .
- Kleinmann RLP 2000 . Prediction of water quality at surface coal mines . Morgantown, West Virginia : The National Mine Land Reclamation Centre .
- Leask , WL . 1993 . Brunner Coal Measures at Golden Bay, Nelson: An Eocene fluvial-estuarine deposit . New Zealand Journal of Geology and Geophysics , 36 : 37 – 50 .
- Lindsay P , Kingsbury M , Pizey M 2003 . Impact of mining on the Lower Ngakawau River. Opportunities for the New Zealand Mining and Minerals Industry . Crown Minerals, MED, 3 Sept–5 Sept 2003, Greymouth, not paginated .
- McCauley C , O'Sullivan A , Weber PA , Trumm D 2010 . Variability of Stockton mine drainage chemistry and its treatment potential with biogeochemical reactors . New Zealand Journal of Geology and Geophysics 53 : 213 227 .
- McCauley , CA , O'Sullivan , AD , Milke , MW , Weber , PA and Trumm , DA . 2009 . Sulphate and metal removal in bioreactors treating acid mine drainage dominated with iron and aluminium . Water Research , 43 : 961 – 970 .
- Nathan , S . 1986 . Cretaceous and Cenozoic sedimentary basins of the West Coast region, South Island New Zealand. Wellington, New Zealand Geological Survey . Report nr Basin Studies , 1 : 99p
- Nathan , S . 1996 . Geology of the Buller Coalfield , Lower Hutt : Institute of Geological & Nuclear Sciences Limited .
- Nathan S , Rattenbury MS , Suggate RP 2002 . Geology of the Greymouth Area, 1:250000 Geological Map. QMap 12. Greymouth Area, Institute of Geological & Nuclear Sciences Limited .
- Newman NA 1988 . Mineral matter in Coal of the West Coast, South Island, New Zealand . PhD thesis, University of Canterbury , Christchurch .
- Newman , J and Newman , NA . 1992 . “ Tectonic and paleo-environmental controls on the distribution of Upper Cretaceous coals on the West coast of the South Island, New Zealand ” . In Controls on the distribution and quality of cretaceous coals , Edited by: McCabe , PJ and Parish , JT . Boulder : Geological Society of America .
- Perry EF , Rauch HW 2006 Water quality evolution in flooded and unflooded coal mine pools . ICARD, 27 March–29 March 2006. American Society of Mining and Reclamation, Lexington . 1565 1581 .
- Plumlee G , Logsdon M 1999 . The environmental geochemistry of mineral deposits . Part A: processes, techniques and health issues . Chelsea : Society of Economic Geologists .
- Pope J , Singh B , Thomas D 2005 . Mining related environmental database for West Coast and Southland: data structure and preliminary geochemical results . AusIMM 2005 Annual Conference, 13 November–16 November, Auckland . 7 .
- Pope J , Newman N , Craw D 2006 . Coal mine drainage geochemistry, West Coast, South Island – a preliminary water quality hazard model . AusIMM Annual Conference, 29 August–1 September, Waihi . 1 12 .
- Pope J 2007 . Interpretation of ARD evolution trends in solid energy free draining kinetic leach columns . Volume 1. Christchurch, CRL Energy Ltd. Report nr 07-41102 . 1 36 .
- Pope J , Weber P , MacKenzie A , Newman N , Rait R 2010 . Correlation of acid base accounting characteristics with the Geology of commonly mined coal measures, West Coast and Southland, New Zealand . New Zealand Journal of Geology and Geophysics 53 : 153 166 .
- Raine , JI . 1984 . Outline of palynological zonation of Cretaceous to Paleogene terrestrial sediments in the West Coast region, South Island, New Zealand. Wellington, New Zealand Geological Survey . Report nr Report , 109 : 82p
- Skousen , J , Rose , A , Geidel , G , Foreman , J , Evans , R and Hellier , W . 1998 . A handbook of technologies for the avoidance and remediation of acid mine drainage , Morgantown : National Mine Reclamation Centre .
- Smart R , Skinner WM , Levay G , Gerson AR , Thomas JE , Sobieraj H , Schumann R , Weisener CG , Weber PA , Miller SD , Stewart WA 2002 . ARD test handbook: Project P387. A prediction and kinetic control of acid mine drainage Melbourne, , Australia : AMIRA, International Ltd, Ian Wark Research Institute .
- Stumm , W and Morgan , JJ . 1996 . Aquatic chemistry , New York : John Wiley and Sons Inc .
- Suggate , RP . 1959 . New Zealand coals, their geological setting and its influence on their properties. Wellington, New Zealand Geological Survey . Report nr Bulletin , 134 : 113p
- Titheridge DG 1992 . The depositional setting of the Brunner Coal Measures, Buller Coalfield . Wellington, Ministry of Commerce. Report nr Resource Information Report 13 . 1 40 .
- Trumm , D , Watts , M , Pope , J and Lindsay , P . 2008 . Using pilot trials to test geochemical treatment of acid mine drainage on Stockton Plateau . NZ Journal of Geology and Geophysics , 51 : 175 – 186 .
- Weber , PA , Skinner , WM , Hughes , J , Lindsay , P and Moore , T . 2006 . Source of Ni in coal mine acid rock drainage, West Coast, New Zealand . International Journal of Coal Geology , 67 : 214 – 220 .
- Weisener C , Weber P 2010 . Preferential oxidation of pyrite as a function of morphology and relict texture . New Zealand Journal of Geology and Geophysics 53 : 167 176 .