ABSTRACT
The Prohibition mine processing site was abandoned in 1951. Part of the site was remediated in 2016, but some remaining processing residues exposed at the surface have elevated As, Cu, Hg, Pb and Zn. Most of these residues consist of haematitic sandy tailings with oxidised As material (As = 4000–9000 mg/kg), and that As is largely immobile in the environment. Minor As-rich flue residues have As contents of 600–4000 mg/kg, from which As may be mobilised into the environment, but dissolved As <0.3 mg/L prevails in associated waters. Lead-rich residues (locally >5 wt% Pb) from an assay office including arsenic-rich glassy slags (locally >3 wt% As) were also deposited. Naturally colonising plants have absorbed low As contents, with <2 mg/kg (dry weight) in shrubs and up to 20 mg/kg (dry weight) in grasses. This natural revegetation appears to be adequate for site rehabilitation.
Introduction
Many historical mining areas around the world were abandoned with no rehabilitation, and have since become popular sites for tourism and recreation (Ashley and Lottermoser Citation1999; Hardesty Citation2001; Potts et al. Citation2002; Conesa et al. Citation2008; Power et al. Citation2009; Corriveau et al. Citation2011; Hudson-Edwards et al. Citation2011). There are general conflicts between site preservation for historical and/or biodiversity purposes, free public access, potential health risks to visitors and the potential for ecological degradation from elevated levels of metals (Hardesty Citation2001; Batty Citation2005; Conesa et al. Citation2008). Hence, it is desirable to obtain the scientific background to the mineralogy and geochemistry of mining residues, and consequently evaluate how the physical and chemical properties of the mine wastes interact with the surficial environment, so that appropriate management of sites can be undertaken to balance the conflicting demands for site utilisation (Ashley and Lottermoser Citation1999; Hardesty Citation2001; Batty Citation2005; Conesa et al. Citation2008; Corriveau et al. Citation2011).
The Reefton goldfield of Westland (A–C) has been the focus of mining in orogenic gold deposits since the late nineteenth century, and the most recent gold mine, at Globe-Progress (A) closed in 2015. Parts of the Reefton goldfield are both a draw for tourists and repositories for a range of potentially toxic materials. In particular, the Waiuta historic town site (C), now a ‘ghost town’, is a popular destination for visitors, although some parts of the immediate area around the town have elevated levels of potentially toxic elements, particularly arsenic (As) (Craw et al. Citation2007; Haffert and Craw Citation2008a, Citation2008b; Haffert et al. Citation2010a, Citation2010b). Considerable scientific effort has been spent on defining the nature of various mining areas in the Reefton goldfield, especially at mineral processing sites that included crushing facilities (batteries) and gold-extraction plants. These processing sites were the principal places where a range of potentially toxic materials were liberally distributed in the environment during historical activity (e.g. ). Examples of mining areas that have been characterized previously, indicated in (A–C), include the historical underground mines at Globe-Progress (Hewlett et al. Citation2005), Big River processing wastes (Druzbicka and Craw Citation2015), the Alexander battery and related sites (Malloch and Craw Citation2017; Malloch et al. Citation2017), Golden Lead battery site (Malloch and Craw Citation2017) and several sites in the Waiuta area (A–C) (Noble et al. Citation2002; Craw et al. Citation2007; Haffert and Craw Citation2008a, Citation2008b; Rait et al. Citation2010; Haffert et al. Citation2010a, Citation2010b). The Prohibition processing site at Waiuta (C) is the locus of this study.
Figure 1. Location maps for the Waiuta town and associated mining and processing sites. A, Waiuta in relation to some other gold mines in the Reefton Goldfield. B, The Blackwater and Snowy River catchments that drain the area, with Prohibition and Blackwater mine shaft sites and Snowy River processing site. C, Waiuta ghost town, showing the local drainage and topography in the region of Prohibition site of this study.
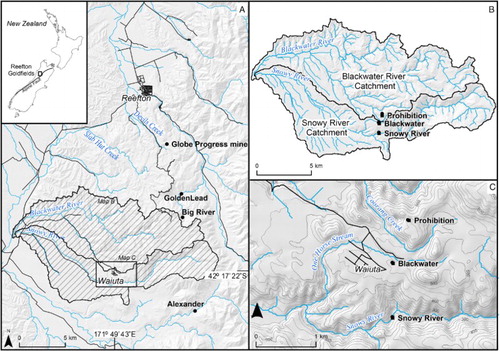
Figure 2. Flow chart showing the pathways for processing of the ore (entering from centre right) from ore separation into silicate and sulphide-rich portions (purple, right), and the downstream wastes and products of the mine. Mine wastes are around perimeter of diagram, with those relevant to this study in red boxes.
When mining ceased at Waiuta in 1951, all the mine processing wastes were left in an unremediated state and casual visitors originally had unrestricted access to the sites and associated wastes that were exposed at the surface. In particular, some of the processing wastes at the Prohibition site (C) were extremely rich in As (Craw et al. Citation2007; Haffert and Craw Citation2008a, Citation2008b; Rait et al. Citation2010; Haffert et al. Citation2010a, Citation2010b), and those parts of the processing site have recently (2016) been remediated to limit the surface environmental exposure of As and potential As mobilisation into discharging waters. Despite recent activity, not all the Prohibition site has been remediated, and some remnants of mine processing residues remain (A). These residues are highly diverse in character, as they were deposited from different parts of the processing system with different ore-related and added chemical components. This paper characterises the remaining residues according to their mineralogy and geochemistry, and relates the different mineral components to potential environmental mobilisation of metals and metalloids at the site. Further, the site has undergone some natural revegetation, so we evaluate the mobility of As into that vegetation where it has developed on As-rich substrates. We integrate these observations to suggest that natural processes have been adequate to effectively rehabilitate the tailings site, and these natural processes are on-going, so that there is no requirement for engineered intervention.
Figure 3. Physical setting of the Prohibition mine wastes. A, Aerial view of the Prohibition site before the 2016 rehabilitation, showing the locations of tailings, slag and assay debris of this study in relation to the main processing site. Field X-ray fluorescence measurement sites from this study (Table S1) are indicated, with representative spots in the rehabilitation area from Haffert and Craw (Citation2009) for comparison. B, Photograph of a block of slag. C, Photograph from the rehabilitated processing plant area, viewed towards the rehabilitated wetland (yellow arrow in A), taken in November 2016. D, Photograph of natural revegetation of the red tailings, at site indicated in A.

General setting
Geology and physical setting
The Waiuta mining area, like the Reefton goldfield in general, is hosted in the Paleozoic Greenland Group, which consists of greenschist facies metagreywacke and argillite (Rattenbury & Stewart Citation2000; Christie and Brathwaite Citation2003). Mining at Waiuta focused on deep underground excavations into the Birthday Reef, a steeply dipping gold-rich quartz vein system that was mined between 1906 and 1951 (Christie and Brathwaite Citation2003; Hamisi et al. Citation2017). The Birthday Reef contained abundant free gold within quartz, and additional gold was encapsulated in sulphide minerals, mainly pyrite and arsenopyrite in the vein and the immediately adjacent host rock (Hamisi et al. Citation2017). Minor chalcopyrite, sphalerite and galena are distributed through the sulphidic parts of the ore (Hamisi et al. Citation2017).
The Prohibition site was operational from 1938 to 1951, during which time ore was extracted from the Prohibition shaft and gold extraction was carried out close to the shaft, in extensive processing facilities. The Prohibition processing site was situated on a plateau in steep terrain c. 560 m above sea level and c. 120 m above the surrounding valley. The site experiences high average annual rainfall of c. 2300 mm distributed throughout the year, and a mean temperature of 12 °C (Mew and Ross Citation1994). Because of the site’s position, there is little run-off onto the main processing and shaft site, but rainfall onto the site runs off onto the tailings impoundment and flue areas because they are stepped down from the rest of the site. The site is within the Blackwater catchment and run-off from the site is into the Blackwater Creek, a tributary to the Grey River which terminates in the Tasman Sea.
Mineral processing system
The Prohibition processing plant extracted free gold from crushed quartz by gravity settling, and then used flotation to separate gold-bearing sulphide minerals from crushed ore (). Free gold particles in crushed ore underwent amalgamation with Hg and the gold was removed from that amalgam in a retort (). Gold in sulphides was encapsulated as microparticles and in solid solution within pyrite and arsenopyrite (Hamisi et al. Citation2017), and this refractory gold was liberated in an Edwards roaster for cyanidation (). Gold was saved after cyanidation using Zn dust precipitation facilitated by Pb compounds. The Edwards roaster had a chamber to condense the As that was volatilised by roasting of arsenopyrite in the ore, and the resultant arsenolite (AsIII2O3) was sold as a by-product (Hutton Citation1947).
Following separation of the free gold and sulphide concentrate from the ore, the silicate tailings were disposed of into a tailings impoundment downslope of the site (A). Likewise, the roaster tailings and cyanidation tailings were disposed of into the tailings impoundment, and all these tailings became mixed during discharge and sedimentation (). Exhaust gases, including uncondensed As, were released from the Edwards roaster via a chimney flue system that was partially buried in a trench that followed the ground surface. The flue system initially extended for c. 150 m downslope past the tailings impoundment (A). Some As-rich residues formed within that flue and remain where the flue has now largely decomposed (A; ). A longer flue was constructed along a different pathway at a later date.
Table 1. Characteristics of the mine wastes which remains on site; tailings material, flue residue, assay debris and slag.
During roasting of the sulphides concentrate, glassy slag was formed in the Edwards roaster ( and B; ), and this was discarded at the top of the slope leading to the tailings impoundment (A). Some sulphide concentrate and residues from an assaying office () were discarded in the same area as the slag (A,B). Assaying residues included Pb-rich wastes including Pb-contaminated assaying cupels (crucibles).
Revegetation and rehabilitation
The processing plant site remained largely barren of vegetation apart from minor colonisation by exotic grasses and moss species (A; Craw et al. Citation2007). These grasses and mosses could not colonise the area around the Edwards roaster, although some moss grew in a wetland below the roaster where high-As residues were deposited anthropogenically and with subsequent erosion (Craw et al. Citation2007; Haffert and Craw Citation2008a, Citation2008b; Haffert et al. Citation2010a). The areas with high-As residues were remediated in 2016 in a major government-funded project managed by the Department of Conservation. Rehabilitation involved removal of material with the highest As concentrations and burial of the rest of these substrates under a layer of imported gravel, with no addition of vegetation (C). This remediation activity included the wetland, most of the slag, and parts of the assay office residues ( and A,C).
Vegetation was largely removed from the area surrounding the processing site while the mine was active, but since then much of that surrounding area has become naturally revegetated with native and exotic species (Craw et al. Citation2007) (A,D). Most of the vegetation on the tailings area, which is a principal topic of this study, consists of native shrubs with some exotic grasses (A,D).
Methods
Characterisation of solid material
The metal and As contents of Prohibition mine wastes were determined in the field with an Olympus Innov-X Systems model XPD-4000 field portable X-ray fluorescence instrument (FP-XRF). All measurements were done in soil mode and the acquisition time was 45 s for most measurements, with some follow-up measurements at 30 s acquisition time. The FP-XRF model is similar to the one used elsewhere at the Prohibition processing site by Haffert and Craw (Citation2009) and the same instrument that was used at other Reefton goldfield sites (Druzbicka and Craw Citation2015; Malloch and Craw Citation2017; Malloch et al. Citation2017). As results were calibrated against laboratory results in the same manner as in these previous studies and these As results, corrected for field conditions and moisture content, are provided in Table S1. Calibration data used for the As corrections are listed in Table S2, along with the calibration equation. Elements of interest other than As (Cu, Pb, Zn, Hg) are mostly at relatively low levels (and only relative differences are of interest in this study), and no specific calibration of these metals was carried out.
Minerals in the mine wastes were identified using standard incident and transmitted light microscopy, X-ray diffraction (XRD), and a scanning electron microscope (SEM) with energy-dispersive spectroscopy (EDS) analytical facility. XRD analysis used CuKα radiation with a PANalytical X’Pert Pro MPD PW3040/60 instrument housed in the Geology Department, University of Otago, and resultant diffraction patterns were analysed using the PANalytical High Score software package. Samples were mounted into epoxy blocks and carbon coated to limit charging prior to examination with a Zeiss Sigma VP (variable pressure) SEM fitted with a HKL INCA Premium Synergy Integrated EDS system at the Otago Centre for Electron Microscopy (OCEM), University of Otago. Elemental spot analyses were obtained, and element maps were constructed to determine elemental distributions.
Water and plant sampling and analysis
Surface waters were syringed from the water body and filtered through a 0.45 µm filter into acidified (redistilled nitric acid) sample bottles, stored in a chilly bin with cooler packs then analysed by Hills Laboratories, an internationally accredited laboratory, using inductively coupled plasma-mass spectrometry (ICP-MS) analysis for As, Sb, Cu, Pb and Zn with respective detection limits of 0.0010 mg/L (As), 0.0002 mg/L (Sb), 0.0005 mg/L (Cu), 0.00010 mg/L (Pb) and 0.0010 mg/L (Zn).
Samples (c. 500 g) of shrub leaves and stems were collected above waist height on tailings to avoid contamination by the underlying substrate. Each shrub sample was a composite of different branches of the same plant. Grass samples were collected by pulling the tops off blades to avoid contamination. Sampling occurred after several days of rain, so dust contamination is considered minimal, and the plants were not washed before drying and submission for analysis. Vegetation samples were oven dried at c. 35 °C for 4 days and sent to Hills Laboratories, where the samples were ground. Preparation for analysis was done with nitric and hydrochloric acid micro-digestion and filtration, and samples were then analysed for As by ICP-MS.
Laboratory leaching experiments
Surface sediment samples of mine tailings and flue residues were collected to a maximum depth of 15 cm, oven dried at 35 °C in the laboratory at the University of Otago Geology Department. Then, 20 g of the dried material was added to Schott 50 ml screw top jars and 30 ml of distilled water was added to the jars, the contents were stirred, lids replaced tightly and placed undisturbed in a dark cupboard for 1 and 6 months. No attempt was made to control the pH of dissolved oxygen contents during these experiments. The ratio of 20 g sediment to 30 ml water is the same as that used by Mains et al. (Citation2006). To investigate the effects of potential fertiliser addition to the site, various phosphorus amendments up to 1000 mg/kg (Table S3) were added to some of the jars according to the methods outlined by Mains et al. (Citation2006). The tailings samples had As c. 6500 mg/kg and the flue residue samples had As c. 2500 mg/kg, as determined by calibration-corrected FP-XRF analyses. All samples were duplicated for both the 1- and 6-month experiments, except the control for the flue residue. After 1 and 6 months, the waters from a set of samples were analysed. The samples were prepared by pouring the leachate off the sample and filtering it through a 0.45 µm filter into acidified (nitric acid) sample bottles. The samples were analysed by Hill Laboratories using ICP-MS analysis for As, Cu, Pb and Zn.
Descriptions of processing residues
Tailings
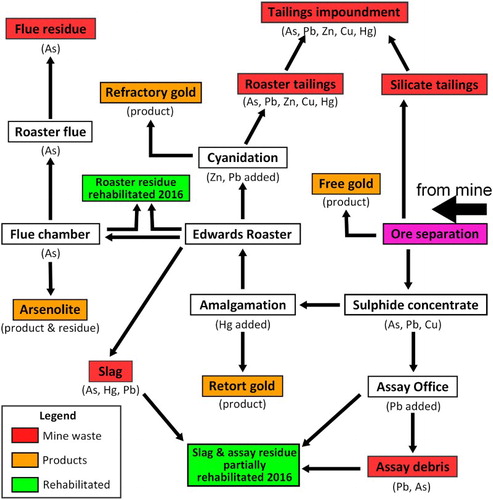
The tailings impoundment material consists of bedded, silty red-coloured roaster tailings and sandy silicate tailings from which the sulphides ore was concentrated (A). The tailings impoundment overflowed during production and the run-off was to the southeast towards the flue, covering an area of c. 1400 m2 (A). Tailings deposits are up to 50 cm deep in places but are predominantly a veneer c. 10 cm thick on the hillside. Tailings deposits are generally saturated with water where they have pooled in topographic depressions; the pH of the saturating water is between 4.5 and 5.5.
Figure 4. Images of red tailings material. A, Hole dug in tailings, showing the bedding and varying arsenic concentrations down the hole. B, Scanning electron microscopy backscatter image showing rhombic-shaped pseudomorphs of arsenopyrite (aspy), cubic-shaped pseudomorphs of pyrite (py), quartz (qtz), and some non-porous maghemite. C, Energy-dispersive spectroscopy arsenic map of red tailings, showing the heterogeneous distribution of arsenic on the micron scale with the high arsenic cements and metal(loid) oxides.
The tailings are red in colour and dominated by quartz from the original ore and porous haematite particles from the roaster (; ). Bedding in the tailings is defined by centimetre-scale laminations with differing silicate contents, silicate particle sizes and proportions of haematite (A). Some of the haematite is pseudomorphous after arsenopyrite and pyrite (A,B), and some particles have less porous maghemite rims. Relict sulphide mineral particles (pyrite, arsenopyrite) are rare in the tailings, and these commonly have oxidised rims.
Bulk As concentrations typically range between 4000 and 9000 mg/kg ( and A; Table S1). Porous haematite has variable As concentrations, typically <3 wt%, whereas non-porous maghemite contains up to 17 wt% As. Arsenic is also distributed irregularly throughout the rest of the tailings material (C), with rare scattered scorodite particles. Much of the As occurs dispersed at low levels (<1 wt%) in brown fine-grained amorphous Fe oxyhydroxide material, and more rarely at higher concentrations (>5 wt%) in similar brown fine-grained material. Minor amounts of Fe oxyhydroxides contain other metals such as Pb, Zn and/or Cu. For convenience, we refer herein to low-As Fe oxyhydroxide as HFO and As-rich Fe oxyhydroxide as HFA (following Malloch et al. Citation2017 who report on similar materials at a nearby site), while recognising that at least some of the low-As Fe oxyhydroxide may not be HFO according to the stricter definitions of, for example, Dzombak and Morel (Citation1990).
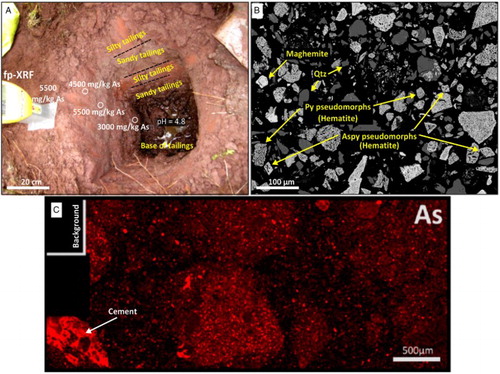
The tailings are generally uncemented, although minor formation of HFO helps to bind some of the material. Localised As-bearing cementing material in the tailings (C) is primarily HFA. This cementing material has variable As and Fe contents, and at least two generations of this cement can be identified in . Some low-As, high-Fe cement has developed between neighbouring Fe-rich tailings particles, and this has overgrown smaller intervening clastic particles (, upper). More widespread cement in this portion of tailings occurs as hydrous As-bearing HFO that has developed extensive desiccation cracking on dehydration after sampling (, upper). Minor sulphur occurs in the former cement but is largely absent from the latter ().
Figure 5. Scanning electron microscopy images of the cement in the red tailings, with electron backscatter image at top showing a particle from roaster with a partially oxidised rim and two generations of cementation. Energy-dispersive spectroscopy element maps (middle and bottom) show the distribution of Fe, S, As and Si in the immediate vicinity of the roaster particle (indicated in boxes).
Roaster flue deposits
The flue was originally an elongate wooden box-like structure in a shallow trench that passed across the lower portion of the tailings depositional area (A). This structure has now largely decomposed, and the interior contents have had some contamination with tailings from up-slope. Consequently, the deposit is highly variable in mineralogy and texture, and consists largely of tailings haematite and silicates, and variable amounts of organic material (A–E). Water-saturated flue deposits have pH values of 4.2 to 4.8.
Figure 6. Scanning electron microscopy images of flue residues. A, Backscatter electron image of porous haematite and quartz from the tailings material in the flue residues. B, Backscatter electron image of typical flue residues with detrital feldspars, organic material, and As, Fe oxides. C, Backscatter electron image of a scorodite particle, with As and Fe element maps, D, Backscatter electron image and As, Mn maps of Fe–Mn–As-oxide, showing localised distribution of As in the surrounding material. E, Backscatter electron image and As, Fe maps of skeletal Fe–As material in a detrital particle.
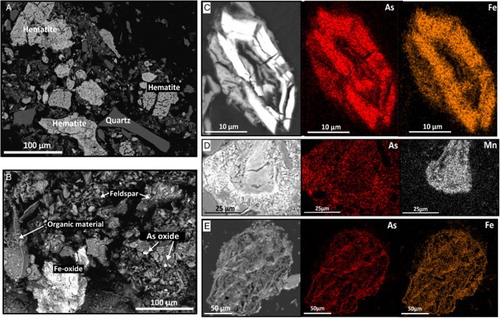
Bulk As concentrations range from 600 to 4000 mg/kg (A; Table S1). The As distribution through the material is highly variable with As contained mainly within haematite, HFO and HFA. Discrete As mineral clasts of arsenolite (As2O3) and scorodite (FeAsO4·2H2O) are scattered through the deposits (B,C). Minor cementation has occurred around some As-rich clasts (D). In contrast, partial dissolution of As has left skeletal particles (E). Cu, Zn, Pb and/or Mn oxyhydroxide material is rare (D).
Slag deposits
The slag material originated during roasting of the sulphide concentrate and was discarded as hard metre-scale blocks (A,B). These blocks, which are now a rusty brown colour, have a vesicular clinker texture with abundant slag clasts cemented by glassy material ( and ). Clasts range from angular to rounded and vary in size from micrometres to centimetres ( and ). Fine-grained (<0.5 mm) relict sulphide minerals, mainly pyrite and arsenopyrite, are scattered throughout the slag (C). There is a wide range of Fe oxide and oxyhydroxide minerals throughout the slag, in clasts and cement, including crystalline haematite, maghemite, magnetite and goethite (; A–C).
Figure 7. Scanning electron microscopy images of slag material. A, Backscatter electron image showing quartz particles (pale grey) and Fe-oxyhydroxides (white). B, Backscatter electron image of crystalline Fe oxide formed in voids, and at least two compositions of Fe oxyhydroxides as indicated by the variation in greyscale. C, Backscatter electron image of relict arsenopyrite (aspy, pale grey) embedded in Fe oxyhydroxide, with energy-dispersive spectroscopy maps of As and S.
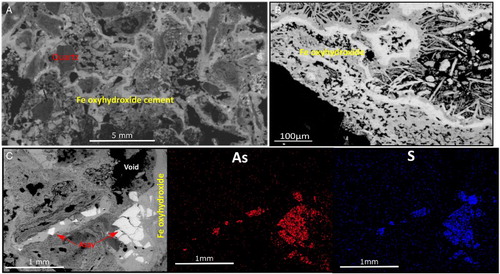
The slag typically contains c. 2 wt% As, although some weathered debris spalling from the slag has up to 3.6 wt% As. In contrast, crystalline Fe oxide cements within the slag contain <1 wt% As, and weathering decomposition of this cement yields lower As contents in the debris. Some of the slag has elevated mercury contents, with Hg concentrations up to 800 mg/kg, and Pb up to 0.5 wt%.
Assay office debris
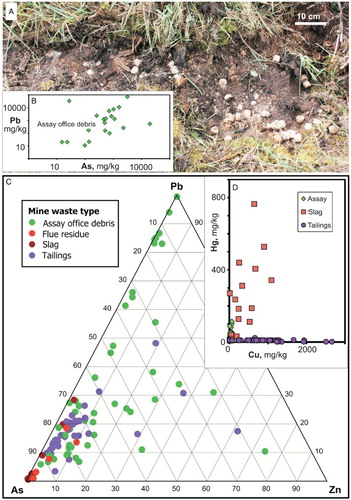
Debris from the assay office at the site () was discarded in the same general area as the slag (A). Larger fragmental material includes c. 1–4 cm of hard dark green glassy pebbles with a grey–white external alteration skin. Abundant used assaying cupels are scattered liberally in some areas. Both types of debris have elevated As and Pb contents, commonly >1 wt% for both elements. The hard pebbles contain Pb carbonate, possibly cerussite as detected by XRD, but most of the material in the pebbles is amorphous to X-rays. Finer grained sediment, which was at least partially derived by decomposition of the coarse debris, forms a centimetre-scale veneer on the ground surface. This veneer has elevated As and Pb contents as well, with up to 7 wt% Pb locally ( and ; Table S1). Elevated Pb levels predominate locally over As in some of this debris, in contrast to the other types of mine waste at the Prohibition site (C).
Figure 8. Assay office debris. A, Photograph of cupels and associated fine-grained veneer in soil. B, Plot (log scale) of As vs Pb for the fine-grained veneer in and around deposits in A. C, Ternary diagram of relative As, Pb and Zn contents of assay office debris compared with the mine wastes. D, Plot of Cu vs Hg for assay office debris (green) compared with nearby slag and tailings.
Environmental arsenic and metal mobility
Field environment
Most of the mine wastes are not saturated with water, and there are no streams directly draining these wastes. However, some of the tailings are water-saturated, and water accumulates in holes dug into these tailings (A). Two samples of this water were collected: one from a hole dug several months prior to collection, and one from a hole dug immediately before collection (). An additional sample was taken from the nearest creek (), which flows out of a mine waste rock dam below the wetland in the rehabilitated zone (sample collected prior to rehabilitation; A). Both of the tailings water samples had similar dissolved As concentrations, and low but variable dissolved metal contents.
Table 2. Surface water samples collected from Prohibition tailings area in this study (first three samples), with some other Prohibition waters and waters from Globe historic mine areas for comparison.
Because As is the principal element of environmental interest in the tailings area, a selection of naturally colonised plant species was sampled for analysis of As uptake into the plant structures from the underlying tailings in which they were growing. Two samples each of kanuka, celery pine and horopito () were collected for analysis in and near the area illustrated in (D). One of each of these samples contained leaves only, and the other sample contained leaves and fine stems. One sample of manuka leaves, and one sample of shoots and stems of gorse were collected from the same area (). One sample of a mixture of grasses growing on saturated tailings in the same area (D) was also collected. Analytical results (; A) show that shrubs have absorbed <2 mg/kg As (dry weight), whereas grasses have absorbed 20 mg/kg As (dry weight). Shrub samples with only leaves have slightly higher As contents than those containing a component of stems with the leaves ().
Figure 9. Environmental mobility of As and metals in Prohibition mine wastes. A, Arsenic uptake by various plant species growing on tailings. B, Experimental leaching of As (after 1 and 6 months) from samples of tailings and flue residues with a range of phosphorus amendments. C, Comparison of leaching of As and Zn from tailings and flue residue samples, in relation to the As and Zn contents of the substrates.
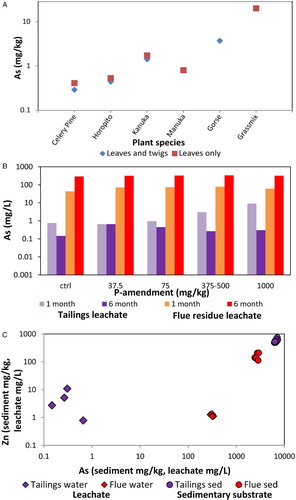
Table 3. Arsenic concentrations (dry weight) in a selection of plants growing directly in Prohibition tailings.
Leaching experiments
Mobilisation of arsenic and metals from tailings and flue residues has been further evaluated using laboratory leaching experiments. Samples of the two different types of substrate were immersed in water, and the waters were analysed for their As and metal contents after 1 month and again after 6 months. Extra leaching samples were set up for phosphorus (P) amendments, at a range of P concentrations (B). These P amendments were done to evaluate the potential effects of dissolved phosphate on As mobility, because phosphate can substitute for arsenate in minerals and mineral adsorption sites, and thereby contribute to As mobility (Elbaz-Poulichet et al. Citation2000; O’Reilly et al. Citation2001; Smith et al. Citation2002; Mains et al. Citation2006). Addition of P to waters in the tailings and flue residues could occur via long-term natural dissolution of accessory phosphate minerals in the substrates (), or as anthropogenic mediation to enhance plant colonisation and growth or the immobilisation of Cu, Pb or Zn (Ruttens et al. Citation2006; Mains et al. Citation2006; Kumpiene et al. Citation2008; Mendez and Maier Citation2008).
The results of the leaching experiments (B) show that there were at least two orders of magnitude more As leached from the flue residues than from the tailings during these experiments, with up to 335 mg/L As leached from one flue residue sample (Table S3). For the tailings material, there was a decrease of nearly one order of magnitude in the leachate As concentrations from the 1- to the 6-month experiment for most of the experimental runs (B), possibly because of re-adsorption to the solids. In contrast, the flue residue showed an increase in As leached of nearly one order of magnitude between 1 and 6 months (B). There was little or no consistent effect of P amendment on these results (B).
Despite the substantial dissolution of As from the flue residue experiments, there was only minor dissolution of other metals, the most significant of which was Zn (C; Table S3). The tailings samples used in the experiments contained substantially more Zn (several hundred mg/kg) than the c. 100 mg/kg Zn in flue residues (C). Mobilisation of this substrate Zn resulted in up to 10 mg/L dissolved Zn in leachate from tailings, whereas flue residues yielded <1 mg/L Zn (C).
Discussion
Mine processing controls on metals in environment
Most of the environmental issues in the Reefton goldfield are related to the elevated As concentrations that occur at mine and processing sites (Hewlett et al. Citation2005; Haffert and Craw Citation2008a; Druzbicka and Craw Citation2015; Malloch et al. Citation2017). In addition, some of the mines in the goldfield have elevated Sb (Ashley et al. Citation2003; Milham and Craw Citation2009; Druzbicka and Craw Citation2013, Citation2015). Antimony levels are generally low at the Prohibition site, with <20 mg/kg in the ore, mainly in solid solution in arsenopyrite, and stibnite is absent (Hamisi et al. Citation2017). However, processing-related sulphide concentration locally raised the roaster residues to 100–1000 mg/kg Sb (Craw et al. Citation2007). Elevated metalloid (As, Sb) concentrations reflect the presence of arsenopyrite, As-bearing pyrite and stibnite in Reefton goldfield ores, and these are natural phenomena that have been exacerbated by mine and processing activities (Milham and Craw Citation2009; Druzbicka and Craw Citation2013, Citation2015; Kerr et al. Citation2015). The resulting metalloid-related environmental issues are typical of orogenic gold deposits around the world (Plumlee et al. Citation1999; Craw et al. Citation2015; Desbarats et al. Citation2015). Minor chalcopyrite in the ore (typically <50 mg/kg Cu; Hamisi et al. Citation2017) was apparently concentrated in the sulphide fraction and roasted, so that variable but locally elevated Cu concentrations have persisted associated with Fe oxyhydroxide in the tailings (D; ).
In addition to the above natural aspects of ore mineralogy, the processing systems at gold mines can introduce additional metals and materials that can also contribute to environmental issues, as outlined for the nearby Alexander and Golden Lead (A) processing systems by Malloch and Craw (Citation2017). The Prohibition processing site of this study has some of these issues as a result of historical usage of Hg, Zn and Pb on the site (). These additional metals are not uniformly distributed on the site but are largely confined to the general area in which they were used, or downstream discharge sites.
Lead is one of the most widespread additional metals at the Prohibition site (A–C; Table S1), as it was used as part of the cyanidation process and extensively in the assay office. The original ore contained only minor Pb (<100 mg/kg), principally as galena (Hamisi et al. Citation2017). Lead added at the cyanidation stage has caused some contamination of the tailings, where it occurs with metal-bearing Fe oxyhydroxides (). Most of the Pb contamination occurs as a result of disposal of material from the assay office, where some substrates contain >1 wt% Pb (A–C; Table S1). Some of this elevated Pb resides in lead carbonate, but most is associated with discarded cupels (A,B) and the mineralogical nature is unknown.
Zinc metal was used extensively in the cyanidation process at Prohibition site, as at the nearby Alexander site (Malloch et al. Citation2017), and some of that Zn has continued through to the Prohibition tailings (C) where it now occurs associated with metal-bearing Fe oxyhydroxides (). Zn contamination is at generally low levels of a few hundred mg/kg (C) compared with the original gold-bearing ore which contains <100 mg/kg Zn, mostly in traces of sphalerite (Hamisi et al. Citation2017). Likewise, Hg contamination from the processing plant, where liquid mercury was used mainly for amalgamating free gold (), is at very low levels in the tailings (D). However, some Hg from this part of the processing system apparently continued to the Edwards roaster and ended up in the discarded slag, where some Hg levels between 100 and 800 mg/kg have persisted (D).
Long-term As mineral stability
The diverse mine wastes at the Prohibition site have been lying exposed at the surface for c. 70 years, so they provide an opportunity to examine the environmental stability of a range of As-bearing minerals on that time scale. Previous work in the vicinity of the roaster (now remediated; A) has shown that the two most common As minerals are arsenolite and scorodite (Haffert and Craw Citation2008a, Citation2008b; Haffert et al. Citation2010a, Citation2010b). Arsenolite is a residue from by-product production (), and scorodite was formed in the more oxidised part of the roaster system followed by further formation as a cement as the arsenolite residues were weathered (Haffert and Craw Citation2008a). In that As-rich, Fe-poor setting, arsenolite was readily dissolved in rainwater, the As(III) became oxidised and formed scorodite, an As(V) mineral, as a relatively stable product (Krause and Ettel Citation1989; Langmuir et al. Citation2006). Scorodite was further stabilised in that area by lowering of the pH (<5) during arsenolite dissolution (Haffert and Craw Citation2008a; Haffert et al. Citation2010a).
Most of the As in the tailings examined in this study is hosted in haematite and maghemite (cf. Paktunc et al. Citation2006), and these minerals have remained inert and unaltered since initial deposition ( and ). Some scorodite and As-bearing iron oxyhydroxide material (HFO and HFA) occur in the tailings, derived directly from the roaster and possibly formed in situ during oxidation of relict sulphide minerals (). Some dissolution and reprecipitation of As occurred within the tailings, associated with the HFO and HFA, and this has led to minor cementation of the tailings ( and ). However, the spatial scale of As mobility for this cementation appears to be small (<1 mm; ).
The flue residues are partly made up of redeposited tailings and have some of the same As-bearing minerals (A; ). In addition, small grains of arsenolite are still preserved in the flue residues (B), along with some particles of scorodite, and both these minerals were probably derived directly from the roaster. Considering the high solubility of arsenolite, it may have been more abundant in the flue residues when first deposited and has since partially dissolved into the saturating soil water in a similar manner to arsenolite at the roaster (Haffert and Craw Citation2008a). Conversely, preservation of angular particles of scorodite (C) in this water-saturated environment attest to the long-term stability of this mineral (Paktunc and Bruggeman Citation2010).
Arsenic minerals in the slag have remained largely intact within the glassy blocks that were originally discarded at the site, and even arsenopyrite has been preserved with little oxidation inside the slag blocks (C). Crumbling and spalling exterior surfaces of the blocks created high-As zones in the substrate, but that As appears to have been restricted to chips of slag, rather than migrating and cementing the substrate. Similarly, As in the assay debris is still confined to the small fragments of debris that have accumulated beneath the larger material (A).
Arsenic mobility in the biosphere
The low dissolved As in waters saturating the tailings attests to the long-term stability of As minerals in those tailings, as outlined above, compared with the much higher levels of dissolved As that have been mobilised from more soluble materials elsewhere at the Prohibition site (). Likewise, the leaching experiments conducted in this study confirm that only low levels (typically <1 mg/L) of dissolved As can be extracted from the tailings on a time scale of months (B,C). Hence, mobilisation of dissolved As into plants from the tailings is only minor. Shrubs also appear to largely exclude As from their structures despite high levels of solid As in the substrates in which they grow (A; ). Slightly higher levels of As uptake into grasses (>20 mg/kg dry weight; ; Craw et al. Citation2007) may enhance the mobility of As into the biosphere. Mosses are the most effective organisms for moving As into the biosphere in this environment, and Craw et al. (Citation2007) report one species, Pohlia wahlenbergii, that contained 3 wt% As in the Prohibition wetland. This species is not present in the tailings area of this study.
Relatively high levels of dissolved As (c. 100 mg/L) can be extracted from the flue residues on a time scale of months in the laboratory (B), although the volume of this material at the Prohibition site is small (A). These laboratory-based high dissolved As levels are similar to those observed in on-site waters where arsenolite dissolution has been the principal source (Haffert and Craw Citation2008b; Haffert et al. Citation2010a), and minor mobilisation of arsenolite from the flue residues on site (B) into the biosphere is probable.
Conclusion
The principal processing and roasting areas at the Prohibition mine site were remediated in 2016. This study has shown that additional residues of material around the old assay office at his site have elevated As and Pb, particularly associated with discarded assay cupels. Likewise, glassy slag deposits near the assay office have elevated As and Pb. Much of this As–Pb-rich material has been removed or covered during remediation of the adjacent site, and most of the remaining material has been colonised naturally by vegetation.
Other mining and processing residues that have ore-related and added chemical components, including As, Cu, Hg, Pb and Zn, persist on the site beyond the rehabilitated areas. The most voluminous of these residues is the tailings material, which is broadly uniform in composition and has As levels between 4000 and 9000 mg/kg. The As is predominantly associated with oxides and oxyhydroxides of iron, including haematite, maghemite, and amorphous Fe oxyhydroxide material containing variable amounts of As. Laboratory leaching experiments show that the As in the tailings is difficult to mobilise in water and in solutions with variable amounts of P amendment, yielding <2 mg/L dissolved As. Although this potential level of dissolved As is high from a regulatory perspective, water sampled from the tailings in the field contains <0.3 mg/L dissolved As.
The roaster flue residues are highly variable in composition and have As levels between 600 and 4000 mg/kg. The As occurs with iron oxides and hydroxides, and As minerals that include scorodite and arsenolite. The volume of these residues is small, and they are present only along the line of now-deteriorated wooden flue boxing. In contrast to the tailings material, the flue residues have very high As leaching potential (>300 mg/L) from water and P-amended water experiments. These experiments demonstrate that the stability of As within the substrates is related to the chemistry and mineralogy of the material rather than to the substrate As concentrations.
The tailings area has been recolonised naturally with predominantly native shrubs and exotic grasses during the c. 70 years since the site was abandoned. Mobilisation of As into the shrubs growing directly in the high As tailings material is minor (<2 mg/kg dry weight) with uptake into grasses slightly higher (>20 mg/kg dry weight). The combination of relatively insoluble minerals and recolonisation by vegetation means that almost all the remnants of the processing residues at the Prohibition site have low potential to mobilise significant metals and metalloids into the biosphere. Hence, natural rehabilitation has occurred at this site to a level that avoids the necessity of engineered intervention of the type that was recently undertaken in adjacent areas.
Supplementary Tables
Download MS Excel (27.3 KB)Acknowledgements
The Department of Conservation (DOC) provided logistical assistance and we are particularly grateful for the enthusiastic support of Jim Staton. Discussions with James Pope and Dave Trumm were helpful throughout the project. Gemma Kerr provided field assistance, expert knowledge, and assistance with X-ray diffraction. Brent Pooley provided expert sample preparation of difficult material, and Kat Lilly assisted with SEM work at the Otago Centre for Electron Microscopy. Constructive comments from two anonymous reviewers significantly improved the presentation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ashley PM, Craw D, Graham BP, Chappell DA. 2003. Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and Southern New Zealand. Journal of Geochemical Exploration. 77:1–14. doi: 10.1016/S0375-6742(02)00251-0
- Ashley PM, Lottermoser BG. 1999. Arsenic contamination at the Mole River mine northern New South Wales. Australian Journal of Earth Sciences. 46:861–874. doi: 10.1046/j.1440-0952.1999.00748.x
- Batty LC. 2005. The potential importance of mine sites for biodiversity. Mine Water and the Environment. 24:101–103. doi: 10.1007/s10230-005-0076-0
- Christie AB, Brathwaite RL. 2003. Hydrothermal alteration in metasedimentary rock-hosted orogenic gold deposits, Reefton goldfield, South Island, New Zealand. Mineralium Deposita. 38:87–107. doi: 10.1007/s00126-002-0280-9
- Conesa HM, Schulin R, Nowack B. 2008. Mining landscape: a cultural tourist opportunity or an environmental problem?: The study case of the Cartagena–La Unión Mining District (SE Spain). Ecological Economics. 64:690–700. doi: 10.1016/j.ecolecon.2007.06.023
- Corriveau MC, Jamieson HE, Parsons MB, Campbell JL, Lanzirotti A. 2011. Direct characterization of airborne particles associated with arsenic-rich mine tailings: particle size, mineralogy and texture. Applied Geochemistry. 26:1639–1648. doi: 10.1016/j.apgeochem.2011.04.021
- Craw D, Cavanagh J, Druzbicka J, Harding JS, Kerr G, Pope J, Trumm D. 2015. A geoenvironmental model for orogenic gold deposits to predict potential environmental effects. Mine Water and the Environment. 34:388–403. doi: 10.1007/s10230-015-0358-0
- Craw D, Rufaut C, Haffert L, Paterson L. 2007. Plant colonization and arsenic uptake on high arsenic mine wastes, New Zealand. Water, Air, and Soil Pollution. 179:351–364. doi: 10.1007/s11270-006-9238-3
- Desbarats AJ, Parsons MB, Percival JB. 2015. Arsenic mobility in mildly alkaline drainage from an orogenic lode gold deposit, Bralorne mine, British Columbia. Applied Geochemistry. 57:45–54. doi: 10.1016/j.apgeochem.2014.11.015
- Druzbicka J, Craw D. 2013. Evolving metalloid signatures in waters draining from a mined orogenic gold deposit, New Zealand. Applied Geochemistry. 31:251–264. doi: 10.1016/j.apgeochem.2013.01.011
- Druzbicka J, Craw D. 2015. Metalloid attenuation from runoff waters at an historic orogenic gold mine, New Zealand. Mine Water and the Environment. 34:417–429. doi: 10.1007/s10230-014-0316-2
- Dzombak DA, Morel FMM. 1990. Surface complexation modeling: Hydrous Ferric Oxide. New York: John Wiley, 416 pp.
- Elbaz-Poulichet F, Dupuy C, Cruzado A, Velasquez Z, Achterberg EP, Braungardt CB. 2000. Influence of sorption processes by iron oxides and algae fixation on arsenic and phosphate cycle in an acidic estuary (Tinto River, Spain). Water Research. 34:3222–3230. doi: 10.1016/S0043-1354(00)00073-7
- Haffert L, Craw D. 2008a. Processes of attenuation of dissolved arsenic downstream from historic gold mine sites, New Zealand. The Science of the Total Environment. 405:286–300. doi: 10.1016/j.scitotenv.2008.06.058
- Haffert L, Craw D. 2008b. Mineralogical controls on environmental mobility of arsenic from historic mine processing residues, New Zealand. Applied Geochemistry. 23:1467–1483. doi: 10.1016/j.apgeochem.2007.12.030
- Haffert L, Craw D. 2009. Field quantification and characterisation of extreme arsenic concentrations at a historic mine processing site, Waiuta, New Zealand. New Zealand Journal of Geology and Geophysics. 52:261–272. doi: 10.1080/00288300909509890
- Haffert L, Sander SG, Hunter KA, Craw D. 2010a. Evidence for arsenic-driven redox chemistry in a wetland system: a field voltammetric study. Environmental Chemistry. 7:386–397. doi: 10.1071/EN10019
- Haffert L, Craw D, Pope J. 2010b. Climatic and compositional controls on secondary arsenic mineral formation in high-arsenic mine wastes, South Island, New Zealand. New Zealand Journal of Geology and Geophysics. 53:91–101. doi: 10.1080/00288306.2010.498403
- Hamisi J, MacKenzie D, Pitcairn I, Blakemore H, Zack T, Craw D. 2017. Hydrothermal footprint of the birthday reef, Reefton goldfield, New Zealand. New Zealand Journal of Geology and Geophysics. 60:59–72. doi: 10.1080/00288306.2016.1274332
- Hardesty DL. 2001. Issues in preserving toxic wastes as heritage sites. The Public Historian. 23:19–28. doi: 10.1525/tph.2001.23.2.19
- Hewlett L, Craw D, Black A. 2005. Comparison of arsenic and trace metal contents of discharges from adjacent coal and gold mines, Reefton, New Zealand. Marine and Freshwater Research. 56:983–995. doi: 10.1071/MF05018
- Hudson-Edwards KA, Jamieson HE, Lottermoser BG. 2011. Mine wastes: past, present, future. Elements. 7:375–380. doi: 10.2113/gselements.7.6.375
- Hutton GP. 1947. Mineral dressing and gold extraction of Blackwater Mines (Reefton) Ltd. NZ. [PhD thesis]. University of Otago, Dunedin, New Zealand.
- Kerr G, Pope J, Trumm D, Craw D. 2015. Experimental metalloid mobilisation from a New Zealand orogenic gold deposit. Mine Water and the Environment. 34:404–416. doi: 10.1007/s10230-015-0332-x
- Krause E, Ettel VA. 1989. Solubilities and stabilities of ferric arsenate compounds. Hydrometallurgy. 22:311–337. doi: 10.1016/0304-386X(89)90028-5
- Kumpiene J, Lagerkvist A, Maurice C. 2008. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments – A review. Waste Management. 28:215–225. doi: 10.1016/j.wasman.2006.12.012
- Langmuir D, Mahoney J, Rowson J. 2006. Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4.2H2O) and their application to arsenic behaviour in buried mine tailings. Geochimica et Cosmochimica Acta. 70:2942–2956. doi: 10.1016/j.gca.2006.03.006
- Mains D, Craw D, Rufaut CG, Smith CMS. 2006. Phytostabilization of gold mine tailings from New Zealand. part 2: experimental evaluation of arsenic mobilization during revegetation. International Journal of Phytoremediation. 8:163–183. doi: 10.1080/15226510600742559
- Malloch KR, Craw D. 2017. Comparison of contrasting gold mine processing residues in a temperate rain forest, New Zealand. Applied Geochemistry. 84:61–75. doi: 10.1016/j.apgeochem.2017.05.027
- Malloch KR, Craw D, Trumm D. 2017. Arsenic mineralogy and distribution at the historic Alexander gold mine, Reefton goldfield, New Zealand. New Zealand Journal of Geology and Geophysics. 60(2):129–144. doi: 10.1080/00288306.2017.1292924
- Mendez MO, Maier RM. 2008. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environmental Health Perspectives. 116:278–283. doi: 10.1289/ehp.10608
- Mew G, Ross CW. 1994. Soil variation on steep greywacke slopes near Reefton, Western South Island. Journal of the Royal Society of New Zealand. 24:231–242. doi: 10.1080/03014223.1994.9517467
- Milham L, Craw D. 2009. Antimony mobilization through two contrasting gold ore processing systems, New Zealand. Mine Water and the Environment. 28:136–145. doi: 10.1007/s10230-009-0071-y
- Noble C, Webster-Brown J, Brown K. 2002. The source and fate of arsenic in selected West Coast catchments, South Island, New Zealand. Australasian Institute of Mining and Metallurgy NZ Branch Annual Conference Proceedings, Parkville, Victoria, Australia.
- O’Reilly SE, Strawn DG, Sparks DL. 2001. Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Science Society of America Journal. 65:67–77. doi: 10.2136/sssaj2001.65167x
- Paktunc D, Bruggeman K. 2010. Solubility of nanocrystalline scorodite and amorphous ferric arsenate: implications for stabilization of arsenic in mine wastes. Applied Geochemistry. 25:674–683. doi: 10.1016/j.apgeochem.2010.01.021
- Paktunc D, Kingston D, Pratt A, McMullen J. 2006. Distribution of gold in pyrite and in products of its transformation resulting from roasting of refractory gold ore. The Canadian Mineralogist. 44:213–227. doi: 10.2113/gscanmin.44.1.213
- Plumlee GS, Smith KS, Montour MR, Ficklin WH, Mosier EL. 1999. Geologic controls on the composition of natural waters and mine waters draining diverse mineral-deposit types. Reviews in Economic Geology. 6:373–432.
- Potts PJ, Ramsey MH, Carlisle J. 2002. Portable X-ray fluorescence in the characterisation of arsenic contamination associated with industrial buildings at a heritage arsenic works site near Redruth, Cornwall, UK. Journal of Environmental Monitoring. 4:1017–1024. doi: 10.1039/b207259a
- Power MR, Pirrie D, Camm GS, Anderson JCO. 2009. The mineralogy of efflorescence on As calciner buildings in SW England. Mineralogical Magazine. 73:27–42. doi: 10.1180/minmag.2009.073.1.27
- Rait R, Trumm D, Pope J, Craw D, Newman N, MacKenzie H. 2010. Adsorption of arsenic by iron rich precipitates from two coal mine drainage sites on the West Coast of New Zealand. New Zealand Journal of Geology and Geophysics. 53:177–193. doi: 10.1080/00288306.2010.500320
- Rattenbury MS, Stewart M. 2000. Structural setting of the Globe-Progress and Blackwater gold mines, Reefton goldfield, New Zealand. New Zealand Journal of Geology and Geophysics. 43:435–445. doi: 10.1080/00288306.2000.9514900
- Ruttens A, Colpaert J V, Mench M, Boisson J, Carleer R, Vangronsveld J. 2006. Phytostabilization of a metal contaminated sandy soil. II: influence of compost and/or inorganic metal immobilizing soil amendments on metal leaching. Environmental Pollution. 144:533–539. doi: 10.1016/j.envpol.2006.01.021
- Smith E, Naidu R, Alston AM. 2002. Chemistry of inorganic arsenic in soils. Journal of Environment Quality. 31:557–563. doi: 10.2134/jeq2002.5570
