ABSTRACT
Inorganic carbon, nitrogen and phosphorus are the main elements required by seaweeds for photosynthesis and growth. This review focusses mainly on nitrogen, but the roles of carbon and phosphorus, which may interactively affect seaweed physiological processes, are also explored. Fundamental concepts such as limiting nutrients, sources, and ratios, mechanisms of nutrient uptake, nutrient assimilation and storage, patterns of uptake and preferences for different nitrogen sources are discussed. The roles of abiotic (water motion, light, temperature, salinity and desiccation) and biotic (life stages and age class) factors in nutrient (nitrogen, phosphorous, carbon) uptake are also reviewed. Understanding species-specific nitrogen physiologies and nitrogen source preferences will enable polyculture of different seaweed species and the use of seaweeds as biofilters in integrated multitrophic aquaculture systems.
INTRODUCTION
Inorganic carbon, light and nutrients are required for seaweed photosynthesis and growth, and interactively regulate rates of seaweed production. Nitrogen is the element most frequently observed to limit growth, although in some cases phosphorus may be limiting. Moreover, because inorganic carbon (Ci) in seawater occurs primarily as bicarbonate (HCO3−), the inability of some species to use HCO3− as a Ci source may lead to carbon limitation, especially among subtidal and tide pool species. This review will focus on nitrogen nutrition of seaweeds and will also consider phosphorus and carbon physiology which may interactively affect nitrogen uptake and assimilation, and consequently, seaweed photosynthesis and growth. This review is not intended for be comprehensive but to build on the earlier reviews of Harrison & Hurd (Citation2001) and Hurd et al. (Citation2014, ch. 6). Here, we outline fundamental concepts of natural nutrient sources to seaweeds, the mechanisms by which seaweeds take up and assimilate nutrients, and the utility of ‘kinetic curves’ in understanding mechanisms and rates of nutrient uptake. Next, we discuss how nutrient uptake and growth are regulated by abiotic and biotic factors using both classical and contemporary literature examples; the context is that seaweed growth can be enhanced by providing optimal environmental factors such as light, water motion, and nutrient supply. We then explain how concepts might be applied to seaweed polyculture and integrated multitrophic aquaculture (IMTA).
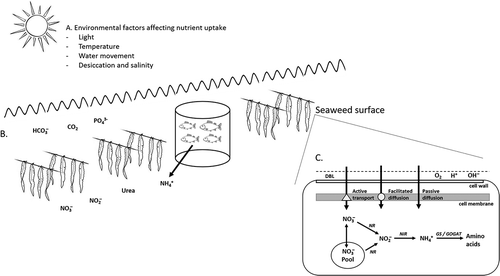
Fig. 1. Schematic of (A) environmental factors regulating uptake nutrient by seaweeds; (B) inorganic carbon, phosphorous and nitrogen sources available in seawater for seaweeds, including the organic form of nitrogen, urea. Ammonium is naturally available in seawater at relatively low concentrations, but levels can be enhanced via excretion from marine animals, including in an IMTA situation (e.g. salmon); (C) for seaweeds to take up nutrients, they must first cross the diffusion boundary layer (DBL) and cell wall. Within the DLB, products released via photosynthesis, respiration and nutrient uptake may accumulate (e.g. O2, H+ and OH−). Once they have crossed the cell wall, nutrients are taken into the cell via active transport, and/or facilitated transport, and/or passive diffusion across the cell membrane. Within the cell, nutrients may be stored in various pools or are assimilated. The example given is for nitrate uptake and assimilation; nitrate may be stored in an inorganic pool or converted to ammonium via the enzymes NR and nitrite reductase (NiR). Unlike nitrate, ammonium storage is limited in seaweeds and it is rapidly converted to amino acids via glutamine synthetase (GS) and glutamate synthase (GOGAT).
LIMITING NUTRIENTS, SOURCES, AND RATIOS
A fundamental understanding of the sources of nutrients (nitrogen, phosphorus, carbon) available to seaweeds and the seaweed’s nutrient requirements for optimal growth is essential to enhance the growth conditions in any production system (Hurd et al. Citation2014). When a seaweed’s demand for a nutrient is greater than its supply, the nutrient becomes ‘limiting’; that is, it limits growth (Harrison & Hurd Citation2001). Liebig’s law of the minimum states that ‘the nutrient available in the smallest quantity with respect to the requirements of the plant will limit its rate of growth’ (see Hurd et al. Citation2014, ch. 6, p. 240): if the limitation by a particular nutrient (e.g. nitrogen) is overcome by increasing the supply, then a different nutrient may become limiting (e.g. phosphorous). There are also interactions among nutrients; for balanced growth, they are required in certain ratios (see below). For example, alleviating nitrogen limitation in Fucus vesiculosus Linnaeus triggered an increase in the uptake of phosphate (PO43−; Perini & Bracken Citation2014).
In natural systems, nitrogen is the nutrient that most commonly limits seaweed growth, with phosphorous being the second most common limiting nutrient. Nitrogen is available in the inorganic forms nitrate (NO3−) and ammonium (NH4+) and the organic form urea. Nitrate-based growth is termed ‘new production’ because NO3− is externally supplied; for example, from below the thermocline or from upwelling (Boyd & Hurd Citation2009). Primary production based on NH4+ and urea is termed ‘recycled production’ because it is internally regenerated within the system by invertebrates and fish associated with the seaweeds (Boyd & Hurd Citation2009; Taylor & Rees Citation1998). The relative preference index can be used to determine the preference of a seaweed for NO3− vs NH4+ vs urea, for example, between different seasons (Phillips & Hurd Citation2003).
Seawater nutrients have different seasonal cycles depending on geographic location, and it is important to have baseline data to understand when a particular nutrient might become limiting for seaweed growth. In many temperate regions, seawater NO3− concentrations in surface waters have a strong seasonal cycle due to seasonal thermoclines. In winter, NO3− concentrations are maximal, ranging from 5 to 20 µM depending on geographic location. With increased light in spring, temperature rises and the thermocline forms, separating the surface mixed layer from the cooler, NO3−-rich waters below. Biological activity – mostly phytoplankton – removes NO3− from surface waters which become NO3− deplete (Boyd & Hurd Citation2009). However, in other systems, nitrate supply is driven by seasonal upwelling events; for example, the California current (Jacox et al. Citation2015). PO43− follows a seasonal pattern similar to NO3−, but concentrations rarely reach ‘zero’; that is, below the detection limit. In summer, recycled production predominates, with seaweeds using NH4+ excreted by zooplankton, invertebrates and fish. In tropical waters, concentrations of seawater NO3− are comparatively low year-round, and the systems are based largely on regenerated NH4+. Tropical systems tend to be dominated by symbiotic corals, and seaweed biomass is naturally low due to higher levels of herbivory (Briggs et al. Citation2018; Lewis Citation1985). However, under eutrophic conditions, seaweed biomass can increase at the expense of corals (Schaffelke Citation1999).
In natural systems, seaweeds can take up a substantial proportion of their nitrogen from invertebrates and fish with which they are associated (Taylor & Rees Citation1998). For example, the relationship between Macrocystis pyrifera (Linnaeus) C.Agardh and hydrozoans is mutualistic: growth rates of M. pyrifera are enhanced by hydrozoans which excrete NH4+ that is taken up by the underlying seaweed (Hepburn & Hurd Citation2005; Hepburn et al. Citation2012). From an aquaculture perspective, however, such ‘fouling’ by invertebrates can be detrimental to offshore production (Buck et al. Citation2017; Stévant et al. Citation2017). Urea is also an important source of nitrogen for seaweeds: in southern New Zealand urea provided c. 30% of nitrogen to four species of intertidal seaweed in summer (Phillips & Hurd Citation2003), and in California, USA, urea represents an important year-round supply of nitrogen to M. pyrifera (Smith et al. Citation2018). Seaweeds can utilise organic phosphorous via the external enzyme alkaline phosphatase, which breaks down organic matter on the seaweed’s surface into PO43−, which is then taken up (Schaffelke Citation2001).
Dissolved inorganic carbon (DIC, such as CO2 and HCO3−) is usually considered in terms of photosynthesis, rather than as a nutrient per se. Because the concentration of HCO3− in seawater is high (2000 µM) relative to dissolved CO2 (14 µM), seaweeds that use HCO3− are unlikely to be limited by Ci supply (Raven et al. 2011). However, around 35% of seaweeds cannot utilise HCO3− (Kübler & Dudgeon Citation2015) and they may be carbon limited under certain conditions, such as high light levels which may cause an increase in carbon requirement (Cornwall et al. Citation2015). In addition, seaweed photosynthesis removes DIC from the surrounding seawater, which modifies the seawater carbonate system such that pH increases and the supply of both CO2 and HCO3− decreases: at pH 9.0 most DIC is available as carbonate which cannot be used in photosynthesis (Björk et al. Citation2004). Therefore, in highly productive systems, carbon may limit growth of some seaweeds, although this will depend on the mechanisms of DIC uptake and their carbon requirements for growth (van der Loos et al. Citation2019).
For balanced growth, seaweeds require essential nutrients (C, N, P) in species-specific ratios (Duarte Citation1992). Using literature values from 46 seaweeds, Duarte (Citation1992) found that the percentage tissue carbon (per unit tissue dry weight) is 10%–50% with a median value of 25%; tissue nitrogen range is 0.2%–4.2% with a median range of 0.6%–2.2%, and phosphorous from 0.1% to 0.5% with a median of 0.1%. The ratios of these elements (C:N:P) are often used to infer nutrient limitation. For example, for the giant kelp M. pyrifera, a C:N of < 20 indicates nitrogen sufficiency; whereas, > 25 indicates nitrogen-limited growth (Hurd et al. Citation1996). However, to confirm whether or not a particular nutrient is limiting requires experimental testing, in which seaweeds are grown at a range of nutrient concentrations and the relationship between percentage N (or percentage P) and growth is determined (i.e. growth kinetics; cf. Hanisak Citation1979; reviewed in Hurd et al. Citation2014).
MECHANISMS OF NUTRIENT UPTAKE
Nutrient uptake kinetics is a useful method to infer the mechanisms of uptake, to determine the uptake rate at a range of concentrations (Hurd et al. Citation2014), and to examine the potential of a seaweed for IMTA (Kang et al. Citation2013). Inorganic nutrients move across the cell membrane via three general mechanisms: (1) passive transport in which nutrients move via passive diffusion down a concentration gradient; (2) facilitated diffusion which is also by transport down an electrochemical gradient but carrier or channel proteins are involved; and (3) active transport against a concentration gradient, which, unlike 1 and 2, requires energy to fuel membrane transport systems; for example, carrier proteins (see Harrison & Hurd Citation2001; Hurd et al. Citation2014). To obtain a ‘kinetic curve’ for a seaweed, uptake rate is measured at a range of nutrient concentrations using either a time course of depletion or multiple flask method: the relative utility of each method is discussed in Harrison & Druehl (Citation1982), Hurd & Dring (Citation1990), and Pedersen (Citation1994).
A plot of nutrient uptake rate against concentration yields various patterns, which can be used to infer the mechanism of uptake. If a linear regression is observed, then uptake is most likely via passive diffusion. If uptake rate follows a hyperbolic curve whereby the rate is linear at low concentrations and then reaches a plateau (termed saturated uptake), then the mechanism is either active transport or facilitated diffusion: it is not possible to categorically determine which of these mechanisms is operating without further mechanistic experiments using inhibitors (e.g. Barr et al. Citation2004). A hyperbolic curve is often called a ‘Michaelis-Menten’ curve from which two ‘kinetic parameters’ are derived: maximum uptake rate (Vmax) and half-saturation constant (Ks). A low Ks value indicates a high affinity for the nutrient at low concentrations, but because its value is dependent on Vmax, other parameters are used to determine uptake abilities at low concentrations: (1) ‘uptake efficiency’, the ratio Vmax/Ks (Perini & Bracken 2016; Smit Citation2002), and (2) ‘initial slope’ (Is, also termed alpha) of a rectangular hyperbola which can be determined directly by plotting a linear regression to the linear part of the Michaelis-Menten curve (Nishihara et al. Citation2005; Smit Citation2002). The advantage of the latter method is that it allows a direct comparison with seaweeds that exhibit linear (i.e. diffusive) uptake. Finally, both passive and saturated uptake systems can occur simultaneously, which is termed ‘biphasic’ or ‘multiphasic’ uptake, and indicates that two or more uptake systems are operating (Rees Citation2014; Taylor et al. Citation1998).
NO3− uptake by macroalgae is thought to be via active transport, although this has been confirmed (using inhibitors) for very few species. NH4+ uptake tends to be via passive diffusion, although for Scytothamnus australis (J.Agardh) J.D.Hooker & Harvey, uptake was linear in summer but showed saturating kinetics in winter. In addition, when very high concentrations of NH4+ were supplied (700 µM) in a time course experiment, saturating uptake kinetics were observed for Undaria pinnatifida (Harvey) Suringar, Ecklonia cava Kjellman, Gracilaria incurvata Okamura, Porphyra yezoensis [= Pyropia yezoensis (Ueda) M.S.Hwang & H.G.Choi] and Ulva compressa Linnaeus (Kang et al. Citation2013): this study illustrates that if concentrations are sufficiently high, NH4+ uptake will become saturated; that is, the supply is greater than the metabolic demand.
For DIC acquisition, CO2 is taken up via passive diffusion which is energetically ‘inexpensive’ compared to HCO3− uptake which is via active transport and requires energy (Raven et al. Citation2011). Mechanisms of HCO3− uptake have been investigated for relatively few species and can include proton pumps, H+-ATPase, direct HCO3− uptake via an anion exchange (AE) protein and external HCO3− dehydration mediated by CAext (Fernández et al. Citation2014). Seaweeds growing in light-limited habitats can increase uptake of CO2 relative to HCO3− as a mechanism of reducing energetic costs (Cornwall et al. Citation2015; Hepburn et al. Citation2011).
NUTRIENT ASSIMILATION AND STORAGE
NO3− uptake and assimilation require energy from the light reactions of photosynthesis (Pritchard et al. Citation2015; Raven et al. Citation1992). NO3− is assimilated to NH4+, which requires the synthesis and maintenance of two enzymes (nitrate and nitrite reductase) and energy from eight electrons (Boyd & Hurd Citation2009). However, NO3− can be stored in its inorganic form in cellular vacuoles. In temperate regions, NO3− storage may occur during winter when both temperature and light levels are low, and the stores are used in spring when light and temperature increase (e.g. Hurd et al. Citation1996). Urea is broken down into NH4+ and CO2 via the enzyme urease (Phillips & Hurd Citation2004). NH4+ is not typically stored in large concentrations within the cell and is rapidly metabolised to amino acids via glutamine and glutamate synthetase (Taylor & Rees Citation1999).
PATTERNS OF UPTAKE AND PREFERENCES FOR DIFFERENT NITROGEN SOURCES
Understanding patterns of uptake and preferences of seaweeds for particular sources of a nutrient are key in optimising growth in aquaculture systems: some species prefer NH4+ over NO3−; whereas, others show no preference and grow equally well on both. Seaweeds may show ‘surge uptake’, a term used to describe a rapid uptake of a nutrient over a relatively short period of time (10–60 min), often following a period of reduced nutrient supply. Surge uptake of Ni has been reported for several species. When NH4+ was the sole nitrogen source for Kappaphycus alvarezii (Doty) Doty ex P.C.Silva, increasing concentrations resulted in surge uptake for the first 30 min: the rate of surge uptake was greater with a higher initial NH4+ concentration (Dy & Yap Citation2001). Similarly, surge NH4+ uptake was reported for Stictosiphonia arbuscula (=Bostrychia arbuscula W.H.Harvey) and Scytothamnus australis (Phillips & Hurd Citation2003). Interestingly, surge NH4+ uptake also occurs when both NO3− and NH4+ are supplied together: NO3− uptake by Laminaria groenlandica [= Saccharina latissima (Linnaeus) C.E.Lane, C.Mayes, Druehl & G.W.Saunders] was completely suppressed in the first 30 min while surge NH4+ uptake occurred (Harrison et al. Citation1986). Thereafter, S. latissima took up both nitrogen sources simultaneously, at similar rates that were also equal to the rates when only NO3− or NH4+ was present in the medium (Harrison et al. Citation1986).
Seaweeds often take up NO3− and NH4+ simultaneously, but rates may be different for each nitrogen source. For example, NH4+ was taken up more rapidly than NO3− in both Hypnea musciformis (Wulfen) J.V.Lamouroux and Macrocystis pyrifera, but at high NH4+ concentrations NO3− uptake was partially inhibited for Hypnea but not by Macrocystis (Haines & Wheeler Citation1978). In contrast, when NO3−, NH4+ and urea were provided simultaneously, four intertidal seaweed species (Bostrychia arbuscula, Apophlaea lyallii J.D.Hooker & Harvey, Scytothamnus australis, and Xiphophora gladiata (Labillardière) Montagne ex Kjellman) were capable of simultaneous uptake of all N forms at different rates (Phillips & Hurd Citation2003).
In phytoplankton and higher plants, NH4+ has a range of interactive effects with other aspects of cellular metabolism; it can inhibit NO3− uptake, stimulate or depress primary production and growth, and, at high concentrations or when supplied as an exclusive source of Ni, cause toxicity (Britto & Kronzucker Citation2002; Collos & Harrison Citation2014). In seaweeds, such interactions are not well understood. For Codium fragile (Suringar) Hariot, the ability of NH4+ to inhibit NO3− uptake depends on the NH4+ concentration: NH4+ concentrations of 1 µM can reduce the uptake of both NO3− and NO2− by 26% and 31%, respectively; whereas, at higher concentrations (10 µM), NH4+ completely inhibited NO3− and NO2− uptake (Hanisak & Harlin Citation1978). For Gracilaria vermiculophylla [= Agarophyton vermiculophyllum (Ohmi) Gurgel, J.N.Norris et Fredericq], in the presence of high concentrations of both NH4+ (150 µM) and NO3− (450 µM), a higher NO3− uptake rate was observed than for NH4+ (Abreu et al. Citation2011a). However, following a single addition of each Ni source at a lower concentration (50 µM), the NH4+ uptake rate was higher than that of NO3−. It is unclear whether the NH4+ supplied at a high concentration facilitated NO3− uptake or caused a reduction in its own uptake as a control mechanism against toxicity (Abreu et al. Citation2011a). Furthermore, the ability of NH4+ to inhibit NO3− uptake appears to be life-stage dependent: the presence of NH4+ inhibited NO3− uptake in mature plants but not in germlings of Fucus distichus Linnaeus (Thomas et al. Citation1985).
Algal growth and biomass production under different Ni forms can be used as a proxy for nitrogen uptake and use efficiency. For example, Fucus spiralis Linnaeus grew similarly on either NH4+ or NO3− (Topinka & Robbins Citation1976); whereas, Gracilaria foliifera (Forsskål) Børgesen and Neoagardhiella baileyi [= Agardhiella subulata (C.Agardh) Kraft & M.J.Wynne] grew faster in NH4+-enriched cultures than in NO3−- or sewage-enriched cultures (DeBoer et al. Citation1978). Higher growth rate and consistently higher biomass yield was also reported in Ulva lactuca Linnaeus fertilised with NH4+ than with NO3− (Ale et al. Citation2011). These examples show species-specific differences in the preference of NH4+ and NO3− and corresponding growth responses.
Research on nitrogen nutrition in seaweeds has focused on inorganic sources NO3− and NH4+, the mechanisms and rates of uptake, and the relative preference of one source over the other. However, some species are able to take up a third inorganic source, nitrite (NO2−); for example, Codium fragile subsp. tomentosoides [= Codium fragile subsp. fragile (Suringar) Hariot], which may be responsible for its competitive advantage over other algae (Hanisak & Harlin Citation1978) and its invasive success. However, NO2− uptake and assimilation by seaweeds has rarely been studied because NO2− is not considered a major form in natural seawater: this view may have hampered our understanding of the relative importance of NO2− as an inorganic source for seaweeds. Moreover, NO2− can be an important component of discharge from some marine animal hatcheries; for example, concentrations of 8 mM were observed in effluent from a shrimp farm in Malaysia (Rabiei et al. Citation2016). Therefore, knowledge of the rates and mechanisms of NO2− uptake are needed for further development of some IMTA systems.
ABIOTIC FACTORS AFFECTING NUTRIENT UPTAKE
Knowledge of seaweed nutrient physiology and the environmental factors affecting nutrient uptake and metabolism is important to evaluate for the enhancement of commercial biomass production. Here, we explore the effects of the key abiotic factors on seaweed nutrient uptake.
Water motion
Water motion is a fundamental driver of nutrient uptake and seaweed productivity because it regulates both the larger scale supply of nutrients and DIC via advection to the seaweed surface and the thickness of the velocity and diffusion boundary layers (DBLs) that form at the seaweed surface (Hurd Citation2017). Nutrients move across the DBL via molecular diffusion; therefore, in slow flows, where thicker boundary layers form, the supply of nutrients may be reduced compared to fast flows, termed ‘mass transfer limitation’ of growth (Hurd Citation2000). Many laboratory studies illustrate how NO3− and NH4+ uptake rates increase with seawater velocity, until a maximum rate is reached (see review by Hurd Citation2017). Simulating wave action can also cause increased growth rates (Barr et al. Citation2008). The velocity at which the uptake rate saturates will depend on the species’ requirement for a nutrient and also on the seaweed’s morphology and growth form. Seaweeds growing in dense beds create a ‘canopy boundary layer’, an additional layer across which nutrients must travel in order to reach the seaweed surface (Hurd Citation2017).
Increasing velocities do not always cause an increase in uptake rate. For the red seaweed Adamsiella chauvinii (Harvey) L.E.Phillips & W.A.Nelson, increasing water motion caused an increase in NH4+ uptake but not NO3− (Kregting et al. Citation2008). In addition, if the concentration of nutrients within the water column is low (e.g. in summer in temperate systems), water velocity may have no effect on growth rate. For example, growth rates of the giant kelp M. pyrifera were enhanced by water velocity in autumn, at which time the seaweeds were nitrogen limited and the supply of nitrogen in the water column was relatively high. However, water motion had no effect on growth in the other seasons. In winter, light was the factor limiting growth; in spring the seaweeds were nitrogen sufficient, and thus, increasing N supply had no effect on growth, and in summer there was no Ni in the seawater (Hepburn et al. Citation2007; Hurd Citation2017).
In general, in both onshore and offshore seaweed cultivation systems, optimal seawater flow is beneficial for maximal nutrient and DIC supply; that is, DBLs are as thin as possible. For example, line cultivation of Undaria pinnatifida in Galicia, northwest Spain, yielded higher biomass when grown in a moderately exposed compared to a wave-sheltered site (Peteiro & Freire Citation2011). Water motion also generates drag forces on seaweed thalli, which may enhance productivity via increased DIC uptake (Kraemer & Chapman Citation1991), but too much water motion damages thalli and can rip seaweeds off the substratum (Kawamata Citation2001).
Light
Seaweeds are exposed to a diurnally variable and dynamic light regime which drives photosynthesis and growth; likewise, light intensity affects nutrient uptake. Consequently, under suboptimal light levels, addition of nutrients will have minimal effect on growth rate. Diurnal regulation in Ni uptake by phototrophs has significance in terms of the overall energy budget, for example, the amount of energy that can be allocated to growth. It is generally considered that nitrogen incorporation during the day is energetically less expensive than at night. This is because during daylight, the energy and carbon necessary for the assimilation process are provided directly by photosynthesis; whereas, in the dark, accumulated carbohydrates are the energy source (Huppe & Turpin Citation1994; Turpin Citation1991). Several studies have revealed higher daytime uptake rates of NH4+ and NO3− compared to nighttime. For example, in Laminaria longicruris [= Saccharina longicruris (Bachelot de la Pylaie) Kuntze], NO3− uptake in the dark is 20%–40% lower than that in light (Harlin & Craigie Citation1978). In Hypnea musciformis, nighttime uptake of NO3− and NH4+ is also reduced by 41% and 64%, respectively, than under light (Haines & Wheeler Citation1978). The same light response has been reported in other brown and red seaweeds (e.g. Gordillo et al. Citation2002; Harrison et al. Citation1986; Pereira et al. Citation2008). Moreover, NO3− (and PO43−) uptake rates increase with increasing daylength (Gordillo et al. Citation2002). Conversely, in F. spiralis, light has no effect on uptake of either NH4+ or NO3−, but it did stimulate NO2− uptake (Topinka Citation1978). The effect of light on Ni transport is likely related to active transport which is an energy-related process, as opposed to passive diffusion, which does not require a light-dependent energy source.
It is more energetically expensive to incorporate NO3− than NH4+ because NO3− must be reduced to NH4+ (see above; Gordillo Citation2012). The strong preference for NH4+ and restricted use of NO3− in the slow-growing red macroalga Anotrichium crinitum (Kützing) Baldock inhabiting low-light habitats indicates the direct contribution of photosynthesis in providing energy for nutrient assimilation (Pritchard et al. Citation2015). Similarly, diffusive uptake of CO2 by seaweeds increases relative to active uptake of HCO3− in low-light environments, and this, too, is considered a mechanism for reducing energetic costs when light is a limiting factor (Cornwall et al. Citation2015; Hepburn et al. Citation2011).
Temperature
Temperature affects all aspects of seaweed physiology through its regulation of enzyme activity, rate constants of chemical reactions, and the rate of diffusion of nutrients across boundary layers. For nutrients that are taken up by active transport, temperature is likely to affect rates because it will affect the activity of membrane transporters; whereas, there may be less effect on uptake via passive diffusion. For example, NO3− uptake by Laurencia brongniartii J.Agardh was higher at higher temperatures; whereas, NH4+ uptake was unaffected by temperature, probably because uptake is by passive diffusion (Nishihara et al. Citation2005). In temperate regions, seasonal patterns are also observed in uptake rates likely to be driven by monthly temperature changes. For summer-adapted S. longicruris, maximum NO3− uptake was observed at 15 °C, and at lower temperatures – 10, 5 and 0 °C – uptake was 13%, 34%, and 70% lower, respectively respectively, than at 15 °C (Harlin & Craigie Citation1978). Seasonal variation in nutrient uptake is tightly coupled to temperature acclimation. For example, nutrient uptake of winter-adapted (10 °C) S. longicruris was 27% lower than that of summer adapted (15 °C) tissue; however, uptake at 10 °C was 32% higher in winter-adapted sporophytes than summer-adapted sporophytes exposed to winter temperature; that is, 10 °C (Harlin & Craigie 1976). An exception is the red seaweed A. chauvinii, for which uptake rates of NO3− and NH4+ were lower in summer than in winter (Kregting et al. Citation2008).
Cellular metabolism is reduced at temperatures above and below the optimum range, which can affect nutrient uptake. Dose–response curves, whereby seaweeds are grown at a range of temperatures and their nutrient uptake rates measured, have rarely been studied for seaweeds. C. fragile exposed to five different temperatures (6, 12, 18, 24 and 30 °C) showed different optimum temperature ranges for different Ni sources. An optimal temperature range for Vmax for NO3− was 18–24 °C; whereas, the optimal temperature range for NO2− and NH4+ was 12–24 °C (Hanisak & Harlin Citation1978). At a lower temperature (15 °C), Gracilaria gracilis (Stackhouse) Steentoft, L.M.Irvine & Farnham also has a higher affinity for NH4+ than for NO3− than at 20 °C (Smit Citation2002). The efficient NH4+ uptake at lower temperatures may also be related to its relative importance as the primary N source in winter for some cold-temperate seaweed species (Phillips & Hurd Citation2003). The seasonal preference for specific nutrient sources of four intertidal seaweeds from New Zealand was NH4+ > NO3− > urea during winter and NH4+ = NO3− > urea in summer (Phillips & Hurd Citation2003). The above examples support the theory that assimilating NO3− is energetically expensive; as such, its metabolism is favoured at seasonally higher temperatures and in a saturating light environment.
Carbon dioxide
Because both carbon and nitrogen are required for balanced growth, the concentration of DIC in seawater can affect seaweed nitrogen uptake. For example, in Hizikia fusiformis [= Sargassum fusiforme (Harvey) Setchell], NO3− uptake was higher in CO2-enriched culture than ambient CO2 (Zou Citation2005). Moreover, the higher CO2 concentration also enhanced nitrate reductase (NR) activity during the light period. Greater maximum NR activity, higher affinity for NO3−, and a higher Vmax/Km ratio were observed in high CO2-grown thalli than in ambient CO2-grown thalli, indicating efficient enzyme activity under high CO2 (Zou Citation2005).
In M. pyrifera, uptake rates of both NO3− and NH4+ were higher under higher CO2 concentrations when seawater was enriched with NH4+ compared to NO3− (Fernández et al. Citation2017a). Moreover, irrespective of the seaweed’s initial N status, NO3− uptake rates and NR activity increased under higher CO2 but there was no enhancement of photosynthesis rates and growth (Fernández et al. Citation2017b). This suggests that higher [H+]/reduced pH under higher CO2 concentrations plays a role in regulating N metabolism. Similarly, enhanced NR activity was observed in Ulva rigida C.Agardh when it was grown under a combination of high CO2 and NO3− concentrations; however, when grown under low NO3−, NR activity was reduced regardless of CO2 concentration (Gordillo et al. Citation2001). This suggests that the effects of CO2 on N metabolism of U. rigida are likely associated with de novo synthesis of NR rather than with changes in C metabolism. Together with higher CO2, optimum light and temperature may also play important roles in translating higher NO3− uptake and NR activity into higher growth rates.
Salinity
Freshwater input from rainfall and rivers to coastal areas can bring nutrients from agricultural activities, thereby increasing nutrient levels in the sea. Seasonal and/or recurrent exposure to nutrient-rich, low-salinity water can potentially affect seaweed farm sites and corresponding biomass production.
Very few studies have investigated the effect of salinity on nutrient uptake. In the tropical Kappaphycus alvarezii, NO3− uptake was not affected under different salinities (20, 25, 30, 35, 40) but temperature seems to have a synergistic effect on uptake rate: at lower salinities (20 and 25; nutrient uptake was highest at 20 °C; whereas, at higher salinities, uptake was higher at 30 °C; Mandal et al. Citation2015).
When cold-temperate Saccharina latissima and Laminaria digitata (Hudson) J.V.Lamouroux were cultivated in nutrient-enriched (NO3− and PO43−) brackish water (salinity 18), total tissue N (as a proxy for nutrient uptake) increased by 47% and 33%, respectively, compared to the same species that were grown in nutrient-sufficient seawater (salinity 34) from a deep fjord (Mortensen Citation2017). Although NO3− concentrations between brackish and full-salinity seawater were not comparable, the study showed that both species are able to survive for up to 13 days under lower salinity when nutrient concentrations are elevated. Reducing seawater salinity to 50% of ambient increased NO3− uptake in Fucus serratus Linnaeus by c. 40%, which resulted in a 20% higher growth rate than 100% seawater (Gordillo et al. Citation2002). Moreover, Enteromorpha intestinalis (= Ulva intestinalis Linnaeus), which thrives in nutrient-rich estuaries, rapidly takes up available nutrients under low salinity for growth and short-term osmoregulation (Cohen & Fong Citation2004). This mechanism is thought to be responsible for the outbreak of opportunistic algal blooms – for example, Gracilaria tenuistipitata [= Agarophyton tenuistipitatum (C.F.Chang et B.-M.Xia) Gurgel, J.N.Norris & Fredericq] – in brackish waters (Wang et al. Citation2014). The few studies available suggest that interactive effects of high nutrient concentrations in seawater and low salinity may compensate for any independent negative effects of low salinity. Further studies are required to test this hypothesis.
Desiccation
When intertidal seaweeds are exposed to air, they are removed from their sources of nitrogen and phosphorous (Hurd & Dring Citation1990; Thomas et al. Citation1987a) but they are still able to acquire DIC as CO2. Seaweeds growing higher in the intertidal zone – for example, Porphyra, Ulva and Fucus spp. – are adapted to desiccation. Upon resubmergence into seawater containing nutrients, an increase in nitrogen (NO3− and NH4+) uptake rates has been reported in several species, including Gigartina papillata [= Mastocarpus papillatus (C.Agardh) Kützing], Gracilaria pacifica I.A.Abbott, Ulva intestinalis, Fucus distichus, and Pelvetiopsis limitata (Setchell) N.L.Gardner (Thomas et al. Citation1987a, Citation1987b). Similar patterns have been observed for PO43− uptake with high-shore F. spiralis showing a surge uptake of PO43− (Hurd & Dring Citation1991). Relative to vertical distribution pattern and emersion duration, Bostrychia arbuscula inhabiting the upper intertidalzone is competitively superior in N uptake than the same species and other species inhabiting the mid- to lower intertidalzone (Phillips & Hurd Citation2003, Citation2004). Mild (< 30%) desiccation enhances photosynthesis, because fluxes of CO2 in air are 10,000 times greater than those in seawater (Bell Citation1993; Dring & Brown Citation1982; Madsen & Maberly Citation1990; Oates Citation1985). However, greater levels of desiccation are typically detrimental to photosynthesis. Seaweeds that are desiccation tolerant have various cellular mechanisms for rapidly repairing cellular membranes and metabolic processes upon rehydration (Burritt et al. Citation2002; Im et al. Citation2017; Kim et al. Citation2009, Citation2013; Kumar et al. Citation2011).
Farming methods for commercially valuable intertidal seaweeds have taken advantage of their desiccation tolerance to reduce the incidence of disease and growth of competing algal species (Blouin et al. Citation2007; Food and Agriculture Organization Citation2005). For the cultivation of nori – that is, Porphyra and Pyropia spp. – fixed net cultivation systems (also known as ‘pole systems’) in the intertidal are often preferred over floating or semifloating cultivation systems in deep water, because they ensure periodic exposure of the seaweed to air (Pereira & Yarish Citation2010; Tseng Citation1981).
BIOLOGICAL FACTORS AFFECTING NUTRIENT UPTAKE
Life stages and age class
Different age classes of perennial seaweeds exhibit different nitrogen uptake kinetics; however, there has been no systematic study on nutrient requirements, kinetics, and metabolism in early life history stages of seaweeds. Among the few available studies, NO3− and NH4+ uptake rates of germlings of Fucus distichus were higher than those of mature thalli (20 to 40 times for NO3− and 8 times for NH4+; Thomas et al. Citation1985). Germlings are physiologically similar to sectional meristematic tissue – for example, apical fronds of Fucus and basal lamina of Saccharina – and because they are actively growing they have correspondingly higher nutrient requirements. In contrast, older fronds/lamina and stipes of mature seaweeds have a lower nutrient uptake rate which reflects their relatively low physiological activity and low N demands for maintenance of non- or slow-growing parts (Topinka Citation1978).
Comparison between different age classes of kelp sporophytes showed that first-year S. latissima sporophytes are able to take up twice as much Ni than older age classes (Harrison et al. Citation1986). Moreover, first-year kelps showed diel periodicity – that is, light:dark response in NO3− and NH4+ uptake – whereas, second- and third-year sporophytes did not (Harrison et al. Citation1986). Consequently, sporophytes with higher nutrient uptake and higher tissue N can contain higher protein (e.g. Mortensen Citation2017). Changes in total protein content and amino acid composition have implications for industrial applications of wild and cultivated biomass. Furthermore, for bioremediation initiatives, young and physiologically active plants will be more efficient than adult plants in removing excess nutrients.
To date, no comparative study has looked into the nutrient physiology of different life history stages (e.g. spores, gametophytes, sporophytes) of kelps, which are cultivated primarily for food and other industrial applications. Despite this shortfall, a study showed that NO3− enrichment during indoor cultivation of rope-seeded U. pinnatifida gametophytes until juvenile sporophytes developed produced larger sporophytes after 2- to 3-month outgrowth in the field than the control group (Gao et al. Citation2013). This suggests that growth and biomass production in adult sporophytes are enhanced when the early life history stages are nitrogen replete.
APPLICATION TO SEAWEED CULTIVATION
The sections above describe fundamental information on seaweed nutrient uptake and its regulation by abiotic and biotic factors. Here, we apply this knowledge to the production of commercial seaweed products other than biomass (e.g. polysaccharides) and in aquaculture systems.
Effect of tissue nitrogen status on commercial products
For agarophytes and carrageenophytes, the flow of photosynthate into various end products (e.g. carbohydrate and protein) is dependent on tissue nitrogen status (Bird Citation1988; Bird et al. Citation1981; Chopin et al. Citation1990, Citation1995; Macler Citation1986). Nitrogen fertilisation increases N-based tissue compounds (e.g. amino acids, phycobiliprotein and chlorophyll), thereby increasing photosynthetic activity and growth but reducing phycocolloid content (e.g. agar and carrageenan). Conversely, nitrogen limitation reduces photosynthesis and growth and induces an altered C allocation towards N-free macromolecules; for example, storage of carbohydrates and/or lipids (Bird et al. Citation1981; Li et al. Citation1990; Macler Citation1986; Roleda et al. Citation2013). The partitioning to different carbohydrates in Gelidium coulteri Harvey was also affected by tissue nitrogen status, where N-enriched algae had higher floridoside levels and significantly lower amounts of agar and starch than found in N-limited plants (Macler Citation1986). Therefore, optimising the yield of valuable products requires knowledge of the interactions between nitrogen, phosphorous and carbon metabolism.
Seaweed polyculture: Complementary use, competition and facilitation
Co-cultivation of different seaweed species with different nitrogen physiologies – that is, those preferring NH4+ and those preferring NO3− – may be beneficial because niche partitioning can reduce competition (Bracken & Stachowicz Citation2006). For example, when eight species of intertidal seaweeds [five reds: Mastocarpus papillatus, Mazzaella flaccida (Setchell & N.L.Gardner) Fredericq, Microcladia borealis Ruprecht, Porphyra perforata [= Pyropia perforata (J.Agardh) S.C.Lindstrom], and Prionitis lanceolata (Harvey) Harvey; one brown: Fucus gardneri (= Fucus distichus Linnaeus); and two greens: Cladophora columbiana Collins and Ulva taeniata (Setchell) Setchell & N.L.Gardner)] were cultivated in mono- and polycultures in the presence of both NO3− and NH4+, uptake by the diverse assemblages was 22% greater than that in the monoculture (Bracken & Stachowic Citation2006).
The choice of seaweed species in a polyculture should consider their complementary use of different nitrogen forms because some species – for example, Porphyra and Ulva – may not be suitable for co-cultivation with other species because both have high N demand and, consequently, they perform best in monoculture (Bracken & Stachowicz Citation2006). Ulva linza Linnaeus was also observed to outcompete Gracilaria lemaneiformis [= Gracilariopsis lemaneiformis (Bory de Saint-Vincent) E.Y.Dawson, Acleto & Foldvik] when grown together due to its fast nutrient uptake and also through allelopathy (Gao et al. Citation2014). Co-cultivation of Ulva species with different morphologies – that is, sheet-like and tubular – may result in competitive exclusion. For example, U. intestinalis can outcompete Ulva expansa (Setchell) Setchell & N.L.Gardner for nutrients and negatively affect growth rate of the latter (Fong et al. Citation1996). Moreover, the release or leaking of dissolve organic nitrogen (DON) from U. expansa was readily available for assimilation by U. intestinalis, which facilitated growth and dominance of the latter species (Fong et al. Citation1996).
Integrated multitrophic aquaculture: Seaweed as biofilters
Among the different environmental concerns related to intensive fish farming, such as the effects of farmed fish escapees on wild populations, heavy metal leaching from fish cages, and effects of unregulated use of antibiotics, coastal eutrophication is a primary concern. Decomposition of excessive fish feeds and animal excretion can increase dissolved nutrients, primarily nitrogen, into the water column, which can lead to harmful algal blooms and deterioration of coastal environments (e.g. Buschmann et al. Citation2008a; Chopin et al. 2001; Domingues et al. Citation2015). IMTA is a farming method that aims to mitigate the impact of eutrophication associated with fish farming and enhance the sustainability of aquaculture by driving ecological efficiency, environmental acceptability, product diversity, profitability and societal benefit (Kleitou et al. Citation2018). IMTA refers to the integrated farming of several organisms from different trophic levels, where one species complements another. For example, in an IMTA system where seaweed is cultivated in close proximity to fish, the seaweed serves as a biofilter, assimilating excess nutrients from the fish farm and converting them into valuable biomass (; Fernández et al. Citation2019).
IMTA can be established in land-based fish farms where nutrient-rich seawater from fish tanks is supplied to separate tanks where seaweed is either cultivated (e.g. Abreu et al. Citation2011b; Corey et al. Citation2014; Domingues et al. Citation2015) or seeded on longlines and deployed proximate to fish and mussel farms (e.g. Buschmann et al. Citation2008b; Marinho et al. Citation2015). Species with efficient nutrient uptake/removal capacity – for example, Gracilaria spp., Macrocystis pyrifera and Saccharina latissima – have proved suitable for reducing excess nitrogen in fish farms (Abreu et al. Citation2011b; Buschmann et al. Citation2008b; Marinho et al. Citation2015).
Light and temperature are the primary environmental factors that affect the capacity of seaweeds to remove nutrients, which, consequently, control seaweed growth and productivity. For example, in a land-based IMTA system, Agarophyton vermiculophyllum is more efficient in removing nitrogen from fish farms in northern Portugal from spring to summer (April–August) and less efficient during winter (Abreu et al. Citation2011b). Conversely, integration of Palmaria palmata (Linnaeus) F.Weber & D.Mohr with Atlantic halibut in Nova Scotia for nitrogen removal is feasible below 10 °C, but not in summer due to the seasonal life cycle of P. palmata, when reproductive maturation leads to thallus disintegration (Corey et al. Citation2014). At higher temperatures (17–21 °C) in northern Portugal, both P. palmata and Chondrus crispus Stackhouse are able to remove approximately seven times more nitrogen in a cascade IMTA system (Matos et al. Citation2006) than that reported in Nova Scotia (Corey et al. Citation2014). The success of an IMTA system is dependent not only on the biology of the seaweed species and its stocking density but also on several environmental factors controlling nutrient uptake and assimilation.
In summary, knowledge of a seaweed’s nutritional requirements and the regulation of nutrient uptake and assimilation by abiotic and biotic factors are key to bringing new species into aquaculture. In addition to the economic value of seaweeds, seaweed farming can have positive environmental impacts because it makes use of nutrient emissions from fish farms and other anthropogenic nitrogen and phosphorus sources that enter the ocean. Seaweed aquaculture can also take up anthropogenic carbon dioxide emissions that cause ocean acidification. Therefore, sustainable seaweed farming is good not only for the economy but also for the health of the ocean.
Acknowledgement
We dedicate this article to Professor Paul J. Harrison, a gifted algal nutrient physiologist and inspiring mentor.
References
- Abreu M.H., Pereira R., Buschmann A.H., Sousa-Pinto I. & Yarish C. 2011a. Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. Journal of Experimental Marine Biology and Ecology 407: 190–199. DOI: 10.1016/j.jembe.2011.06.034.
- Abreu M.H., Pereira R., Yarish C., Buschmann A.H. & Sousa-Pinto I. 2011b. IMTA with Gracilaria vermiculophylla: productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312: 77–87. DOI: 10.1016/j.aquaculture.2010.12.036.
- Ale M.T., Mikkelsen J.D. & Meyer A.S. 2011. Differential growth response of Ulva lactuca to ammonium and nitrate assimilation. Journal of Applied Phycology 23: 345–351. DOI: 10.1007/s10811-010-9546-2.
- Barr N.G., Kloeppel A., Rees T.A.V., Scherer C., Taylor R.B. & Wenzel A. 2008. Wave surge increases rates of growth and nutrient uptake in the green seaweed Ulva pertusa maintained at low bulk flow velocities. Aquatic Biology 3: 179–186. DOI: 10.3354/ab00079.
- Barr N.G., Tijsen R.J. & Rees T.A.V. 2004. Contrasting effects of methionine sulfoximine on uptake and assimilation of ammonium in Ulva intestinalis (Chlorophyceae). Journal of Phycology 40: 697–704. DOI: 10.1111/j.1529-8817.2004.04004.x.
- Bell E.C. 1993. Photosynthetic response to temperature and desiccation of the intertidal alga Mastocarpus papillatus. Marine Biology 117: 337–346. DOI: 10.1007/BF00345679.
- Bird K.T. 1988. Agar production and quality from Gracilaria sp. strain G-16: effects of environmental factors. Botanica Marina 31: 33–39. DOI: 10.1515/botm.1988.31.1.33.
- Bird K.T., Hanisak M.D. & Ryther J. 1981. Chemical quality and production of agars extracted from Gracilaria tikvahiae grown in different nitrogen enrichment conditions. Botanica Marina 24: 441–444. DOI: 10.1515/botm.1981.24.8.441.
- Björk M., Axelsson L. & Beer S. 2004. Why is Ulva intestinalis the only macroalga inhabiting isolated rockpools along the Swedish Atlantic coast? Marine Ecology Progress Series 284: 109–116. DOI: 10.3354/meps284109.
- Blouin N., Xiugeng F., Peng J., Yarish C. & Brawley S.H. 2007. Seeding nets with neutral spores of the red alga Porphyra umbilicalis (L.) Kützing for use in integrated multi-trophic aquaculture (IMTA). Aquaculture 270: 77–91. DOI: 10.1016/j.aquaculture.2007.03.002.
- Boyd P.W. & Hurd C.L. 2009. Ocean Nutrients. In: Surface ocean:lower atmosphere processes (Ed. by C. Le Quéré & E.S. Saltzman), pp. 36–97. American Geophysical Union, Washington D.C., USA.
- Bracken M.E.S. & Stachowic J.J. 2006. Seaweed diversity enhances nitrogen uptake via complementary use of nitrate and ammonium. Ecology 87: 2397–2403. DOI: 10.1890/0012-9658(2006)87[2397:SDENUV]2.0.CO;2.
- Briggs C.J., Adam T.C., Holbrook S.J. & Schmitt R.J. 2018. Macroalgae size refuge from herbivory promotes alternative stable states on coral reefs. PLoS ONE 13(9): e0202273. DOI: 10.1371/journal.pone.0202273.
- Britto D.T. & Kronzucker H.J. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159: 567–584. DOI: 10.1078/0176-1617-0774.
- Buck B.H., Nevejan N., Wille M., Chambers M.D. & Chopin T. 2017. Offshore and multi-use aquaculture with extractive species: seaweeds and bivalves. In: Aquaculture perspective of multi-use sites in the open ocean (Ed. by B. Buck & R. Langan), pp. 23–69. Springer, Cham, Switzerland.
- Burritt D.J., Larkindale J. & Hurd C.L. 2002. Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 215: 829–838. DOI: 10.1007/s00425-002-0805-6.
- Buschmann A.H., Hernández-González M.C., Aranda C., Chopin T., Neori A., Halling C. & Troell M. 2008a. Mariculture waste management. Ecological Engineering 3: 2211–2217.
- Buschmann A.H., Varela D.A., Hernández-González M.C. & Huovinen P. 2008b. Opportunities and challenges for the development of an integrated seaweed-based aquaculture activity in Chile: determining the physiological capabilities of Macrocystis and Gracilaria as biofilters. Journal of Applied Phycology 20: 571–577. DOI: 10.1007/s10811-007-9297-x.
- Chopin T., Buschmann A.H., Halling C., Troell M., Kautsky N., Neori A., Kraemer G.P., Zertuche-Gonzalez J.A., Yarish C. & Neefus C. 2001. Integrating seaweeds into marine aquaculture systems: a key toward sustainability. Journal of Phycology 37: 975–986. DOI: 10.1046/j.1529-8817.2001.01137.x.
- Chopin T., Gallant T. & Davison I. 1995. Phosphorus and nitrogen nutrition in Chondrus crispus (Rhodophyta): effects on total phosphorus and nitrogen content, carrageenan production, and photosynthetic pigments and metabolism. Journal of Phycology 31: 283–293. DOI: 10.1111/j.0022-3646.1995.00283.x.
- Chopin T., Hanisak M.D., Koehn F.E., Mollion J. & Moreau S. 1990. Studies on carrageenans and effects of seawater phosphorus concentration on carrageenan content and growth of Agardhiella subulata (C. Agardh) Kraft and Wynne (Rhodophyceae, Solieriaceae). Journal of Applied Phycology 2: 3–16. DOI: 10.1007/BF02179764.
- Cohen R.A. & Fong P. 2004. Physiological responses of a bloom-forming green macroalga to short-term change in salinity, nutrients, and light help explain its ecological success. Estuaries 27: 209–216. DOI: 10.1007/BF02803378.
- Collos Y. & Harrison P.J. 2014. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Marine Pollution Bulletin 80: 8–23. DOI: 10.1016/j.marpolbul.2014.01.006.
- Corey P., Kim J.K., Duston J. & Garbary D.J. 2014. Growth and nutrient uptake by Palmaria palmata integrated with Atlantic halibut in a land-based aquaculture system. Algae 29: 35–45. DOI: 10.4490/algae.2014.29.1.035.
- Cornwall C.E., Revill A.T. & Hurd C.L. 2015. High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynthesis Research 124: 181–190. DOI: 10.1007/s11120-015-0114-0.
- DeBoer J.A., Guigli H.J., Israel T.L. & D’Elia C.F. 1978. Nutritional studies of two red algae. I. Growth rate as a function of nitrogen source and concentration. Journal of Phycology 14: 262–266. DOI: 10.1111/j.1529-8817.1978.tb00296.x.
- Domingues B., Abreue M.H. & Sousa-Pinto I. 2015. On the bioremediation efficiency of Mastocarpus stellatus (Stackhouse) Guiry, in an integrated multi-trophic aquaculture system. Journal of Applied Phycology 27: 1289–1295. DOI: 10.1007/s10811-014-0414-3.
- Dring M.J. & Brown F.A. 1982. Photosynthesis of intertidal brown algae during and after periods of emersion– A renewed search for phycological causes of zonation. Marine Ecology Progress Series 8: 301–308. DOI: 10.3354/meps008301.
- Duarte C.M. 1992. Nutrient concentration of aquatic plants: patterns across species. Limnology and Oceanography 37: 882–889. DOI: 10.4319/lo.1992.37.4.0882.
- Dy D.T. & Yap H.T. 2001. Surge ammonium uptake of the cultured seaweed, Kappaphycus alvarezii (Doty) Doty (Rhodophyta: gigartinales). Journal of Experimental Marine Biology and Ecology 265: 89–100. DOI: 10.1016/S0022-0981(01)00325-2.
- Fernández P.A., Hurd C.L. & Roleda M.Y. 2014. Bicarbonate uptake via an anion exchange protein is the main mechanism of inorganic carbon acquisition by the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) under variable pH. Journal of Phycology 50: 998–1008. DOI: 10.1111/jpy.12247.
- Fernández P.A., Leal P.P., & Henríquez L.A. 2019. Co-culture in marine farms: macroalgae can act as chemical refuge for shell-forming molluscs under an ocean acidifi cation scenario. Phycologia 58: 542–551. DOI: 10.1080/00318884.2019.1628576.
- Fernández P.A., Roleda M.Y., Leal P.P., Hepburn C.D. & Hurd C.L. 2017b. Tissue nitrogen status does not alter the physiological responses of Macrocystis pyrifera to ocean acidification. Marine Biology 164: 177. DOI: 10.1007/s00227-017-3204-z.
- Fernández P.A., Roleda M.Y., Leal P.P. & Hurd C.L. 2017a. Seawater pH, and not inorganic nitrogen source, affects pH at the blade surface of Macrocystis pyrifera: implications for responses of the giant kelp to future oceanic conditions. Physiologia Plantarum 159: 107–119. DOI: 10.1111/ppl.2017.159.issue-1.
- Fong P., Boyer K.E., Desmond J.S. & Zedler J.B. 1996. Salinity stress, nitrogen competition, and facilitation: what controls seasonal succession of two opportunistic green macroalgae? Journal of Experimental Marine Biology and Ecology 206: 203–221. DOI: 10.1016/S0022-0981(96)02630-5.
- Food and Agriculture Organization. 2005. Cultured Aquatic Species Information Programme. Porphyra spp. In: FAO fisheries and aquaculture department [online] (Ed. by J. Chen & P. Xu), Rome, Italy. Updated 18 February 2005. http://www.fao.org/fishery/culturedspecies/Porphyra_spp/en; searched on 08 June 2018.
- Gao X., Agatsuma Y. & Taniguchi K. 2013. Effect of nitrate fertilization of gametophytes of the kelp Undaria pinnatifida on growth and maturation of the sporophytes cultivated in Matsushima Bay, northern Honshu, Japan. Aquaculture International 21: 53–64. DOI: 10.1007/s10499-012-9533-5.
- Gao Z., Xu D., Meng C., Zhang X., Wang Y., Li D., Zhou J., Zhuang Z. & Ye N. 2014. The green tide-forming macroalga Ulva linza outcompetes the red macroalga Gracilaria lemaneiformis via allelopathy and fast nutrients uptake. Aquatic Ecology 48: 53–62. DOI: 10.1007/s10452-013-9465-9.
- Gordillo F.J.L. 2012. Environment and algal nutrition. In: Seaweed biology: novel insights into ecophysiology, ecology and utilization (Ed. by C. Wiencke & K. Bischof), pp. 67–86. Springer, Heidelberg, Germany.
- Gordillo F.J.L., Dring M.J. & Savidge G. 2002. Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity. Marine Ecology Progress Series 234: 111–118. DOI: 10.3354/meps234111.
- Gordillo F.J.L., Niell F.X. & Figueroa F.L. 2001. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213: 64–70. DOI: 10.1007/s004250000468.
- Haines K.C. & Wheeler P.A. 1978. Ammonium and nitrate uptake by the marine macrophytes Hypnea musciformis (Rhodophyta) and Macrocystis pyrifera (Phaeophyta). Journal of Phycology 14: 319–324. DOI: 10.1111/j.1529-8817.1978.tb00305.x.
- Hanisak M.D. 1979. Nitrogen limitation of Codium fragile subsp. tomentosoides as determined by tissue analysis. Marine Biology 50: 333–337. DOI: 10.1007/BF00387010.
- Hanisak M.D. & Harlin M.M. 1978. Uptake of inorganic nitrogen by Codium fragile subsp. tomentosoides (Chlorophyta). Journal of Phycology 14: 450–454. DOI: 10.1111/j.1529-8817.1978.tb02467.x.
- Harlin M.M. & Craigie J.S. 1978. Nitrate uptake by Laminaria longricruris (Phaeophyceae). Journal of Phycology 14: 464–467. DOI: 10.1111/j.1529-8817.1978.tb02470.x.
- Harrison P.J. & Druehl L.D. 1982. Nutrient uptake and growth in the Laminariales and other macrophytes: a consideration of methods. In: Synthetic and degradative processes in marine macrophytes (Ed. by L.M. Srivastava), pp. 99–120. Walter de Gruyter, Berlin, New York, USA.
- Harrison P.J., Druehl L.D., Lloyd K.E. & Thompson P.A. 1986. Nitrogen uptake kinetics in three year-classes of Laminaria groenlandica (Laminariales: phaeophyta). Marine Biology 93: 29–35. DOI: 10.1007/BF00428652.
- Harrison P.J. & Hurd C.L. 2001. Nutrient physiology of seaweeds: application of concepts to aquaculture. Cahiers de Biologie Marine 42: 71–82.
- Hepburn C.D., Frew R.D. & Hurd C.L. 2012. Uptake and transport of nitrogen derived from sessile epifauna in the giant kelp Macrocystis pyrifera. Aquatic Biology 14: 121–128. DOI: 10.3354/ab00382.
- Hepburn C.D, Holborow J.D, Wing S.R, Frew R.D, & Hurd C.L. 2007. Exposure to waves enhances the growth rate and nitrogen status of the giant kelp Macrocystis pyrifera. Marine Ecology Progress Series 339: 99–108. DOI: 10.3354/meps339099.
- Hepburn C.D. & Hurd C.L. 2005. Conditional mutualism between giant kelp Macrocystis pyrifera and colonial epifauna. Marine Ecology Progress Series 302: 37–48. DOI: 10.3354/meps302037.
- Hepburn C.D., Pritchard D.W., Cornwall C.E., McLeod R.J., Beardall J., Raven J.A. & Hurd C.L. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biology 17: 2488–2497. DOI: 10.1111/j.1365-2486.2011.02411.x.
- Huppe H.C. & Turpin D.H. 1994. Integration of carbon and nitrogen metabolism in plant and algal cells. Annual Review of Plant Physiology and Plant Molecular Biology 45: 577–607. DOI: 10.1146/annurev.pp.45.060194.003045.
- Hurd C.L. 2000. Water motion, marine macroalgal physiology, and production. Journal of Phycology 36: 453–472. DOI: 10.1046/j.1529-8817.2000.99139.x.
- Hurd C.L. 2017. Shaken and stirred: the fundamental role of water motion in resource acquisition and seaweed productivity. Perspective in Phycology 4: 73–81. DOI: 10.1127/pip/2017/0072.
- Hurd C.L. & Dring M.J. 1990. Phosphate uptake by intertidal algae in relation to zonation and season. Marine Biology 107: 281–289. DOI: 10.1007/BF01319827.
- Hurd C.L. & Dring M.J. 1991. Desiccation and phosphate uptake by intertidal fucoid algae in relation to zonation. British Phycological Journal 26: 327–333. DOI: 10.1080/00071619100650291.
- Hurd C.L., Harrison P.J., Bischof K. & Lobban C.S. 2014. Seaweed ecology and physiology, ed. 2. Cambridge University Press, Cambridge, UK. 551 pp.
- Hurd C.L., Harrison P.J. & Druehl L.D. 1996. The effect of seawater flow velocity on nutrient uptake by morphologically distinct forms of Macrocystis integrifolia from sheltered and exposed sites. Marine Biology 126: 205–214. DOI: 10.1007/BF00347445.
- Im S., Lee H.-N., Jung H.S., Yang S., Park E.-J., Hwang M.S., Jeong W.-J. & Choi D.-W. 2017. Transcriptome-based identification of the desiccation response genes in marine red algae Pyropia tenera (Rhodophyta) and enhancement of abiotic stress tolerance by PtDRG2. Chlamydomonas. Marine Biotechnology 19: 232–245. DOI: 10.1007/s10126-017-9744-x.
- Jacox M.G., Bograd S.J., Hazen E.L. & Fiechter J. 2015. Sensitivity of the California Current nutrient supply to wind, heat, and remote ocean forcing. Geophysical Research Letters 42: 5950–5957. DOI: 10.1002/2015GL065147.
- Kang Y.H., Hwang J.R., Chung I.Y. & Park S.R. 2013. Development of a seaweed species-selection index for successful culture in a seaweed-based integrated aquaculture system. Journal of Ocean University of China 12: 125–133. DOI: 10.1007/s11802-013-1928-z.
- Kawamata S. 2001. Adaptive mechanical tolerance and dislodgement velocity of the kelp Laminaria japonica in wave-induced water motion. Marine Ecology Progress Series 211: 89–104. DOI: 10.3354/meps211089.
- Kim J.K., Kraemer G.P. & Yarish C. 2009. Comparison of growth and nitrate uptake by New England Porphyra species from different tidal elevations in relation to desiccation. Phycological Research 57: 152–157. DOI: 10.1111/j.1440-1835.2009.00533.x.
- Kim J.K., Kraemer G.P. & Yarish C. 2013. Emersion induces nitrogen release and alteration of nitrogen metabolism in the intertidal genus Porphyra. PLoS ONE 8: e69961. DOI: 10.1371/journal.pone.0069961.
- Kleitou P., Kletou D. & David J. 2018. Is Europe ready for integrated multi-trophic aquaculture? A survey on the perspectives of European farmers and scientists with IMTA experience. Aquaculture 490: 136–148. DOI: 10.1016/j.aquaculture.2018.02.035.
- Kraemer G.P. & Chapman D.J. 1991. Effects of tensile force and nutrient availability on carbon uptake and cell wall synthesis in blades of juvenile Egregia menziesii (Turn.) Aresch. (Phaeophyta). Journal of Experimental Marine Biology and Ecology 149: 267–277. DOI: 10.1016/0022-0981(91)90049-3.
- Kregting L.T., Hurd C.L., Pilditch C.A. & Stevens C.L. 2008. The relative importance of water motion on nitrogen uptake by the subtidal macoalga Adamsiella chauvinii (Rhodophyta) in winter and summer. Journal of Phycology 44: 320–330. DOI: 10.1111/j.1529-8817.2008.00484.x.
- Kübler J.E. & Dudgeon S.R. 2015. Predicting effects of ocean acidification and warming on algae lacking carbon concentrating mechanisms. PLoS ONE 10(7): e0132806. DOI: 10.1371/journal.pone.0132806.
- Kumar M., Gupta V., Trivedi N., Kumari P., Bijo A.J., Reddy C.R.K. & Jha B. 2011. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environmental and Experimental Botany 72: 194–201. DOI: 10.1016/j.envexpbot.2011.03.007.
- Lewis S.M. 1985. Herbivory on coral reefs: algal susceptibility to herbivorous fishes. Oecologia 65: 370–375. DOI: 10.1007/BF00378911.
- Li R., Li J.J. & Wu C.Y. 1990. Effect of ammonium on growth and carrageenan content in Kappaphycus alvarezii (Gigartinales, Rhodophyta). Hydrobiologia 204: 499–503.
- Macler B.A. 1986. Regulation of carbon flow by nitrogen and light in the red alga, Gelidium coulteri. Plant Physiology 82: 136–141. DOI: 10.1104/pp.82.1.196.
- Madsen T.V. & Maberly S.C. 1990. A comparison of air and water as environments for photosynthesis by the intertidal alga Fucus spiralis (Phaeophyta). Journal of Phycology 26: 24–30. DOI: 10.1111/j.0022-3646.1990.00024.x.
- Mandal S.K., Ajay G., Monisha N., Malarvizhi J., Temkar G. & Mantri V.A. 2015. Differential response of varying temperature and salinity regimes on nutrient uptake of drifting fragments of Kappaphycus alvarezii: implication on survival and growth. Journal of Applied Phycology 27: 1571–1581. DOI: 10.1007/s10811-014-0469-1.
- Marinho G.S., Holdt S.L., Birkeland M.J. & Angelidaki I. 2015. Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. Journal of Applied Phycology 27: 1963–1973. DOI: 10.1007/s10811-014-0519-8.
- Matos J., Costa S., Rodrigues A., Pereira R. & Pinto I.S. 2006. Experimental integrated aquaculture of fish and red seaweeds in northern Portugal. Aquaculture 252: 31–42. DOI: 10.1016/j.aquaculture.2005.11.047.
- Mortensen L.M. 2017. Remediation of nutrient-rich, brackish fjord water through production of protein-rich kelp S. latissima and L. digitata. Journal of Applied Phycology 28: 3089–3096. DOI: 10.1007/s10811-017-1184-5.
- Nishihara G.N., Terrada R. & Noro T. 2005. Effect of temperature and irradiance on the uptake of ammonium and nitrate by Laurencia brongniartii (Rhodophyta, Ceramiales). Journal of Applied Phycology 17: 371–377. DOI: 10.1007/s10811-005-5519-2.
- Oates B.R. 1985. Photosynthesis and amelioration of desiccation in the intertidal saccate alga. Colpomenia peregrina. Marine Biology 89: 109–119. DOI: 10.1007/BF00392882.
- Pedersen M.F. 1994. Transient ammonium uptake in the macroalga Ulva lactuca (Chlorophyta) – nature, regulation, and the consequences for choice of measuring technique. Journal of Phycology 30: 980–986. DOI: 10.1111/j.0022-3646.1994.00980.x.
- Pereira R., Kraemer G., Yarish C. & Sousa-Pinto I. 2008. Nitrogen uptake by gametophytes of Porphyra dioica (Bangiales, Rhodophyta) under controlled-culture conditions. European Journal of Phycology 43: 107–118. DOI: 10.1080/09670260701763393.
- Pereira R. & Yarish C. 2010. The role of Porphyra in sustainable culture systems: physiology and applications. In: Seaweeds and their role in a globally changing environment (Ed. by A. Israel & R. Einav & J. Seckbach), pp. 339–354. Springer, Dordrecht, Netherlands.
- Perini V. & Bracken M.E.S. 2014. Nitrogen availability limits phosphorus uptake in an intertidal macroalga. Oecologia 175: 667–676. DOI: 10.1007/s00442-014-2914-x.
- Peteiro C. & Freire O. 2011. Effect of water motion on the cultivation of the commercial seaweed Undaria pinnatifida in a coastal bay of Galicia, northwest Spain. Aquaculture 314: 269–276. DOI: 10.1016/j.aquaculture.2011.02.009.
- Phillips J.C. & Hurd C.L. 2003. Nitrogen ecophysiology of intertidal seaweeds from New Zealand: N uptake, storage and utilization in relation to shore position and season. Marine Ecology Progress Series 264: 31–48. DOI: 10.3354/meps264031.
- Phillips J.C. & Hurd C.L. 2004. Kinetics of nitrate, ammonium, and urea uptake by four intertidal seaweeds from New Zealand. Journal of Phycology 40: 534–545. DOI: 10.1111/jpy.2004.40.issue-3.
- Pritchard D.W., Hurd C.L., Beardall J. & Hepburn C.D. 2015. Restricted use of nitrate and a strong preference for ammonium reflects the nitrogen ecophysiology of a light-limited red alga. Journal of Phycology 51: 277–2287. DOI: 10.1111/jpy.12272.
- Rabiei R., Phang S.M., Lim P.E., Salleh A., Sohrabipour J., Ajdari D., & Zarshenas G.A. 2016. Productivity, biochemical composition and biofiltering performance of agarophytic seaweed, gelidium elegans (red algae) grown in shrimp hatchery effluents in Malaysia. Iranian Journal Of Fisheries Sciences 15: 53–74.
- Raven J.A., Giordano M., Beardall J. & Maberly S.C. 2011. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynthesis Research 109: 281–296. DOI: 10.1007/s11120-011-9632-6.
- Raven J.A., Wollenweber B. & Handley L.L. 1992. A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytologist 121: 19–32. DOI: 10.1111/nph.1992.121.issue-1.
- Rees T.A.V. 2014. Scaling and transport kinetics in aquatic primary producers. Marine Ecology Progress Series 509: 103–112. DOI: 10.3354/meps10883.
- Roleda M.Y., Slocombe S.P., Leakey R.J.G., Day J.G., Bell E.M. & Stanley M.S. 2013. Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresource Technology 129: 439–449. DOI: 10.1016/j.biortech.2012.11.043.
- Schaffelke B. 1999. Short-term nutrient pulses as tools to assess responses of coral reef macroalgae to enhanced nutrient availability. Marine Ecology Progress Series 182: 305–310. DOI: 10.3354/meps182305.
- Schaffelke B. 2001. Surface alkaline phosphatase activities of macroalgae on coral reefs of the central Great Barrier Reef, Australia. Coral Reefs 19: 310–317. DOI: 10.1007/s003380000128.
- Smit A.J. 2002. Nitrogen uptake by Gracilaria gracilis (Rhodophyta): adaptations to a temporally variable nitrogen environment. Botanica Marina 45: 196–209. DOI: 10.1515/BOT.2002.019.
- Smith J.M., Brzezinski M.A., Melack J.M., Miller R.J., & Reed D.C. 2018. Urea as a source of nitrogen to giant kelp (Macrocystis pyrifera). Limnology and Oceanography Letters 3: 365–373. DOI: 10.1002/lol2.10088.
- Stévant P., Rebours C. & Chapman A. 2017. Seaweed aquaculture in Norway: recent industrial developments and future perspective. Aquaculture International 25: 1373–1390. DOI: 10.1007/s10499-017-0120-7.
- Taylor M.W. & Rees T.A.V. 1999. Kinetics of ammonium assimilation in two seaweeds, Enteromorpha sp. (Chlorophyceae) and Osmundaria colensoi (Rhodophyceae). Journal of Phycology 35: 740–746. DOI: 10.1046/j.1529-8817.1999.3540740.x.
- Taylor R.B., Peek J.T.A. & Rees T.A.V. 1998. Scaling of ammonium uptake by seaweeds to surface area: volumeratio: geographical variation and the role of uptake by passive diffusion. Marine Ecology Progress Series 169: 143–148. DOI: 10.3354/meps169143.
- Taylor R.B. & Rees T.A.V. 1998. Excretory products of mobile epifauna as a nitrogen source for seaweeds. Limnology and Oceanography 43: 600–606. DOI: 10.4319/lo.1998.43.4.0600.
- Thomas T.E., Harrison P.J. & Taylor E.B. 1985. Nitrogen uptake and growth of the germlings and mature thalli of Fucus distichus. Marine Biology 84: 267–274. DOI: 10.1007/BF00392496.
- Thomas T.E., Harrison P.J. & Turpin D.H. 1987b. Adaptations of Gracilaria pacifica (Rhodophyta) to nitrogen procurement at different intertidal locations. Marine Biology 93: 569–580. DOI: 10.1007/BF00392795.
- Thomas T.E., Turpin D.H. & Harrison P.J. 1987a. Desiccation enhanced nitrogen uptake rates in intertidal seaweeds. Marine Biology 94: 293–298. DOI: 10.1007/BF00392943.
- Topinka J.A. 1978. Nitrogen uptake by Fucus spiralis (Phaeophyceae). Journal of Phycology 14: 241–247. DOI: 10.1111/jpy.1978.14.issue-3.
- Topinka J.A. & Robbins J.V. 1976. Effects of nitrate and ammonium enrichment on growth and nitrogen physiology in Fucus Spiralis. Limnology and Oceanography 21: 659–664. DOI: 10.4319/lo.1976.21.5.0659.
- Tseng C.K. 1981. Marine phycoculture in China. Proceedings of the International Seaweed Symposium 10: 124–152.
- Turpin D.A. 1991. Effects of inorganic N availability on algal photosynthesis and carbon metabolism. Journal of Phycology 27: 14–20. DOI: 10.1111/j.0022-3646.1991.00014.x.
- van der Loos L.M., Schmid M., Leal P.P., McGraw C.M., Britton D., Revill A.T., Virtue P., Nichols P.D. & Hurd C.L. 2019. Responses of macroalgae to CO2 enrichment cannot be inferred solely by their inorganic carbon uptake strategy. Ecology and Evolution 9: 125–140. DOI: 10.1002/ece3.4679.
- Wang C., Lei A., Zhou K., Hu Z., Hao W. & Yang J. 2014. Growth and nitrogen uptake characteristics reveal outbreak mechanism of the opportunistic macroalga Gracilaria tenuistipitata. PLoS ONE 9(10): e108980. DOI: 10.1371/journal.pone.0101343.
- Zou D. 2005. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250: 726–735. DOI: 10.1016/j.aquaculture.2005.05.014.
