ABSTRACT
Background: Maternal cannabis use in pregnancy is linked with long-term adverse behavioral outcomes in offspring. Epigenetic processes established in utero that affect dopaminergic (reward) signaling may mediate risks. Associations between cannabis use and offspring DNA methylation have not been investigated; however, maternal tobacco smoking in pregnancy is associated with distinct patterns of DNA methylation at birth and beyond. Objectives: To determine whether maternal cannabis use is associated with methylation of the dopamine receptor gene DRD4 promoter in infants. Methods: Mothers in the Triple B study provided detailed information on drug use in each trimester of pregnancy. Buccal swabs were collected from neonates at 8 weeks (n = 804, 51.7% male, and 48.3% female). DRD4 promoter DNA methylation was measured using SEQUENOM MassARRAY. Results: Fifty-seven of the women in the study reported drug use during pregnancy, of whom 44 used cannabis. Of 19 cytosine-phosphate-guanine dinucleotides (CpG) units tested in DRD4, gestational cannabis use was associated with offspring methylation at 1 CpG unit in multivariate models (β + 1.48, CI: 0.02 to 2.93, and p = 0.047). At another site there was weak evidence that both cannabis and other drug use were independently associated with increased methylation, while the association with tobacco was in the reverse direction (cannabis use β + 0.67, CI: −0.12 to 1.46, and p = 0.09; other drug use β + 1.11, CI: 0.17 to 2.05, and p = 0.02; tobacco use β −0.41, CI: −0.85 to 0.03, and p = 0.07). None of the associations would remain significant after correction for multiple testing. Conclusion: There is no strong evidence that maternal cannabis use in pregnancy is associated with offspring DRD4 methylation.
Introduction
Drug or “substance” use during pregnancy is relatively common, reported by up to 5% of women in the general population, with cannabis the most frequently used (Citation1). Given the changing prevalence of use related to legislative changes in some countries (i.e., increasing use with decriminalization), studies concerning maternal cannabis use are an important consideration for public health (Citation2,Citation3).
Although research into the effects of prenatal exposure to maternal cannabis use on offspring is still in its infancy, adverse effects have been reported. A recent meta-analysis involving 24 studies found that cannabis-exposed infants had a higher risk of anemia, decreased birth weight, and a greater chance of being placed in intensive care (Citation4). Subsequent studies have reported similar findings (Citation5) as well as longer-term detrimental effects. Mothers who used cannabis daily in the first trimester had offspring with deficits in verbal reasoning and short-term memory in childhood (Citation6) and any in utero exposure has been associated with attention problems and increased aggression in 18-month-old girls (Citation7). Heavy cannabis use (one or more joints a day) during the first trimester of pregnancy has also been associated with lower reading, spelling, maths, and comprehension skills in 10-year-old primary school students (Citation8), which may be due in part to an increased risk of mood disorders such as anxiety and depression.
The detrimental effects of cannabis could result from the transmission of Δ9-tetrahydrocannabinol (THC), the psychoactive constituent within cannabis, to the fetus (Citation9). THC can readily cross the placenta, it has been shown to increase the diameter of umbilical veins and arteries, and THC stimulation of endocannabinoid signaling may induce apoptosis of developing placental cytotrophoblasts (Citation10). Thus biologically available THC can have a negative impact on the fetus in utero. Given its key role in prenatal development and association with several postnatal phenotypes in humans, epigenetic mechanisms may help mediate these short-term and long-term detrimental effects (Citation11). Broadly, epigenetics refers to environmentally sensitive alterations in gene expression that do not change the underlying DNA sequence (Citation12). DNA methylation, particularly methylation of cytosine-phosphate-guanine dinucleotides (CpG), is the most commonly studied epigenetic mechanism.
Most epigenetic studies of cannabis have focused on the effects of cannabis use in adults, primarily investigating the THC cannabinoid receptor type 1 (CNR1) which encodes the receptor CB1R. Elevated methylation of the CNR1 promoter and downregulation of CB1R mRNA, as measured in peripheral blood, is associated with cannabis use in schizophrenia patients (Citation13). Methylation levels were also related to the severity and frequency of cannabis cravings. CB1R is known to be co-expressed with dopamine receptors (Citation14) and cannabis, like all recreational drugs, causes an increase in dopamine levels in the terminal regions of the mesolimbic dopamine system. Dopamine signaling is linked to memory, cognition, and is one of the major reward pathways in the brain implicated in drug addiction (Citation15). Little is known about the effects of maternal cannabis use on methylation of genes involved in dopamine signaling in offspring.
The primary aim of this study was to determine the association between maternal gestational cannabis use and offspring DNA methylation. Of the set of genes controlling neuro-signaling within dopaminergic pathways, this study focused on offspring DRD4 methylation patterns given that this gene has not only been associated with substance use (Citation16) and addiction (Citation17,Citation18), but importantly has also been linked to infant health outcomes, including birth weight, behavior, and neurodevelopment (Citation19,Citation20). Differential methylation of this gene could thus help mediate the association between maternal substance use and poor infant health outcomes, although no studies to date have yet measured DRD4 methylation in this regard.
The secondary aim was to investigate how maternal tobacco smoking could influence the association between cannabis and infant methylation. Most studies to date do not differentiate cannabis from tobacco use, despite the fact that cannabis is often smoked together with tobacco, and tobacco has similar effects on infant health. This is problematic because maternal smoking during pregnancy has been consistently shown to influence infant DNA methylation in peripheral tissue and to mediate the association between prenatal exposure (Citation21,Citation22) and infant birth weight (Citation23). Furthermore, a very recent study found that cannabis alone had no independent effect on perinatal outcomes, but augmented the risk associated with tobacco smoking (Citation24). Studies examining effects associated with tobacco and cannabis in isolation and in combination are needed to elucidate unique effects.
Methods
Triple B pregnancy cohort
This longitudinal pregnancy cohort of 1,634 families is focused on perinatal maternal lifestyle factors and infant health outcomes. Families were recruited through general public and specialist drug and alcohol antenatal services at major hospitals in New South Wales and Western Australia (Citation25). Mother–infant dyads with major medical complications were excluded. Written informed consent was provided by all participants, and all relevant human research ethics committees approved the study. In-depth health, lifestyle, and sociodemographic data were collected across pregnancy and postpartum. This included detailed questionnaires about drug use in each trimester of pregnancy and the first 8 weeks postpartum.
Methylation analysis
The DRD4 methylation assay covered a 396 base pair region of the gene promoter spanning chr11:635,510–636,905 (UCSC hg38), previously shown to be differentially methylated in association with attention deficit hyperactivity disorder (ADHD) (Citation26). Infant buccal swabs were collected at 8 weeks of age. Extracted DNA underwent bisulfite conversion (EZ-96 DNA Methylation-LightningTM MagPrep kit (Irvine, CA)), followed by PCR amplification in triplicate with primers 5ʹ-GGACCCCCTGCCCAGGGTCAGAGG-3ʹ and 5ʹ-TGCCAGATACCAGGTGGACTAGGGTG-3ʹ, and Sequenom MassARRAY analysis (Citation27). Average DRD4 methylation was calculated after excluding any triplicate samples deviating by ≥10% from the median. Outlying data (>10% outside 1.5xIQR) were excluded, as were CpG units (n = 4) and participants with <85% data retained after quality control (n = 92). Health, lifestyle, and sociodemographic data did not differ significantly between excluded participants and those included in this analysis.
Statistical analysis
To investigate the association between prenatal maternal substance use (yes/no) with infant DRD4 promoter methylation, t-tests were used for univariate analysis, followed by multivariate linear regression models adjusted for potential confounding factors, and Sequenom batch effects.
Results
shows the maternal and infant characteristics of the population according to cannabis use during pregnancy. Cannabis use was the most commonly used drug (n = 44, 77.2% of drug users) and was most common among mothers in trimester 1 (), likely prior to knowledge of pregnancy. Of those who reported cannabis use, eight used cannabis daily throughout trimester 1, with the dose ranging between (the equivalent of) 0.5 and 7 joints. A total of 29 women used other types of drugs, with the type and quantity varying quite considerably. Of these women, 16 also used cannabis.
Table 1. Maternal and infant population characteristics according to maternal cannabis use during pregnancy (N = 804*).
Table 2. Maternal substance use across pregnancy and at 8 weeks postpartum.
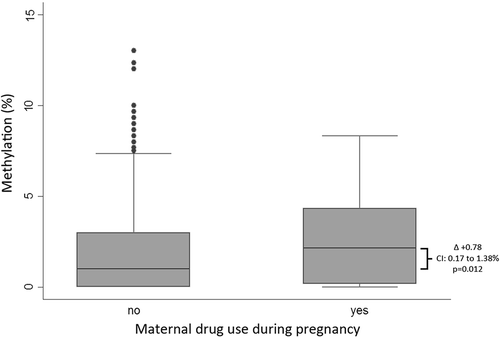
We first investigated the association between any type of substance use during pregnancy and infant DRD4 promoter methylation. Of 19 CpG units tested, the only evidence of an association was a very small increase in methylation at CpG.32 (Δ + 0.78, 95% CI: 0.17–1.38%, and p = 0.012) (, Table S1). Cannabis use was similarly associated with differential methylation at the same site (Δ + 0.79, 95% CI: 0.11–1.46%, and p = 0.023) (, Table S2). These associations remained even after adjustment for covariates, including drug use at 8 weeks postpartum, given the potential for effects resulting from exposure during breastfeeding or second-hand exposure. Smoking tobacco during pregnancy was itself associated with DRD4 methylation at this same site, but in the reverse direction (cannabis: β + 0.77, 95% CI: 0.06 to 1.48, and p = 0.032; tobacco: β −0.41, 95% CI: −0.85 to 0.03, and p = 0.072). However, when adjusting for other substance use as a covariate, which was also associated with increased CpG.32 methylation, the association with cannabis becomes nonsignificant (cannabis: β + 0.67, 95% CI: −0.12 to 1.46, and p = 0.098; other substance use: β + 1.11, 95% CI: 0.17 to 2.05, and p = 0.020). Cannabis use was also associated with increased methylation at CpG.21.22.23 when we adjusted for tobacco use in the multivariate model (β + 1.48, 95% CI: 0.02 to 2.93, and p = 0.047, unadjusted Δ + 1.03, 95% CI: −0.29% to 2.36%, and p = 0.13), and regardless of other drug use. Out of interest, adding alcohol use during pregnancy to these models did not alter the associations seen.
Figure 2. Mean infant DRD4 promoter methylation according to maternal cannabis use during pregnancy. Bars indicate 95% CI.
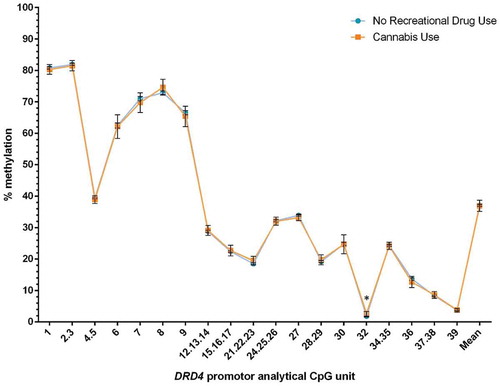
Given that cannabis is frequently taken together with tobacco, we further compared DRD4 promoter methylation levels across four groups: no drug or tobacco use (n = 658), tobacco alone (n = 96), cannabis alone (n = 9), and both cannabis and tobacco (n = 35). Only cannabis use appeared to increase methylation compared to no substance use (). In addition, exclusive cannabis use was associated with 2.08% higher methylation at CpG.32 compared to tobacco use alone (95% CI: 0.19–3.96% and p = 0.024). Exclusive cannabis use compared to combined cannabis and tobacco showed no significant difference in DNA methylation (p = 0.48).
Table 3. Infant DRD4 CpG.32 methylation when comparing substance use groups to no substance use during pregnancy.
Discussion
Here we report a nominally significant association between cannabis use during pregnancy and increased DRD4 promoter methylation at one CpG site (CpG11:636796, hg38) in infants, which remained after adjustment for concurrent tobacco use. We also found some, although nonsignificant, evidence that both other drug use and cannabis use appeared to increase methylation at another site, while tobacco smoking had a reverse effect (decreased methylation). This is despite reports that cannabis and tobacco have similar (Citation28) and even cumulative detrimental effects on infant health (Citation24). However, the overall effect sizes observed are very small and the strength of association would not remain if corrected for multiple testing (using a Bonferroni corrected significance level of 0.0026 given the 19 CpG units examined).
Dopamine signaling is involved in many cognitive processes, including memory and reward (Citation15). Cannabis dependence is linked to a reduction in striatal dopamine release which is also correlated with poor working memory and learning performance (Citation29). Prenatal cannabis exposure has been associated with decreased dopamine receptor D2 expression in human fetal brain specimens (Citation30). Dopamine receptor deficiency has been linked to increased choice and immediacy to use drugs, and is observed in people with substance use disorders (Citation31). DRD4, a D2-like dopamine receptor, was chosen as a strong candidate gene in this study due to its key role in dopamine signaling, and its links to drug use in genetic association studies. A DRD4 variable number tandem repeat has been associated with increased problematic cannabis use (Citation32), and a heightened risk of externalizing behavior problems that contribute to substance use disorders and addiction (Citation33). DRD4 genetic variants can also moderate the effect of prenatal stress on children’s antisocial behavior (Citation34). Furthermore, increased DRD4 methylation in saliva has been associated with increased severity of ADHD symptoms in children (Citation35).
Our study is strengthened by the in-depth data concerning substance use and tobacco smoking across pregnancy and the large sample size. The percentage of women using drugs during pregnancy (7.03%) was higher than that reported in the general Australian population (2.2%) (Citation36). Our study had over 80% power (α = 0.05, two-sided) to detect a minimum effect size of 0.385 standard deviation (SD) difference in the mean methylation between groups. For CpG.32 for example, with a group SD of 2.2, our study could detect a methylation difference of 0.86% between groups defined by drug use. The power of the analysis was nevertheless limited, particularly in regard to the timing or dose of exposure during pregnancy, which could not be investigated, or specific drugs other than cannabis, which are likely to have varying effects on methylation and infant health outcomes (Citation37). Tissue specificity of DNA methylation marks may contribute to the largely negative findings of our study. Dopamine signaling is prominent in the brain, but due to the limitations in accessing such tissue, we chose to investigate buccal cell DNA methylation, given that (Citation1) buccal epithelial cells have the same developmental origins to neuronal cells (i.e., derive from ectoderm) and (Citation2) previous studies have provided some support for the use of buccal cells as a proxy for neurodevelopmental phenotypes (Citation38). The power of the study may have been limited due to underreporting of maternal substance use, which could help explain the predominantly negative findings (if a proportion of women classified as nonusers had in fact used drugs during pregnancy). However, to address this limitation in the Triple B study, a random selection of 85 participants provided urine samples for analysis in the third trimester of pregnancy. There was 97% agreement between self-report substance use and urine analysis, indicating the overall high accuracy of the self-report data. Other limitations are that we could not properly investigate the independent effects of tobacco from cannabis, as only nine cannabis users in the study did not smoke tobacco.
Conclusion and future research
We found no convincing evidence of an association between prenatal substance use overall or cannabis use and differential infant DRD4 methylation. However, given the reported effects of maternal drug use on infant health outcomes, as well as consistent evidence that smoking tobacco during pregnancy influences infant peripheral DNA methylation, it is possible that genes other than DRD4 may be involved, such as CNR1. Further studies could investigate other components of dopaminergic signaling, or use a more unbiased discovery approach such as an epigenome-wide association study. Both approaches would benefit from a substantially larger sample of maternal drug users. Such approaches are essential to provide a better understanding of the biological mechanisms underlying the association between prenatal drug exposure and infant health outcomes.
Declaration of interest
The authors declare no conflicts of interest.
IADA_A_1314488_Supplementary_Data.docx
Download MS Word (26.6 KB)Acknowledgments
We gratefully acknowledge NDARC and NDRI, the research staff and students who assisted with collection of the data, the hospitals and antenatal clinics for their assistance with recruitment, and the study participants and their families. We also gratefully acknowledge the investigators of the Triple B Pregnancy Cohort Study not listed as authors. Finally, we acknowledge the Cannabis Cohorts Research Consortium (NHMRC Project Grants: AAP1009381, AAP1064893), the Biobank at the Murdoch Childrens Research Institute, and Dr Benjamin Ong for assistance with the Sequenom MassARRAY platform.
Funding
This current study is funded by a grant from the Financial Markets Foundation for Children (Australia) (2015-252). The Triple B study was funded by an Australian National Health and Medical Research Council (NHMRC) Project Grant #GNT630517 for $2,196,179 and was financially supported by the National Drug and Alcohol Research Centre (NDARC), University of New South Wales. NDARC and the National Drug Research Institute (NDRI), Curtin University, are funded by the Australian Government under the Substance Misuse Prevention and Service Improvements Grants Fund. We also acknowledge financial support from Australian Rotary Health and the Foundation for Alcohol Research and Education (FARE).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s
Additional information
Funding
References
- Roth CK, Satran LA, Smith SM. Marijuana use in pregnancy. Nurs Women’s Health 2015;19:431–437.
- Calvigioni D, Hurd YL, Harkany T, Keimpema E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry 2014;23:931–941.
- Cook J, Lloyd-Jones DM, Ogden E, Bonomo Y. Medical use of cannabis: an addiction medicine perspective. Intern Med J 2015;45:677–680.
- Gunn JKL, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, Ehiri JE. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016;6:e009986.
- Brown SJ, Mensah FK, Ah Kit J, Stuart-Butler D, Glover K, Leane C, Weetra D, et al. Use of cannabis during pregnancy and birth outcomes in an Aboriginal birth cohort: a cross-sectional, population-based study. BMJ Open 2016;6:e010286.
- Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry 2008;47:254–263.
- El Marroun H, Hudziak JJ, Tiemeier H, Creemers H, Steegers EA, Jaddoe VW, Hofman A, et al. Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug Alcohol Depend 2011;118:470–474.
- Goldschmidt L, Richardson GA, Cornelius MD, Day NL. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol 2004;26:521–532.
- Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry 2012;7:149–156.
- Costa MA. The endocannabinoid system: a novel player in human placentation. Reprod Toxicol 2016;61:58–67.
- Vaiserman AM. Long-term health consequences of early-life exposure to substance abuse: an epigenetic perspective. J Dev Orig Health Dis 2013;4:269–279.
- Lan N, Chiu MP, Ellis L, Weinberg J. Prenatal alcohol exposure and prenatal stress differentially alter glucocorticoid signaling in the placenta and fetal brain. Neuroscience 2015; 342:167–179.
- Liu J, Chen J, Ehrlich S, Walton E, White T, Perrone-Bizzozero N, Bustillo J, et al. Methylation patterns in whole blood correlate with symptoms in schizophrenia patients. Schizophr Bull 2014;40:769–776.
- Morris CV, DiNieri JA, Szutorisz H, Hurd YL. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur J Neurosci 2011;34:1574–1583.
- Rice ME, Patel JC. Somatodendritic dopamine release: recent mechanistic insights. Philos Trans R Soc Lond B Biol Sci 2015;370:1672.
- Bobadilla L, Vaske J, Asberg K. Dopamine receptor (D4) polymorphism is related to comorbidity between marijuana abuse and depression. Addict Behav 2013;38:2555–2562.
- Di Ciano P, Pushparaj A, Kim A, Hatch J, Masood T, Ramzi A, Khaled MA, et al. The impact of selective Dopamine D2, D3 and D4 ligands on the rat gambling task. Plos One 2015;10: e0136267.
- Baker TE, Stockwell T, Barnes G, Haesevoets R, Holroyd CB. Reward sensitivity of ACC as an intermediate phenotype between DRD4-521T and substance misuse. J Cogn Neurosci 2016;28:460–471.
- Wazana A, Moss E, Jolicoeur-Martineau A, Graffi J, Tsabari G, Lecompte V, Pascuzzo K, et al. The interplay of birth weight, dopamine receptor D4 gene (DRD4), and early maternal care in the prediction of disorganized attachment at 36 months of age. Dev Psychopathol 2015;27:1145–1161.
- Money KM, Stanwood GD. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci 2013:7:260.
- Markunas CA, Xu Z, Harlid S, Wade PA, Lie RT, Taylor JA, Wilcox AJ. Identification of DNA methylation changes in newborns related to maternal smoking during pregnancy. Environ Health Perspect 2014;122:1147–1153.
- Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, Reese SE, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet 2016;98:680–696.
- Kupers LK, Xu X, Jankipersadsing SA, Vaez A, La Bastide-Van Gemert S, Scholtens S, Nolte IM, et al. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol 2015;44:1224–1237.
- Chabarria KC, Racusin DA, Antony KM, Kahr M, Suter MA, Mastrobattista JM, Aagaard KM. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol 2016;215:506.e1–7.
- Hutchinson D, Wilson J, Allsop A, Elliott E, Najman J, Burns L, Bartu A, et al. The Triple B Pregnancy Cohort Study: a longitudinal study of the relationship between alcohol, tobacco and other substance use during pregnancy and the health and wellbeing of Australian children and families. 2017;epub.
- Van Mil NH, Steegers-Theunissen RPM, Bouwland-Both MI, Verbiest MMPJ, Rijlaarsdam J, Hofman A, Steegers EA, et al. DNA methylation profiles at birth and child ADHD symptoms. J Psychiatr Res 2014;49:51–59.
- Januar V, Ancelin ML, Ritchie K, Saffery R, Ryan J. BDNF promoter methylation and genetic variation in late-life depression. Transl Psychiatry 2015;5:e619.
- Ion R, Bernal AL. Smoking and preterm birth. Reprod Sci 2015;22:918-926.
- Van De Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R, Ojeil N, et al. Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry 2017; 22:68–75.
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL. Maternal cannabis use alters ventral striatal Dopamine D2 gene regulation in the offspring. Biol Psychiatry 2011;70:763–769.
- Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, Dean AC, London ED. Low Dopamine D2/D3 receptor availability is associated with steep discounting of delayed rewards in methamphetamine dependence. Int J Neuropsychopharmacol 2015;18: pyu119.
- Olsson CA, Moyzis RK, Williamson E, Ellis JA, Parkinson-Bates M, Patton GC, Dwyer T, et al. Gene-environment interaction in problematic substance use: interaction between DRD4 and insecure attachments. Addict Biol 2013;18:717–726.
- Mallard TT, Doorley J, Esposito-Smythers CL, McGeary JE. Dopamine D4 receptor VNTR polymorphism associated with greater risk for substance abuse among adolescents with disruptive behavior disorders: preliminary results. Am J Addict 2016;25:56–61.
- Zohsel K, Buchmann AF, Blomeyer D, Hohm E, Schmidt MH, Esser G, Brandeis D, et al. Mothers’ prenatal stress and their children’s antisocial outcomes: a moderating role for the dopamine D4 receptor (DRD4) gene. J Child Psychol Psychiatry 2014;55:69–76.
- Dadds MR, Schollar-Root O, Lenroot R, Moul C, Hawes DJ. Epigenetic regulation of the DRD4 gene and dimensions of attention-deficit/hyperactivity disorder in children. Eur Child Adolesc Psychiatry 2016;25:1081–1089.
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey Detailed Report 2013. Australian Institute of Health and Welfare; 2014.
- El Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, Huizink AC. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J Am Acad Child Adolesc Psychiatry 2009;48:1173–1181.
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A, Moffitt TE, Mill J. A longitudinal study of epigenetic variation in twins. Epigenetics 2010;5:516–526.