ABSTRACT
Chronic pain substantially impacts patient function and quality of life and is a burden to society at large in terms of increased health care utilization and loss of productivity. As a result, there is an increasing recognition of chronic pain as a public health crisis. However, there remains wide variability in clinical practices related to the prevention, assessment, and treatment of chronic pain. Certain fundamental aspects of chronic pain are often neglected including the contribution of the psychological, social, and contextual factors associated with chronic pain. Also commonly overlooked is the importance of understanding the likely neurobiological mechanism(s) of the presenting pain and how they can guide treatment selection. Finally, physicians may not recognize the value of using electronic medical records to systematically capture data on pain and its impact on mood, function, and sleep. Such data can be used to monitor onset and maintenance of treatments effects at the patient level and evaluate costs at the systems level. In this review we explain how these factors play a critical role in the development of a coordinated, evidence-based treatment approach tailored to meet specific needs of the patient. We also discuss some practical approaches and techniques that can be implemented by clinicians in order to enhance the assessment and management of individuals with chronic pain in primary care settings.
Introduction
Acute pain plays a protective role by alerting a person to actual or potential physical injury. Acute pain typically lasts less than 3 months with resolution upon healing of the underlying injury.[Citation1] In contrast, chronic pain persists long after we would expect the apparent cause of the pain to have resolved.[Citation2,Citation3] Chronic pain can present without evidence of, and out of proportion to, overt physical damage. Thus, chronic pain may be viewed as a distinct condition associated with pathological changes occurring in the central nervous system (CNS) rather than merely a symptom of another disorder.[Citation4]
Poorly managed chronic pain is associated with significant deteriorations in physical and emotional functioning, overall quality of life, and is a burden to society at large. Society experiences the burden of chronic pain in terms of high health-care utilization, reduced productivity, disability payments, and lost tax revenue. In the United States, both the prevalence of chronic pain and the associated costs ($560–635 billion annually as of 2010) are estimated to be in excess of those for cardiovascular disease, cancer, or diabetes.[Citation4–Citation7] The estimated prevalence of chronic pain, however, varies as there is no consensus definition of chronic pain with respect to its frequency, duration, or severity. Data from the 2012 National Health interview survey indicate that 126.1 million adults (56%) reported some pain in the previous 3 months, including 25.3 million adults (11%) who reported daily (chronic) pain and 23.4 million (10.3%) who reported ‘a lot’ of pain.[Citation8] Some individuals affected by chronic pain exhibit substantial restriction of participation in work-related, social, and self-care activities for 6 months or more. Such disabling pain is termed ‘high-impact chronic pain’ in the National Pain Strategy.[Citation9]
Recently, there has been some increase in the recognition of chronic pain as a public health crisis. However, there remains wide variability in clinical practices related to the prevention, assessment, and treatment of chronic pain.[Citation4,Citation9] Chronic pain is a complex biologic, psychological, and social phenomenon that may be influenced by individual patient genetics, life experiences, and current circumstances. Unfortunately, clinicians, especially those in primary care, sometimes receive insufficient education and training with respect to chronic pain and its treatment.[Citation10–Citation12] Even with proper education primary-care practitioners (i.e. internists, family physicians, osteopaths, nurse practitioners, and physician assistants) may find it difficult to translate this knowledge into clinical practice for a number of reasons, including the lack of reliable and validated objective measures of pain, concerns about adverse effects, and limitations in patient contact time. Currently available measures are based upon patient self-report, associated with great variability, and exhibit only modest, at best, associations with objective signs. Additionally, the misuse and diversion of prescription opioid medications have contributed to significant increases in opioid addiction, abuse, and overdose that sometimes overshadow other clinical and social issues pertinent to effective pain care, lead to prescriber confusion, and put pressure on the prescriber to limit opioid use.[Citation13,Citation14] A significant impediment is that attention to issues associated with the complexity of chronic pain requires increased time commitment from already tightly scheduled providers.
The purpose of this review is to promote broader understanding and wider use of existing knowledge regarding chronic pain and its management as recommended by the Institute of Medicine’s Report on Pain and the National Pain Strategy.[Citation4,Citation9] We highlight several known, but often unheeded, approaches that can help enhance the understanding of pain and improve the treatment of individuals with chronic pain. The simple, evidence-based framework described here is consistent with the latest understanding of the pathophysiology and neurobiology of pain, recent clinical guidelines for the management of chronic pain, and recent public policy reports.[Citation4,Citation9] Since the majority of people with chronic pain seeks and receives care in the primary-care setting,[Citation15,Citation16] the framework was created with primary-care providers in mind. The principles and approaches discussed here should assist clinicians in addressing many of the challenges listed above and can make the assessment and management of patients with chronic pain both more effective and more efficient. As a result, implementation of this framework may improve clinical outcomes, increase patient satisfaction, and help lower the costs associated with the treatment of chronic pain.
Factors often overlooked with respect to chronic pain
Multiple guidelines describe appropriate assessment and management of patients with different chronic pain conditions.[Citation17–Citation22] However, several important factors noted in these guidelines and known to most clinicians are not consistently addressed or implemented, particularly in the primary-care setting.
The importance of understanding the likely mechanism(s) underlying the presenting pain is often minimized. All pain is not the same and identifying the presumed basic type(s) that may be contributing to the clinical presentation of a particular patient plays an important role in the selection of appropriate treatment because pharmacological agents and certain interventional therapies produce their therapeutic effects by targeting specific neurobiological (or psychological) mechanisms.
The importance of the psychosocial aspects of pain may also be frequently discounted. An understanding of the psychological, social, and contextual factors helps explain variability in response to both pain and the treatment of pain among different individuals. It can also guide the selection of pharmacologic and non-pharmacologic treatments and self-management strategies. The benefit of a collaborative, interdisciplinary, and multidisciplinary approach that integrates patient education and psychological services to supplement multimodal pharmacologic and rehabilitative strategies is also commonly overlooked, underutilized (due to time constraints or lack of trained personnel), or frequently unavailable.
Finally, the importance of systematic capture of data beyond basic pain characteristics (location, duration, and severity) is often underestimated. These data could include descriptive characteristics of the presenting pain, the effect of pain on sleep, mood, and function, and modulating factors such as activity and social support. Such data capture, preferably in electronic medical records (EMRs), is essential to supporting effective patient management by tracking changes in pain characteristics and responses to treatment over time, particularly if the patient is seen by more than one practitioner.
Together, these factors play a critical role in the development of a coordinated, evidence-based treatment approach tailored to meet specific needs of the patient. We describe these factors in greater detail below, followed by an overview of some practical approaches and techniques clinicians can implement in order to enhance the assessment and effective management of individuals with chronic pain treated in a primary-care setting.
Understanding chronic pain
Establishing the likely type(s) of pain is as important as identifying the cause(s) of pain
The term chronic pain is often used to refer to multiple clinical conditions associated with persistent and recurrent pain such as arthritis and joint pain, chronic low back pain (CLBP), headaches, chronic widespread pain, painful diabetic peripheral neuropathy (pDPN), and pain associated with metastatic disease among others. Traditionally, a distinction has been made between chronic noncancer pain and pain associated with life-threatening oncologic conditions. The reasons to view cancer-related pain as a separate category include its complex etiology, often higher level of pathology and/or severity of pain, complexity of patient care, and more aggressive treatment approaches in an end of life setting. However, there is no evidence to suggest that the neural mechanisms involved in pain associated with cancer are distinct from other chronic pain conditions.[Citation23,Citation24] Historically, chronic pain conditions have each been considered distinct disorders and pain has been viewed predominantly, if not exclusively, as a symptom of peripheral pathology. This view is reflected in the numerous International Classification of Disease codes and separate classifications and treatment guidelines for these conditions that are often based solely on signs, symptoms, body location, and information on structural pathology. Thus, these diagnostic classifications are rarely helpful in selecting appropriate treatments that target underlying neurobiological and psychosocial mechanisms of pain that may be present.[Citation25]
Although the neurobiological and psychosocial mechanisms of pain, particularly at an individual level, are not well understood and may be difficult to assess, viewing chronic pain as a single homogenous entity is clearly not helpful.[Citation26] For example, all types of pain do not respond to the same pharmacotherapy. Nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to relieve pain due to inflammation or tissue injury in some chronic pain conditions but are generally ineffective for neuropathic pain. Similarly, most opioids can provide limited relief for some patients with trauma, inflammation, and neuropathic pain, but accumulating data suggest that they are not beneficial in patients with fibromyalgia (FM) and similar conditions (see below).[Citation27,Citation28] Thus, understanding the likely causes and, more importantly, the basic pathophysiology of chronic pain as much as possible can help guide the selection of appropriate therapy for each patient.
Three main types of pain pathophysiology, separately or together, are thought to be responsible for the majority of presentations of chronic pain
Nociceptive pain is associated with tissue damage due to trauma, inflammation, or other nonhealing injury. Damage generally does not involve the central or peripheral nervous system itself. Nociceptive pain is the predominant type of pain in individuals with osteoarthritis (OA), rheumatoid arthritis (RA), neck and back pain with structural pathology, and chronic tendonitis or bursitis, among others ().[Citation3]
Figure 1. (A) The three main types of pain pathophysiology give rise to chronic pain conditions. (B) These types of pain may present separately or in combination to contribute to the overall pain experience.
PHN = postherpetic neuralgia; pDPN = painful diabetic peripheral neuropathy.
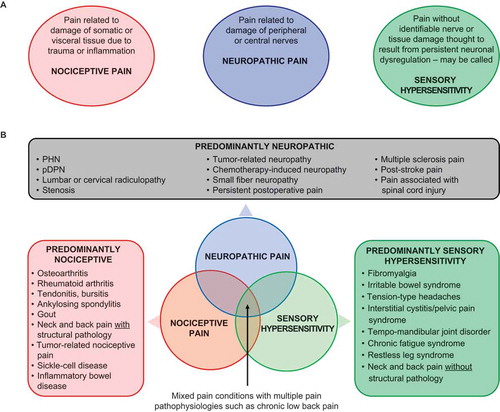
Prolonged nociceptive pain can lead to peripheral sensitization, which describes a process whereby tissue damage causes release of chemical substances such as bradykinin, prostaglandins, leukotrienes, and substance P that increase the sensitivity of peripheral neurons to noxious stimuli by lowering neuronal activation thresholds and increasing firing rates. The clinical consequences are hyperalgesia (greater levels of perceived pain in response to painful stimuli) and allodynia (pain in response to stimuli such as touch which normally do not evoke pain) in tissues sensitized by this process.[Citation29]
Neuropathic pain is associated with damage to the nervous system.[Citation30,Citation31] Neuropathic pain is classified as central or peripheral depending on the primary location of the damaged nerves. Peripheral neuropathic pain is the predominant type of pain in pDPN, postherpetic neuralgia, lumbar or cervical radiculopathy, stenosis, tumor-related neuropathy, chemotherapy-induced neuropathy, small-fiber neuropathy, and persistent postoperative pain. Neuropathic pain associated with spinal cord injury and poststroke pain are examples of central neuropathic pain ().
Persistent neuropathic pain (nerve injury) and persistent nociceptive input (tissue injury) can trigger central sensitization, a long-lasting increase in the excitability and responsiveness of neurons in the CNS that results in hyperalgesia and allodynia.[Citation30]
Sensory hypersensitivity or centralized pain describes a type of pain that exists without an identifiable nerve or tissue damage (FM is its prototypical example) (). This type of pain is hypothesized to be a result of persistent dysfunction of neurons throughout the CNS that leads to lowering of pain thresholds and amplification of sensory signals. It is referred to by many names including centralized, dysfunctional, or idiopathic pain, central sensitization, and central sensitivity syndromes.[Citation32–Citation35] While no clear consensus presently exists on the terminology, the hallmark of this type of pain appears to be generalized hypersensitivity to a variety of stimuli including mechanical, thermal, olfactory, auditory, and visual cues.[Citation36–Citation39]
Unlike traditional central sensitization triggered by persistent nociceptive and/or neuropathic input, it is often impossible to pinpoint the cause of sensory hypersensitivity. It is thought to occur throughout the CNS and may involve additional mechanisms such as a decrease in descending inhibition and neuroplasticity in pain processing areas of the brain.[Citation34,Citation40,Citation41] Contributing factors are thought to include genetics, environment, history of physical or psychological trauma, and emotional stress.[Citation42]
In addition to FM, Irritable Bowel Syndrome, tension-type headaches, interstitial cystitis, temporomandibular joint disease (TMD), chronic fatigue syndrome, and possibly restless leg syndrome are thought to be sensory hypersensitivity conditions ().[Citation43–Citation46] These conditions often share features typical of FM including diffuse pain in multiple body regions, fatigue, sleep disorders, memory difficulties, mood disturbance, individual or familial history of chronic pain, and increased sensitivity to sensory input in the presence of an otherwise generally normal physical examination. Furthermore, sensory hypersensitivity conditions often co-present with each other suggesting a possible common pathogenesis.[Citation47,Citation48]
Most patients have mixed pain state because they have more than one type of pain pathophysiology
More than one mechanism and type of pain can, and often does, contribute to an individual’s pain experience (). Such mixed pain states are frequently present in patients with CLBP, pain associated with malignancy, OA, RA, and other chronic pain conditions traditionally viewed as nociceptive. In individual studies, for example, neuropathic or neuropathic-like pain was evident in 37% of patients with CLBP,[Citation49] 26–67% of patients with failed back surgery syndrome,[Citation50] and 19% of patients with OA.[Citation51] Likewise, in one study, as many as 42% of patients with nociceptive axial back pain met diagnostic criteria for FM.[Citation52] Further, FM-like symptoms frequently occur in patients with RA, OA, and regional musculoskeletal pain, and the degree of these FM-like symptoms predicts pain intensity and degree of disability in these patients.[Citation53–Citation56] In studies of OA and RA, 11% of patients with OA and 15% of patients with RA also had FM.[Citation57,Citation58]
Other classifications of chronic pain
Traditionally, classification of chronic pain has been based on signs, symptoms, body location, or information on structural pathology (e.g. musculoskeletal, visceral, ischemic, chronic regional pain syndrome, etc.) rather than on underlying mechanism(s). While such classifications may be essential for a definitive diagnosis and coding, they may also group conditions with different neurobiological and/or psychosocial mechanisms and may not aid the selection of appropriate therapy. Musculoskeletal pain, for example, affects bones, muscles, ligaments, tendons, and nerves, and can be localized or widespread. This classification includes predominantly nociceptive conditions such as RA and OA but also includes neuropathic conditions and FM, which is the prototypical sensory hypersensitivity condition. Visceral pain arises from internal organs but is usually perceived in somatic tissues of the body wall that receive the same sensory innervation as an internal organ. Examples of this classification include chronic pancreatitis, prostatitis, and endometriosis. Although some of these conditions are predominantly nociceptive in nature and respond to NSAIDs, many represent a mixed pain condition.[Citation3] Ischemic pain is defined as pain associated with insufficient blood flow due to obstruction, injury, or trauma. This classification of pain is present in conditions such as peripheral arterial disease and can progress from predominantly nociceptive in nature to having a significant neuropathic component.[Citation59] Complex regional pain syndrome (CRPS) has been viewed as predominantly neuropathic in nature but patients with CRPS often have inflammatory nociceptive and sensory hypersensitivity mechanisms along with autonomic and immune components.[Citation31,Citation60]
Pain is not a simple biologic sensory phenomenon
For many patients the success of therapy depends on recognition by both the patient and clinician that pain is more than a sensory experience. Pain is an output of the brain and not a simple estimation of the magnitude of sensory input. Many factors influence the severity of experienced pain, including an individual’s genetics, comorbid medical conditions, prior history, current psychological state, and socioeconomic circumstances. These factors also affect how patients react to pain and respond to treatment.[Citation61] Although chronic pain can contribute to the development of depression, anxiety, stress, and sleep disorders, these factors exhibit a bi-directional relationship with pain and their presence can lower pain thresholds, influence perception, and increase the risk of developing chronic pain.[Citation62–Citation68]
Even basic steps to assess and address an individual’s psychological and social factors can significantly increase the likelihood of treatment success. This requires an individualized treatment approach that shifts from predominantly biomedical strategies (i.e. additional testing or dose escalation due to poor response) to a more biopsychosocial perspective with greater focus on patient expectations of treatment, self-management, and referrals for coordinated multidisciplinary and interdisciplinary care when appropriate. This shift may be especially important for the successful management of patients with ‘high-impact chronic pain’ who, arguably, represent the greatest challenge for clinicians.[Citation9]
Important elements of the appropriate assessment of patients with chronic pain
There is a considerable degree of alignment among many condition-specific treatment guidelines with respect to the assessment of patients with chronic pain. They include a few simple and structured questions to establish the severity, location, duration, and likely causes of pain as well as questions related to patient health status, medical history, comorbid illnesses, prior and current medications, and family and social history.[Citation69] The importance of the physical exam cannot be overestimated as it can reveal muscle stretch reflex changes and key sensory features such as allodynia, myofascial trigger points, patterns of referred pain, and weak or inhibited muscles. It can also guide the need for non-pharmacologic interventions such as physical and occupational therapy or mind–body techniques. Disease-specific diagnostic criteria such as those for OA or FM simplify identification of pain characteristics and treatment decisions for those individual conditions, and their broad utilization in everyday practice is recommended.[Citation70,Citation71]
The overwhelming majority of guidelines also share recommendations regarding (1) the utility of properly identifying pain type(s), pathophysiology, and mechanisms to guide treatment selection and (2) the assessment and management of an individual’s psychosocial characteristics, including expectations of treatment (). However, these elements are not always part of the assessment and management approach in clinical practice and are, therefore, discussed in greater detail below.
Table 1. Consensus among guidelines for the treatment of chronic pain.
Evaluating the types of pain potentially contributing to the individual experience
As noted, chronic pain can persist after the apparent cause of the pain has resolved or, in some cases, in the absence of a discernable cause. This underscores the importance of evaluating the pain itself, in terms of its potential neurobiological mechanisms or types of pathophysiology, since various treatments are designed to target specific neurobiological and/or psychosocial mechanisms (see section below on selecting pharmacological treatment). Clinicians can use a few simple steps to identify the broad type(s) of pain that may be present in a particular patient.
The ways in which individuals describe and experience their pain () can be used to help identify the pain types present as they tend to differ among nociceptive pain (sore, throbbing, dull, tender, aching, cramping), neuropathic pain (hot, burning, electric shock, stabbing, painful cold, tingling, prickling, pins and needles, numbness), and sensory hypersensitivity (widespread and/or associated with mood disturbance, cognitive dysfunction, or general hypersensitivity to sensory input such as bright lights, loud noises, or smells).[Citation1,Citation35,Citation37,Citation41,Citation80–Citation83] In addition, hyperalgesia and allodynia are often found in patients with neuropathic pain or sensory hypersensitivity, but less so with nociceptive pain.
Table 2. Patient pain descriptors/reported symptoms among the three types of pain.
Pain descriptors and symptoms form the basis for several fairly general as well as disease-specific measures developed and validated to help assess the type(s) of pain in an individual with chronic pain. Some are sufficiently short and straightforward to be useful in the primary-care setting. For example, the 6-item IDPain® can be used for patients with non-headache chronic pain to help distinguish between nociceptive and neuropathic pain. In a multicenter study of 586 patients with chronic pain, IDPain was shown to reliably predict a subsequent diagnoses of neuropathic pain by pain specialists.[Citation84] Likewise, the 9-item PainDETECT can identify, with high probability, the presence of a neuropathic pain component in patients with CLBP.[Citation49] An example of a brief, more disease specific measure, is the 2011 Fibromyalgia Survey (FS) criteria, which can detect FM-like pain with a sensitivity, accuracy, and specificity of over 90%.[Citation53] Though specifically designed for FM, the FS is useful as a measure for the presence of sensory hypersensitivity in general. In several patient populations, it has been shown to be highly predictive of nonresponsiveness to surgical interventions intended to relieve pain and of levels of opioid consumption in the perioperative period.[Citation85–Citation87] Notably, the FS is predictive of nonresponse to surgery and opioid consumption at levels well below the threshold used to diagnose FM.[Citation85–Citation87]
The use of brief, simple instruments described above, or other currently available validated tools, can help determine which types of pain are present and play a key role in informing treatment decisions. (For a more extended discussion of disease-specific measures see [Citation69].)
Evaluating psychosocial aspects of chronic pain
Patients’ self-report of pain location and intensity, while important, is insufficient in and of itself to fully understand how they experience pain and to inform an effective treatment strategy. Clinicians also need to gain an understanding of psychosocial risk factors and the impact of pain on the patient’s function, attitude, mood, sleep, and interaction with others.[Citation69] The initial psychosocial assessment need not be complex or time-consuming. Ideally, however, it should be conducted on every individual presenting with chronic pain. Even a single question for each of these impact domains can help differentiate among patients reporting similar pain intensity, help establish individual treatment goals, and point to additional therapeutic approaches that may contribute to overall treatment success.
An example of a brief screening tool that asks these important questions is the Brief Psychosocial Screening: ACT-UP approach.[Citation61] It contains five simple questions to assess how pain affects patient Activity, how patients Cope with their pain, how patients Think about their pain, how pain Upsets their mood, and how pain affects their interaction with People.
The results of the initial screening can inform the need for more in-depth patient assessment. For example, individuals reporting significant effects on mood may require more detailed assessments of anxiety and depression using validated disease-specific tools or referral to a mental health professional. Likewise, patients reporting marked impact on function may require a more in-depth assessment of their functioning either by the provider or referral for physical or occupational therapy for evaluation. Patients reporting disturbed sleep may benefit from further assessment of their insomnia. Patients with poor coping skills may think that their pain will never get better leading to an exaggerated perception of and reaction to pain, a persistent focus on pain, and feelings of hopelessness and despair; a phenomenon known as pain catastrophizing. Pain catastrophizing, along with depression and anxiety, has been identified as an important predictor of poor treatment outcomes. Therefore, it is critical that these elements be properly assessed before the initiation of treatment so appropriate pharmacological and non-pharmacological therapies can be utilized. We describe this in more detail below.
Selection of treatments for chronic pain
Currently, pharmacotherapy is the most commonly utilized approach to chronic pain management. As discussed, however, pain is more than a biological phenomenon and a treatment plan should include both pharmacological and non-pharmacological therapies to address both the physical and psychosocial elements of chronic pain. Unfortunately, non-pharmacological treatments are often underutilized. Many factors influence treatment decisions in patients with chronic pain including cost of treatment, access to treatment, local regulations, and public policy. Ideally, however, treatment of patients with chronic pain should primarily be based on the underlying types of pain present and the psychosocial profile of the patient. These two elements are described here in more detail.
Selecting pharmacologic treatment based on the type(s) of pain pathophysiology
There is broad agreement that the classes of pharmacotherapy recommended to treat specific types of chronic pain should be based on pathophysiology whenever possible ().
Figure 2. Recommended medication classes for the three types of pain pathophysiology.
aBased on strength of clinical evidence. NSAIDs = nonsteroidal anti-inflammatory drugs; AEDs = antiepileptic drugs; SNRIs = serotonin-norepinephrine reuptake inhibitors; TCAs = tricyclic antidepressants.
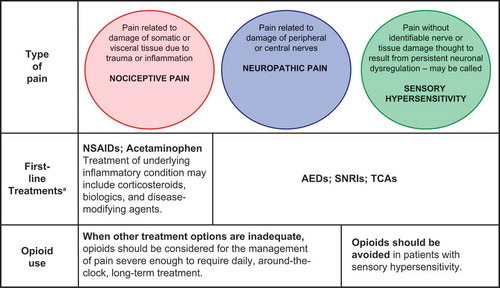
For conditions that are predominantly nociceptive in nature due to ongoing tissue damage or inflammation (e.g. arthritis), NSAIDs or acetaminophen are recommended and may be used in combination with corticosteroids, biologics, or disease-modifying agents for the treatment of the underlying inflammation. Notably, older patients may have increased risk of cardiovascular, renal, and hematological adverse events with NSAIDs and are more likely to take additional medications that may interact with NSAIDs. Thus, patient-specific risk mitigation in older adults should include, among others, the use of the lowest effective systemic dose of NSAIDs for the shortest time possible (including use of topical NSAIDs and other analgesics), careful monitoring of adverse events and concomitant medications, prescription of gastro-protective agents, and patient and caregiver education.[Citation88]
For predominantly neuropathic conditions and for sensory hypersensitivity, recommended first-line treatments include antiepileptic drugs, such as the α2δ ligands pregabalin and gabapentin, and serotonin–norepinephrine reuptake inhibitors such as duloxetine.[Citation44,Citation89,Citation90] NSAIDs are not recommended in these instances as they have not shown efficacy against pain that is non-nociceptive in nature.
As described previously, one should not assume that a patient’s pain experience has only one type of pathophysiology, particularly in patients with conditions traditionally viewed as nociceptive or inflammatory (i.e. CLBP, OA, RA, and possibly headache). Other types of pain may develop in such patients secondary to the persistent nociceptive input or may represent an independent comorbidity. For example, up to 40% of patients with CLBP and about 20% of patients with OA may have a significant neuropathic or sensory hypersensitivity component that is not likely to respond to medications that treat nociceptive pain.[Citation49,Citation51] Similarly, chronic nociceptive input may contribute to the pain of a patient with a sensory hypersensitivity condition such as FM and such patients will likely feel more pain than would be expected based on the magnitude of nociceptive input.
Many patients with chronic pain have more than one type of pain pathophysiology and are likely to be partial responders, at best, to treatments that target only a single type. These patients will often require a multimodal analgesic approach including pharmacological and non-pharmacological therapies to achieve adequate pain relief. Unfortunately, there are few controlled studies evaluating combination pharmacotherapy or a combination of pharmacological and non-pharmacological treatments in patients with chronic pain. However, identifying candidates who would benefit from multimodal therapy and optimizing their treatment accordingly may shorten time to adequate pain relief and may allow the clinician to avoid the inappropriate utilization of opioids in some cases.
Considerations for the trial of opioid analgesics
Opinions are mixed regarding the relative risk to benefit ratio of opioid analgesics for chronic pain, primarily because of the significantly higher risks for misuse, abuse, addiction, diversion, overdose, and death compared with other analgesics. Moreover, there is an absence of long-term data regarding the effectiveness on reducing pain, improving physical and emotional functioning, and safety of opioids, though the same concern can be raised in regard to other analgesic treatments. Due to the unique risks, however, greater caution must be exercised when considering initiating opioid therapy or prescribing it as part of an ongoing medication regimen.[Citation14,Citation27,Citation91,Citation92] Recent treatment guidelines, pain medicine thought leaders, the National Pain Strategy, the FDA, and the CDC agree that opioids may be medically appropriate and safe for acute pain and for selected patients with chronic pain when such pain cannot be adequately managed with other methods. In fact, the FDA has recently updated the indication for extended release (ER) opioids to read: ‘…only for patients with pain severe enough to require daily, around-the-clock, long-term treatment and for whom alternative treatment options are inadequate.’ Thus, a thorough review of a patient’s medical history is required to verify that they have failed to respond to, or could not tolerate, an adequate trial of the recommended first-line non-opioid medications and appropriate non-pharmacologic therapies before an opioid analgesic is considered.[Citation93–Citation95]
In addition, both the FDA and CDC agree that opioid-naïve patients, who meet the requirements above, should be first prescribed immediate release opioids. Further, if it is deemed necessary to prescribe an ER opioid, a lower dose should be selected for opioid-naïve compared with opioid-tolerant patients.[Citation95] Patients are considered opioid-tolerant if they have been taking, for ≥1 week, at least 60 mg of morphine daily, at least 30 mg of oral oxycodone daily, at least 8 mg of oral hydromorphone daily, or an equianalgesic dose of another opioid.[Citation95] Moreover, if a patient is started on opioid therapy (or any therapy for that matter), the impact of the treatment on pain and physical and emotional functioning, as well as any adverse events, should be routinely monitored and used as the basis for decisions regarding continuation, modification, or termination of treatment.
A recent survey of clinical practice in the United States, however, does not appear to reflect some of the recommendations described above. Based on national claims data from March 2008 to February 2012, 42–52% of patients with pDPN, FM, or arthritis were prescribed opioids as part of a first-line treatment, likely immediately following a trial of an over-the-counter NSAID (IMS data on file 2012). Factors contributing to high opioid prescribing rates may include (1) the perception that they are an ‘easy’ choice (i.e. they are thought to relieve various types of pain thereby eliminating the need to consider pain pathophysiology); (2) relatively greater access to opioids and formulary availability of generic opioids compared with non-pharmacologic therapies or non-opioid analgesics; and (3) an underestimation of the risks associated with significant dose escalation of opioids when used as prescribed, and the potential for prescription opioid abuse and diversion. In some cases, even when a patient is identified as being at ‘high risk’ with respect to opioid treatment, there is limited implementation of recommended risk-mitigation strategies.[Citation96,Citation97]
In view of the challenges of predicting, with any degree of certainty, who will become a problematic user, a decision to prescribe an opioid analgesic should trigger the implementation of a set of risk management principles and strategies.[Citation98] These principles include careful evaluation and documentation of a patient’s risk for opioid abuse and aberrant behaviors (including misuse, abuse, addiction, and diversion); regular assessment of efficacy, tolerability, and aberrant behaviors; and defining specific clinical procedures should abuse, addiction, or both become evident (). These risk management approaches are described in greater detail elsewhere.[Citation72,Citation75,Citation93,Citation99,Citation100] It should be noted, however, that a ‘trial’ of opioid medication implies an understanding between patient and physician that they are not committing to indefinite or long-term opioid use. If no significant analgesia or functional improvement (within the context of patient comorbidities and functional limitations) is achieved within a specified period of weeks to months, or if adverse effects appear or persist despite careful dose adjustment and titration, opioid analgesics should be slowly tapered down and discontinued.[Citation101]
Figure 3. A 4-step approach to universal precautions for opioid prescribing in patients with chronic pain.
Universal precautions in opioid prescribing for chronic pain are recommended. Used with permission from www.rethinkopioids.com[Citation72] Copyright ©2014 Pfizer Inc.
![Figure 3. A 4-step approach to universal precautions for opioid prescribing in patients with chronic pain.Universal precautions in opioid prescribing for chronic pain are recommended. Used with permission from www.rethinkopioids.com[Citation72] Copyright ©2014 Pfizer Inc.](/cms/asset/4c56d4e2-67c0-4488-be19-a9f46e85087e/ipgm_a_1188319_f0003_b.gif)
Finally, emerging data suggest that long-term opioid use may be particularly inappropriate in some patients with sensory hypersensitivity conditions such as FM due to the unique pathophysiologic characteristics of these conditions.[Citation28,Citation102] Opioid use in this patient population may cause paradoxical CNS changes increasing central and peripheral sensitization manifested by pain amplification.[Citation28] Because FM and related disorders are viewed as syndromes of central pain amplification, patients may be at a higher risk for developing tolerance to opioids and/or, hypothetically, opioid-induced hyperalgesia.[Citation28,Citation103] In addition, anxiety and mood disorders are common comorbidities in patients with FM but are also known risk factors for nonmedical use of opioids.[Citation104] Conversely, potential adverse effects of opioid use such as cognitive impairment, increased irritability, depressed mood, disturbed sleep, fatigue, and sedation are also potential clinical concerns in patients with FM.[Citation105,Citation106] Similarly, greater caution is called for when considering opioids for the treatment of mixed pain conditions where the contribution of sensory hypersensitivity may be significant, such as patients with CLBP or headache disorder.[Citation27] In this subset of patients, opioid initiation or continuation should be avoided.
Ultimately, the goal in patients with chronic pain is to optimize non-pharmacologic therapy and appropriate non-opioid medications as part of an initial comprehensive treatment approach targeting specific pathophysiologic changes and psychological aspects of pain. This type of structured approach may help clinicians avoid potential pitfalls resulting from inappropriate prescribing of opioids and should be viewed as a fundamental part of responsible opioid prescribing since it may lead to a reduction in the number of patients with chronic pain who are exposed to opioid analgesics and their associated risks. Reducing the amount of prescription opioids in circulation (i.e. unfinished prescriptions poorly monitored and left in unsecure locations within the home) will help in reducing the risk to non-patients (i.e. family members, spouses, friends, recreational users, and substance abusers). Thus, the decision to prescribe an opioid analgesic for the treatment of any patient with chronic pain may not be made lightly and, once it is made, the risk reduction strategies described above and in should be followed consistently.
Non-pharmacologic treatment(s) for chronic pain
Non-pharmacologic intervention(s) should be an integral part of a comprehensive treatment approach and should be tailored to the patients’ needs as identified by the clinician. Such interventions include self-help and self-management educational materials, psychological therapies, physical therapies, complementary and alternative medicine (CAM), family education, vocational counseling, and combinations of these.[Citation23,Citation107] Clearly, in some primary-care settings clinicians may not have access to any or all of the therapies listed above. However, treating patients with chronic pain with a purely pharmacological approach often leads to poor analgesic response, little functional improvement, and both patient and clinician frustration. We recommend, therefore, that clinicians work to identify available networks, potential collaborators, and opportunities for referral in order to expand their, and their patients’, access to non-pharmacological therapies. Moreover, significant others, when appropriate, should be educated and included as allies.
Examples of psychological interventions are cognitive-behavior therapy (CBT), relaxation training, hypnosis, and biofeedback. The benefits of CBT for individuals with chronic pain are supported by the greatest amount of research evidence.[Citation108,Citation109] The goal of CBT is to decrease maladaptive thoughts, help patients develop a greater sense of control, and reduce pain catastrophizing patterns of thinking and behavior.[Citation110] Some people with chronic pain fear that movement and exercise will increase their pain or even result in paralysis. However, fear of pain or injury related to exercise is one of the strongest predictors of disability for people with CLBP; about two-thirds of patients who avoid activity they are capable of, do so because they believe that they might (re)injure their back.[Citation111] CBT directly targets these fears by discussion and in vivo performance of feared activities. Coping skills training is a specific type of CBT that focuses on developing skills to help patients better express themselves and manage their stress and pain through group therapy and relaxation techniques.[Citation112–Citation114]
The known impact of pain on sleep may trigger a conversation about sleep hygiene, behavioral modification(s), selecting a pain treatment that does not further disrupt sleep, and ultimately, a consideration of a sleep aid. Since long-term benzodiazepine use is associated with a potential for serious adverse effects such as dependence and overdose, clinicians should carefully weigh the risks and benefits of different classes of hypnotics and/or refer the patient to a sleep disorder specialist to help select sleep aids with the lowest risk of adverse outcomes.[Citation115,Citation116]
Finally, the marked impact of pain on function or physical activities may trigger a referral for physical or occupational therapy. The intent of rehabilitative therapy is to restore functions that may have been impacted by pain including walking, stretching, posture, endurance, and flexibility. CAM includes practices such as massage therapy, spinal manipulation, meditation, breathing exercises, guided imagery, yoga, and acupuncture.[Citation117]
Other treatments for chronic pain
Though oral agents are the cornerstone of pharmacologic therapy for chronic pain their use may be limited in certain patients, particularly the elderly.[Citation118] Topical therapies have shown efficacy against chronic pain and offer several advantages over systemic medications including the requirement for a lower total systemic daily dose, site-specific drug delivery, the potential to avoid first-pass metabolism, and fewer major drug interactions and systemic side effects.[Citation118–Citation120] Nonselective NSAIDs, for example, have been formulated as patches and topical gel solutions for the treatment of predominantly nociceptive pain conditions such as OA, whereas lidocaine and capsaicin have been formulated as patches for the treatment of predominantly neuropathic pain conditions such as pDPN.
In some patients, interventional pain procedures, neuromodulation, or surgery may be beneficial if the etiology of pain is clearly established and if the pain cannot be adequately managed by less invasive optimized therapy. Even though no more than 40–60% of patients with refractory neuropathic pain obtain lasting, albeit partial, relief with most interventional measures, these measures can be viewed as part of a more comprehensive approach that also includes pharmacologic and non-pharmacologic treatments.[Citation121]
Disease management approach to pain care
The National Pain Strategy and current guidelines for the management of chronic pain recommend a better collaborative, multidisciplinary approach in the primary-care setting that integrates psychological services and patient education to supplement pharmacological therapies or interventional pain management techniques.[Citation7] To succeed, the care model must shift from the current fragmented, fee-for-service approach to one based on better incentives for pain prevention (primary, secondary, and tertiary) and on collaborative care along the continuum of the pain experience at all levels and settings of care.[Citation9]
Establishing a multidisciplinary team (including the primary-care physician, nurse practitioners/physician assistants, physical therapists, pharmacists, dieticians, social workers, psychologists, clinical and occupational pharmacologists, and neurologists) may be a luxury in some settings but it is the goal to strive toward for the management of patients with chronic pain.[Citation122] Indeed, collaborative care models for the management of pain have been developed that stress an integration of care between the primary-care physician, the patient, and the pain specialist in order to stratify patients and develop personalized evidence-based treatment approaches based on risk factors and comorbidities present.[Citation123,Citation124] A collaborative treatment approach would foster communication between patients and clinicians enabling establishment of realistic treatment goals and expectations and helping patients become more involved in their own care.
The value of documenting pain and its impact
Appropriate data need to be systematically collected and captured to effectively monitor changes in pain characteristics, treatment impact, maintenance of effects, and costs at the systems level. While such data are often collected in specialty pain clinics and secondary/tertiary referral centers, data on pain severity, impact on function, mood and sleep, and pain type and pathophysiology are rarely documented in the primary-care setting in part because there are no simple EMR modules available to support such data collection. Efforts to develop and validate such modules, compatible with currently available EMR systems, are currently ongoing.[Citation125]
Such a detailed record would improve patient–clinician dialogue and help patients better understand their specific pain. This would allow patients to experience a greater level of engagement in their care that may lead to better self-management practices. Additionally, clinicians would be able to better identify subtle or sudden changes that could call for more in-depth assessments or referral to specialists. At the systems level, the use of such EMR module would provide access to information on the number of patients with chronic pain within the system and their demographics, medical history, comorbidities, concomitant medications, treatment(s) for chronic pain, adherence to guidelines, duration of therapy, and health-care utilization and costs. Changes in therapeutic approaches could be correlated with patient outcomes and health-care costs to develop more efficient algorithms, assess impact of special interventions such as patient and/or physician educational programs, identify and address gaps in clinical practice, and evaluate overall best practices.
The importance of detailed documentation is highlighted by the fact that management of chronic pain tends to be palliative and rehabilitative in nature rather than curative. Thus, in addition to their primary-care physician, the patient may see a variety of providers over the course of many years and a detailed, readily available patient record is extremely helpful in maintaining treatment continuity.
Conclusions and recommendations
We have reviewed several aspects of appropriate assessment and management of individuals with chronic pain that are recognized in current treatment guidelines but are not consistently addressed or implemented, particularly in the primary-care setting. By adopting a number of straightforward principles and using the practical approaches described in this manuscript, primary-care clinicians and other health-care providers should be able to improve the care of their patients and reduce the incidence of adverse effects. These principles are summarized below.
Because chronic pain is a biopsychosocial phenomenon and not purely a biological one, clinicians must consider a patient’s history, comorbid conditions, and psychological, socioeconomic, and contextual factors when developing a treatment plan.
A critical first step is to conduct a thorough, focused physical exam to establish both the potential cause of pain and the likely pathophysiologic type(s) of pain present in a particular patient (). Brief validated questionnaires and screening tools are available to assist the clinician in this assessment.
Table 3. Key elements regarding the assessment of the patient with chronic pain.
Clinicians should use their assessment of psychosocial factors and pathophysiologic pain type(s) to develop an individualized treatment plan incorporating non-pharmacologic therapy and appropriate first-line, non-opioid pain medications. Clinicians should set realistic patient-specific treatment goals with continual monitoring of the treatment effects on pain, function, mood, sleep, along with adverse effects. With any intervention, lack of improvement in pain and function, loss of effect over time, the appearance of significant adverse events, or inability to establish the cause and type of pain may be a reason for treatment change or discontinuation and/or referral to a pain specialist.
For patients who have not achieved acceptable levels of pain relief from appropriate non-pharmacologic therapy and first-line non-opioid pharmacologic medications during an adequate trial, clinicians may consider a brief trial of an opioid analgesic unless contraindicated (e.g. due to patient risk for misuse, abuse, addiction or diversion, or due to medical comorbidity) and unless the individual is not expected to benefit based on the likely type of pain pathophysiology present (e.g. sensory hypersensitivity). Clinicians should universally apply a set of risk management principles and strategies developed to support appropriate opioid prescribing (), including treatment discontinuation when warranted (e.g. if significant benefits are not observed within a few weeks of treatment). Clinicians should be aware of the correlation between higher opioid doses and the likelihood of serious adverse outcomes such as respiratory depression and death even in those using opioids as prescribed. Clinicians should be cautious of prescribing opioids in combination with drugs such as benzodiazepines and in patients with significant comorbid psychiatric disorders.
A multidisciplinary, collaborative approach to the management of chronic pain should be implemented whenever possible and to the fullest extent possible. Such an approach may include integrating pharmacotherapy with patient education, non-pharmacologic interventions such as physical and occupational therapy, behavioral health interventions such as CBT, counseling, and relaxation training, as well as acupuncture, and aerobic exercise. In certain cases, interventional pain procedures and neuromodulation may also be considered if the pain cannot be adequately managed by less invasive, optimized therapy.
All pain assessments and pain management decisions should be documented, preferably in an EMR, to facilitate individual patient monitoring over time and to enable tracking changes in pain prevalence, impact, treatment, and costs over time at the systems level.
It is important to acknowledge that implementation of these principles will require an upfront investment of time and effort on the part of the primary-care provider, including additional training, changes in workflow, and technological advances, among others. We believe, however, that the introduction of even one or some of the above approaches into the everyday practice may improve clinical outcomes and increase patient satisfaction, enhance efficiency throughout the treatment process, and in the long run may help decrease morbidity and disability and lower the costs associated with the treatment of chronic pain.
Declaration of interests
Development of this manuscript was funded by Pfizer Inc. Editorial support was provided by Matt Soulsby, PhD, CMPP, of Engage Scientific Solutions and was funded by Pfizer Inc. M Brodsky, S Donevan, PW Park, and S Watt are fulltime employees of, and own stock in, Pfizer Inc. S Stanos serves or has recently served as a consultant to Daiichi Sankyo, Endo, MyMatrixx, Pfizer, and Scillex; and has received research support from Grunenthal. C Argoff serves or has served as a consultant to Pfizer, Teva, Collegium, Purdue, Endo, Depomed, Xenoport, Astra‐Zeneca, Daichi‐Sakyo, Scilex, and Semnur Pharma; serves or has served as a speaker for Allergan, Teva, Xenoport, Depomed, Astra‐Zeneca, and Daiichi‐Sakyo; has received grants from Endo, Lilly, and Forest; and owns stock in Pfizer and Depomed. DJ Clauw serves or has served recently as a consultant for Pfizer, Forest, Eli Lilly, Pierre Fabre, Cypress Biosciences, Wyeth, UCB, Astra Zeneca, Merck, Johnson and Johnson, Nuvo, Jazz, Abbot, Cerephex, Iroko, Tonix, Theravance, IMC, Zynerba, Sammumed, Astellas, and Aptinyx; and has received research support from Pfizer, Cypress Biosciences, Forest, Merck, Nuvo, and Cerephex. Y D’Arcy serves or has recently served as a consultant to Pfizer, Depo‐Med, Charleston Labs, Astra Zeneka, and Ortho‐Mcneil; receives book royalties from Springer publishing; serves as a peer reviewer for Elsevier and Lippincott; and serves on the editorial boards for Nursing and The Nurse Practitioner Journal. KB Gebke has served as a consultant for Pfizer. MP Jensen has served as a consultant for Pfizer, Analgesic Solutions, Goalistics, and Malinckrodt previously, and his institution currently receives funding from Zogenix. E. Lewis&Clark is a past employee of, advisory board consultant to, and owner of stock in Pfizer Inc. B McCarberg serves or has recently served as a consultant to Pfizer, Collegium, Millennium, Takeda, Depomed, Janssen, Kaleo, and Astra Zeneca; and owns stock in Johnson and Johnson, Protein Design Labs, Biospecific Technologies, Nektar Therapeutics, Galena, and Collegium. DC Turk serves or has recently served as a consultant to Pfizer, Nektar, Develco, Ironwood, Mallinckrodt, Orexo, and Xydnia. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors would like to thank Dr John Markman for helpful discussions during early development of this manuscript.
References
- Mirchandani A, Saleeb M, Sinatra R. Acute and chronic mechanisms of pain. New York (NY): Springer Science and Business Media; 2011.
- Bonica JJ. The management of pain. Philadelphia (PA): Lea & Febiger; 1953.
- Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007.
- The Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education and research. Washington (DC): The National Academies Press; 2011.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012 [cited 2015 Nov 23]. Available from: http://seer.cancer.gov/csr/1975_2012
- Schiller JS, Lucas JW, Ward BW, et al. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012;10:1–207. Data from the National Health Survey.
- Health, United States, 2011: with special feature on socioeconomic status and health. National Center for Health Statistics; 2012 [cited 2015 Nov 23]. Available from: http://www.cdc.gov/nchs/data/hus/hus11.pdf
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780.
- The National Pain Strategy. National Institutes of Health [Last accessed 2016 Mar 23]. Available from: http://iprcc.nih.gov/docs/HHSNational_Pain_Strategy.pdf
- Mezei L, Murinson BB. Pain education in North American medical schools. J Pain. 2011;12:1199–1208.
- O’Rorke JE, Chen I, Genao I, et al. Physicians’ comfort in caring for patients with chronic nonmalignant pain. Am J Med Sci. 2007;333:93–100.
- McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119–130.
- Center for Disease Control. Morbidity and Mortality Weekly Report. 2013;62:234.
- National Survey on Drug Use and Health. 2013. U.S. Department Of Health and Human Services [cited 2015 Dec 14]. Available from: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf
- Americans talk about pain. Peter D. Hart Research Associates; 2003 [cited 2015 Dec 14]. Available from: http://www.researchamerica.org/sites/default/files/uploads/poll2003pain.pdf
- Harle CA, Bauer SE, Hoang HQ, et al. Decision support for chronic pain care: how do primary care physicians decide when to prescribe opioids? A qualitative study. BMC Fam Pract. 2015;16:48.
- Singh JA, Saag KG, Bridges SL Jr., et al. American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2015;2016(68):1–26.
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and rehabilitation. Neurology. 2011;76:1758–1765.
- Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:S3–S14.
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e88.
- Carville SF, Arendt-Nielsen L, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–541.
- Fitzcharles MA, Ste-Marie PA, Goldenberg DL, et al. Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag. 2012;2013(18):119–126.
- Berry PH, Chapman CR, Covington EC, et al. Pain: current understanding of assessment, management, and treatments [cited 2015 Dec 15]. Available from: http://www.npcnow.org/system/files/research/download/Pain-Current-Understanding-of-Assessment-Management-and-Treatments.pdf
- Turk DC. Remember the distinction between malignant and benign pain? Well, forget it. Clin J Pain. 2002;18:75–76.
- Fillingim RB, Dworkin RH, Turk DC. The ACTTION-APS pain taxonomy initiative: response to Henriques et al. J Pain. 2014;15:1201–1202.
- Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744.
- Franklin GM. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 2014;83:1277–1284.
- Painter JT, Crofford LJ. Chronic opioid use in fibromyalgia syndrome: a clinical review. J Clin Rheumatol. 2013;19:72–77.
- Schaible H-G, Ebersberger A, Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther. 2011;13:210.
- IASP Pain Taxonomy. International Association for the study of pain [cited 2015 Dec 14]. Available from: http://www.iasp-pain.org/Taxonomy
- Jay GW, Barkin RL. Neuropathic pain: etiology, pathophysiology, mechanisms, and evaluations. Dis Mon. 2014;60:6–47.
- Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract Res Clin Rheumatol. 2003;17:685–701.
- Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15.
- Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457–464.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states–maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–154.
- Wilbarger JL, Cook DB. Multisensory hypersensitivity in women with fibromyalgia: implications for well being and intervention. Arch Phys Med Rehabil. 2011;92:653–656.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–422.
- Jay GW, Barkin RL. Fibromyalgia. Dis Mon. 2015;61:66–111.
- Petersel DL, Dror V, Cheung R. Central amplification and fibromyalgia: disorder of pain processing. J Neurosci Res. 2011;89:29–34.
- Clauw DJ, Arnold LM, McCarberg BH, et al. The science of fibromyalgia. Mayo Clin Proc. 2011;86:907–911.
- Harris RE, Clauw DJ. How do we know that the pain in fibromyalgia is “real”? Curr Pain Headache Rep. 2006;10:403–407.
- Kato K, Sullivan PF, Evengård B, et al. Chronic widespread pain and its comorbidities: a population-based study. Arch Intern Med. 2006;166:1649–1654.
- Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547–1555.
- Kato K, Sullivan PF, Evengård B, et al. A population-based twin study of functional somatic syndromes. Psychol Med. 2009;39:497–505.
- Watson NF, Buchwald D, Goldberg J, et al. Neurologic signs and symptoms in fibromyalgia. Arthritis Rheum. 2009;60:2839–2844.
- Clauw DJ. Fibromyalgia and related conditions. Mayo Clin Proc. 2015;90:680–692.
- NIH workshop on chronic overlappin pain condition. National Institute of Neurological Disorders and Stroke [cited 2015 Dec 14]. Available from: http://www.ninds.nih.gov/news_and_events/events/meeting-summary-chronic-pain.htm
- Freynhagen R, Baron R, Gockel U, et al. pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920.
- Markman JD, Kress BT, Frazer M, et al. Screening for neuropathic characteristics in failed back surgery syndromes: challenges for guiding treatment. Pain Med. 2015;16:520–530.
- Hochman JR, Gagliese L, Davis AM, et al. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–654.
- Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65:3285–3292.
- Wolfe F, Clauw DJ, Fitzcharles M-A, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122.
- Wolfe F, Michaud K. Anemia and renal function in patients with rheumatoid arthritis. J Rheumatol. 2006;33:1516–1522.
- Mease PJ, Hanna S, Frakes EP, et al. Pain mechanisms in osteoarthritis: understanding the role of central pain and current approaches to its treatment. J Rheumatol. 2011;38:1546–1551.
- Meeus M, Vervisch S, De Clerck LS, et al. Central sensitization in patients with rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2012;41:556–567.
- Hawker GA, French MR, Waugh EJ, et al. The multidimensionality of sleep quality and its relationship to fatigue in older adults with painful osteoarthritis. Osteoarthritis Cartilage. 2010;18:1365–1371.
- Yunus MB. The prevalence of fibromyalgia in other chronic pain conditions. Pain Res Treat. 2012;2012:584573.
- Ruger LJ, Irnich D, Abahji TN, et al. Characteristics of chronic ischemic pain in patients with peripheral arterial disease. Pain. 2008;139:201–208.
- Rockett M. Diagnosis, mechanisms and treatment of complex regional pain syndrome. Curr Opin Anaesthesiol. 2014;27:494–500.
- Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19–25.
- Buenaver LF, Smith MT. Sleep in rheumatic diseases and other painful conditions. Curr Treat Options Neurol. 2007;9:325–336.
- Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(s1):S9–S27.
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133.
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132.
- Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–116.
- Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep. 2013;15:421.
- Jordan KD, Okifuji A. Anxiety disorders: differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother. 2011;25:231–245.
- Turk DC, Melzack R. Handbook of pain assessment. New York (NY): The Guilford Press; 2011.
- Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–489.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600–610.
- Universal precautions in opioid prescribing for chronic pain: the time has come [cited 2015 Dec 14]. Available from: http://rethinkopioids.com/applying-universal-precautions
- Management of opioid therapy for chronic pain [cited 2016 Jan 11]. Available from: http://www.healthquality.va.gov/guidelines/Pain/cot/COT_312_Full-er.pdf
- Washington State Interagency Guideline on Prescribing Opioids for Pain [cited 2016 Jan 11]. Available from: http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf
- Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: Potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clin Proc. 2012;87:683–694.
- Rolfs RT, Johnson E, Williams NJ, et al. Utah clinical guidelines on prescribing opioids for treatment of pain. J Pain Palliat Care Pharmacother. 2010;24:219–235.
- Hooten WM, Timming R, Belgrade M, et al., Institute for Clinical Systems Improvement. 2013. Assessment and management of chronic pain [ updated 2013; cited 2016 Jan 11]. Available from: https://www.icsi.org/_asset/bw798b/ChronicPain.pdf
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–474.
- Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46:328–346.
- Epstein JB, Wilkie DJ, Fischer DJ, et al. Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol. 2009;1:26.
- Gilron I, Watson CP, Cahill CM, et al. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006;175:265–275.
- Gulati A, Loh J. Assessment of pain: complete patient evaluation. New York (NY): Springer; 2011.
- Arnold LM, Clauw DJ, Dunegan LJ, et al. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc. 2012;87:488–496.
- Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22:1555–1565.
- Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122:1103–1111.
- Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology. 2013;119:1434–1443.
- Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67:1386–1394.
- Barkin RL, Beckerman M, Blum SL, et al. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging. 2010;27:775–789.
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173.
- Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251.
- Salsitz EA. Chronic pain, chronic opioid addiction: a complex nexus. J Med Toxicol. 2016;12:54–57.
- Nadeau SE. Opioids for chronic noncancer pain: to prescribe or not to prescribe-what is the question? Neurology. 2015;85:646–651.
- Argoff CE, Viscusi ER. The use of opioid analgesics for chronic pain: minimizing the risk for harm. Am J Gastroenterol. 2014;2:3–8.
- Katz MH. Mitigating the dangers of opioids. JAMA Inter Med. 2015;175:616–16.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States. MMWR Recomm Rep. 2016;65(1):1–49.
- Starrels JL, Becker WC, Weiner MG, et al. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958–964.
- Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467–483.
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508.
- Gourlay DL, Heit HA. Universal precautions revisited: managing the inherited pain patient. Pain Med. 2009;10(S2):S115–S123.
- Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2–guidance. Pain Phys. 2012;15:S67–S116.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. J Pain. 2009;10:113–130.e22.
- Peng X, Robinson RL, Mease P, et al. Long-term evaluation of opioid treatment in fibromyalgia. Clin J Pain. 2015;31:7–13.
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496.
- Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47.
- Barkhuizen A. Rational and targeted pharmacologic treatment of fibromyalgia. Rheum Dis Clin North Am. 2002;28:261–290.
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11:35–46.
- ACPA Resource Guide to Chronic Pain Medication and Treatment. American Chronic Pain Association; 2015 [cited 2015 Dec 14]. Available from: http://www.theacpa.org/uploads/documents/ACPA_Resource_Guide_2015_Final%20edited%20(3).pdf
- Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009;2:CD007407.
- Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26:1–9.
- Jensen MP. Psychosocial approaches to pain management: an organizational framework. Pain. 2011;152:717–725.
- Crombez G, Vlaeyen JW, Heuts PH, et al. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339.
- Roditi D, Robinson ME. The role of psychological interventions in the management of patients with chronic pain. Psychol Res Behav Manag. 2011;4:41–49.
- Keefe FJ, Shelby RA, Somers TJ. Catastrophizing and pain coping: moving forward. Pain. 2010;149:165–166.
- Jackson T, Wang Y, Fan H. Self-efficacy and chronic pain outcomes: a meta-analytic review. J Pain. 2014;15:800–814.
- Cheatle MD, Shmuts R. The risk and benefit of benzodiazepine use in patients with chronic pain. Pain Med. 2015;16:219–221.
- Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry. 2014;26:214–224.
- National Center for Complementary and Integrative Health website [cited 2015 Dec 15]. Available from: https://nccih.nih.gov/health/pain/chronic.htm
- Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med. 2013;125:25–33.
- Anitescu M, Benzon HT, Argoff CE. Advances in topical analgesics. Curr Opin Anaesthesiol. 2013;26:555–561.
- Moody ML. Topical medications in the treatment of pain. Pain Med News. 2010;8:15–21.
- Dworkin RH, O’Connor AB, Kent J, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154:2249–2261.
- Turk DC, Stanos SP, Palmero TM, et al. Interdisciplinary pain management white paper [cited 2015 Dec 14]. Available from: http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white-paper.pdf
- Peppin JF, Cheatle MD, Kirsh KL, et al. The complexity model: a novel approach to improve chronic pain care. Pain Med. 2015;16:653–666.
- Kroenke K, Krebs E, Wu J, et al. Stepped Care to Optimize Pain care Effectiveness (SCOPE) trial study design and sample characteristics. Contemp Clin Trials. 2013;34:270–281.
- Coyne K, Currie B, Donevan S, et al. Psychometric validation of the electronic chronic pain questions in a primary care setting. Postgrad Med. 2015;127:S49.
