ABSTRACT
The study was undertaken to develop an effective bio-sanitation treatment using essential oils for the highly perishable leafy vegetable lettuce. The combination of thyme oil and tea tree oil mixed together had a significantly higher total phenolic content as well as higher antioxidant scavenging activity than other single oils or oil mixtures. Lettuce inoculated with Escherichia coli O157:H7 showed significantly reduced population counts in the presence of the sanitation treatment of a combination of thyme + tea tree oil applied by dipping at 0.75% + 0.75% or 1.5% + 1.5% concentrations compared to commercial sodium hypochlorite (200 mg/L NaOCl) washing after 5 days shelf life at 10°C. These results indicate that thyme + tea tree oil dipping treatment could be used to control E. coli O157:H7 contamination in lettuce as an alternative to NaOCl.
Introduction
Lettuce, Lactuca sativa L., can be contaminated by pathogens from the soil, manure or irrigation water during production (Islam et al. Citation2004) as well as during harvesting, field handling and transporting, sorting and packaging operations. Outbreaks related to consumption of lettuce contaminated with Escherichia coli have been reported by Ethelberg et al. (Citation2010). Lettuce is generally eaten raw and is classified under the ‘highest concern for microbiological safety’ group of fresh produce (FAO/WHO Citation2008). Post-harvest washing in water containing 50–200 mg/L of chlorine is sometimes used to sanitise fruits and vegetables. However, this treatment has been reported to have health risks associated with the formation of trihalomethanes and haloacetic acids (Olmez & Kretzschmar Citation2009).
Concerns regarding the safety of chlorine have resulted in research on alternative treatments, often with natural sanitisers, including the use of essential oils (EOs) (Tornuk et al. Citation2011; Sagdic et al. Citation2013). The EOs are concentrated hydrophobic volatile aromatic compounds extracted from plants. Cardile et al. (Citation2009) reported that pathogenic microorganisms are less likely to develop resistance to EOs while their plant origin is generally recognised as being safe (GRAS) and eco-friendly. The food industry prefers using compounds such as EOs as antioxidants, antimicrobials and flavouring agents due to their free radical scavenging activity (Ghazghazi et al. Citation2013). EOs in combination are also more effective against targeted bacteria than when applied individually (Stojković et al. Citation2013). Undesirable effects of EOs at higher concentrations, especially due to their strong aroma components influencing sensory properties, can be reduced by combining different EOs at lower concentrations (Ponce et al. Citation2011). However, limited reports are available regarding the stand-alone or combined application of natural antimicrobials on commonly consumed vegetables (Scollard et al. Citation2013).
Therefore, the objectives of this study were: (1) To evaluate the total phenol content and antioxidant scavenging activity of four EOs alone or in combination, as indicators of their antimicrobial and preservative quality; and (2) To investigate E. coli O157:H7 population counts on lettuce inoculated with this bacterial strain and dipped in thyme and tea tree oils, alone or in combination.
Material and methods
Essential oils
The pure EOs were obtained via hydro-distillation of dried samples of sage (Salvia dolomitica), tea tree (Melaleuca alternifolia), thyme (Thymus vulgaris) and ylang-ylang (Cananga odorato).
Gas chromatography/mass spectrometry (GC–MS)
EOs were analysed according to Sellamuthu et al. (Citation2013) with a GC Agilent 7890A coupled to an MS Agilent 5973N MSD (Chemetrix Ltd, Johannesburg, South Africa) with a split/splitless injector equipped with an HP-5MS column (30 m × 0.25 mm i.d. × 0.25 μm) (Agilent part number 19091S-433). The GC/MS was coupled with a flame ionisation detector. The GC conditions were column pressure, 65 kPa (9.43 psi) with a helium flow rate of 1 mL/min; oven temperature 60–240°C at 3°C/min with a 60 min analysis time for component separation. The injection volume was 1 µL in the oven. The GC-MS injector temperature was kept at 250°C. A mass spectrometer was run by positive impact electronisation at 70 eV with a spectra range of 50–550 amu, 2 min solvent delay and 300°C transfer line. The mass spectra were compared to both the National Institute of Standards Technology (NIST) and the Mass Spectra Library as well as those reported in literature data.
Preparation of inoculum
Escherichia coli O157:H7 (ATCC8739) cultures were obtained from the Biotechnology and Food Technology Department, Tshwane University of Technology. These cultures were stored at −70°C in 20% glycerol. E. coli O157:H7 was grown in trypticase soy broth (Merck, Johannesburg, South Africa) supplemented with 0.6% of yeast extract (Merck, Johannesburg, South Africa) (TSB-YE). Pure cultures of E. coli O157:H7 were activated in the brain heart infusion (BHI) broth at 37°C for 24 h. One millilitre of the fresh culture (24 h) in BHI broth was transferred to 1 L sterile distilled water and stirred. The average count of E. coli O157:H7 in the dipping solution was 9.2 log CFU/mL.
Determination of total phenolic content and DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay
The total phenol contents of stand-alone EOs (thyme, tea tree, ylang-ylang, sage oils) and EO combinations (thyme + tea tree, thyme + ylang-ylang, thyme + sage oil) were determined using the Folin-Ciocalteu method (Singleton et al. Citation1999) with some modifications. EO (2 mL) was mixed with methanol (2 mL). Thereafter, the EO-methanol mixture (9 µL) was mixed with 109 µL Folin-Ciocalteu reagent (Sigma Aldrich, South Africa) and incubated for 2 min at 25°C. For EO combinations, 1 mL thyme oil and 1 mL of specific EO were mixed in methanol (2 mL). Thereafter, 180 µL of freshly prepared 7.5% Na2CO3 reagent was added to neutralise the reaction. The mixture was then incubated in the dark for 4 min at 50°C and absorbance read at 760 nm (Anthos Zenyth 2000 Rt Spectrophotometer (Biochrom Cambridge-UK)). Gallic acid was used as the standard and the results are expressed in mg/L.
The antioxidant activity of different concentrations of EO samples was determined according to the hydrogen donating or radical scavenging ability, using stable free radical 2,2′-diphenyl-1-picrylhydrazyl (DPPH) (Brand-Williams et al. Citation1995). Twenty-five microlitres of different concentrations (0.015–25 mg/mL) of EOs prepared in methanol were added to 210 µL DPPH (90 µmol/L) solutions (Sigma Aldrich, Johannesburg, South Africa).
The mixture was sonicated and kept for 60 min in the dark. Absorbance was read at 515 nm (Anthos Zenyth 2000 Rt Spectrophotometer (Biochrom Cambridge-UK)). The results were expressed as IC50 (sample required to reduce the absorbance of the radical by 50%) in milligrams of gallic acid equivalent per millilitre of EO.
Lettuce inoculation and storage
Freshly harvested green loose lettuce var. Accqurel was obtained from a commercial farm and the leaves were washed in deionised water in order to remove the dust and soil particles. Thereafter, the leaves were dried using sterile paper towels (Gündüz et al. Citation2010). The dip inoculation method was adopted in this investigation as described by Moore-Neibel et al. (Citation2011). A total of 200 g lettuce leaves were dipped in E. coli O157:H7 diluted solution (2 L) for 10 min (Moore-Neibel et al. Citation2011) and thereafter, the lettuce leaves were transferred into sterile glass bottles placed under a bio-safety cabinet for 2 h (Gündüz et al. Citation2010) at 20°C.
The final concentrations of stand-alone thyme or tea tree oils for 1.0%, 1.5% and 3.0% or EOs in combinations for low (0.5% thyme + 0.5% tea tree), intermediate (0.75% thyme + 0.75% tea tree) and high (1.5% thyme + 1.5% tea tree) concentrations were prepared in distilled water. The suspension was mixed thoroughly by shaking vigorously for 5 min at 25°C before use. Subsequently, the lettuce inoculated with E. coli O157:H7 was dipped for 5 min in the above-mentioned dipping solutions (thyme or tea tree or thyme + tea tree oil) with continued shaking. The leaves were aseptically removed, drained and then packed in standard commercial packaging (a bi-orientated polypropylene bag 20 cm × 35 cm × 35 µm thick with two 1 mm holes (Modern Packaging, Pretoria, South Africa)) in order to reduce water loss from the leaves. The control lettuce samples were dipped in 200 mg/L NaOCl (Niemira Citation2008) (adjusted to pH 6.5 with citric acid) at 25°C and sterile water (control) was included for comparison. Following water removal from the surface of the lettuce leaves, the samples were transferred into the packaging, heat-sealed and the samples were held at 10°C for 5 days. The storage temperature chosen was representative of the retail display market conditions in South Africa. Lettuce samples were removed from the market simulation conditions after 1, 3 and 5 days for microbiological analysis.
The survival of E. coli O157:H7 was determined after 1, 3 and 5 days. For the numeration of E. coli O157:H7, 10 g of lettuce leaves were transferred into a sterile stomacher bag (Nasco Whirl-Park, USA) and filled with 90 mL of buffered peptone water (0.1% w/v, pH 7.0) and macerated for 1–2 min with a paddle blender (Stomacher 400 Circulator, Seward, UK). The stomached sample (1 mL) was serially diluted in 9 mL of sterile peptone water (1.0 g/L). Serially diluted samples were spread-plated (0.1 mL) to the surface of a Sorbitol Macconkey Agar plate in triplicate. Following 24 h incubation at 37°C, colonies were counted and results were transformed to log colony forming units (CFU) per g of lettuce.
Statistical analysis
The experiments were arranged in complete randomised designs with six replicate samples per treatment for the total phenolic content and the antioxidant activity. The data were subjected to one-way analysis of variance (ANOVA). Counts of E. coli O157:H7 after different EO treatments and storage times were subjected to a two-way ANOVA. Five replicate samples per treatment were used in this study. Means of significant effects were separated using Fisher’s protected t-test for LSD (least significant differences) at the 5% significance level. Data were analysed using GenStat (2010 edition).
Results
Effect of EOs on total phenolics and radical scavenging activity
Sage (Salvia dolomitica), tea tree (Melaleuca alternifolia), thyme (Thymus vulgaris) and ylang-ylang (Cananga odorato) were selected in this study due to their antibacterial properties based on their minimum inhibitory concentration (MIC) from our previous findings (unpublished data). The predominant components of the EOs used in this study are presented in .
Table 1. Chemical constituents of the essential oils used in this study.
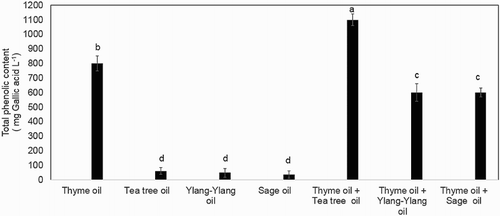
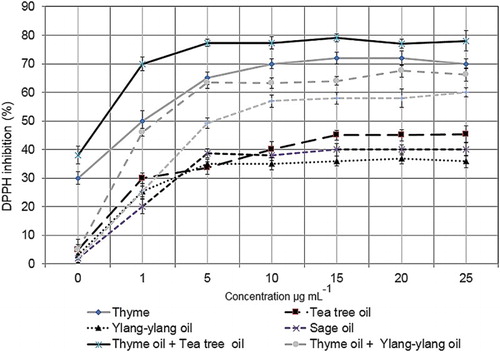
The total phenolic compound was significantly higher (p < .05) in thyme oil than the three other EOs (tea tree, ylang-ylang and sage) tested (). The total phenolic content was higher in tea tree oil, ylang-ylang oil and sage oil mixed with thyme oil. The thyme + tea tree oil mixture had a higher total phenolic content than thyme oil alone. The EOs showed different antioxidant scavenging activities with thyme oil reaching the highest value (). No significant difference (p > .05) in scavenging activity was observed between the ylang-ylang oil, sage oil and tea tree oil regardless of the concentration assayed. The IC50 values, that is, the concentration that inhibits 50% of the DPPH radical, were 1 mg/mL for thyme oil, 65 mg/ mL for tea tee oil, 70.7 mg/mL for ylang-ylang oil and 73.7 mg/mL for sage oil, respectively. Among the EO combinations or mixtures, thyme + tea tree oil showed the strongest radical scavenging activity (IC50 0.5 mg/mL) followed by thyme + ylang-ylang oil (IC50 2 mg/mL) and then by thyme + sage oil (IC50 5 mg/mL). GC/MS analysis identified thymol (31.2%) as the major compound present in thyme oil ().
Effect of EOs on Escherichia coli inoculated lettuce
Two EOs (thyme oil and tea tree oil) were selected for this investigation due to their higher total phenolic content and antioxidant scavenging activity. Dipping in distilled water yielded the least reduction of E. coli O157:H7 population with increasing storage time (). However, dipping in NaOCl solution reduced the E. coli O157:H7 population to log CFU/g 5.21 (2.60 log reduction) after 5 days at 10°C. Increasing the concentration of thyme oil or tea tree oil in the dipping solution resulted in a gradual reduction of E. coli O157:H7 population with increasing storage time. Thyme or tea tree oils alone at 3% concentration in the dipping solution significantly reduced the E. coli O157:H7 population on infected lettuce to 3.8 log CFU/g (4.01 log reduction) after 5 days on the retail shelf, demonstrating their effectiveness as a sanitation treatment compared to the NaOCl solution (). There were no significant differences (p > .05) between thyme oil or tea tree oil treatments at 1.5% and 3% concentrations ().
Table 2. Interaction effect of thyme oil and tea tree oil combination treatment and storage time on reduction of Escherichia coli O157:H7 at the retail shelf condition (10°C).
Thyme oil and tea tree oil combinations at 0.5% + 0.5% or 1.5% + 1.5%, or 0.75% + 0.75% concentrations did not show any significant reduction (p > .05) in the E. coli O157:H7 population after 24 h (Day 1) storage at the retail shelf (). However, among the different concentrations of thyme oil + tea tree oil combinations, reductions in E. coli O157:H7 populations after 5 days at the retail shelf showed the following trend: thyme + tea tree oil (0.5% + 0.5%) < thyme + tea tree oil (0.75% + 0.75%) < thyme + tea tree oil (1.5% + 1.5%) (). Thyme + tea tree oil (1.5% + 1.5%) significantly reduced (p < .05) the E. coli O157:H7 population in infected lettuce after 5 days on the retail shelf to 1.72 log CFU/g (6.09 log reduction). It was also evident that thyme + tea tree oil at 0.75% + 0.75% or 1.5% + 1.5% reduced the E. coli O157:H7 population on inoculated lettuce more effectively than the commercially used NaOCl solution after 5 days on the retail shelf ().
Discussion and conclusions
Lettuce is consumed in fresh form as salads and washed in water or chlorinated water and is a highly perishable commodity with a limited shelf life of approximately 5 days at 10°C. Washing with water with mechanical action was reported to physically remove microorganisms attached to the plant surface. Chlorinated water is used widely as a disinfecting agent due to its availability and relatively low cost (Fabrozio & Cutter Citation2003). Although washing in chlorinated water helps to reduce the cross contamination of pathogens from a healthy plant surface to the infected surface (Artés et al. Citation2009), the effectiveness of chlorinated water on microbial activity is limited (Richardson et al. Citation1998). Many researchers have shown that natural antimicrobial solutions can replace the currently used chlorinated water as disinfecting agents for fresh produce (Wan et al. Citation1998; Singh et al. Citation2002; Xu et al. Citation2007). In this investigation, dip treatment with NaOCl showed the lowest reduction of the E. coli O157:H7 population after 5 days storage at retail shelf conditions than the combined thyme and tea tree oil dip treatments at 0.75% + 0.75% or 1.5% + 1.5% concentrations.
Our finding suggests a synergistic action between the chemical components of the two EOs in our mixture in enhancing the reduction of E. coli populations in lettuce. The antimicrobial activity of EOs is linked to the active components and their functional groups. It has been shown by several researchers that the hydroxyl group of phenolic rings and the presence of delocalised electrons are important for antimicrobial activity (Ultee et al. Citation2002; Aleksic & Knezevic Citation2014; Cabarkapa et al. Citation2016). The direct attachment of the hydroxyl group on the phenolic rings in thymol improves the effectiveness of antibacterial activity of thyme oil (Nazzaro et al. Citation2013). Tea tree oil contains oxygenated terpene and α-terpineol as the major components (Carson et al. Citation2006). Although the cell wall of Gram-negative E. coli O157:H7 has an outer membrane, it is not totally impermeable to hydrophobic molecules such as EOs (Nazzaro et al. Citation2013). Antibacterial activity of thymol causes structural and functional modifications in the outer and inner membranes, which affects membrane permeability and causes release of K + and ATP (Xu et al. Citation2007). In addition, the α-terpineol component in the tea tree oil was shown to cause a bactericidal effect against Gram-negative bacteria by affecting the membrane systems and resulting in loss of cytoplasmic constituents (Oyedemi et al. Citation2009). However, the E. coli population was reported to show tolerance to tea tree oil during its stationary phase rather than during its exponential phase (Gustafson et al. Citation1998). Gustafson et al. (Citation1998) suggests that the survival of the E. coli population could be due to the expression of genes surA, surB and katF responsible for the survival of E. coli. The total phenolic compounds play a major role in contributing to the antibacterial activity and therefore, they can be considered to be an indicator of the antibacterial activity of EOs (Viuda-Martos et al. Citation2011). Phenolic compounds show antimicrobial properties by affecting the membrane function (electron transfer, enzyme activity or nutrient absorption), or forming complexes with extracellular soluble proteins and with bacterial cell wall or direct inhibition of DNA gyrase enzymes (Aleksic & Knezevic Citation2014). Antioxidant scavenging activities of EOs or plant extracts are also strongly correlated with their phenolic content. The higher redox potential of phenolic compounds enables them to act as radical scavengers or hydrogen donors. Our observations support the suggestion of Muchuweti et al. (Citation2007) that scavenging activity is specific to each EO and depends on the structure and position of the HO− group of the phenolic compound, as indicated by the efficiency of scavenging DPPH free radicals. Combined thyme and tea tree oils with high total phenolic content and antioxidant scavenging activity can be recommended for food preservation (Allahghadri et al. Citation2010). The synergistic bactericidal efficacy of thyme + tea tree oil combinations in this study was consistent with the findings of Stojkojić et al. (Citation2013), who suggested that a mixture of EOs from different species could increase the concentration of EOs to effective levels at the same time as reducing the undesirable sensorial impact.
Although a combination sanitation treatment with thyme and tea tree oils will be more costly compared to the chlorination treatment, the thyme oil + tea tree oil lack residual hazardous compounds on the surface of the lettuce and provides a safer product for the consumer.
The EO combinations were applied as dip treatments in this investigation because of their effectiveness in reducing the bacterial population when compared with a spray treatment (Ponce et al. Citation2011). A dip treatment can be easily adopted by the industry since leafy vegetables are washed before packing. However, the antimicrobial effectiveness of the EOs also depends on the leaf morphology, texture of the surface or hydrophobicity or hydrophilicity, presence of antimicrobial secondary metabolites and the nutrition composition which can affect the attachment of the bacteria (Moore-Neibel et al. Citation2011). The thyme oil + tea tree oil were mixed in water during the dipping treatment in this study without the use of organic solvents because this method is considered safe for consumer health.
However, in this study, the type of application method (dipping) of the combined EOs affected the E. coli O157:H7 counts after the fifth day, but not directly after day 1. The antimicrobial activity of the EOs is higher in the volatile phase (López et al. Citation2007; Perricone et al. Citation2015). Gas phase (volatile phase) decontamination shows advantages over currently adopted dipping or spraying, mainly due to greater penetration of EO into the plant surface (Vurma et al. Citation2009; Warriner & Namvar Citation2013). Therefore, application of thyme oil (1.5%) + tea tree oil (1.5%) during the post-harvest vacuum cooling treatment (pre cooling treatment) (Vurma et al. Citation2009; Warriner & Namvar Citation2013) can be tested to significantly reduce the E. coli O157:H7 counts after day 1.
Therefore, mixed thyme and tea tree oils (antimicrobial sanitisers) combined as a dip solution in the washing water will help to reduce the E. coli O157:H7 population due to their antimicrobial properties compared to the conventional chlorinated water treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aleksic V, Knezevic, P. 2014. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiological Research. 169:240–254. doi: 10.1016/j.micres.2013.10.003
- Allahghadri T, Rasooli I, Owlia P, Nadooshan MJ, Ghazanfari T, Taghizadeh M, Astaneh SDA. 2010. Antimicrobial property, antioxidant capacity and cytotoxicity of essential oil from cumin produced in Iran. Journal of Food Science. 75:H54–H61. doi: 10.1111/j.1750-3841.2009.01467.x
- Artés F, Gómez P, Aguayo A, Escalona V, Artés-Hernández F. 2009. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biology and Technology. 51:287–296. doi: 10.1016/j.postharvbio.2008.10.003
- Brand-Williams W, Cuvelierand ME, Berset C. 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology. 28:25–30. doi: 10.1016/S0023-6438(95)80008-5
- Cabarkapa I, Djuragic O, Kostadinović, L. 2016. Essential oils: mode of antimicrobial activity and potential application in food systems. Agro Food Industry Hi Tech. 27:61–64.
- Cardile V, Russo A, Formisano C, Rigano D, Senatore F, Arnold NA, Piozzi F. 2009. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. Journal of Ethnopharmacology. 126:265–272. doi: 10.1016/j.jep.2009.08.034
- Carson CF, Hammer KA, Riley TV. 2006. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clinical Microbiology Reviews. 19:50–62. doi: 10.1128/CMR.19.1.50-62.2006
- Ethelberg S, Lisby M, Böttiger B, Schultz AC, Vilif A, Jensen T, Olsen KE, Scheutz F, Kjelso C, Müller L. 2010. Outbreaks of gastroenteritis linked to lettuce, Denmark. Euro Surveillance [cited 2016 May 9]. Available from: http://www.eurosurveillance.org/images/dynamic/EE/V15N06/art19484.pdf15
- Fabrizio KA, Cutter CN. 2003. Stability of electrolyzed oxidizing water and its efficacy against cell suspensions of Salmonella typhimurium and Listeria monocytogenes. Journal of Food Protection. 66:1379–1384. doi: 10.4315/0362-028X-66.8.1379
- FAO/WHO. 2008. Microbiological hazards in fresh fruits and vegetables: microbiological risk assessment series – Meeting Report, 19–21 October 2007. Agriculture Organization of the United Nations/World Health Organization; [cited 2013 Oct 18]. Available from: http://www.who.int/foodsafety/publications/micro/MRA_FruitVeges.pdf
- Ghazghazi H, Chedia A, Weslati M, Trakhna F, Houssine S, Abderrazak M, Brahim H. 2013. Chemical composition and in vitro antimicrobial activities of Mentha pulegium leaves extracts against food borne pathogens. Journal Food Safety. 33:239–246. doi: 10.1111/jfs.12045
- Gündüz TG, Gönül SA, Karapinar M. 2010. Efficacy of oregano oil in the inactivation of Salmonella typhimurium on lettuce. Food Control 21:513–517. doi: 10.1016/j.foodcont.2009.07.016
- Gustafson, JE, Liew YC, Chew S, Markham J, Bell HC, Wyllie SG, Warmington, JR. 1998. Effects of tea tree oil on Escherichia coli. Letters in Applied Microbiology. 26:194–198. doi: 10.1046/j.1472-765X.1998.00317.x
- Islam M, Doyle MP, Phatak SC, Millner P, Jiang, X. 2004. Persistence of enterohaemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. Journal of Food Protection. 67:1365–1370. doi: 10.4315/0362-028X-67.7.1365
- López, P., Sánchez, C., Batlle, R., Nerín, C. 2007. Vapor-phase activities of cinnamon, Thyme, and Oregano essential oils and key constituents against foodborne microorganisms. Journal of Agricultural and Food Chemistry. 55:4348–4356. doi: 10.1021/jf063295u
- Moore-Neibel K, Gerber C, Patel J, Friedman M, Ravishankar S. 2011. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. Journal of Applied Microbiology. 112:485–492. doi: 10.1111/j.1365-2672.2011.05222.x
- Muchuweti M, Kativu E, Mupure CH, Chidewe C, Ndhlala AR, Benhura MAN. 2007. Phenolic composition and antioxidant properties of some spices. American Journal of Food Technology. 2:414–420. doi: 10.3923/ajft.2007.414.420
- Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. 2013. Effect of essential oils on pathogenic Bacteria. Pharmaceuticals 6:1451–1474. doi: 10.3390/ph6121451
- Niemira BA. 2008. Irradiation compared with chlorination for elimination of Escherichia coli O157:H7 internalized in lettuce leaves: influence of lettuce variety. Journal of Food Science. 73:M208–M213. doi: 10.1111/j.1750-3841.2008.00746.x
- Olmez H, Kretzschmar U. 2009. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT - Food Science and Technology. 42:686–693. doi: 10.1016/j.lwt.2008.08.001
- Oyedemi SO, Okoh AI, Mabinya LV, Pirochenva G, Afolayan AJ. 2009. The proposed mechanism of bactericidal action of eugenol, (-terpineol and (-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. African Journal of Biotechnology. 8:1280–1290.
- Perricone M, Arace E, Corbo MR, Sinigaglia M, Bevilacqua A. 2015. Bioactivity of essential oils: a review on their interaction with food components. Frontiers in Microbiology. 6:1–7. doi: 10.3389/fmicb.2015.00076
- Ponce A, Sara I, Roura A, Moreira, MDR. 2011. Essential oils as biopreservatives: different methods for the technological application in lettuce leaves. Journal of Food Science. 76:M34–M40. doi: 10.1111/j.1750-3841.2010.01880.x
- Richardson SD, Thruston AD Jr, Caughran TV, Collette TW, Patterson KS, Lykins BW Jr. 1998. Chemical by-products of chlorin and alternative disinfectants. Food Technology. 52:58–61.
- Sagdic O, Ozturk I, Törnük F. 2013. Inactivation of non-toxigenic and toxigenic Escherichia coli O157:H7 inoculated on minimally processed tomatoes and cucumbers: utilization of hydrosols of Lamiaceae species as natural food sanitizers. Food Control 30:7–14. doi: 10.1016/j.foodcont.2012.07.010
- Scollard J, Francis GA, O’Beirne D. 2013. Some conventional and latent anti-listerial effects of essential oils, herbs, carrot and cabbage in fresh-cut vegetable systems. Postharvest Biology and Technology. 77:87–93. doi: 10.1016/j.postharvbio.2012.11.011
- Sellamuthu P.S, Sivakumar D, Soundy P, Korsten L. 2013. Enhancing the defence related and antioxidant enzymes activities in avocado cultivars with essential oil vapours. Postharvest Biology and Technology. 81:66–72. doi: 10.1016/j.postharvbio.2013.02.007
- Singh S, Singh RK, Bhunia AK, Stroshine RL. 2002. Efficacy of chlorine dioxide, ozone and thyme essential oil or a sequential washing in killing E. coli O157:H7 on lettuce and baby carrots. LWT-Food Science and Technology. 35:720–729. doi: 10.1006/fstl.2002.0933
- Singleton VL, Orthofer R, Lamuela-Raventos, RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 299:152–178. doi: 10.1016/S0076-6879(99)99017-1
- Stojković D, Glamočlija Ćirić A, Nikolić M, Ristić M, Šiljegović J, Soković, M. 2013. Investigation of antibacterial synergism of Origanum vulgare and Thymus vulgaris essential oils. Archives of Biological Sciences. 65:639–643. doi: 10.2298/ABS1302639S
- Tornuk F, Cankurt H, Ozturk I, Sagdic O, Bayram O, Yetim H. 2011. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella typhimurium on fresh cut carrots and apples. International Journal of Food Microbiology. 148:30–35. doi: 10.1016/j.ijfoodmicro.2011.04.022
- Ultee A, Bennik MHJ, Moezelaar R. 2002. The Phenolic Hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Applied and Environmental Microbiology. 68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002
- Viuda-Martos M, Mohamady MA, Fernández-López J, AbdElrazik KA, Omer EA, Pérez-Álvarez JA, Sendra E. 2011. In vitro antioxidant and antibacterial activities of essential oils obtained from Egyptian aromatic plants. Food Control. 22:1715–1722. doi: 10.1016/j.foodcont.2011.04.003
- Vurma M, Pandit RB, Sastry SK Yousef AE. 2009. Inactivation of Escherichia coli O157:H7 and natural microbiota on spinach leaves using gaseous ozone during vacuum cooling and simulated transportation. Journal of Food Protection. 72:1538–1546. doi: 10.4315/0362-028X-72.7.1538
- Wan J, Wilcock A, Coventry MJ. 1998. The effect of essential oils of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. Journal of Applied Microbiology. 84:152–158. doi: 10.1046/j.1365-2672.1998.00338.x
- Warriner K, Namvar A. 2013. Recent advances in fresh produce post-harvest decontamination technologies to enhance microbiological safety. Stewart Postharvest Review. 1:3. [cited 2013 Nov]. Available from: www.stewartpostharvest.com/Archive/Volume9_2013/Issue1/Warriner.pdf
- Xu W, Qu W, Huang K, Guo F, Yang J, Zhao H, Luo Y. 2007. Antibacterial effect of grapefruit seed extract on food-borne pathogens and its application in the preservation of minimally processed vegetables. Postharvest Biology and Technology. 45:126–133. doi: 10.1016/j.postharvbio.2006.11.019