Abstract
Objective: Recent evidence suggests the α2-adrenoreceptor agonist dexmedetomidine may promote metastasis of cancer cells. In this study we sought to evaluate the impact of dexmedetomidine administration on the survival of children and adolescents with cancer.
Design: Retrospective chart review.
Setting: Comprehensive cancer center.
Patients: Children and adolescents who had undergone cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis.
Intervention: Intraoperative and/or early postoperative (within 24 hours of surgery) administration of dexmedetomidine.
Measurements: Multivariable cox proportional hazard models were used to assess the association between dexmedetomidine administration and progression free survival (PFS) or overall survival (OS).
Main results: Ninety-three patients were identified. The median age was 12 years, 42% were female, and 35% received dexmedetomidine. There were no significant differences between the baseline and perioperative characteristics of patients who received dexmedetomidine and those who did not. In the multivariable analysis, the administration of dexmedetomidine was not associated with PFS (HR = 1.20, 95% CI [0.60–2.41], p = .606) or OS (HR = 0.81, 95% CI [0.35–1.85], p = .611).
Conclusion: In this retrospective study of children and adolescents who had undergone a major oncologic surgery, the intraoperative and/or early postoperative administration of dexmedetomidine was not associated with survival.
Introduction
Dexmedetomidine is a selective α2-adrenoreceptor agonist with sedative, anxiolytic and analgesic properties [Citation1]. In pediatrics, dexmedetomidine is used for procedural sedation, for sedation in the intensive care setting, or for the prophylaxis and treatment of emergence delirium after anesthesia [Citation2]. Dexmedetomidine is also used as a component of perioperative multimodal analgesia, and in children with severe pain [Citation3,Citation4].
Evidence suggests the physiologic response to surgical stress along with the choice of anesthetic agents might influence tumor progression [Citation5,Citation6]. The proposed mechanisms are complex and dependent on the type of anesthetic and cancer cells [Citation7]. With regard to dexmedetomidine, the results of animal studies suggest metastasis promoting effects in breast, lung and colon cancer cell lines [Citation8–10]. The proposed mechanisms include the direct activation of tumor α2-adrenoreceptors [Citation9], and changes in capillary diameter or permeability to tumor cells [Citation8]. Further evidence also suggests dexmedetomidine may induce microstructural changes in the tumor environment which may drive tumor cell proliferation, local invasion and metastasis [Citation11]. Clinically, the intraoperative use of dexmedetomidine has been associated with worse overall survival in adults undergoing lung cancer surgery [Citation12]. The current evidence raises concerns about the perioperative use of dexmedetomidine in patients with cancer, especially those who may require the drug intraoperatively and during the early postoperative period.
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is an extensive procedure which offers a chance for cure in patients with peritoneal tumor spread [Citation13]. In children, this procedure has been performed in patients with peritoneal tumors of various origins including desmoplastic round cell tumor, rhabdomyosarcoma and colorectal cancer [Citation14]. At our institution, dexmedetomidine is used as an anesthetic adjunct during CRS-HIPEC, and for the purposes of postoperative sedation in the intensive care unit. To date, the impact of dexmedetomidine on the survival of children who have undergone CRS-HIPEC is unclear.
The aim of this retrospective study was to evaluate the impact of the intraoperative and/or early postoperative administration of dexmedetomidine on the progression free survival (PFS) and overall survival (OS) of children and adolescents who had undergone CRS-HIPEC. Based on the results of previous studies, our hypothesis was that children who received dexmedetomidine would have worse survival compared to those who did not.
Methods
This retrospective study was approved by the Institutional Review Board (IRB) of the University of Texas MD Anderson Cancer Center (PA 16-0160). Based on the retrospective nature of the study, a waiver of informed consent was granted. After IRB approval, baseline, perioperative and survival data of patients ≤19 years of age who had undergone CRS-HIPEC between January 2006 and September 2017 were extracted from the medical records. Patients who had undergone a repeat CRS-HIPEC for disease recurrence (n = 7) were excluded from the study.
The primary outcomes of interest were PFS and OS. PFS was defined as the length of time from the date of surgery to the date of first evidence of new peritoneal disease or an increase in the size of any residual peritoneal lesions, or the date of death (whichever occurred first). OS was defined as the length of time from the date of surgery to the date of death or last follow-up date. Comparisons were made between patients who received dexmedetomidine intraoperatively and/or within 24 hours of surgery (Dex group), and those who did not receive any dexmedetomidine (no-Dex group).
Perioperative use of dexmedetomidine
The intraoperative use of dexmedetomidine was not standardized, but based on the preference or clinical judgment of the attending anesthesiologist. In cases where it was utilized, an infusion of dexmedetomidine was initiated shortly after induction, and continued until closure of the abdominal fascia. Infusion rates ranged between 0.1 and 0.7 mcg/kg/hr. In the intensive care unit, infusions of dexmedetomidine were used in extremely anxious children, in children with severe pain and in those who required postoperative ventilation. The infusion rates were similar to those administered in the operating room.
Statistical analysis
Descriptive statistics including mean, standard deviation, median and range for continuous variables were calculated. Frequency counts and percentages were calculated for categorical variables. The Fisher’s exact test or Chi-square test was used to evaluate the association between two categorical variables. The Wilcoxon rank sum test or Kruskal–Wallis test was used to evaluate the difference in a continuous variable between or among patient groups. The Kaplan–Meier method was used for time-to-event analysis including progression free survival and overall survival. The Median time to event in months with the 95% confidence interval was calculated. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups. Univariate cox proportional hazards models were fitted to evaluate the effects of continuous variables on time-to-event out comes. The variables with at least marginally significant association with PFS/OS from the univariate analysis (p value <.1) were included in the initial multivariable model. The backward selection method was used. Dexmedetomidine was introduced into the final multivariable model with adjustment of the other covariates. The Schoenfeld residual was used to check the proportional hazards assumption [Citation15]. Statistical software SAS 9.4 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for all the analyses.
Results
Ninety-three children and adolescents were identified. The median age (interquartile range [IQR]) was 12 years (8–16), 42% were female and 35% were in the Dex group. Baseline and perioperative variables were similar between the Dex and no-Dex groups ().
Table 1: Baseline and perioperative characteristics of study population.
Progression free survival
The median PFS of the entire study population was 12 months (95% confidence interval [CI], 10–22). There was no statistically significant difference between the PFS of the Dex and no-Dex groups (median: 12 months in both the Dex and no-Dex groups, p = .448), (). In the multivariable analysis, the presence of extra-abdominal disease (hazard ratio [HR] = 2.40, 95% CI [1.03–5.59], p = .043) and incomplete cytoreduction (HR = 2.49, 95% CI [1.04–6.00], p = .042) were associated with an increased risk of disease progression. The administration of dexmedetomidine was not associated with PFS (HR = 1.20, 95% CI [0.60–2.41], p = .606), ().
Figure 1: Kaplan–Meier curves showing the recurrence free survival of patients who received dexmedetomidine (Yes) and those who did not (No).
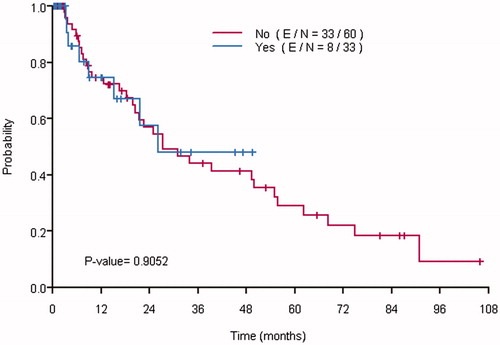
Table 2: Univariate and multivariable cox proportional hazard models for progression free survival.
Overall survival
The median OS of the entire study population was 27 months (95% CI, 22–56). There was no statistically significant difference between the OS of the Dex and no-Dex groups (median: 26 months in the Dex group, versus 27 months in the no-Dex group, p = .905), (). Similar to PFS, the multivariable analysis demonstrated an increased risk of death in patients with incomplete cytoreduction (HR = 3.46, 95% CI [1.31–9.16], p = 0.013). The administration of dexmedetomidine was not associated with OS (HR = 0.81, 95% CI [0.35–1.85], p = .611), ().
Figure 2: Kaplan–Meier curves showing the overall survival of patients who received dexmedetomidine (Yes) and those who did not (No).
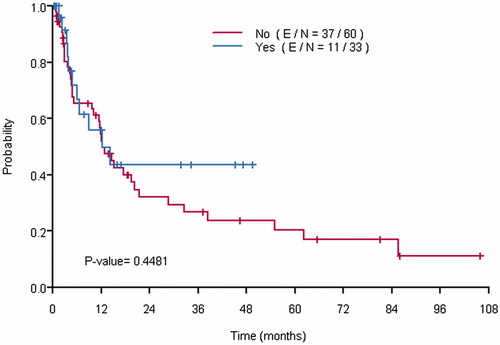
Table 3: Univariate and multivariable cox proportional hazard models for overall survival.
Discussion
In this retrospective study of children and adolescents who had undergone CRS-HIPEC, the intraoperative or early postoperative administration of dexmedetomidine was not associated with PFS or OS. To our knowledge, this is the first study evaluating the association between dexmedetomidine and the survival of children undergoing cancer surgery.
There may be several reasons why our results did not demonstrate any association between dexmedetomidine and survival. Firstly, the effects of dexmedetomidine are yet to be evaluated on the cancer cell lines typically found in our patient population. Due to the small number of patients with colorectal cancer in our study population (n = 4), we could not evaluate the impact of dexmedetomidine on the survival of this sub-group of patients. It is also worth noting that whereas dexmedetomidine has been associated with pro-tumoral effects in breast, lung and colon cancers [Citation8–10,Citation12], the results of studies in ovarian cancer cell lines suggest dexmedetomidine may inhibit the activation of chemotherapy resistance pathways, and thus improve treatment outcomes in patients with ovarian cancer [Citation16]. This finding raises the possibility that the pro-tumoral effects of dexmedetomidine may not be demonstrable in all cancer cell lines.
Secondly, the tumors reported in our study population are either of a very aggressive nature, or typically diagnosed at an advanced stage. For example, desmoplastic round cell tumors often remain asymptomatic until the tumor burden has significantly increased [Citation17]. In patients with rhabdomyosarcoma, overtly metastatic disease as seen in children presenting for CRS-HIPEC is associated with less than a 30% chance of a curative outcome [Citation18]. With regard to colon cancer, children and adolescents tend to present with very aggressive disease associated with genetic syndromes, or with more advanced stages of disease due to the lack of screening in this patient population [Citation19]. The implications of the aforementioned factors could be that any survival impact of dexmedetomidine may have been insignificant in this patient population due to the advanced stage of diseases at the time of diagnosis, or the aggressive nature of the diseases described.
Lastly, the metastasis promoting effects of dexmedetomidine in animal studies have been shown to be variable, with mixed results observed at lower doses (2.5–10 mcg/kg/hr), and consistently deleterious effects at moderate and higher doses (10–20 mcg/kg/hr) [Citation8]. It is unclear how this dosing translates to the clinical setting, but these findings suggest the metastasis promoting effects of dexmedetomidine in humans may be dose dependent. Of note, the doses of dexmedetomidine used in our patient population (0.1–0.7 mcg/kg/hr) are similar to those commonly used in the adult population [Citation20]. The observed difference in findings between ours and previous adult studies may be another example of mixed survival effects of dexmedetomidine at low doses [Citation12]. Similar to the results of previous studies, incomplete cytoreduction and the presence of extra-abdominal disease were significantly associated with worse survival in our patient population. In order to attain maximum benefit from CRS-HIPEC, aggressive tumors such as desmoplastic round cell tumor require resection of all visible tumor, or gross residual disease of less than 2.5 cm2 [Citation13,Citation21]. The presence of extra-abdominal disease often precludes a complete cytoreduction and is associated with a poorer prognosis [Citation22].
A major limitation of our study is the small sample size and the heterogeneity of diagnoses of the study population. This may have limited our ability to detect any significant differences between our study groups. Furthermore, the retrospective nature of our study means some study covariates may have been inaccurately recorded. A larger prospectively designed study may be able to overcome some of the limitations stated.
Conclusion
In this retrospective study of children and adolescents who had undergone cytoreductive surgery with hyperthermic intraperitoneal chemotherapy, the administration of dexmedetomidine was not associated with a significant impact on survival. Further studies are required to evaluate the survival impact of dexmedetomidine in children with cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268.
- Yuen VM. Dexmedetomidine: perioperative applications in children. Paediatr Anaesth. 2010;20:256–264.
- Gorges M, West N, Deyell R, et al. Dexmedetomidine and hydromorphone: a novel pain management strategy for the oncology ward setting during anti-GD2 immunotherapy for high-risk neuroblastoma in children. Pediatr Blood Cancer. 2015;62:29–34.
- Sadhasivam S, Boat A, Mahmoud M. Comparison of patient-controlled analgesia with and without dexmedetomidine following spine surgery in children. J Clin Anesth. 2009;21:493–501.
- Divatia JV, Ambulkar R. Anesthesia and cancer recurrence: what is the evidence?. J Anaesthesiol Clin Pharmacol. 2014;30:147–150.
- Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213–226.
- Kurosawa S. Anesthesia in patients with cancer disorders. Curr Opin Anaesthesiol. 2012;25:376–384.
- Lavon H, Matzner P, Benbenishty A, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120:188–196.
- Bruzzone A, Pinero CP, Castillo LF, et al. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155:494–504.
- Xia M, Ji NN, Duan ML, et al. Dexmedetomidine regulate the malignancy of breast cancer cells by activating alpha2-adrenoceptor/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20:3500–3506.
- Szpunar MJ, Burke KA, Dawes RP, et al. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila). 2013;6:1262–1272.
- Cata JP, Singh V, Lee BM, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33:317–323.
- Sugarbaker P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treatment Rev. 2016;48:42–49.
- Hayes-Jordan A, Green H, Lin H, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for children, adolescents, and young adults: the first 50 cases. Ann Sur Oncol. 2015;22:1726–1732.
- Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67:145–153.
- Cai QH, Tang Y, Fan SH, et al. In vivo effects of dexmedetomidine on immune function and tumor growth in rats with ovarian cancer through inhibiting the p38MAPK/NF-kappaB signaling pathway. Biomed Pharmacother. 2017;95:1830–1837.
- Bulbul A, Fahy BN, Xiu J, et al. Desmoplastic small round blue cell tumor: a review of treatment and potential therapeutic genomic alterations. Sarcoma. 2017;2017:1278268.
- Hettmer S, Li Z, Billin AN, et al. Rhabdomyosarcoma: current challenges and their implications for developing therapies. Cold Spring Harb Perspect Med. 2014;4:a025650.
- Oyeniyi J, Wu J, Liu D, et al. Treatment of carcinomatosis using cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in adolescents and young adults. Am J Surg. 2015;209:610–615.
- Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009;43:2064–2074.
- Hayes-Jordan A, Green HL, Lin H, et al. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann Surg Oncol. 2014;21:220–224.
- Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Oncol. 2007;14:128–133.
