Abstract
Purpose
To investigate the effect of applying stereotactic radiofrequency thermocoagulation in the anterior limbs of patients’ internal capsules in treating intractable tic disorders.
Materials and methods
Patients diagnosed with intractable tic disorders were prospectively enrolled and treated using stereotactic radiofrequency thermocoagulation in the anterior limbs of the internal capsules. Periprocedural complications, effects, and follow-up outcomes were then analyzed.
Results
Fifty patients were enrolled, including 38 with Tourette syndrome and 12 with persistent refractory vocal or motor tic disorders. The radiofrequency thermocoagulation procedure was performed successfully in all patients. Five participants (10%) experienced periprocedural complications, including one having a slight hemiplegia, two developing fevers (4%), and two developing urination disorders (4%). The participants underwent a follow-up for 12 months, with excellent effects being achieved in 23 patients (46%), prominent results in 13 (26%), good results in 10 (20%), and invalid results in 4 (8%), reaching an efficacy rate of 92% (46/50). Thirty-six patients experienced excellent and prominent effects, with no additional management after the radiofrequency ablation being needed, achieving a success rate of 72%. After radiofrequency thermocoagulation, the Yale Global Tic Severity Scale (YGTSS) scores were significantly reduced (p < .01) when compared with those before the procedure. Following this procedure, participants’ serum dopamine levels (SDA) significantly decreased (p < .05), while their serotonin levels were significantly elevated (p < .05) when compared to the measurements taken before the procedure.
Conclusion
Stereotactic radiofrequency thermocoagulation applied to the anterior limbs of patients’ internal capsules may be effective for treating intractable tic disorders, without risk of serious complications.
Introduction
As a developmental neuropsychiatric disease, Tourette syndrome is characterized by vocal and motor tics that are frequently accompanied by various behavioral comorbidities, including obsessive-compulsive disorder, impulse control problems, attention-deficit hyperactivity syndrome, and autism spectrum disorder [Citation1,Citation2]. This disease is diagnosed according to the persistent occurrence of one vocal and two motor tics, starting before the age of 18 years, and lasting longer than a year, without the presence of any other diseases [Citation3]. This disorder’s incidence is estimated as 4–6 in every 1000 children and adolescents per year, with it occurring 3–4 times more in males than females [Citation4,Citation5]. Tics are sudden, intermittent, and short movements and vocalizations preceded by a premonitory impulse or urge, with other accompanying psychiatric comorbidities including anxiety, depression, impulsivity, learning and sleeping disorders, and, in certain cases, self-injurious behaviors [Citation1,Citation6]. Most patients diagnosed with Tourette syndrome experience decreases in the frequency and severity of tics between the ages of 15 and 17 years, with approximately three quarters of patients showing significant improvements in their symptoms during early adulthood [Citation3]. Although this syndrome does not affect peoples’ intellect or cognition, it may still result in serious social and functional burdens, interfering with the normal development of both professional and schooling activities. A variety of psychoactive medications that interact with dopamine and non-dopamine systems, as well as psychoeducative interventions, have a treatment response efficacy of 30%-85% [Citation7]; however, some patients do not respond to these therapies and continue to have persistent tics, becoming treatment-intractable and severely disabled [Citation8,Citation9].
The exact pathophysiological mechanism underlying the development and occurrence of tics is not yet clear, with the associated system dysfunctions suggesting overactivities in the basal ganglia thalamo-cortical loop involving different brain networks, ranging from the mesolimbic and ventral structures to the sensory-motor dorsolateral segments of this same circuit [Citation10–12]. Stimulation of a person’s internal capsule’s anterior limb is a favored therapeutic approach for treating people with obsessive-compulsive disorder who are resistant to both medication and cognitive behavioral treatments [Citation13]. Different studies have found significant effects of deep brain stimulation in the anterior limb of the brain’s internal capsule [Citation14–17]. Sun et al. [Citation18] reported improved capsulotomy results for treating refractory Tourette syndrome, with better effects being achieved when targeting the posterior portion of the anterior limb of patients’ internal capsules as this section has greater white matter fibers projecting to the frontal cortex. As such, blocking the connection between the basal ganglia and the limbic lobes with the frontal cortex may be a more effective approach. The use of radiofrequency waves in stereotactic surgery for humans in treating diseases like epilepsy was first reported in 1965 with various positive effects [Citation19]. Even though some lesioning techniques have also been suggested, radiofrequency thermocoagulation is more popular because of the intrinsic advantages it possesses. Stereotactic radiofrequency thermocoagulation is primarily used for treating focal epilepsy, as an alternative to conventional surgical procedures [Citation20]. We hypothesized that radiofrequency thermocoagulation applied in the anterior limb of a patient’s internal capsule is more effective in treating tic disorders that are resistant to medications and psychoeducative interventions. Thus, the purpose of this research was to study the effects and safety levels of stereotactic radiofrequency thermocoagulation applied in the anterior limb of a patient’s internal capsule for the treatment of intractable tic disorders.
Materials and methods
The ethics committee of Shijiazhuang First Hospital, Hebei Medical University, approved this study, with all participating patients, or their legal guardians, providing signed informed consent. All methods were performed in accordance with the relevant guidelines and regulations. Between May 2010 and August 2016, patients with intractable tic disorders were prospectively enrolled. The inclusion criteria specified patients diagnosed with intractable tic disorders that seriously affect their daily lives, social functioning, and work, while also being unresponsive to treatments like psychotherapy or medication, without the presence of any other organic illnesses.
After admission, the patient had a physical examination, chest radiography, electrocardiography, head Computed tomography and magnetic resonance imaging (MRI) scan, and laboratory test for detection of the health conditions and possible contraindication for the procedure. Before the radiofrequency procedure, anti-tic medication was continued. The radiofrequency thermocoagulation procedure was conducted using local or general anesthesia, according to individuals’ disease severity, with general anesthesia being utilized for those with more serious tics. Each patient was initially positioned on the procedure table and fixed with a LEKSELL localizer (Elekta AB, Stockholm, Sweden) after shaving and, then, a head magnetic resonance imaging scan was applied to locate the anterior limb of the internal capsule. On top of each patient’s forehead, a twist-drill hole was made. After the dura was cauterized and cut open, an anchor bolt was fixed into the drilled hole. An Elekta electrode was then applied to the targeted region and was then fixed. Radiofrequency was produced using the Fischer Neuro N50 generator system (Fischer-Leibinger, Germany), with the radiofrequency needle indicating thermocoagulation being at the electrode tip, with a size of 1.6 mm × 3.0 mm of thermocoagulation. The bilateral anterior limbs of the internal capsule were selected for the radiofrequency thermocoagulation, with the thermocoagulation temperature being maintained at 60 °C–80 °C for 30s–50s.
The width of the internal capsule anterior limb was 2.4 mm–5.2 mm (mean 3.7 mm) on the axial plane of MRI imaging and 14.3–18.4 mm (mean 16.5 mm) on the coronal plane (). Before radiofrequency thermocoagulation, the width and length of the internal capsule anterior limb were measured for determining the volume of thermocoagulation. The width of the anterior limb on the axial plane was the cross sectional area to be destroyed, and the length on the coronal plane was the height of the volume to be destroyed. Based on the width and length of the internal capsule anterior limb, the radiofrequency needle of either 1 mm or 2 mm in diameter was chosen. If the anterior limb width was about 3 mm, a 1 mm radiofrequency needle would be chosen to result in a sphere of thermocoagulation around 3 mm in diameter. If it was over 3 mm, a 2 mm radiofrequency needle would be chosen to reach a sphere of thermocoagulation around 5 mm in diameter. The radiofrequency thermocoagulation was started from the lowest location of the anterior limb, using firstly electric stimulation to check if normal function was injured and, then, performing the thermocoagulation. One minute later, the radiofrequency needle was raised to the next location in the anterior limb for further thermocoagulation. Radiofrequency thermocoagulation was repeated 3–4 times for complete thermocoagulation of the anterior limb on one side, and then, the other side ().
Figure 1. Measurement of the width and length of the anterior limb of the internal capsule on magnetic resonance imaging (MRI). (A&B) On the axial plane of MRI, the width of the anterior limb of the internal capsule was shown. On the axial plane, the width of the anterior limb of the internal capsule was 2.4 mm–2.8 mm In one patient (A) and 3.7 mm–3.8 mm in another patient (B). (C&D) On the coronal plane of MRI, the length of the anterior limb of the internal capsule was shown. The length of the anterior limb of the internal capsule was 14.3 mm–15.2 mm in one patient (C) and 17.2 mm–18.4 mm in another patient (D).
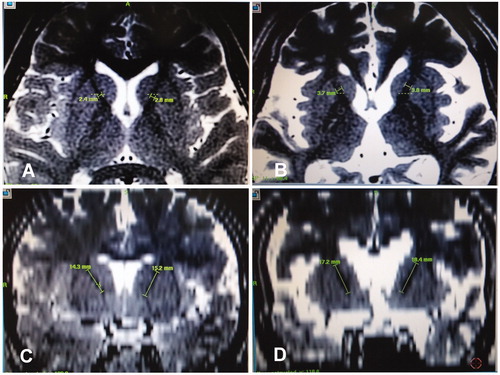
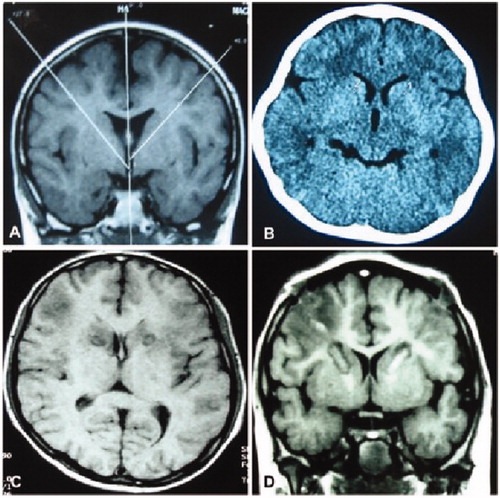
Figure 2. Radiofrequency thermocoagulation in the anterior limbs of internal capsule. (A) The angle of anterior limbs of the internal capsule was shown for location of thermocoagulation. (B) Radiofrequency thermocoagulation was performed in the anterior limbs of internal capsule. (C&D) Eight days after the thermocoagulation, the thermocoagulation lesions were shown in the anterior limbs of the internal capsules.
Immediately after the radiofrequency coagulation, a computed tomography scan was conducted in order to confirm the exact coagulation site. On the first day after radiofrequency thermocoagulation, Cefuroxime was applied once in the dose of 1.5 g, and mannitol was applied once every 12 h to prevent possible cerebral edema around the anterior limb of the internal capsule. If severe cerebral edema existed around the thermocoagulation area, Methylprednisolone was administered twice daily. Ringer’s solution of sodium acetate was administered in the dose of 1000 ml per day to increase intracranial pressure. If the intracranial pressure was within normal range, the sodium acetate solution was stopped. After operation, antipsychotic drugs were continued, and if the conditions were stable with no abnormality in the nervous and psychiatric system but with good control of the tic symptoms, the patient would be discharged home. One week later, a cerebral imaging scan was repeated to measure the coagulation effect in the targeted area. If the symptoms were in good control, the antipsychotic drugs were reduced gradually until complete withdrawal.
All patients were evaluated using the Yale Global Tic Severity Scale (YGTSS) [Citation21], with their serum dopamine (SDA) and serotonin also being measured before and after the radiofrequency ablation procedure. Clinical effects and possible complications were also recorded periprocedurally. Participants’ SDA and serotonin were measured using reversed phase high-performance liquid chromatography, with the kit provided by Donglin Scientific Development Co. Ltd (Wuxi, China), and which was used according to the manufacturer’s instructions. The venous blood of each patient was drawn and centrifuged for 10 min, at 20,000 g at 4 °C, with the serum then being used for testing. In brief, the serum samples and antigen standards were each applied to a well of a 96-well plate that was coated with primary antibodies. The biotin and enzyme conjugate reagents were added to each well, with the plate then being incubated at 37 °C for 30 min. Following this, distilled water was used to rinse each plate four times before it underwent a chromogenic reaction, with the absorbance measurement being performed at 450 nm within 15 min using a microtiter plate reader.
Clinical effects were classified into four categories: excellent, prominent, good, and invalid. Excellent effects indicated complete relief of both vocal and motor tics, as well as in daily functioning, with no need of any further therapy. Prominent effects referred to marked relief of vocal and motor tics, with no interference in routine life nor need for further therapy. Good effects meant that there was some relief in the vocal and motor tic symptoms, but with normal functioning remaining affected, thus resulting in an interference in daily life and the necessitating of further disease management. Finally, invalid results referred to neither relief nor aggravation of the vocal and motor tic symptoms. The total effectiveness rate was an indication of the total excellent, prominent, and good effects achieved.
Statistical analysis
SPSS 21.0 software (IBM, Chicago, IL, USA) was used for all statistical analyses, with continuous data presented as mean ± standard deviation (SD) and a Chi square test being applied. The p-value was set at <.05 as indicating statistical significance.
Results
Fifty participants were enrolled in the study, including 31 male and 19 female patients with an age range of 13–32 years (mean 19.4 ± 1.8) and a disease course of 6–18 years (mean 8.4 ± 1.6). Thirty-eight patients had Tourette syndrome diagnoses, while the remaining 12 had persistent refractory vocal or motor tic disorders. All patients had three rounds of formal treatment with antipsychotic medication for over two years, however, the persistent tic symptoms could not be controlled before the radiofrequency thermocoagulation. No other abnormalities were present in the patients except for the persistent tic symptom. No operation contraindication.
The radiofrequency thermocoagulation procedure was successfully performed in all patients (). Five participants (10%) had periprocedural complications, including one patient having slight hemiplegia, two developing fevers (4%), and the remaining two developing urination disorders (4%). These five patients, however, successfully recovered following appropriate management. No deaths, intracranial hemorrhage, or other complications occurred. The patients were followed up on for 12 months, with excellent effects achieved in 23 (46%), prominent in 13 (26%), good in 10 (20%), and invalid in 4 (8%), with a total efficacy rate of 92% (46/50). Thirty-six patients experienced excellent and prominent effects, with no further additional management being needed following radiofrequency ablation, resulting in a total rate of 72%. After radiofrequency thermocoagulation, the YGTSS scores significantly decreased (p < .01) when compared with those before the radiofrequency procedure (). At six months following the radiofrequency procedure, the YGTSS scores remained significantly improved (p < .01) when compared with those before the procedure, with the greatest improvement rate being found in motor tics (40%), followed by the total severity score (35%). At 12 months, the YGTSS scores remained significantly improved (p < .01), with the greatest improve rate also being shown in motor tics (35%), followed again by the total severity score (29%). After the thermocoagulation procedure, participants’ SDA significantly decreased (p < .05), while their serotonin remained significantly elevated (p < .05) when compared with the measurements taken before the procedure ().
Table 1. YGTSS scores and effective rates before and after surgery (mean ± SD).
Table 2. SDA and serotonin before and after radiofrequency thermocoagulation (mean ± SD, ng/ml).
Discussion
In this study, we investigated the effects of radiofrequency thermocoagulation applied in the anterior limb of patients’ internal capsules in the treatment of intractable tic disorders. Thirty-six patients (72%) experienced excellent and prominent effects, with no further additional management needed following the administration of radiofrequency thermocoagulation, with their YGTSS scores being significantly improved at both the six and 12 months follow-up, with no severe complications being reported in this group. The radiofrequency thermocoagulation procedure also significantly reduced (p < .05) participants’ SDA, while increasing their serotonin.
Stereotactic radiofrequency thermocoagulation became popular in the field of intracranial application in the second half of the twentieth century, possibly because it produces sharply defined lesions, performs both recording and stimulation simultaneously, and allows for the monitoring of impedance [Citation22]. Radiofrequency thermocoagulation takes advantage of the heat produced by the high radiofrequencies, allowing it to destroy local tissue. As such, it has long been used for treating drug-resistant epileptic patients who are ineligible for conventional surgical excisions of their ictal onset zones [Citation20]. In our study, we used this particular technique to destroy the anterior limb of patients’ internal capsule for the treatment of intractable tic disorders. The anterior limb of a person’s internal capsule has often been used as a target for deep brain stimulations [Citation3,Citation23,Citation24]. Sun et al. reported an improvement in capsulotomies for refractory Tourette syndrome, with the destruction of the posterior one third of the patient’s anterior limb of the internal capsule achieving a better outcome in the controlling of their tics [Citation18]. Destruction of the entire anterior limb of a person’s internal capsule achieves even better results because more white matter fibers are destroyed, which are those that project to the frontal cortex, connecting the basal ganglia, limbic lobes, and frontal cortex. On radiofrequency thermocoagulation, the whole structure of the anterior limb of the internal capsule should be destroyed. Based on the size of the anterior limb measured on the MRI imaging, personalized thermocoagulation should be performed because the size of the anterior limbs varied from person to person.
Tic disorders are a disease set involving multiple neurotransmitters within the basal ganglia-thalamo-cortical circuits [Citation25,Citation26]. Studies have found that the metabolic substances of SDA, serotonin, and gamma-amino butyric acid are all associated in the pathophysiology of this disorder [Citation27–29]. As a key monoamine neurotransmitter in the brain, dopamine plays a regulatory role in a persons’ limbic and motor functions, as well as their moods, movement, problem solving, and neurobehavioral abilities [Citation5], indicating that dopamine may be a common neurobiological pathway for the development and symptomology of tic disorders [Citation30]. Dopamine transporters are able to undergo reuptake and, subsequently, transform dopamine into homovanillic acid inside a person’s neurons, then allowing dopamine to be released into the bloodstream, meaning that its serum concentration can be used to investigate the function of central dopamine in psychiatric diseases. Serotonin is another neurotransmitter that has an inhibiting action in the brain and is deeply involved in regulating both behavior and emotion, including the inhibition of aggression [Citation31–33]. Serotonergic dysfunction is thus linked with the pathology of Tourette syndrome [Citation34]. Our study found that SDA was significantly decreased, while serotonin was significantly increased, indicating that functional changes in the brain are related to the mechanism underlying the development of tic disorders. In studying the effects of the Chinese herbal medicine Ningdong Granule on regulating the concentration of dopamine, serotonin, and gamma-amino butyric acid in patients with Tourette syndrome [Citation5], Wang et al. found that this herb functions through increasing the serum content of dopamine and gamma-amino butyric acid, while decreasing that of serotonin. This is shown in participants’ significantly decreased YGTSS scores at the 8-week follow-up, without the occurrence of either severe sequela or other complications. Their study also outlined the role that SDA and serotonin play in the development of tic disorders.
This study possesses some limitations, including the fact that was composed of one center study, it only enrolled people of Chinese ethnicity, its inherent retrospective nature, the lack of randomization, and the fact that it did not use a control except for a short-term follow-up. Future studies should aim to resolve these issues in order to provide more substantial outcomes based on the data presented here.
In conclusion, applying stereotactic radiofrequency thermocoagulation to a patient’s anterior limbs of the internal capsules may be effective in treating intractable tic disorders, without the risk of serious complications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in tourette syndrome. Front Psychiatry. 2016;7:97.
- Jankovic J. Tourette’s syndrome. N Engl J Med. 2001;345(16):1184–1192.
- Casagrande SCB, Cury RG, Alho EJL, et al. Deep brain stimulation in tourette’s syndrome: evidence to date. Neuropsychiatr Dis Treat. 2019;15:1061–1075.
- Peterson BS, Leckman JF. The temporal dynamics of tics in gilles de la tourette syndrome. Biol Psychiatry. 1998;44(12):1337–1348.
- Wang S, Qi F, Li J, et al. Effects of chinese herbal medicine ningdong granule on regulating dopamine (da)/serotonin (5-th) and gamma-amino butyric acid (gaba) in patients with tourette syndrome. Biosci Trends. 2012;6(4):212–218.
- Leckman JF. Tourette’s syndrome. Lancet. 2002;360(9345):1577–1586.
- Eddy CM, Rickards HE, Cavanna AE. Treatment strategies for tics in tourette syndrome. Ther Adv Neurol Disord. 2011;4(1):25–45.
- Freeman RD, Fast DK, Burd L, et al. An international perspective on tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42(7):436–447.
- Malaty IA, Akbar U. Updates in medical and surgical therapies for tourette syndrome. Curr Neurol Neurosci Rep. 2014;14(7):458.
- Da Cunha C, Boschen SL, Gomez AA, et al. Toward sophisticated basal ganglia neuromodulation: review on basal ganglia deep brain stimulation. Neurosci Biobehav Rev. 2015;58:186–210.
- Muller-Vahl KR, Grosskreutz J, Prell T, et al. Tics are caused by alterations in prefrontal areas, thalamus and putamen, while changes in the cingulate gyrus reflect secondary compensatory mechanisms. BMC Neurosci. 2014;15(1):6.
- Nordstrom EJ, Bittner KC, McGrath MJ, et al. Hyperglutamatergic cortico-striato-thalamo-cortical circuit” breaker drugs alleviate tics in a transgenic circuit model of tourettes syndrome. Brain Res. 2015;1629:38–53.
- Mian MK, Campos M, Sheth SA, et al. Deep brain stimulation for obsessive-compulsive disorder: past, present, and future. Neurosurg Focus. 2010;29(2):E10.
- Beszłej J, Siwicki D, Fila-Witecka K, et al. Deep brain stimulation in obsessive-compulsive disorder – case report of two patients. Psychiatr Pol. 2019;53(4):807–824.
- Gupta A, Khanna S, Jain R. Deep brain stimulation of ventral internal capsule for refractory obsessive-compulsive disorder. Indian J Psychiatry. 2019;61(5):532–536.
- Menchon JM, Real E, Alonso P, et al. A prospective international multi-center study on safety and efficacy of deep brain stimulation for resistant obsessive-compulsive disorder. Mol Psychiatry. 2019. DOI:10.1038/s41380-019-0562-6.
- Park HR, Kim IH, Kang H, et al. Electrophysiological and imaging evidence of sustained inhibition in limbic and frontal networks following deep brain stimulation for treatment refractory obsessive compulsive disorder. PLoS One. 2019;14(7):e0219578.
- Sun B, Krahl SE, Zhan S, et al. Improved capsulotomy for refractory tourette’s syndrome. Stereotact Funct Neurosurg. 2005;83(2–3):55–56.
- Schwab RS, Sweet WH, Mark VH, et al. Treatment of intractable temporal lobe epilepsy by stereotactic amygdala lesions. Trans Am Neurol Assoc. 1965;90:12–19.
- Bourdillon P, Rheims S, Catenoix H, et al. Surgical techniques: stereoelectroencephalography-guided radiofrequency-thermocoagulation (seeg-guided rf-tc). Seizure. 2019. DOI:10.1016/j.seizure.2019.01.021.
- Leckman JF, Riddle MA, Hardin MT, et al. The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573.
- Cosman ER, Jr., Cosman ER. Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6(6):405–424.
- Johnson KA, Fletcher PT, Servello D, et al. Image-based analysis and long-term clinical outcomes of deep brain stimulation for tourette syndrome: a multisite study. J Neurol Neurosurg Psychiatry. 2019;90(10):1078–1090.
- Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, et al. Deep brain stimulation for tourette syndrome: target selection. Stereotact Funct Neurosurg. 2012;90(4):213–224.
- Albin RL, Mink JW. Recent advances in tourette syndrome research. Trends Neurosci. 2006;29(3):175–182.
- Jimenez-Shahed J. Tourette syndrome. Neurol Clin. 2009;27(3):737–755. vi
- Grados MA, Walkup JT. A new gene for tourette’s syndrome: a window into causal mechanisms? Trends Genet. 2006;22(6):291–293.
- Muller-Vahl KR, Meyer GJ, Knapp WH, et al. Serotonin transporter binding in tourette syndrome. Neurosci Lett. 2005;385(2):120–125.
- Seo D, Patrick CJ, Kennealy PJ. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav. 2008;13(5):383–395.
- Singer HS. Tourette’s syndrome: from behaviour to biology. Lancet Neurol. 2005;4(3):149–159.
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289(5479):591–594.
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15(4–6):603–616.
- Yan Z. Regulation of gabaergic inhibition by serotonin signaling in prefrontal cortex: molecular mechanisms and functional implications. Mol Neurobiol. 2002;26(2–3):203–216.
- Wong DF, Brasic JR, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in tourette syndrome: clues from an in vivo neurochemistry study with pet. Neuropsychopharmacol. 2008;33(6):1239–1251.
