ABSTRACT
Background: US FDA guidance recommends measuring the degree of effort needed to manipulate abuse-deterrent (AD) opioids. The ALERRT® instrument (PinneyAssociates; Bethesda, MD) uses visual analog scales to assess the labor, effort, and resources necessary to physically compromise AD product candidates in standardized settings. Objective: Use the ALERRT® instrument for testing morphine abuse-deterrent, extended-release, injection-molded tablets (ADER-IMT) 60 and 100 mg and the comparators immediate-release (IR) morphine sulfate 30 mg and extended-release (ER) morphine sulfate 60 mg. Methods: Four technicians tested the products using 10 household tools. The ALERRT instrument quantified effort (all tools) and time (3 preselected tools) required for manipulation. Results: Morphine-ADER-IMT 60 and 100 mg were difficult to manipulate, as demonstrated by high scores (mean range, 71.0−99.0 and 77.0−99.5, respectively). IR and ER morphine sulfate were easy to manipulate (low scores; mean range, 2.0−14.8 and 2.3−17.5, respectively). Statistically significant mean differences between morphine-ADER-IMT and comparators’ ALERRT scores were observed. Manipulations of morphine-ADER-IMT 60 and 100 mg for 300 seconds failed to produce substantial powdering. Manipulations of IR morphine sulfate (mean range, 65.5−175.8 seconds) and ER morphine sulfate (49.3−163.0 seconds) produced substantial to complete powdering in 92% of tablets. Conclusions: Morphine-ADER-IMT was extremely difficult to manipulate versus non-AD formulations of morphine. The ALERRT system differentiated the degree of effort for manipulation of morphine-ADER-IMT and non-AD morphine formulations, indicating sensitivity of this instrument as part of Category 1 testing. By measuring the degree of effort required for manipulation, the ALERRT instrument provides an empirical assessment into the relative difficulty of manipulating opioid analgesics for abuse.
Introduction
Widespread prescription use of opioids for chronic pain has led to greater opioid availability, with the majority of nonmedical users obtaining the drug from a friend or relative (Citation1). In 2014 in the United States, 4.3 million people aged ≥12 reported nonmedical use of pain relievers within the previous month (Citation2); similar numbers were reported in 2011, 2012, and 2013 (4.5, 4.9, and 4.5 million, respectively) (Citation3). In the United States, overdose with opioids, including heroin, resulted in 28,647 deaths in 2014, with a 14% increase in the age-adjusted rate of drug overdose deaths compared with 2013 (Citation4). Prescription drugs were associated with 22,767 overdose deaths in 2013; 71.3% of these deaths (16,235) involved the use of opioids, the most frequently prescribed drug category in the United States and the most common treatment for chronic pain (Citation3,Citation5).
Some formulations of immediate-release (IR) and extended-release (ER) opioids can be manipulated to achieve more rapid drug release and to facilitate alternate routes of administration (i.e., injecting, snorting, and smoking) (Citation6,Citation7). Abuse-deterrent (AD) formulations have been developed to substantially reduce misuse and abuse of opioids by making manipulation more difficult or rendering the manipulated product less attractive and less amenable to alternate routes of administration (Citation8). In vitro laboratory-based studies (i.e., Category 1) designed to determine the relative degree of difficulty of compromising the AD properties of a formulation are a key component in the evaluation and labeling of AD opioids by the US Food and Drug Administration (FDA) (Citation8). Because of differing AD technologies, these studies use individually tailored physical and chemical manipulation procedures to evaluate the different AD product candidates along with their appropriate comparators. Typical outcomes include the characteristics of the test formulation after crushing, grinding, or milling, and changes in the formulation that would limit dissolution of the manipulated product (Citation8).
To date, the focus of Category 1 testing has been on characterizing the output resulting from different attempts at manipulating AD product candidates. These tests essentially attempt to reproduce common abuse practices in laboratory settings under standardized conditions. Another important dimension of Category 1 testing is the input aspect or the “degree of effort required to bypass or defeat the abuse-deterrent properties” (Citation8).
The amount of time, effort, and resources required to manipulate a specific formulation affects its attractiveness for misuse and abuse. For example, an easily crushed opioid formulation that can be readily prepared for injection within minutes is likely to be preferred over a hardened tablet that resists extraction and takes considerable effort and time to manipulate. In a previous study, 40.0% to 53.3% of participants (experienced users of opioids for nonmedical purposes) stated that they were willing to spend a maximum of 10 minutes tampering with oxycodone (10 or 40 mg) (Citation9). A comparison of two formulations of oxymorphone found that the maximum time participants were willing to spend tampering with the products was approximately 15 minutes (Citation10). In a similar study, participants spent an average of 3 to 7 minutes to manipulate oxycodone or tapentadol for intranasal or intravenous administration even though they were allowed up to 60 minutes (Citation11). The latest report from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS) system showed that ease of manipulation was a main factor for a strong preference for IR over ER opioids (Citation12). Therefore, AD technology that requires time-consuming manipulation to achieve drug recovery in clinically important quantities could be a useful deterrent against drug misuse and abuse.
In this concept, the measurement of effort, time, and resources can be evaluated with visual analog scale (VAS) measurements in a similar manner to the assessment of subjective behaviors (e.g., Drug Liking and Take Drug Again VASs used in human abuse potential studies). Similar use of VASs can provide routine assessment of the degree of effort for physical manipulations of new and existing opioid products.
The ALERRT® (PinneyAssociates, Bethesda, MD) instrument, which uses VASs and was developed to assess the labor, effort, and resources required for tampering, measures quantitatively the amount of effort associated with physical manipulation attempts commonly used by drug users. The overall concept is that of an “input” and “output” functional relationship. In addition to Category 1 studies that focus on the “output” of the manipulation effort, the ALERRT instrument was developed to capture information about the “input” (time, effort, and resources necessary to convert the product into an abusable form). If the degree of effort required is perceived by drug users to be onerous, it is possible that this property may in and of itself deter abuse (Citation9,Citation11). In this respect, it is important to note that this dimension is not captured in Category 3 clinical studies designed to evaluate the abuse potential of a new drug. Because the investigational product is prepared in a standardized manner in a clinical pharmacy without subject involvement, subjects do not go through the process of manipulating the product before responding to the clinical endpoints (e.g., Drug Liking and Take Drug Again). Category 3 studies focus solely on assessing the subjective responses predictive of the likelihood of abuse, with a manipulated preparation of the test products, without regard to how much work is required to render the drug into an abusable form (Citation8). As more AD products are developed, and because the instruments that measure drug liking and other pharmacodynamic endpoints are not very sensitive or discriminating, the “input” or level of effort to get a drug into an abusable form will become a more critical factor in the overall assessment of abuse potential to reflect real-world conditions and the impact that AD formulations could have.
The morphine abuse-deterrent, extended-release injection-molded tablet (ADER-IMT) is a novel AD, ER morphine product candidate (EG-001; Egalet Corporation, Wayne, PA) that incorporates a proprietary matrix technology (Guardian™; Egalet) in combination with a novel manufacturing process, plastic injection molding, using conventional and safe pharmaceutical polymers. This combination of formulation and process results in tablets with controlled-release properties as well as physical and chemical barriers that have been demonstrated to resist common and rigorous methods of manipulation in Category 1 studies, thereby limiting particle size reduction (Citation13). Furthermore, in oral and intranasal Category 3 human abuse potential studies, overall drug liking and other key indicators of abuse potential were significantly lower after administration of oral manipulated morphine-ADER-IMT or after insufflation of morphine-ADER-IMT compared with a marketed formulation of ER morphine that had been crushed (Citation14,Citation15).
Using the ALERRT instrument, the degree of effort required to physically compromise morphine-ADER-IMT was compared with the degree of effort required to compromise 2 marketed formulations without AD properties (IR morphine sulfate [generic; Roxane Laboratories, Columbus, OH] and ER morphine sulfate [MS Contin®; Purdue Pharma LP, Stamford, CT]).
Material and methods
ALERRT instrument
In accordance with the 2015 FDA guidance on AD opioids, and to better assess the degree of effort needed to manipulate these product candidates, the ALERRT instrument was developed to quantify and standardize this aspect of Category 1 testing (Citation8). Four trained technicians from an independent laboratory (Drugscan, Horsham, PA) performed standardized physical manipulations of four opioid formulations for a maximum of 300 seconds and assessed the amount of effort (all tools) and time (3 preselected tools) using the ALERRT instrument. The opioid formulations tested were morphine-ADER-IMT (60 mg), morphine-ADER-IMT (100 mg), and the comparator tablets IR morphine sulfate (30 mg) and ER morphine sulfate (60 mg). Unfortunately, the unique feature and look of each of the formulations prevented the blinding of the technicians with respect to AD status. The 10 household tools used were spoons, mortar/pestle, pill crusher, hammer, food grater, foot file, razor blade, spice grinder, and two coffee grinders.
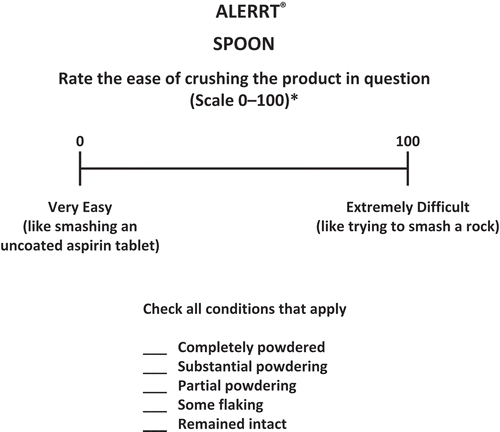
The ALERRT instrument uses a series of 100-mm VASs (0 = very easy, 100 = extremely difficult; example shown in ) specific to 10 household tools commonly used by recreational users of prescription drugs to manipulate opioid products. Each scale is tailored for a specific tool so that the scales vary as a function of each tool. For example, for the spoon, ease of crushing is measured and for the knife ease of cutting is measured. The mean overall ALERRT score is defined as the mean value for all of the tools. Scores on the high end of the scale denote “extremely difficult to defeat,” and scores on the low end denote “very easy to defeat.” Prior to the start of each testing day, the technicians used a mortar and pestle to perform a calibration procedure. The calibration procedure consisted of placing one metal nut into the mortar and used a pestle to attempt to crush the nut for 30 seconds. This assessment represented the extreme right side of the VAS which is labeled “Extremely Difficult.” After removing the nut, an uncoated aspirin tablet was placed in the mortar and a pestle was used to attempt to crush the tablet for 30 seconds. This assessment represented the extreme left side of the VAS which is labeled “Extremely Easy.” The calibration procedures reminded the technicians of the VAS anchors. Technicians typically started with simple compression tools (e.g., spoons), then progressed to more complex tools and finally to high-powered electrical tools (e.g., coffee grinders). In addition to the quantitative measurement of time and effort/input required to manipulate opioid products, some predefined descriptive terms were used (such as, but not limited to, “completely powdered,” “substantial powdering,” “partial powdering,” “some flaking,” and “remained intact”) to further elaborate on and provide a qualitative component for the results/output of the manipulations.
Statistical analysis
Four opioid formulations were nested within 10 tools, which were nested within four technicians. A simple random permutation was used to assign tablet order to tool for each technician. The primary analysis examined the difference in means for ALERRT scores of the four opioid formulations for each type of household tool, and descriptive statistics (mean, SD, median, range) were calculated. Mean (SD) differences in ALERRT scores between formulations and between raters were calculated; 95% CIs were used to interpret statistical significance, where applicable. The number of tablets manipulated to different outcomes such as “mostly chunks and flaking” was captured for each tool (except the razor blade because of different descriptive outcomes), and proportions for each outcome were calculated. A correlation analysis was conducted to evaluate reliability among raters, and Pearson product–moment correlation coefficients are reported.
Results
Assessing degree of effort for physical manipulation with the ALERRT instrument
ALERRT scores
ALERRT scores for morphine-ADER-IMT were at the higher end of the scale for all tools, signifying the need for substantial manipulation effort. Scores for the 60 mg tablets (mean range, 71.0–99.0) and 100 mg tablets (mean range, 77.0–99.5) were comparable (). In contrast, scores for IR morphine sulfate and ER morphine sulfate were at the lower end of the scale for all tools, indicating that less effort was needed to manipulate them. Scores were comparable for IR morphine sulfate (mean range, 2.0–14.8) and ER morphine sulfate (mean range, 2.3–17.5).
Table 1. ALERRT scores for each tablet by type of tool.
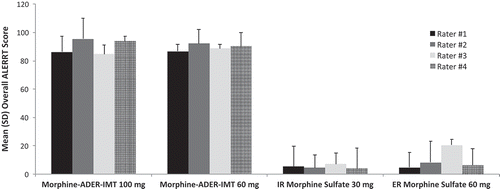
Mean overall ALERRT scores (mean for all of the tools) demonstrated that 60 and 100 mg morphine-ADER-IMT were extremely difficult to manipulate, whereas mean overall ALERRT scores for IR and ER morphine sulfate indicated that these tablets were easily manipulated (). The differences in ALERRT scores between each formulation of morphine-ADER-IMT and the comparators were significant based on 95% CIs. No significant difference was observed between 60 and 100 mg morphine-ADER-IMT (). There was general consistency among the four raters for each of the four formulations () and for the mean overall rater scores, with mean ratings for each rater, when collapsed across all formulations and tools, ranging from 45.8 to 50.3 (data not shown). The Pearson product–moment correlation coefficients in this study among the raters for the 10 tools ranged from 0.90 to 1.00, reflecting excellent reliability among the raters.
Figure 2. Mean (SD) overall ALERRT scores and differences in overall ALERRT scores between tablets. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; ER = extended release; IR = immediate release; VAS=visual analog scale. Values are the mean (SD) of the ALERRT scores for each tablet when collapsed across all 10 tools and all four raters. Tabular values are the differences in mean (SD) overall ALERRT scores for each tablet when collapsed across all 10 tools and all four raters.
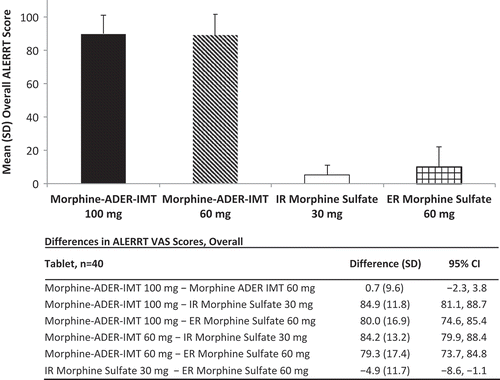
Figure 3. Mean (SD) overall ALERRT scores for individual tablets by rater. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; ER = extended release; IR = immediate release. Values are the mean (SD) of the ALERRT scores for each tablet for each rater when collapsed across all 10 tools.
Duration and outcome of manipulation
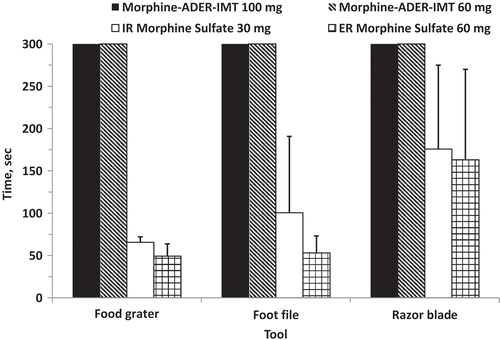
A food grater, foot file, and razor blade were preselected for the determination of the time needed to manipulate each formulation. Morphine-ADER-IMT (60 and 100 mg) withstood 300 seconds (maximum time allowed) of manipulation with all three tools (), with little breakdown or powdering of the product (i.e., no tablets were reduced to a state of substantial or complete powdering). In contrast, the mean duration of manipulation with the same tools ranged from 65.5 to 175.8 seconds for IR morphine sulfate and from 49.3 to 163.0 seconds for ER morphine sulfate. In most instances (92%), these attempts resulted in substantial, complete, and/or fine powdering of the IR and ER morphine sulfate tablets.
Figure 4. Mean (SD) duration of manipulation for individual tablets by preselected tool. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; ER = extended release; IR = immediate release. Values are the mean (SD) of the duration of manipulation for each tablet for each preselected tool when collapsed across all four raters, with a maximum of 300 seconds or until no further reduction in size was achieved.
When the outcomes for all 10 tools were tabulated, substantial to complete powdering was observed in 97.2% of IR morphine sulfate tablets and in 77.1% of ER morphine sulfate tablets () and without damage to any of the tools used for manipulation. In contrast, none of the tools produced complete or substantial powdering of morphine-ADER-IMT (), with 94.4% and 97.3% of 100 mg and 60 mg morphine-ADER-IMT remaining “intact/mostly intact” or with “some flaking/mostly chunks and flaking,” respectively. Furthermore, the hardness of morphine-ADER-IMT produced mechanical failure or damage to some of the tools used for attempting tablet crushing and grinding, including one broken pestle, six cracked coffee grinder lids, and five broken coffee grinders.
Table 2. Number of different outcomes* after manipulation of each tablet.
Discussion
The level of effort and the time required for manipulation of novel morphine-ADER-IMT were compared with manipulation of IR and ER morphine sulfate using the ALERRT instrument as a means of quantifying effort (i.e., input). The assessment was conducted by four independent technicians with various tools as part of the Category 1 characterizations. Key findings demonstrated that morphine-ADER-IMT was extremely hard, and it was difficult to produce particle size reduction, requiring much greater effort and time compared with non-AD IR and ER morphine comparators. Not only was there a high level of effort and time used in attempts to manipulate morphine-ADER-IMT, but the outcomes of the manipulations of morphine-ADER-IMT (as descriptively characterized as a supplement to the ALERRT scoring) indicated a clear differentiation to the outcome of the manipulations of IR and ER morphine sulfate. Some of the attempts to defeat morphine-ADER-IMT resulted in damage to a number of tools without any success in achieving a state of complete or substantial powdering. In contrast, IR and ER morphine sulfate tablets were easily crushed to a fine powder, and no instruments were damaged in the efforts. The combination of the hardness of morphine-ADER-IMT, the high degree of effort needed for physical manipulation as indicated by the ALERRT data, and the resistance of the product to particle size reduction (the first step in getting a product into abusable form) suggests that the potential for misuse and abuse of morphine-ADER-IMT will be lower than with currently marketed non-AD products.
A recent report from the RADARS system showed that ease of manipulation was a main factor in the determination of preference for different formulations of opioids in an abuser population (Citation12). The measurement of the degree of effort needed to defeat or compromise the AD features of different opioid formulations is an important component of Category 1 laboratory studies (Citation8). The results from this ALERRT study demonstrate the practicality and utility of this instrument in Category 1 physical manipulation studies. The consistency in the ALERRT ratings by the four laboratory technicians was high but with some of the variability that would be expected under real-world conditions.
Drug liking is considered a reliable assessment of the likelihood of abuse of a drug, and human abuse potential studies can be used to analyze the likability of various intact and physically manipulated formulations of drugs (Citation16,Citation17). As drug users attempt to manipulate prescription drugs to change the route of administration and produce greater euphoria (Citation17), a significant increase in time and effort required to achieve maximal euphoria may play a role in decreasing the likelihood of abuse of a given drug formulation. This was the rationale for the design of Guardian Technology and its physical/chemical barrier mechanism to deter opioid misuse and abuse. The ALERRT findings serve as complementary data to Category 3 clinical abuse potential studies, in which the products are manipulated in a standardized fashion in a clinical pharmacy, and the subjects do not have to put in the time and effort themselves to prepare the products for abuse. Therefore, similar drug liking data from a clinical abuse potential study may not mean that the products have the same AD profile, especially if it is harder to get one product into an abusable form compared with another.
Limitations
A potential limitation of this study is the use of only four technicians to perform the evaluation of the test and comparator products using the ALERRT instrument. For determination of the overall ALERRT scores, the use of four technicians results in n=40, which is sufficient to perform a statistical comparison between the test and comparator products. However, for the assessment of the time needed to manipulate the formulations with three prespecified tools, the n=4 may have contributed to the variability observed with IR morphine sulfate (foot file and razor blade) and ER morphine sulfate (razor blade). Another potential limitation of this study was the inability to blind the test formulations because of the unique features and looks of each of the products which could lead to bias. The overall ALERRT scores between each of the technicians were consistent for each of the formulations suggesting that potential bias because of the inability to blind the test formulations was small.
Conclusions
In conclusion, both “input” (i.e., physical and chemical work required to try to defeat an AD product) and “output” (i.e., the results of physical and chemical manipulations) affect the level of attractiveness of an opioid for abuse. Therefore, it is essential to measure both factors when evaluating the AD potential of novel formulations. This can be done in a laboratory setting during Category 1 testing, and these findings should be considered in the translation and meaning of Category 3 human abuse potential findings. The ALERRT instrument differentiated between the degree of effort required for manipulation of morphine-ADER-IMT and two non-AD formulations of morphine, indicating the utility of this new tool in assessing the level of effort involved for physical manipulation of AD formulations as an important part of FDA Category 1 studies. The AD profile of morphine-ADER-IMT, based on the hardness of the tablet, the level of effort required to manipulate it, and the limited reduction in particle size after significant time and effort spent, suggests that this product candidate with these AD features could help reduce accidental misuse by chewing and intended abuse by manipulation compared with non-AD morphine ER products currently on the market.
Financial disclosures
Edward J. Cone is an employee of PinneyAssociates and provides consulting services, including the design and interpretation of Category 1 studies, to Egalet and other developers of CNS medications. Financial support was provided by Egalet for the preparation of this manuscript.
August R. Buchhalter is an employee of PinneyAssociates and provides consulting services, including the design and interpretation of Category 1 studies, to Egalet and other developers of CNS medications. Financial support was provided by Egalet for the preparation of this manuscript.
Karsten Lindhardt is an employee of Egalet Corporation and owns stock and stock options in the company.
Torben Elhauge is an employee of Egalet Corporation and owns stock and stock options in the company.
Jeffrey M. Dayno is an employee of Egalet Corporation and owns stock and stock options in the company
Funding
This study was sponsored and funded by Egalet Corporation (Wayne, PA). Medical writing support was provided by Complete Publication Solutions, a CHC Group company (North Wales, PA), and funded by Egalet Corporation.
Additional information
Funding
References
- Bannwarth B. Will abuse-deterrent formulations of opioid analgesics be successful in achieving their purpose? Drugs 2012;72:1713–1723.
- Substance Abuse and Mental Health Services Administration. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD: US Department of Health and Human Services; 2015. HHS Publication No. (SMA) 15–4927.
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: US Department of Health and Human Services; 2014. HHS Publication No. (SMA) 14–4863.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–1382.
- Grady D, Berkowitz SA, Katz MH. Opioids for chronic pain. Arch Intern Med 2011;171:1426–1427.
- Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain 2013;14:351–358.
- Katz N, Dart RC, Bailey E, et al. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse 2011;37:205–217.
- US Food and Drug Administration. Guidance for industry: abuse-deterrent opioids – evaluation and labeling. Silver Spring, MD: US Department of Health and Human Services; 2015.
- Sellers EM, Perrino PJ, Colucci SV, Harris SC. Attractiveness of reformulated OxyContin® tablets: assessing comparative preferences and tampering potential. J Psychopharmacol 2013;27:808–816.
- Vosburg SK, Jones JD, Manubay JM, et al. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug Alcohol Depend 2012;126:206–215.
- Vosburg SK, Jones JD, Manubay JM, et al. A comparison among tapentadol tamper-resistant formulations (TRF) and OxyContin® (non-TRF) in prescription opioid abusers. Addiction 2013;108:1095–1106.
- Cicero TJ, Ellis MS, Harney J. Abuse prevalence and preference of immediate release versus extended release opioids. RADARS System Technical Report, 2015-Q3; 2015.
- Cone EJ, Buchhalter AR, Lindhardt K, Elhauge T, Skak N. Crushing and extraction resistance of EG-001, an abuse-deterrent ER morphine in clinical development [abstract 32]. Presented at: PAINWeek, September 2–6, 2014; Las Vegas, NV.
- Smith MD, Webster LR, Lawler J, Lindhardt K, Dayno JM. Human abuse potential of an abuse-deterrent (AD), extended-release (ER) morphine product candidate (Morphine-ADER Injection-Molded Tablets) versus extended-release morphine administered orally in nondependent recreational opioid users. Pain Med 2016:[Epub ahead of print].
- Webster LR, Smith MD, Lawler J, Lindhardt K, Dayno JM. Human abuse potential of an abuse-deterrent (AD), extended-release (ER) morphine product candidate (Morphine-ADER Injection-Molded Tablets) vs extended-release morphine administered intranasally in nondependent recreational opioid users. Pain Med 2016:[Epub ahead of print].
- Comer SD, Zacny JP, Dworkin RH, et al. Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. Pain 2012;153:2315–2324.
- Turk DC, O’Connor AB, Dworkin RH, et al. Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations. Pain 2012;153:1997–2008.