ABSTRACT
Background: For patients with opioid use disorder, buprenorphine extended-release injection (BUP-XR) achieves sustained therapeutic plasma concentrations, controls craving and withdrawal symptoms, and improves patient outcomes. Given retention challenges during transmucosal buprenorphine (BUP-TM) induction, assessing methods to quickly achieve sustained buprenorphine concentrations is important.
Objectives: This open-label, single-group, single-center pilot study (NCT03993392) evaluated safety and tolerability of initiating BUP-XR following a single BUP-TM 4 mg dose.
Methods: Eligible participants abstained from short and long-acting opioids for 6 and 24 hours, respectively. If the Clinical Opiate Withdrawal Scale (COWS) was ≥8, BUP-TM 4 mg was administered. Participants not exhibiting hypersensitivity, precipitated opioid withdrawal (POW), or sedation symptoms within 1 hour received BUP-XR 300 mg (assessed as inpatients for 48 hours and outpatients to Day 29). Endpoints were COWS score increase ≥6, independent adjudication of POW, and opioid use.
Results: Twenty-six participants (14 male) received BUP-TM, 24 received BUP-XR, and 20 completed the study. After injection, COWS scores decreased from pre-BUP-TM baseline of 14.6 ± 4.1 to 6.9 ± 4.1 at 6 hours and 4.2 ± 3.2 at 24 hours. Most participants (62.5%) experienced maximum COWS scores pre-BUP-XR; 2 experienced a COWS score increase ≥6, occurring at 1 and 2 hours post-BUP-XR. By adjudication, 2/24 participants experienced POW. Irritability, anxiety, nausea, and pain were the most frequent adverse events (AEs) with no serious AEs.
Conclusions: Results support increased flexibility for initiating BUP-XR. Initiating BUP-XR 300 mg following a single BUP-TM 4 mg dose was well tolerated. Although some participants initially experienced withdrawal symptoms after injection, significant symptomatic improvement was observed in all participants within 24 hours.
Introduction
Opioid use disorder (OUD) is a chronic, relapsing disease that has grown to epidemic proportions (Citation1–3). Current standard of care for OUD includes a whole-patient approach using a combination of medications with counseling/behavioral therapy (Citation4). Because OUD typically involves periods of exacerbation and remission, exploring optimal treatment strategies, including long-acting injectable buprenorphine formulations, is warranted. These novel formulations provide a new treatment option for patients as they work toward lasting recovery, a process through which patients improve their health and wellness, live self-directed lives, and strive to reach their full potential.
Currently, there is great interest in initiating treatment using a long-acting depot formulation as rapidly as possible, thus potentially maintaining therapeutic plasma concentrations of buprenorphine from the outset, reducing the need to provide take-home transmucosal buprenorphine (BUP-TM) for outpatient use, improving patient retention and limiting opportunities for misuse and diversion (Citation4–8). This is especially critical during the COVID-19 pandemic when contact with patients may be limited, despite increases in opioid overdose deaths following stay-at-home orders (Citation2,Citation9). In response to the epidemic, care providers have implemented strategies to enhance accessibility to medical treatment by shifting services online, increasing availability of take-home doses of opioid agonist maintenance treatment, and increasing duration of take-home dose prescriptions (Citation10).
Once-monthly buprenorphine extended-release injection (BUP-XR; SUBLOCADE®, Indivior Inc., North Chesterfield, VA, USA) is approved for use in the United States for the treatment of moderate to severe OUD in patients who have initiated treatment with a BUP-TM product, followed by dose adjustment for a minimum of 7 days (Citation11). The efficacy and safety of BUP-XR have been demonstrated in 2 randomized, placebo-controlled multicenter trials (Citation12,Citation13). In these trials, BUP-XR rapidly achieved consistent therapeutic plasma concentrations of buprenorphine, which facilitated rapid stabilization of a patient’s clinical condition. It also provided sustained therapeutic concentrations that blocked drug-liking of opioid agonists and helped control withdrawal and craving symptoms over the entire monthly dosing period (Citation14). Participants receiving BUP-XR reported better health, increased medication satisfaction, and decreased health care utilization (Citation15), and BUP-XR monthly injections for up to 1 year resulted in high treatment satisfaction and improvements in patient-centered outcomes (Citation16).
Because current labeling instructions recommending stabilization with BUP-TM for 7 days (Citation11) do not account for rapid attainment of therapeutic concentrations with BUP-XR, we conducted a pilot study to evaluate the safety and tolerability of administering the first BUP-XR 300 mg injection following a single 4-mg dose of BUP-TM in patients with OUD.
Methods
Study design and procedures
This open-label, single-group, single-center study (NCT03993392) was conducted at the Hassman Research Institute, Berlin NJ from August 2019 to December 2019. It was completed in accordance with International Council for Harmonization Good Clinical Practice. An Institutional Review Board reviewed and approved the protocol and the Informed Consent Form before study initiation. All participants provided written informed consent before screening.
Screening was performed within 30 days to confirm study eligibility. Clinical and laboratory assessments were completed and self-reported illicit drug use over the past 30 days was reviewed. Participants aged ≥18 years were eligible if they had documented history of moderate or severe OUD as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, were seeking buprenorphine treatment for OUD and were appropriate candidates in the opinion of the investigator. They were excluded if they had received medications to treat OUD in the 2 weeks before screening or any current or prior treatment with long-acting buprenorphine. Participants were advised to abstain from short-acting opioids for at least 6 hours and long-acting opioids for 24 hours before arriving at the clinic on the morning of Day 1 and to abstain from alcohol before clinic admission.
Rapid initiation procedures are illustrated in . Upon arriving at the clinic for the inpatient induction, participants provided a sample for a qualitative and quantitative urine drug screen (UDS) and completed an alcohol breathalyzer test. Investigators discussed positive UDS results with participants and used the TimeLine Follow Back (TLFB) assessment to record drug use since the screening visit (Citation17). Participants reporting use of short- or long-acting opioids within 6 or 24 hours, respectively, or who had elevated blood alcohol concentration, could not check-in but could be rescheduled within 30 days of consent. All concomitant medications were reviewed for contraindicated medications; females of childbearing potential also had a urine pregnancy test. All inclusion and exclusion criteria are listed in the Supplemental data.
Figure 1. Flow diagram outlining procedure used to initiate buprenorphine extended-release in study participants.
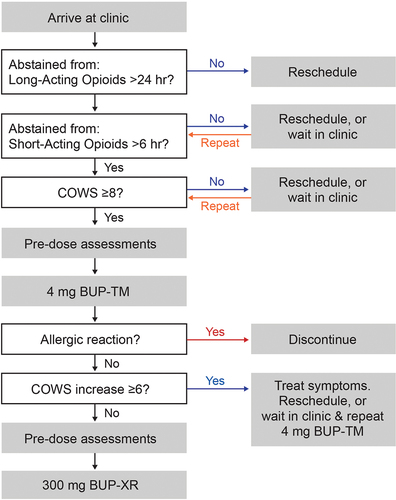
Withdrawal symptoms were assessed using the Clinical Opiate Withdrawal Scale total score (COWS score) (Citation18,Citation19) and opioid craving was assessed using the Opioid Craving Visual Analogue Scale (OC-VAS) (Citation20). If COWS score was <8, participants were either rescheduled for check-in or remained in the clinic for repeat assessments. Once the participant’s COWS score was ≥8, vital signs, liver tests, 12-lead electrocardiograms, adverse events (AEs), and sedation using a VAS were completed. Then a single 4-mg dose of BUP-TM was administered, consistent with the initial recommended dose for BUP-TM induction (Citation11,Citation21,Citation22).
One hour after the 4-mg BUP-TM dose, pre-dose BUP-XR assessments were completed, including COWS and OC-VAS, sedation VAS, AEs, vital signs, and concomitant medications. If the participant did not exhibit hypersensitivity, precipitated withdrawal (POW), or sedation symptoms, BUP-XR 300 mg was administered, and clinical assessments were completed as an inpatient for 48 hours. Rescue medications as outlined in clinical guidelines (Citation4,Citation23) and supplemental BUP-TM were permitted after BUP-XR to treat withdrawal symptoms. If POW from BUP-TM occurred, the BUP-TM induction could be restarted later the same or next day.
Participants were scheduled for weekly clinic visits until 28 days after BUP-XR administration (Day 29) to assess COWS, OC-VAS, AEs, vital signs, concomitant medications, and drug use (by TLFB and UDS). Those who discontinued the study after buprenorphine induction were contacted by telephone 24 to 72 hours after BUP-TM to assess any AEs and concomitant medications. All participants received counseling following local standard of care throughout the study. Participants who completed the study were permitted to enroll in an extension study (NCT04060654) for an additional 5 months of BUP-XR injections, safety monitoring, and assessments of illicit drug use.
Outcomes
Key endpoints evaluated in this study included: (1) number and percentage of participants experiencing POW within 1 hour after BUP-XR administration (because there is no widely accepted definition of POW based on changes in COWS score for a specific period after dosing, POW was defined as an increase in COWS score ≥6 from pre-BUP-XR value); (2) number and percentage of participants with ≥6-point increase in COWS score within the first 6, 12, 24 or 48 hours post-BUP-XR administration; (3) normalized area under the curve (AUC) of COWS score from BUP-XR administration (time 0) through 6, 12, 24 and 48 hours post-BUP-XR administration; (4) total COWS score during the treatment period (defined as each assessment timepoint from BUP-TM administration on Day 1 through Day 29); (5) score on the OC-VAS during the treatment period; and (6) AEs.
Because the definition of POW selected for the key endpoint has not been validated across prior studies of opioid agonist induction, a post-study Event Adjudication Committee (EAC) was incorporated. This group of independent clinical experts (external to the study site and sponsor) viewed all clinical factors including individual components of the COWS scores, drug use history, urine drug screen results and safety data to determine if and when POW occurred. Results from the clinical assessment were compared to outcomes from the key endpoint definition of POW based upon changes in COWS score.
Although efficacy was not an objective of this study, the number and percentage of participants negative for opioids at the Day 29 visit was assessed as an exploratory endpoint.
Safety endpoints included the proportion of participants with concomitant medications and treatment-emergent adverse events (TEAEs) after the first BUP-TM dose, including any drug-related TEAEs, serious TEAEs, and TEAEs leading to discontinuation.
Statistical analyses
Fifteen adult participants with moderate to severe OUD were planned to be administered BUP-XR. With a sample size of 15 and assuming a true event rate of 5% or 10% (based on other published definitions of POW (Citation8,Citation24,Citation25)), the probability of observing ≥1 POW event was 53.7% or 79.4%; respectively.
The number and percentage of participants with COWS score increasing by ≥6 from the pre-BUP-XR value within each key timepoint interval (1, 6, 12, 24 and 48 hours) were summarized and the cumulative event rate was estimated using the Kaplan–Meier method. If a participant did not experience an increase in COWS score by ≥6 from the pre-BUP-XR value and had a missing COWS assessment at 1 or more key timepoints, the participant was censored at the last key timepoint before the first missing timepoint; therefore, no event could be eliminated from the analysis due to censoring. Normalized AUC of COWS from pre-BUP-XR values through the key timepoints was calculated using the linear trapezoidal method. COWS, OC-VAS and sedation scores were summarized for observed data, and a post-hoc analysis evaluated change from pre-BUP-TM (COWS, OC-VAS) or from pre-BUP-XR (sedation VAS) at each timepoint using a t-test without adjustment for multiplicity. Post-hoc analyses of the timepoint and severity of the maximum COWS score were also performed. COWS score and COWS score severity were summarized for 2 subgroups: participants whose maximum score occurred at the pre-BUP-XR timepoint and those whose maximum score occurred at a post-BUP-XR timepoint. These analyses were conducted for all participants who received a BUP-XR injection and had at least 1 COWS assessment before the BUP-XR injection and 1 assessment within 48 hours after BUP-XR injection (Full Analysis Set).
The number and percentage of participants with adjudicated POW following BUP-TM and adjudicated POW within 48 hours post-BUP-XR were summarized.
For safety endpoints, the number and percentage of participants who reported TEAEs and concomitant medications were summarized in the population of participants who received BUP-XR (Safety Analysis Set).
Data from the qualitative UDS and TLFB at each visit were combined to derive the number and percentage of participants positive/negative for opioid use, using observed data. The combined result was positive if either assessment was positive for opioid use, and negative if all non-missing assessments were negative. A post-hoc sensitivity analysis imputed missing assessments as positive.
Results
Disposition and demographics of participants
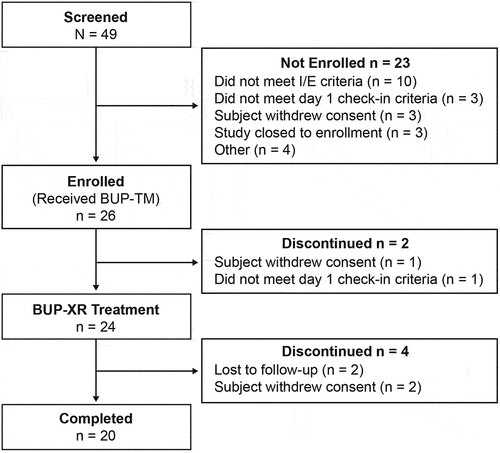
Disposition of participants is shown in . There were 49 participants screened, of which 26 enrolled to ensure that 15 participants would have COWS assessment before and post-BUP-XR injection for all key timepoints (1, 6, 12, 24 and 48 hours), 24 were administered BUP-XR and 20 completed the Day 29 visit. Demographic and OUD characteristics at screening are shown in the Supplemental data. All participants reported opioid use within the previous 30 days, and 27% reported intravenous use. The most frequently reported drugs during the TLFB interview were opioids (including heroin) and oxycodone; only 5 participants reported fentanyl use. Cannabinoids (n = 16) and cocaine (n = 12) were also frequently used. Upon check-in on Day 1, fentanyl was detected in urine for 17/24 (71%) participants; generally, these participants reported use of other opioids by intravenous or nasal routes.
Key endpoints
Of the 24 participants who received BUP-XR, 2 had a COWS score increase of ≥6 from the pre-BUP-XR value; 1 occurred within 1 hour (COWS score = 15 to 22) and the other within 2 hours (COWS score = 3 to 11) post-BUP-XR. The cumulative event rate for POW defined as an increase in COWS score ≥6 from the pre-BUP-XR value at the end of the first hour was 0.043. The cumulative event rate increased to 0.089 at the end of 6 hours and remained the same through 48 hours post-BUP-XR. Normalized AUC for COWS through 1, 6, 12, 24 and 48 hours continuously decreased through 48 hours (). Mean COWS scores are shown in . After injection, COWS scores (mean ± standard deviation [SD]) significantly decreased from a pre-BUP-TM baseline of 14.6 ± 4.1 (moderate withdrawal) to 6.9 ± 4.1 (mild withdrawal) at 6 hours and to 4.2 ± 3.2 (no active withdrawal) at 24 hours post-BUP-XR. Mean COWS remained in the no active withdrawal category until the Day 29 visit.
Figure 3. Clinical opiate withdrawal scale (COWS), opioid craving visual analogue scale (OC-VAS) and sedation visual analogue scale (Sedation VAS) scores over time following initiation of buprenorphine extended-release injection (BUP-XR) one hour following an initial dose of 4-mg transmucosal buprenorphine (BUP-TM). Time: D1 is check-in on the day of initiation (Day 1); PTM is prior to administration of BUP-TM; 0 h is prior to BUP-XR injection; D8, D15, D22 and D29 indicate days after buprenorphine injection. Panel A (upper left), COWS Total Score (Mean ± standard deviation [SD]) (Full Analysis Set; n = 24). A score of 0 to 4 is no active withdrawal, 5 to 12 is mild, 13 to 24 is moderate, 25 to 36 is moderately severe, and >36 is severe withdrawal. *p-value ≤0.05 based on t-test for change from PTM value. After injection, COWS scores significantly decreased from a pre-BUP-TM baseline. Panel B (upper right), COWS Total Score (Mean ± SD) by Maximum COWS Severity Subgroup (Full Analysis Set). Subgroups are based on maximum COWS total score: participants whose maximum occurred at the pre-BUP-XR (MAXpre) timepoint and those whose maximum occurred at a post-BUP-XR (MAXpost). Participants with a maximum severity post-BUP-XR injection tended to have a slower decline in withdrawal severity immediately following BUP-XR injection, but COWS scores still returned to the pre-BUP-XR average between 4 to 6 hours post-BUP-XR. Panel C (lower left), OC-VAS Score (Mean ± SD) (Full Analysis Set). *p-value ≤0.05 based on t-test for change from PTM value. A steady decrease from baseline in opioid craving was measured to 48 h post-BUP-XR and was sustained to Day 29 of treatment. Panel D (lower right),” Sedation VAS Score (Mean ± SD) (Safety Analysis Set). *p-value ≤0.05 based on t-test for change from 0 h value. Mean sedation VAS score tended to be highest between 4 to 8 hours post-BUP-XR but did not change substantially up to 36 hours post-BUP-XR.
![Figure 3. Clinical opiate withdrawal scale (COWS), opioid craving visual analogue scale (OC-VAS) and sedation visual analogue scale (Sedation VAS) scores over time following initiation of buprenorphine extended-release injection (BUP-XR) one hour following an initial dose of 4-mg transmucosal buprenorphine (BUP-TM). Time: D1 is check-in on the day of initiation (Day 1); PTM is prior to administration of BUP-TM; 0 h is prior to BUP-XR injection; D8, D15, D22 and D29 indicate days after buprenorphine injection. Panel A (upper left), COWS Total Score (Mean ± standard deviation [SD]) (Full Analysis Set; n = 24). A score of 0 to 4 is no active withdrawal, 5 to 12 is mild, 13 to 24 is moderate, 25 to 36 is moderately severe, and >36 is severe withdrawal. *p-value ≤0.05 based on t-test for change from PTM value. After injection, COWS scores significantly decreased from a pre-BUP-TM baseline. Panel B (upper right), COWS Total Score (Mean ± SD) by Maximum COWS Severity Subgroup (Full Analysis Set). Subgroups are based on maximum COWS total score: participants whose maximum occurred at the pre-BUP-XR (MAXpre) timepoint and those whose maximum occurred at a post-BUP-XR (MAXpost). Participants with a maximum severity post-BUP-XR injection tended to have a slower decline in withdrawal severity immediately following BUP-XR injection, but COWS scores still returned to the pre-BUP-XR average between 4 to 6 hours post-BUP-XR. Panel C (lower left), OC-VAS Score (Mean ± SD) (Full Analysis Set). *p-value ≤0.05 based on t-test for change from PTM value. A steady decrease from baseline in opioid craving was measured to 48 h post-BUP-XR and was sustained to Day 29 of treatment. Panel D (lower right),” Sedation VAS Score (Mean ± SD) (Safety Analysis Set). *p-value ≤0.05 based on t-test for change from 0 h value. Mean sedation VAS score tended to be highest between 4 to 8 hours post-BUP-XR but did not change substantially up to 36 hours post-BUP-XR.](/cms/asset/24866616-9f6c-4a01-a43a-27a6652cd7b3/iada_a_2106574_f0003_b.gif)
Table 1. COWS total score normalized AUC through 1, 6, 12, 24 and 48 hours post-BUP-XR 300 mg.
Most participants (62.5%) experienced their maximum severity of withdrawal pre-BUP-XR (6 mild, 9 moderate). They demonstrated a steady decline in withdrawal severity post-BUP-XR (). At 2 hours post-BUP-XR, mean COWS scores indicated either mild or no active withdrawal. Participants with a maximum severity post-BUP-XR (n = 9, 37.5%), tended to have a slower decline in withdrawal severity immediately following BUP-XR injection. For these participants, COWS returned to the pre-BUP-XR average between 4 and 6 hours post-BUP-XR, then decreased further. After 12 hours post-BUP-XR, all participants in both subgroups were in mild or no active withdrawal. From 20 to 48 hours onwards, both subgroups showed similar average COWS scores. No one experienced severe withdrawal (COWS score >36) and one participant experienced moderately severe withdrawal (COWS score 25 to 36).
Per adjudication by the EAC, no participant experienced POW following BUP-TM, and 2 of the 24 (8.3%) participants experienced POW following BUP-XR. Both participants reported polydrug use and both had among the highest levels of fentanyl and norfentanyl detected in admission urine samples. One participant reached COWS score = 22 at 1 hour and 27 at 2 hours post-BUP-XR. They received supplemental 4-mg BUP-TM at 1 hour and COWS score fell to 19 at 4 hours post-BUP-XR. A second participant had a COWS score = 19 before BUP-XR and 21 at 1 hour post-BUP-XR and the COWS score fell to 9 at 4 hours post-BUP-XR without supplemental BUP-TM.
Mean OC-VAS score by timepoint is shown in . A steady decrease from the pre-BUP-XR mean [SD] value of 57.0 mm ±28.9 was seen until 48 hours post-BUP-XR (12.1 mm ±12.1) and remained stable until the end of the study (7.3 mm ±9.2).
Mean sedation VAS score by timepoint is shown in . The mean sedation VAS score tended to be highest between 4 and 8 hours post-BUP-XR but did not change substantially up to 36 hours post-BUP-XR. Timepoints with the highest scores did not correlate with the expected buprenorphine time to maximum observed plasma concentration for BUP-XR of 24 hours (Citation11), and no sedation VAS score was considered clinically significant by the investigator.
Exploratory endpoint
All participants who received BUP-XR had a qualitative UDS at every visit. For the 20 participants completing study visits with non-missing results for UDS or TLFB, 6 (30%) participants were negative for opioids at the Day 29 visit. The post-hoc sensitivity analysis, where missing results were considered positive for opioids, indicated 25% (6/24) were negative for opioids at Day 29.
Safety
A total of 20 (83.3%) participants reported at least 1 TEAE. Irritability (n = 12), anxiety (n = 11), nausea (n = 10) and pain (n = 10) were the most commonly reported TEAEs. Injection site bruising (3 participants; 12.5%) was the only TEAE considered to be related to study drug by the Investigator reported more than once. Five (20.8%) participants reported a total of 8 severe TEAEs (irritability [n = 4], pain [n = 2], chills [n = 1] and vomiting [n = 1]) that are typical of opioid withdrawal. All occurred within 48 hours of BUP-XR administration and resolved within one day. There were no clinically significant changes in liver enzymes and no reports of removal of the BUP-XR depot. There were no deaths, serious AEs, or TEAEs leading to study withdrawal.
A total of 15 (62.5%) participants used concomitant medications, the majority of which were started within 48 hours post-BUP-XR. Two participants received a single dose of 4-mg BUP-TM after BUP-XR injection and 15 received other rescue medications (see Supplemental data). The most common concomitant medications were ondansetron for nausea/vomiting (10 [41.7%] participants), clonidine for anxiety/irritability (10 [41.7%] participants), ibuprofen for pain/body aches (9 [37.5%] participants), and trazadone for insomnia (5 [20.8%] participants).
Discussion
The overall goal of buprenorphine induction in OUD is to improve withdrawal symptoms without over-sedation (Citation23). With the ongoing COVID-19 pandemic and the rise in opioid related deaths (Citation1–3,Citation9), rapid initiation of once-monthly BUP-XR could prove beneficial by maintaining therapeutic plasma concentrations of buprenorphine from the outset, and reducing the need for take-home BUP-TM (Citation5,Citation6). In this study, initiation of BUP-XR 300 mg following a single 4-mg dose of BUP-TM did not result in any unexpected safety findings, and the reported AEs and level of sedation indicate that this rapid initiation demonstrated a safety profile comparable to that observed with previous studies of BUP-XR induction and maintenance (Citation12,Citation13). With regards to treatment retention, 17 participants (65%) of the 24 who received a first BUP-XR injection enrolled in the extension study and 11 completed a course of 6 injections, including the 2 participants who were adjudicated to have POW in the first few hours after BUP-XR.
For all participants, COWS scores decreased to <12, representing either mild or no active withdrawal within 16 hours post-BUP-XR; by 48 hours, 5 participants experienced mild withdrawal (COWS score 5 to 12) and all remaining participants had no active withdrawal in the study. Most participants experienced their maximum severity of withdrawal (6 mild, 9 moderate) before BUP-XR.
Precipitated withdrawal can complicate buprenorphine induction and long-term treatment response. Since there is no standardized objective definition of POW, we defined POW as COWS increase ≥6 post-BUP-XR. There was concordance between the protocol definition and adjudication assessment of POW for 25 (97%) of the participants post-BUP-TM and 22 (92%) of the participants post-BUP-XR. An increase of COWS score ≥6 identified those participants with possible risk of clinical POW; however, POW may be associated with multiple factors, such as the level of opioid dependence, time since the last use of opioids, type of opioid use (short-acting or long-acting), and previous experience with medications for OUD or detoxification (Citation24). Note that both participants that experienced POW reported polydrug use and had among the highest levels of fentanyl and norfentanyl detected in admission urine samples. Additionally, during the adjudication EAC members weighted certain COWS categories (i.e., gastrointestinal issues) more than others (i.e., anxiety/irritability). Thus, an increase in COWS score alone should not be used as a substitute for clinical judgment of POW based on presenting opioid withdrawal signs and symptoms.
All COWS score increases of ≥6 and adjudicated POW events occurred within 2 hours of BUP-XR administration and included maximum reported COWS severity of 27, suggesting that after the first BUP-XR injection following rapid initiation, it is important to monitor patients for significant withdrawal during the first 2 hours. The use of concomitant medications is recommended for alleviating any withdrawal symptoms, such as ondansetron for nausea/vomiting, clonidine for anxiety/irritability, and ibuprofen for pain/body aches, in accordance with current guidelines (Citation23). In addition, adjunctive use of BUP-TM in increments of 2 to 4 mg up to a maximum of 12 mg during the first day of the rapid initiation process could help alleviate withdrawal symptoms (Citation21).
An ongoing concern with induction of buprenorphine is that precipitated withdrawal symptoms will emerge when a full opioid agonist is replaced by the partial agonist. For this reason, various low-dose approaches have been proposed for gradually transitioning patients from methadone to buprenorphine (Citation26) or starting buprenorphine treatment over a period of several days (Citation27). Given the challenges of retaining patients during buprenorphine induction and the risk of overdose with patients who do not receive medications for opioid use disorder, we have explored single-day induction with BUP-XR to quickly achieve sustained concentrations of buprenorphine that address symptoms of withdrawal, reduce opioid craving, and block the reinforcing effects of illicit opioids (Citation14). This protocol is more consistent with the procedures proposed by Mariani et al. for clinic inductionCitation7 and Herring et al. for emergency department inductionCitation28. The potential risks of this approach are POW and sedation following the 300-mg dose of BUP-XR. In this sample of 26 subjects, the single-day induction protocol did not result in marked sedation or a protracted period of POW, as illustrated by adverse events reporting and frequent measures of sedation VAS and COWS scores up to 48 hours after BUP-XR injection.
Potential limitations of this study include the absence of a control group with standard BUP-TM induction and the small number of participants treated as inpatients for 48 hours at a single center that might not fully represent the real-world population of patients with OUD. A post-hoc power calculation was performed due to both the uncertain assumed event rate under the novel definition of POW used and enrollment beyond the planned sample size. With 24 participants (increased from the planned 15 participants) and assuming the true event rate is 8.9% (i.e., the estimated cumulative event rate based on the Kaplan–Meier method), the probability of observing at least 1 event is 89.3%.
Withdrawal signs and symptoms were monitored frequently (at 0, 1, 2, 3, 4, 6, 8, 12, 16, 20, 24, 36 and 48 h) in the clinic after the first BUP-XR injection, which is not feasible for monitoring during outpatient induction in clinical practice. This frequency resulted in some missing assessments due to participant sleeping, participant refused because of an AE or other reasons. However, the missing COWS data are unlikely to significantly impact the study findings. First, the primary analysis using Kaplan–Meier estimation accounted for missing data at the key timepoints and applied censoring such that all events would be included independent of any missing data. Second, almost half of the missing COWS assessments were due to the participant sleeping, which would indicate a good clinical status with no POW. Finally any assessments missing due to AEs were evaluated by the EAC and at least 19 COWS assessments were available at every timepoint.
A specific feature of this study was the inclusion of an external adjudication committee that reviewed all data, including missing assessments. Using their clinical judgment, the committee assessed whether POW occurred for each enrolled participant. Given concordance between the protocol definition and adjudication assessment of POW for nearly all participants, the missing assessments did not appear to have a meaningful impact on key study findings.
These data show that BUP-XR 300 mg could be safely initiated following a single 4-mg dose of BUP-TM, indicating a safety profile comparable to that observed with previously with BUP-XR induction. Although some subjects initially experienced withdrawal symptoms after injection, significant improvement or resolution of symptoms was observed in all subjects within 24 hours. The data from this pilot study indicate the potential for flexible initiation of BUP-XR to aid patient-centered treatment of OUD and can be used to plan trials of buprenorphine induction.
Authors’ contributions
HH: Participated in the conception and design of the study, drafting the results and revising critically for important intellectual content, and have approved the manuscript for publication.
SS: Participated in the conception and design of the study; acquisition/collection and analysis/interpretation of the data; drafted the introduction, methods, statistical methods/analysis, results, discussion and revised critically for important intellectual content; and have approved the manuscript for publication.
SNS: Participated in the conception and design of the study, acquisition/collection and analysis/interpretation of the data; drafting the introduction, results, and discussion and revised critically for important intellectual content; and have approved the manuscript for publication.
AH: Participated in the conception and design of the study, acquisition/collection and analysis/interpretation of the data, drafting the introduction, methods, statistical methods/analysis, results, and discussion and revised critically for important intellectual content, and have approved the manuscript for publication.
BB: Participated in the analysis and interpretation of the data, drafting the introduction and discussion and revising critically for important intellectual content, and have approved the manuscript for publication.
RD: Participated in the conception and design of the study, analysis and interpretation of the data, drafting the introduction, methods, statistical methods or analysis, results, discussion and revising critically for important intellectual content, and have approved the manuscript for publication.
Hassman_et_al._Rapid_Induction_Appendix_Revised_6-22-22.docx
Download MS Word (48.5 KB)Acknowledgement
The authors thank the study participants. Editorial assistance was provided by Karen Mittleman, PhD, and funded by Indivior Inc. We thank the External Adjudication Committee members (F. Gerard Moeller, M.D. and Katarina Wiest, Ph.D.). for their clinical expertise and insights into the research findings.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00952990.2022.2106574
Disclosure statement
Howard Hassman is the principal investigator for the site that conducted the clinical study. Stephanie Strafford, Sunita N. Shinde, Amy Heath, and Robert L. Dobbins are employees of Indivior Inc. Brent Boyett is consultant to Indivior Inc. and was a member of the External Adjudication committee for this study.
Additional information
Funding
References
- Hedegaard H, Miniño AM, and Warner M. Drug overdose deaths in the United States, 1999-2019. NCHS Data Brief. 2020;394:1–8.
- Chandler RK, Villani J, Clarke T, McCance-Katz EF, Volkow ND. Addressing opioid overdose deaths: the vision for the HEALing communities study. Drug Alcohol Depend. 2020;217:108329. doi:10.1016/j.drugalcdep.2020.108329.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19: expanded prevention efforts needed [press release]; 2020. Accessed June 3, 2021. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
- Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9:358–67. doi:10.1097/ADM.0000000000000166.
- Farhoudian A, Baldacchino A, Clark N, Gerra G, Ekhtiari H, Dom G, Mokri A, Sadeghi M, Nematollahi P, Demasi M, et al. COVID-19 and substance use disorders: recommendations to a comprehensive healthcare response. an international society of addiction medicine practice and policy interest group position paper. Basic Clin Neurosci. 2020;11:133–50. doi:10.32598/bcn.11.covid19.1.
- Dunlop A, Lokuge B, Masters D, Sequeira M, Saul P, Dunlop G, Ryan J, Hall M, Ezard N, Haber P, et al. Challenges in maintaining treatment services for people who use drugs during the COVID-19 pandemic. Harm Reduct J. 2020;17:26. doi:10.1186/s12954-020-00370-7.
- Mariani JJ, Mahony AL, Podell SC, Brooks DJ, Brezing C, Luo SX, Naqvi NH, Levin FR. Open-label trial of a single-day induction onto buprenorphine extended-release injection for users of heroin and fentanyl. Am J Addict. 2021;30:470–76. doi:10.1111/ajad.13193.
- Jacobs P, Ang A, Hillhouse MP, Saxon AJ, Nielsen S, Wakim PG, Mai BE, Mooney LJ, Potter S, Blaine JD, et al. Treatment outcomes in opioid dependent patients with different buprenorphine/naloxone induction dosing patterns and trajectories. Am J Addict. 2015;24:667–75. doi:10.1111/ajad.12288.
- Mason M, Welch SB, Arunkumar P, Post LA, Feinglass JM. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order - Cook County, Illinois, January 1, 2018-October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:362–63. doi:10.15585/mmwr.mm7010a3.
- Scheibein F, Stowe MJ, Arya S, Morgan N, Shirasaka T, Grandinetti P, Saad NA, Ghosh A, Vadivel R, Ratta-Apha W, et al. Responding to COVID-19: emerging practices in addiction medicine in 17 countries. Front Psychiatry. 2021;12:634309.
- Sublocade injection [package insert]. North Chesterfield, VA: Indivior Inc.; 2021.
- Andorn AC, Haight BR, Shinde S, Fudala PJ, Zhao Y, Heidbreder C, Learned SM, Fox NL, Nadipelli VR, Hassman D, et al. Treating opioid use disorder with a monthly subcutaneous buprenorphine depot injection: 12-month safety, tolerability and efficacy analysis. J Clin Psychopharmacol. 2020;40:231–39. doi:10.1097/JCP.0000000000001195.
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, Greenwald MK, Nadipelli VR, Ling W, Heidbreder C, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393:778–90. doi:10.1016/S0140-6736(18)32259-1.
- Nasser AF, Greenwald MK, Vince B, Fudala PJ, Twumasi-Ankrah P, Liu Y, Jp J 3rd, Heidbreder C. Sustained-release buprenorphine (RBP-6000) blocks the effects of opioid challenge with hydromorphone in subjects with opioid use disorder. J Clin Psychopharmacol. 2016 Feb;36:18–26. doi:10.1097/JCP.0000000000000434.
- Ling W, Nadipelli VR, Solem CT, Ronquest NA, Yeh Y-C, Learned SM, Mehra V, Heidbreder C. Patient-Centered outcomes in participants of a buprenorphine monthly depot (BUP-XR) double-blind, placebo-controlled, multicenter, phase 3 study. J Addict Med. 2019;13:442–49. doi:10.1097/ADM.0000000000000517.
- Ling W, Nadipelli VR, Solem CT, Ronquest NA, Yeh Y-C, Learned SM, Mehra V, Heidbreder C. Effects of monthly buprenorphine extended-release injections on patient-centered outcomes: A long-term study. J Subst Abuse Treat. 2020;110:1–8.
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. J Consult Clin Psychol. 2000;68:134–44. doi:10.1037/0022-006X.68.1.134.
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the clinical opiate withdrawal scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105:154–59. doi:10.1016/j.drugalcdep.2009.07.001.
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253–59. doi:10.1080/02791072.2003.10400007.
- McMillan DE, Gilmore-Thomas K. Stability of opioid craving over time as measured by visual analog scales. Drug Alcohol Depend. 1996;40:235–39. doi:10.1016/0376-8716(96)01218-5.
- Suboxone® sublingual film [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
- Subutex® sublingual tablet [package insert]. Richmond, VA: Reckitt Benckiser Pharmaceuticals Inc.; 2011.
- Medications for opioid use disorder. Treatment Improvement Protocol (TIP) series 63. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2020.
- Whitley SD, Sohler NL, Kunins HV, Giovanniello A, Li X, Sacajiu G, Cunningham CO. Factors associated with complicated buprenorphine inductions. J Subst Abuse Treat. 2010;39:51–57. doi:10.1016/j.jsat.2010.04.001.
- Nielsen S, Hillhouse M, Weiss RD, Mooney L, Sharpe Potter J, Lee J, Gourevitch MN, Ling W. The relationship between primary prescription opioid and buprenorphine-naloxone induction outcomes in a prescription opioid dependent sample. Am J Addict. 2014;23:343–48. doi:10.1111/j.1521-0391.2013.12105.x.
- Soyka M, Groß G. Transition from methadone to subcutaneous buprenorphine depot in patients with opioid use disorder in custodial setting - a case series. Am J Drug Alcohol Abuse. 2021;47:599–604. doi:10.1080/00952990.2021.1963757.
- Tay Wee Teck J, Baldacchino A, Gibson L, Lafferty C. Using microdosing to induct patients into a long-acting injectable buprenorphine depot medication in low threshold community settings: A case study. Front Pharmacol. 2021;12:631784. doi:10.3389/fphar.2021.631784.
- Herring AA, Vosooghi AA, Luftig J, Anderson ES, Zhao X, Dziura J, Hawk KF, McCormack RP, Saxon A, D’Onofrio G, et al. High-Dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi:10.1001/jamanetworkopen.2021.17128.