ABSTRACT
Background: Opioid use disorder during pregnancy is a growing health concern. Methadone maintenance is the treatment of choice but emerging data indicate buprenorphine is a viable alternative. Due to costs and limited accessibility of methadone, pregnant women may require transition from methadone to buprenorphine for maintenance treatment. Objectives: To assess safety and effectiveness of transitioning from methadone to buprenorphine when necessary during pregnancy. Methods: A standardized protocol using low buprenorphine doses to minimize emergent withdrawal symptoms under careful obstetric and psychiatric monitoring was implemented in 20 pregnant women. Outpatient maternal and neonatal outcomes were assessed. Results: Women maintained on an average methadone dose of 44 ± 4.77 (20–100) mg/day (mean±standard error mean (SEM); range) were successfully transitioned to 12.60 ± 0.8 (8–16) mg/day (mean±SEM; range) of buprenorphine. Within 4 weeks of transition, 15% had illicit drugs detected in urine drug screens. Ninety percent of women maintained outpatient follow-up until delivery. At delivery, 38.9% of mothers were exclusively adherent to buprenorphine (without use of illicit substances and/or other psychotropic medications); this resulted in significantly lower rates of neonatal abstinence syndrome (NAS) and shorter hospital stays. Discussion: Pregnant women transitioned from methadone to buprenorphine maintenance showed maternal and neonatal outcomes comparable to studies of women on buprenorphine throughout pregnancy. Infants born to buprenorphine-maintained women who abstained from illicit substances and other prescribed psychotropic medications experienced less severe NAS and shorter hospitalizations compared with women with illicit substance use and other psychotropic medications. These findings suggest women can safely be transitioned from methadone to buprenorphine during pregnancy.
Background
Opioid use disorder during gestation and the perinatal period are growing health concerns as they negatively impact both the pregnant woman and her infant. There are many complications of illicit opioid use during pregnancy. Mothers with opioid use disorder have less frequent healthcare and more chaotic life circumstances that often result in inadequate nutrition and exposure to infectious diseases such as human immunodeficiency virus (HIV) and hepatitis C (Citation1–Citation3). Opioid use disorder during pregnancy is associated with increased risks of abortion, preterm labor, premature rupture of membranes, preeclampsia, intrauterine growth restriction, stillbirth, low birth weight, decreased length and head circumference, and concurrent substance use, and other psychiatric disorders (Citation3–Citation5). Active, stable involvement in a treatment program with maintenance therapy attenuates several of these risks. Infants born to opioid-dependent mothers are also at risk for opioid withdrawal upon delivery which is known as neonatal abstinence syndrome (NAS) (Citation3).
The treatment of opioid use disorder is commonly managed with methadone as maintenance pharmacotherapy. Methadone, a long-acting opioid agonist, is usually taken once daily to alleviate cravings and prevent withdrawal symptoms (Citation6). Methadone clinics are mandated by law to have intense clinical oversight, and methadone is usually distributed on a daily basis. This method of distribution may add a costly and time-consuming burden on the pregnant woman, as this can interfere with normal day-to-day activities. Despite being the standard of care for opioid use disorder, 40% of state medicaid programs do not cover the cost of methadone (Citation7). Buprenorphine, a high-affinity partial mu opioid agonist and kappa opioid antagonist, serves as an alternative pharmacotherapy to address opioid use disorder during pregnancy (Citation3). Interestingly, buprenorphine is on the preferred medication lists for 49 state medicaid programs and the District of Columbia and thus the cost of buprenorphine during pregnancy is covered (buprenorphine is available in one state as a part of a fee for service program). In addition, there is emerging literature that more pregnant women and their healthcare providers are choosing buprenorphine instead of methadone during pregnancy (Citation8).
While in the past, women were encouraged to continue methadone throughout pregnancy, buprenorphine may offer certain benefits compared with methadone for maintenance of pregnant women with opioid use disorder. In an international, randomized controlled clinical trial comparing buprenorphine and methadone, buprenorphine is supported as an acceptable treatment of opioid use disorder during pregnancy (Citation9). In a number of subsequent studies examining methadone and buprenorphine maintenance during pregnancy, the incidence of NAS is equivalent, while buprenorphine maintenance has been associated with decreased severity and duration of NAS compared with methadone maintenance (Citation8–Citation12).
As data supporting buprenorphine maintenance during pregnancy emerge, it is important to develop an approach to safely transfer pregnant women from methadone maintenance to buprenorphine. Transition from methadone to buprenorphine has not been recommended in clinical practice, especially during pregnancy, due to the risks of precipitated withdrawal upon substitution of a partial agonist for a full agonist that is slowly eliminated from the body.
According to best practice in non-pregnant women, methadone is tapered over weeks to months to a maximum of 30 mg methadone daily for at least 1 week and then discontinued prior to the induction of buprenorphine (Citation13). Such an approach is not practical during pregnancy, which is usually a period of psychosocial stress and limited duration. As methadone-maintained pregnant women with opioid use disorder may desire buprenorphine maintenance for a variety of reasons, including access, affordability, and commentary in the lay press, deciding to transition from methadone to buprenorphine and how to optimally accomplish this are often encountered questions in this field.
In this report, we describe a clinically viable and efficacious method to transition pregnant women with opioid use disorder initially maintained on methadone to buprenorphine maintenance, which has become increasingly necessary in our patients. This transition is accomplished with attentive inpatient monitoring and the implementation of a standardized protocol that minimizes opioid withdrawal and cravings and associated risks to the fetus.
Methods
Transition to buprenorphine from methadone maintenance during pregnancy
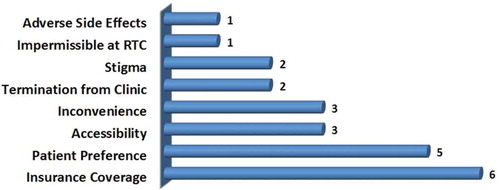
Twenty patients were admitted to the Vanderbilt Addiction Center inpatient service for psychiatric stabilization. As this was a clinical protocol implemented in our hospital as a quality improvement project to allow women who might otherwise not have access to medication-assisted treatment financed by Tenncare (Tennessee Medicaid), the institutional review board/ethics committee deemed that approval was not required. As patients requested to be fully detoxified from methadone, all were individually educated about associated risks as well as the potential benefits of alternative maintenance on buprenorphine by the attending psychiatrist (PRM). As medication-assisted treatment was medically recommended and methadone maintenance was no longer feasible, an informed decision was made by each patient to transition to buprenorphine. The most common reasons for requesting transition from methadone to buprenorphine were: insurance coverage, patient preference, accessibility, and inconvenience ().
Figure 1. Rationale for transitioning from methadone to buprenorphine. Insurance coverage was the most common reason for the transition. RTC, residential treatment center.
Buprenorphine induction may be complicated in pregnancy, particularly if a pregnant woman is either on methadone maintenance or has obtained methadone from the street. As premature administration of buprenorphine can precipitate opioid withdrawal, appreciable withdrawal symptoms must be present before initiating buprenorphine (Citation13). While patients abusing short-acting opioids may experience moderate withdrawal symptoms 6 h after last opioid dose (Citation3), patients taking longer acting opioids such as methadone may need to be monitored for greater than 24 h before manifesting withdrawal signs that would allow buprenorphine induction. In the non-pregnant patient, withdrawal symptoms that are moderate to moderately severe (Clinical Opiate Withdrawal Scale (COWS) score of at least 15) are advised prior to initiation of buprenorphine. However, for induction of buprenorphine during pregnancy, severe opioid withdrawal symptoms are not acceptable, and therefore, withdrawal severity must be carefully monitored and minimized. For safety of the fetus, we used a COWS score of 10 or greater as the threshold for initiation of buprenorphine during pregnancy. The COWS score at which buprenorphine was initiated indicates with some certainty that there are some unoccupied opioid receptors available for the starting low dose of buprenorphine.
As buprenorphine has such high affinity for opioid receptors, a very low dose preferentially (compared with methadone) occupies only the vacant receptors with minimal accentuation of withdrawal symptoms. The initial administration of low-dose buprenorphine was subsequently followed by additional small doses upon the return of moderate withdrawal symptoms (COWS score of 10 or greater). Appropriate fetal monitoring was performed prior and during the transition to buprenorphine.
Protocol
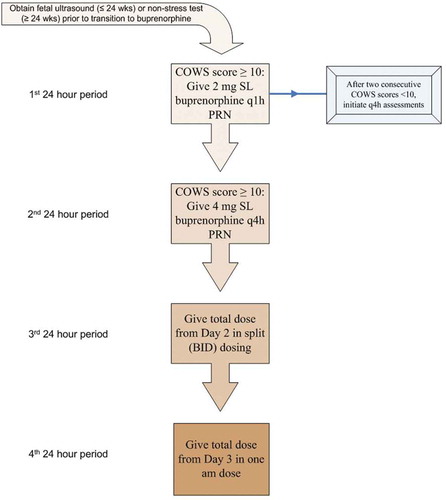
Transition to buprenorphine in the setting of methadone maintenance was implemented in 20 pregnant women using the protocol outlined in . After appropriate counseling and confirmation of obstetric stability, administration of the patient’s daily dose of methadone was discontinued and monitoring began with COWS assessments which measured withdrawal symptoms (Citation14). An obstetric ultrasound (<24 weeks gestation) or fetal non-stress testing (>24 weeks) was obtained with successive fetal monitoring on an individualized basis. Patients were frequently assessed with the COWS protocol (at least hourly). Once each patient exhibited moderate withdrawal symptoms, a 2 mg dose of buprenorphine was administered hourly as needed for the first 24 h. After two consecutive COWS scores <10, assessments were performed less frequently (every 4 h). For the next 24 h period, 4 mg instead of 2 mg doses of buprenorphine was given every 4 h as needed for COWS score ≥10. On the third day of buprenorphine administration, the total amount of buprenorphine given on Day 2 was split into two doses (am and pm). On Day 4, the cumulative dose from Day 3 was administered in one morning dose. Early during this stabilization process, 2 mg doses of buprenorphine were given only when withdrawal symptoms emerged (COWS ≥ 10), but was unlikely after the second day of the protocol.
Given the long half-life of methadone (8–59 h) (Citation15), it was important to continue close monitoring and limited administration of buprenorphine based on tolerability over several days to minimize precipitated opioid withdrawal symptoms related to slow removal of methadone from opioid receptors (tapering of methadone dose is not necessary) due to much greater affinity of buprenorphine to these same receptors. Continued as needed, dosing on Day 2 ensured that patients were able to tolerate higher doses of buprenorphine at once (4 vs. 2 mg) and further established total daily requirement of buprenorphine. The dose split on Day 3 also ensured that patients could tolerate escalated dosages of buprenorphine without abrupt displacement of any remaining methadone. By Day 4, patients were able to tolerate once daily dosing of buprenorphine with little to no concern for precipitating withdrawal symptoms due to time since last methadone use.
Statistical analysis
Pearson correlation analysis was conducted to determine any relationship between characteristics of patients transitioned from methadone to buprenorphine as listed in . The significance of NAS incidence was determined with χ2 test for analysis of contingency. Welch’s t-test was used to ascertain significant differences of duration of hospital stays between groups.
Table 1. Demographic information including age and maintenance doses of methadone and buprenorphine.
Results
Implementation of the protocol () resulted in a safe, smooth transition from methadone to buprenorphine as maintenance treatment of opioid use disorder in pregnancy for 20 patients previously maintained on regular daily methadone doses of 20–100 mg for an average of 6.5 months (). Ninety percent of the patients reported prior adherence with methadone but as mentioned above were unwilling or unable to continue on methadone. Patients were administered the initial buprenorphine dose on average 19 h after their last dose of methadone. The protocol was continued and patients reached stable dosing of buprenorphine in less than 4 days with an average total length of stay of less than 8 days accounting for assessment and/or treatment of psychiatric symptoms ().
Figure 2. Transition to buprenorphine from methadone maintenance during pregnancy. Protocol for the transition from methadone to buprenorphine for opioid maintenance during pregnancy. SL, sublingual; PRN, as needed.
After initial obstetric assessments, seven patients received additional fetal monitoring (non-stress tests, biophysical profiles, tocometry) based on findings of initial fetal monitoring or subjective discomfort. Intermittent absence of fetal breathing was the most notable finding during fetal assessments and there were no unfavorable occurrences such as induction of labor, threatened abortions, or fetal demise. Overall, patients reported effectiveness of buprenorphine in relieving withdrawal symptoms while inpatient. Methadone dose and subsequent dose of buprenorphine were correlated (r = 0.5074; p < 0.05); each (methadone and buprenorphine maintenance doses) were also correlated with maximal COWS scores (r = 0.5813; p < 0.01 and r = 0.5121; p < 0.05, respectively). Age and time to first buprenorphine dose were positively correlated (r = 0.4716; p < 0.05). There was no significant correlation between methadone or buprenorphine maintenance dose and stabilization time or duration of hospitalization.
Outpatient outcomes
Outpatient outcomes for patients transitioned to buprenorphine are shown in . Most patients continued outpatient care within our clinics throughout their pregnancy (90%). Only one patient discontinued buprenorphine due to intolerable nausea and resumed methadone along with illicit substances at time of delivery. Buprenorphine doses were subsequently increased during their pregnancy in four patients. More than 80% of these women reported that buprenorphine was an effective treatment of opioid use disorder and were adherent with buprenorphine at the time of delivery. During the first 4 weeks following the initial transition to buprenorphine, 15% of patients reverted to illicit substance use. There were no re-hospitalizations during this initial 4-week period; however, three patients were hospitalized later during pregnancy for substance use. Illicit substance use was noted in 44% (n = 8/18) of these patients at some point during their pregnancy, and 47% (n = 8/17) had urine drug screens that were positive for illicit substances prior to delivery. The most commonly used illicit substances were opioids (e.g., oxycodone, hydrocodone) in addition to amphetamine, benzodiazepines, cannabis, cocaine, and methadone. Thirty-three percent (n = 6/18) of buprenorphine-maintained women were also prescribed other psychotropic medication at the time of delivery ().
Table 2. Data including compliance, illicit substance use, and reported effectiveness of buprenorphine.
Neonatal outcomes
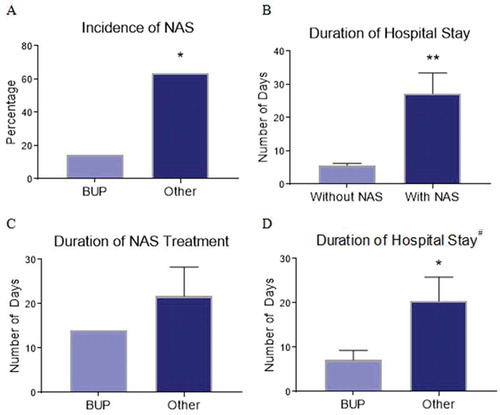
Neonatal outcomes of 18 infants born to buprenorphine-maintained women are illustrated in and . The average gestational age at time of delivery was nearly 39 weeks with two preterm deliveries. Eight neonates were diagnosed with NAS and treated with morphine for an average of 21 days including intensive care and nursery. Among neonates exposed to buprenorphine only (mother exclusively adherent to buprenorphine maintenance without illicit substance use or other prescribed psychotropic medications), only 1 neonate (14.3%) was diagnosed with NAS, whereas 7 (63.6%) of the neonates born to women with buprenorphine non-adherence, illicit substance use proximal to delivery, and/or prescribed psychotropic medications had NAS (, p < 0.05). The duration of hospitalization for infants with NAS was significantly longer compared with infants without NAS (27.25 and 5.6 days, respectively, p < 0.01) as shown in . The average duration of NAS treatment was shorter in neonates exposed only to buprenorphine compared with neonates born to women with buprenorphine non-adherence, illicit substances, and/or psychotropic medications (14 and 21.7 days, respectively) as depicted in . The average duration of total hospitalization was also shorter in neonates exposed only to buprenorphine compared with those exposed to psychotropic medications and illicit substances (7.1 and 20.4 days, respectively, p < 0.05, ). Two neonates (both exposed to buprenorphine only) were admitted to the intensive care unit due to poor feeding/hypoglycemia and respiratory distress/concern for sepsis. Cardiac murmur was noted for two neonates with reassuring echocardiograms (one neonate exposed to buprenorphine, cannabis, and oxcarbazepine; and one neonate exposed to benzodiazepines, opiates, amphetamine, and cocaine).
Table 3. Neonatal outcomes of infants born to buprenorphine-maintained women.
Figure 3. Neonatal outcomes. Data regarding incidence (A) of neonatal abstinence syndrome (NAS), duration of hospitalization comparing infants without and with NAS (B); duration of NAS treatment (C); and total hospitalization duration (D) of infants born to buprenorphine-maintained women. BUP, adherent with buprenorphine only; Other, buprenorphine non-adherence, illicit substance use indicated by urine drug screen proximal to delivery (amphetamines, benzodiazepines, cannabis, cocaine, methadone, and opiates), and/or prescribed psychotropic medication. #Includes all infants both with and without NAS. *P < 0.05; **P < 0.01.
Discussion
Twenty women previously maintained on methadone were successfully transitioned to buprenorphine in the inpatient setting over the course of approximately 4 days. Importantly, all but one of the women who were followed through delivery continued buprenorphine treatment after transition was accomplished, and effectiveness and satisfaction with buprenorphine was reported in more than 80% women, comparable to other reports (Citation9). Neonates whose mothers were exclusively adherent to buprenorphine treatment without exposure to other psychotropic medication or illicit substances had a relatively low rate of NAS (1/7) compared with previous reports (Citation9). However, the development of NAS, duration of NAS treatment, and length of hospital stay of neonates in our sample were influenced by maternal use of illicit substances and/or prescribed psychotropic medications as has been reported in the literature (Citation16). In our report, neonates exposed only to buprenorphine had significantly less NAS and shorter hospitalizations than neonates born to mothers with buprenorphine non-adherence, illicit substance use, and/or other psychotropic medications.
The standard pharmacological treatment for opioid use disorder has been methadone. However, methadone is not always available to patients due to affordability or other limitations. With the recent research on positive neonatal outcomes with buprenorphine maintenance therapy (Citation17), transitioning pregnant women to buprenorphine is an acceptable option. Perhaps because buprenorphine is a partial mu opioid receptor agonist and methadone is a full agonist, there is some evidence that lower buprenorphine doses (16 vs. 24 mg doses) are associated with better outcomes, such as relapse prevention and retention. This is in contrast to reports in methadone maintenance studies where higher methadone doses are associated with better outcomes (Citation13).
In summary, the maternal and neonatal outcomes of pregnant women transitioned from methadone to buprenorphine maintenance were comparable to studies of women with buprenorphine maintenance throughout their pregnancies. Also, neonates born to buprenorphine-maintained women who abstained from illicit substances and did not receive other prescribed psychotropic medications experienced less severe NAS and shorter hospitalizations compared with women with illicit substance use and psychotropic medications. These findings suggest that women previously maintained on methadone can be safely and effectively transitioned to buprenorphine maintenance using the protocol described herein. Buprenorphine is effective in relapse prevention and improving outcomes for both the mother and infant. Buprenorphine is emerging as an evidence-based pharmacotherapy for opioid use disorder during pregnancy and is more often than not the preferred medication by state medicaid programs.
Declaration of interest
One of the authors serves on the Lofexidine Scientific Advisory Board of U.S. WorldMeds in Louisville, Kentucky.
References
- Krans EE, Zickmund SL, Rustgi VK, Park SY, Dunn SL, Schwarz EB. Screening and evaluation of hepatitis C virus infection in pregnant women on opioid maintenance therapy: a retrospective cohort study. Subst Abus [Internet]. 2016 Jan 2 [cited 2016 Nov 11]; 37(1):88–95. Available from: https://www.tandfonline.com/doi/full/10.1080/08897077.2015.1118720.
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years: Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep [Internet]. 2015 May 8 [ cited 2016 Nov 11]; 64(17):453–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25950251.
- Young JL, Martin PR. Treatment of opioid dependence in the setting of pregnancy. Psychiatr Clin North Am [Internet]. 2012 Jun [ cited 2015 May 14]; 35(2):441–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22640765.
- Minnes S, Lang A, Singer L. Prenatal tobacco, marijuana, stimulant, and opiate exposure: outcomes and practice implications. Addict Sci Clin Pract [Internet]. BioMed Central; 2011 Jul [ cited 2016 Nov 11]; 6(1):57–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22003423.
- Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy. Anesthesiology [Internet]. 2014 Dec [ cited 2016 Nov 11]; 121(6):1158–65. Available from: http://anesthesiology.pubs.asahq.org/Article.aspx?doi=10.1097/ALN.0000000000000472.
- Martin P, Patel S, Swift RM. Pharmacology of drugs of abuse. Principles Pharmacology: Pathophysiologic Basis Drug Therapy [Internet]. Lippincott Williams & Wilkins; 2011 [ cited 2015 Dec 9]. Available from: https://books.google.com/books?id=WM7rvNUcrdsC&pgis=1.
- Substance Abuse and Mental Health Services Administration. Medicaid coverage and financing of medications to treat alcohol and opioid use disorders [Internet]. Publication No. SMA-14-4854. Rockville, MD: Department of Health and Human Services; 2014. Available from: http://store.samhsa.gov/shin/content//SMA14-4854/SMA14-4854.pdf.
- Meyer MC, Johnston AM, Crocker AM, Heil SH. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. J Addict Med [Internet]. 2015 Jan [ cited 2015 Jun 15]; 9(2):81–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25622120.
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med [Internet]. 2010 Dec 9 [ cited 2015 Jun 15]; 363(24):2320–31. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3073631&tool=pmcentrez&rendertype=abstract.
- Chen H-H, Chiang Y-C, Yuan ZF, Kuo C-C, Lai M-D, Hung T-W, et al. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatr Dis Treat [Internet]. Dove Press; 2015 Jan 6 [ cited 2015 Jun 15]; 11:609–18. Available from: http://www.dovepress.com/buprenorphine-methadone-and-morphine-treatment-during-pregnancy-behavi-peer-reviewed-fulltext-article-NDT.
- Simmat-Durand L, Lejeune C, Gourarier L. Pregnancy under high-dose buprenorphine. Eur J Obstet Gynecol Reprod Biol [Internet]. 2009 Feb [ cited 2015 Jun 15]; 142(2):119–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19058904.
- Mittal L. Buprenorphine for the treatment of opioid dependence in pregnancy. J Perinat Neonatal Nurs [Internet]. 2014 Jan [ cited 2015 Jun 15]; 28(3):178–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25062519.
- McNicholas L. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treat Improv Protoc Ser 40 DHHS Publ No 04-3939 Subst Abus Ment Heal Serv Adm [Internet]. 2014 [ cited 2015 Dec 9]; Available from: http://www.ncbi.nlm.nih.gov/books/NBK64245/pdf/Bookshelf_NBK64245.pdf.
- Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs [Internet]. 2011 Jan [ cited 2015 Jun 15]; 35(2):253–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12924748.
- Stanhope T, Gill L, Rose C. Chronic opioid use during pregnancy: maternal and fetal implications. Clin Perinatol [Internet]. 2013;40(3):337–50. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23972743.
- Patrick SW, Dudley J, Martin PR, Harrell FE, Warren MD, Hartmann KE, et al. Prescription opioid epidemic and infant outcomes. 2015 May [ cited 2017 Jun 2]; Available from: file:///C:/Users/johnsosv/Downloads/Patrick 2015-Opioid Epidemic (2).pdf.
- Zedler BK, Mann AL, Kim MM, Amick HR, Joyce AR, Murrelle EL, Jones HE. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction [Internet]. 2016 Dec [ cited 2016 Nov 11]; 111(12):2115–28. Available from: http://doi.wiley.com/10.1111/add.13462.
