Abstract
Purpose
Thermal ablation (TA) is a minimally invasive treatment method for symptomatic benign thyroid nodules (BTNs). This study aimed to evaluate the value of TA by comparing the efficacy, safety, and patient satisfaction with conventional/open thyroidectomy (ConT) and endoscopic thyroidectomy (ET) for symptomatic BTNs.
Methods
Patients with symptomatic BTNs who underwent ConT, ET, or TA therapy between January 2018 and January 2020 were included. Pre-operation data of the two comparisons (TA vs. ConT and TA vs. ET) was balanced using propensity score matching. The technique efficacy (volume reduction ratio ≥50%), nodule disappearance, and regrowth rate were calculated after ablation. The operation and hospitalization time, medical cost, complications, post-operative symptoms, and cosmetic scores were recorded and compared. Patient satisfaction was evaluated using a telephone survey.
Results
After a median 19-month follow-up (range, 12–36 months), the technique efficacy rate, nodule disappearance, and regrowth rate were 93.2% (119/129), 6.8% (10/129), and 0.8% (1/129), respectively. Operation time, hospitalization time, and medical costs were less for patients in the TA group than for patients in the ConT and ET groups (all p < 0.001). The incidence of complications, post-operative symptoms, cosmetic scores, and overall satisfaction were not significantly different among groups (all p > 0.05). Post-operative hypothyroidism was less frequent in the TA group than in the ConT and ET groups (all p < 0.05).
Conclusions
Compared to ConT and ET, TA has comparable efficacy, safety, and patient satisfaction and exhibits greater protection of thyroid function for the treatment of symptomatic BTNs.
1. Introduction
Thyroid nodules are detected in 5–7% of adults by physical examination and 60–70% of adults by ultrasound [Citation1–4], with 85–93% of thyroid nodules reported to be benign [Citation2,Citation3]. Conventional/open thyroidectomy (ConT) is currently the primary treatment for symptomatic benign thyroid nodules (BTNs) [Citation2,Citation5]. However, many patients, especially young women, have concerns about permanent scarring on the neck and long-term medication with levothyroxine after surgery. Further, some patients are concerned about the risk of complications associated with surgery and general anesthesia. Thus, non-surgical, minimally invasive techniques for the treatment of BTNs are urgently needed.
Endoscopic thyroidectomy (ET) is a new alternative surgical method for the treatment of BTNs. Patients undergoing ET have been reported to achieve the superior post-operative quality of life and cosmetic and overall satisfaction with treatment compared to those undergoing ConT [Citation6]. The main advantage of ET is the absence of scarring on the neck. However, ET has several disadvantages, such as high surgical complexity and costs. In addition, laparoscopic surgery is relatively invasive and needs to be performed under general anesthesia.
In recent years, percutaneous thermal ablation (TA) has been reported as a safe and effective method for predominantly cystic or solid BTNs [Citation7–12]. Radiofrequency ablation (RFA), microwave ablation (MWA), and laser thermal ablation (LTA) are the most commonly used TA techniques for BTN therapy [Citation13–18]. Compared to ConT, ultrasound-guided TA for BTNs may be advantageous in terms of safety, cosmetic effect, shorter hospitalization time, preservation of thyroid function, and symptom improvement [Citation19–21].
To date, there has been a paucity of studies evaluating the value of TA by comparing treatment outcomes with ConT and ET. Accordingly, we hypothesized that TA may have similar efficacy and safety to ConT and ET for the treatment of patients with BTNs. In this retrospective study, we compared the efficacy and safety of TA, ConT, and ET for the treatment of BTNs and analyzed patient satisfaction with treatment.
2. Materials and methods
2.1. Study design
This retrospective study was approved by our local institutional review board (approval number: SHSY-IEC-4.1/21-366/01), and the need for informed consent from the patients was waived. All patients had provided written informed consent before the operation. All relevant clinical and treatment data were recorded prospectively.
2.2. Patients
We reviewed all consecutive patients who underwent ConT, ET, or TA therapy for symptomatic BTNs between January 2018 and January 2020. The inclusion criteria were as follows: (1) patients with pressure symptom, cosmetic problem, or discomfort in the neck; (2) patients treated with TA (RFA or MWA), ConT, or ET; (3) the nodule was confirmed as benign via fine-needle aspiration (FNA) or core-needle biopsy (CNB) before treatment and in the final resected specimens in the ConT and ET groups; (4) the maximum diameter of the nodule was ≥ 1.5 cm; (5) more than 12-month follow-up duration. The exclusion criteria were: (1) age younger than 18 years; (2) pregnancy; (3) incomplete clinical or follow-up data.
2.3. Pre-operative evaluation
Before the operation, all patients were evaluated using conventional ultrasound, color Doppler ultrasound, laboratory tests, FNA cytology, and physical examination. Contrast-enhanced ultrasound (CEUS) was performed routinely to visualize the vascularity of the nodules before ablation. The laboratory tests included the measurements of thyroid-stimulating hormone (TSH), total triiodothyronine (TT3), total thyroxine (TT4), free thyroxine (FT4), free triiodothyronine (FT3), total antithyroperoxidase antibodies (TPOAb), calcitonin concentration, routine blood examination, prothrombin time, and activated partial thromboplastin time. Patients taking anticoagulant treatment were instructed to stop taking the treatment for 7 days if anticoagulant was taken daily. Nodule volume was calculated using the ellipsoid formula (volume = length × width × depth × 0.524).
Patients were required to evaluate pressure symptoms using a 10-cm visual analog scale (0–10) [Citation22,Citation23] and cosmetic score [Citation24] before treatment. The physicians recorded the cosmetic score: (1) no palpable nodule; (2) no cosmetic problem but palpable nodule; (3) cosmetic problem only on swallowing; (4) readily detected cosmetic problem.
2.4. Ablation procedure
Each patient was placed in the supine position with mild neck extension. A venous catheter was inserted into a forearm vein before ablation. CEUS was conducted in all patients before ablation to assess the blood supply of the targeted thyroid nodules. After local anesthesia with 2% lidocaine at the puncture point, the ablation needle was inserted into the targeted nodules. The selection of RFA or MWA was based on the experience of the operator and the technical conditions of the hospital at the time of surgery. The trans-isthmic approach and moving shot technique were used according to the 2017 Korea guidelines [Citation24,Citation25]. If the nodule was adjacent to vital structures, such as the carotid artery, recurrent laryngeal nerve, trachea, or esophagus, the hydrodissection technique was typically employed to protect these essential structures during the biopsy or ablation [Citation24–26]. The ablation power was reduced or stopped for several seconds if the patient felt intolerable pain. After ablation, CEUS was performed immediately in all patients to evaluate the local treatment response for the targeted nodules. Non-enhancement in the nodule after ablation was defined as complete response, otherwise incomplete response was defined. TA has performed again for the residual portion if CEUS revealed hyper-enhanced viable tissue in the nodule. An ice-block press was used to decrease pain and prevent bleeding post-ablation. Complications were evaluated according to the clinical symptoms and ultrasound imaging during and after the procedure.
2.5. Thermal ablation and ultrasound equipment
RF generators (RF150 and RF300, Apro-Korea, Gyeonggi, Korea) and straight-type modified internally cooled electrodes (18 G) with active tip lengths of 5 or 7 mm (Well-Point RF Electrode, STARmed, Gyeonggi, Korea; CoATherm electrode, Apro-Korea, Gyeonggi, South Korea) were applied for RFA. The generator had an output of 30 W or 35 W. ECO-100 multi-functional microwave therapeutic instrument (Changcheng Microwave System Engineering Co. Ltd., Nanjing, China) and disposable microwave antennas (17 G) with active tip lengths of 3 or 5 mm were applied for MWA. For MWA, the output power setting was 30 or 35 W, the output frequency was 2450 MHz, and an internally cooled microwave antenna with normal saline for cold fluid circulation was used. All ultrasound and CEUS examinations were performed using a commercially available ultrasound system (GE Medical Systems, Milwaukee, WI, USA) equipped with an ML6-15 (frequency range, 6–15 MHz) and a 9 L (frequency range, 2–9 MHz) linear transducer for ultrasound scans and CEUS scans, respectively.
2.6. Surgical procedure
2.6.1. Conventional/open thyroidectomy
For patients undergoing ConT, general anesthesia was adopted. A pillow was placed under the shoulders with the patient in a hyperextension position, and the surgical area was routinely disinfected. A 3–5-cm transverse arc incision was made on the second transverse finger above the sternum. The surgeon cut the skin, subcutaneous tissue, and platysma layer by layer. The skin flaps were separated on the deep surface of the platysma up to the thyroid cartilage and down to the suprasternal fossa, ensuring that both sides reached the front edge of the sternocleidomastoid muscle. A high-frequency electric knife was used to incise the white line of the neck to expose the thyroid gland and remove the nodules and surrounding tissues. Following nodule removal, the incision was washed thoroughly after hemostasis, a negative pressure drainage tube was placed in the incision, and the anterior cervical muscle and subcutaneous tissue were layer-sutured sequentially. Vital signs, vocalization, and incision drainage of patients were observed closely after surgery.
2.6.2. Endoscopic thyroidectomy
For patients undergoing the transthoracic ET approach, general anesthesia was also applied. Patients were positioned in a supine position with hyperextension of the neck, and the surgical area was routinely disinfected. A 0.5–1-cm incision was made in the left and right areolae. Subsequently, a separator was inserted, and the skin flaps were separated up to the thyroid cartilage and the front edge of the sternocleidomastoid muscle on both sides. The troca was inserted, and the operation cavity was established after inflation. The white line of the neck was cut under endoscopy. The surgeon separated the neckband muscles of both sides and the true and false capsules carefully to expose the thyroid. The surgeon lifted the affected thyroid lobe and used an ultrasonic knife to remove the affected thyroid nodule and surrounding tissues carefully and completely. The tissue was extracted, and the specimens were sent for pathological examination. The operation cavity was flushed with saline, and a negative pressure drainage tube was placed in the operation cavity. The neck white thread was sutured with a thread, and the incision was closed sequentially with skin nails. Finally, the surgical incision was pressure-wrapped with a sterile dressing.
2.7. Outcome measurement
Outcomes, including efficacy, treatment-related complication, total treatment time, duration of hospitalization, total hospitalization cost, and cosmetic and symptom scores were evaluated. The total treatment time was defined as the period from the beginning to the end of the operation. After TA, technique efficacy was defined as the volume reduction ratio (VRR) ≥50% after ablation [Citation27]. Nodule regrowth was defined as an increase in nodule volume of ≥50% compared to the minimum recorded volume measured at a given follow-up time point [Citation27].
2.8. Follow-up
Post-ConT and ET, ultrasound examinations, and thyroid function tests were performed at 6-month intervals. All patients who undertook ConT and ET received oral levothyroxine once a day (dose: 50 μg/day) to maintain normal levels of thyroid-stimulating hormone, and the dose was adjusted according to their thyroid function test results. Ablation zones were evaluated at 1, 3, or 6, and 12 months and additional 6-month intervals after TA. Nodule volume, vascularity, echogenicity, symptom, cosmetic score, and thyroid function test were recorded and evaluated during the follow-up period. CEUS was conducted after ablation to evaluate the viability of the ablation zone. The VRR was calculated as VRR (%) = (initial volume − final volume)/initial volume × 100.
2.8.1. Satisfaction survey
Telephone surveys are widely used in public health research worldwide [Citation28]. A telephone survey was conducted to assess patient satisfaction in this study. All selected patients were asked the following questions: (1) is there any discomfort related to the surgery now? (2) are you taking medicine now? (such as levothyroxine); (3) have your symptoms and cosmetic problems alleviated? (4) how is your overall satisfaction with the surgery? The first question was an open question. For the second question, patients were provided two options as possible answers: yes or no. For the remaining questions, patients were provided three options as possible answers: yes, no, or partially. The script of the questionnaire was developed in consideration of ensuring that the telephone survey was concise to avoid the answers being affected by consumer fatigue and that the questions were appropriate for patients who had been treated with three vastly different approaches.
2.8.2. Statistical analysis
Statistical analyses were performed with SPSS statistical software program version 22.0 (SPSS Inc., Chicago, IL, USA) or R software (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria). Propensity score matching (PSM) was used to balance the inherent potential selection biases of two comparisons (TA group vs. ConT group and TA group vs. ET group). The propensity score generated from logistic regression represented the probability of being assigned to the TA or ConT and ET group. In the process of matching, a 1:1 nearest-neighbor matching (with age, sex, nodule size, and volume as covariates) was performed with a caliper value of 0.01. Continuous data are presented as mean ± standard deviation or median (inter-quartile range) values. Continuous variables were compared using a student’s t-test or non-parametric test (Mann–Whitney U test, Wilcoxon Signed Ranks Test). Statistical differences in categorical variables were evaluated using the Pearson Chi-square test or Mann–Whitney U test. p-Values <0.05 (two-sided) were considered statistically significant.
3. Results
3.1. Baseline characteristics
In total, 505 patients that underwent ConT (n = 320), ET (n = 56), or TA (n = 129) were included in this study. Among the 129 patients who underwent TA, 41 patients underwent RFA and 88 patients underwent MWA. presents a flowchart detailing the patient selection process. Compared with patients in the ConT group, patients in the TA group were younger, had a higher proportion of women, and had a smaller nodule size (all p < 0.01). Compared with patients in the ET group, patients in the TA group were older and had higher symptom scores (all p < 0.01). After PSM, two pairing groups (118 patients in the TA group vs. 118 patients in the ConT group; 43 patients in the TA group vs. 43 patients in the ET group) were formed (). Maximum lesion diameter and volume, sex, age, and symptom and cosmetic scores of patients were comparable in the two pairing groups ().
Figure 1. Flow chart of patient inclusion and exclusion in this study. BTNs: benign thyroid nodules; TA: thermal ablation; ConT: conventional/open thyroidectomy; ET: endoscopic thyroidectomy.
Table 1. Comparison of baseline characteristics of patients with BTNs who underwent TA and ConT therapy before and after propensity score matching.
Table 2. Comparison of baseline characteristics of patients with BTNs who underwent TA and ET therapy before and after propensity score matching.
3.2. Treatment outcomes
The complete response rate was 96.9% (125/129) based on CEUS results in 1-month post-TA (). After a median 19-month follow-up (range, 12–36 months), the mean nodule volume in the TA group (n = 129) decreased from 12.1 ± 15.3 ml (range, 0.6–118.7 ml) to 2.6 ± 6.1 ml (range, 0.0–52.1 ml) at the final follow-up point (p < 0.001), with a VRR of 80.7 ± 21.1% (range, −0.0%–100%). The technique efficacy rate was 93.2% (119/129) and 10 (6.8%, 10/129) nodules disappeared completely based on US examination (). Of the patients, 31.0% (40/129) exhibited a VRR < 75% at the final follow-up point (). Only one nodule (0.8%, 1/129) with regrowth was identified at the final follow-up point. Additional ablation was performed in three nodules (2.3%, 3/129). The median symptom score was 3 (range, 0–6) before ablation and 1 (range, 0–2) after ablation at the final follow-up point (p < 0.001). The median cosmetic score decreased from 3 (range, 2–3) to 1 (range, 1–2) after ablation at the final follow-up point (p < 0.001).
Figure 2. Ultrasound images of a 48-year-old woman with a BTN before and after RFA. (A) Before RFA, an ultrasound image reveals a solid mixed echo nodule (arrows), with a nodule volume of 7.06 mL. (B) Before RFA, the nodule shows hyper-enhancement (arrows) on a CEUS image of the early phase. (C) Ultrasound image shows insertion of the needle (short arrows) into the nodule and appearance of a hyperechoic ablation zone during RFA (long arrows). (D). Immediately after RFA, the ablation zone (arrows) shows no enhancement on CEUS images. (E) At 1 month after RFA, the volume of the ablation zone (arrows) is 4.01 mL (VRR = 43.2%). (F) At 1 month after RFA, the ablation zone (arrows) shows no enhancement on CEUS images. (G) At 3 months after RFA, an ultrasound image reveals that the volume of the ablation zone (arrows) has decreased to 2.15 mL (VRR = 69.5%). (H) At 12 months after RFA, the volume of the ablation zone (arrows) has decreased to 0.12 mL (VRR = 98.3%). BTN: benign thyroid nodule; RFA: radiofrequency ablation; VRR: volume reduction ratio; CEUS: contrast-enhanced ultrasound.
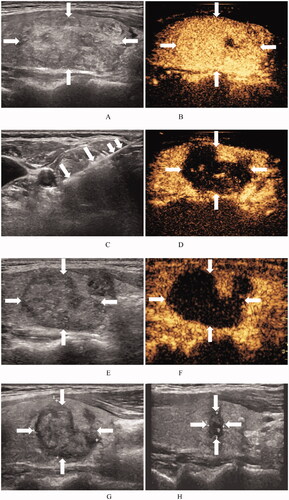
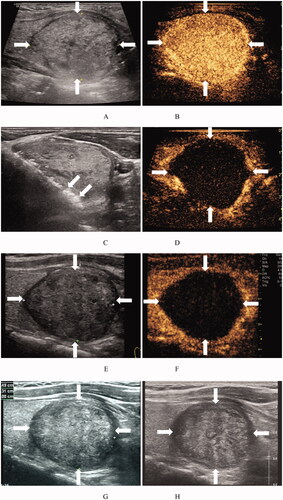
Figure 3. Ultrasound images of a 31-year-old woman with a BTN before and after MWA. (A) Before MWA, an ultrasound image reveals a solid iso-echoic nodule (arrows), with a nodule volume of 23.06 mL. (B) Before MWA, the nodule shows hyper-enhancement (arrows) on CEUS images of the early phase. (C) Ultrasound image shows insertion of the needle (arrows) into the nodule and appearance of a hyperechoic ablation zone (arrows) during MWA. (D) Immediately after MWA, the ablation zone (arrows) shows no enhancement on CEUS images. (E) At 1 month after MWA, an ultrasound image reveals that the volume of the ablation zone (arrows) has decreased to 14.93 mL (VRR = 35.3%). (F) At 1 month after RFA, the ablation zone (arrows) shows no enhancement on CEUS images. (G) At 1 year after MWA, an ultrasound image reveals that the volume of the ablation zone (arrows) is 12.16 mL (VRR = 47.2%). (H) At 1.5 years after MWA, the volume of the ablation zone (arrows) is 12.16 mL (VRR = 47.2%). BTN: benign thyroid nodule; MWA: microwave ablation; CEUS: contrast-enhanced ultrasound; VRR: volume reduction ratio.
Three patients (2.3%, 3/129) with a voice change or cough while drinking recovered within 3 months after ablation. Five patients (3.9%, 5/129) experienced hematoma during the operation, which resolved completely within 1 week. Most patients felt pain or discomfort in the neck while swallowing during and after the ablation for 1 day, but they recovered on the second day. None of the patients dropped out due to pain or discomfort during the ablation.
3.3. Thermal ablation vs. conventional/open thyroidectomy
shows the comparison of treatment outcomes between TA and ConT for BTNs after PSM. The operation time, hospital time, and total hospital cost of patients were 32.6 ± 8.8 min, 1.4 ± 0.6 days, and 2509 ± 244 US dollars, respectively, in the TA group, and 65.8 ± 10.3 min, 4.0 ± 1.0 days, 3001 ± 579 US dollars, respectively, in the ConT group (all p < 0.001). No significant differences were observed in the number of major and minor complications between the ConT group (n = 8) (6.8%, 8/118) and the TA group (n = 4) (3.4%, 4/118) (p = 0.236). Complications included voice change (n = 3), cough while drinking (n = 2), hand numbness (n = 2), and hematoma (n = 1) in the ConT group and voice change (n = 2), cough while drinking (n = 1), and hematoma (n = 1) in the TA group. Post-operative hypothyroidism was observed in 1 (0.8%, 1/118) and 30 (25.4%, 30/118) patients in the TA and ConT groups, respectively (p < 0.001). The post-operative median symptom score in the TA group (2 [range, 2–2]) was comparable to that in the thyroidectomy group (2 [range, 1.5–2]) (p = 0.826). The post-operative median cosmetic score in the TA group (1 [range, 1–1]) was also comparable to that in the ConT group (1 [range, 1–1]) (p = 0.946).
Table 3. Comparison of treatment outcomes between TA and ConT for BTNs after propensity score matching.
3.4. Thermal ablation vs. endoscopic thyroidectomy
shows the comparison of treatment outcomes between TA and ConT for BTNs after PSM. The operation time, hospital time, and total hospital cost of patients were significantly lower in the TA group than in the ET group (29.6 ± 8.0 vs. 71.9 ± 10.0 min, p < 0.001; 0.8 ± 0.6 vs. 3.6 ± 1.1 days, p < 0.001; and 2522 ± 443 vs. 3336 ± 625 US dollars, p < 0.001, respectively). No significant difference was observed in the number of major complications between the TA group (voice change, n = 1) (2.3%, 1/43) and ET group (n = 0) (0%, 0/43) (p = 1.000). However, the number of cases of post-operative hypothyroidism was significantly lower in the TA group (n = 0) (0%, 0/43) than in the ET group (n = 8) (18.6%, 8/43) (p < 0.01). No significant differences were noted in the post-operative median symptom score and cosmetic score between the TA and ET groups (1 [range, 1–2] vs. 1 [range, 0–2], p = 0.356; and 1 [range, 1–2] vs. 1 [range, 1–1], p = 0.196, respectively).
Table 4. Comparison of treatment outcomes between TA and ET for BTNs after propensity score matching.
3.5. Satisfaction survey
3.5.1. Discomfort
After at least 1-year follow-up, the number of patients who experienced post-operative discomfort was significantly lower in the TA group (5.1%, 6/118) than in the ConT group (13.6%, 16/118) (p < 0.05) (), but was similar between the TA and ET groups (4.7 vs. 2.3%, p = 1.000) (). Two patients in the TA group reported symptoms of compression, and one patient reported neck discomfort. The main discomfort in the ConT group was neck discomfort or pain, hand numbness, fatigue, and hoarseness.
Table 5. Comparison of patient satisfaction between TA and ConT for BTNs based on a telephone survey.
Table 6. Comparison of patient satisfaction between TA and ET for BTNs based on a telephone survey.
3.5.2. Symptom and cosmetic relief
The symptom relief rate in the TA group was similar to that in the ConT () and ET groups (). However, more patients in the TA group than in the ConT group were satisfied with cosmetic relief (p < 0.01) (). The main reason for dissatisfaction was a surgical scar in the neck after ConT, especially for patients with scar constitution.
3.5.3. Post-operative levothyroxine use
Post-operative levothyroxine use was noted in 0.8% of patients (1/118) after TA (), 25.4% (30/118) of patients in the ConT group (p < 0.001) (), and 18.6% (8/43) (p < 0.01) of patients in the ET group ().
3.5.4. Overall satisfaction
Overall patient satisfaction was similar among TA, ConT, and ET groups (all p > 0.05) ().
4. Discussion
As the most minimally invasive treatment method compared to ConT or ET, TA has attracted increasing attention from surgeons and patients. In this study, we performed a comprehensive comparison of efficacy, safety, and patient satisfaction between TA, ConT, and ET for treating BTNs. The results showed that the VRR of BTNs was high (80.7%) after TA, and the number of major complications was low (2.3%). Compared with ConT and ET, patients in the TA group achieved similar symptom and cosmetic relief and overall patient satisfaction.
Safety is the main consideration for decision-making regarding surgery selection. Our results indicated that the complication rate was similar between TA, ConT, and ET groups, suggesting that the TA technique is as safe as the other two surgical methods. A multicenter study reported a low complication rate of TA (3.3%) [Citation29], supporting the safety of this procedure. The main complication after TA was a temporary voice change, which was predominantly due to local anesthesia or recurrent laryngeal nerve injury. Hydrodissection technology is a key method for nerve injury prevention [Citation24,Citation25] and should be used routinely during ablation. Moreover, skilled ablation techniques and timely communication with patients during surgery are critical. Intraoperative pain is a common issue during TA. In this regard, lidocaine injection around the thyroid provides pain relief [Citation24,Citation25]. In this study, none of the patients in the TA group withdrew from treatment due to pain, indicating that the mild pain during TA surgery was tolerable by patients.
Efficacy is another key factor for the selection of treatment methods. Our results demonstrated that the complete response rate and technical efficacy were high (> 90%), and the regrowth rate was very low (0.8%). In this study, the VRR for TA was 80.7%, which was consistent with previous studies reporting VRRs of 63–85.4 and 75.8–94% at 12 months post-RFA and MWA, respectively [Citation7,Citation11,Citation30–32]. In addition, patients’ symptoms and cosmetic problems were significantly improved after TA. These findings support the effectiveness of TA. Previous guidelines have also demonstrated that all TA techniques were effective in treating symptomatic BTNs [Citation15,Citation24,Citation27,Citation33,Citation34].
To date, only a few studies have directly compared the treatment outcomes between TA and thyroidectomy for BTNs, and no studies have compared treatment efficacy between TA and ET [Citation19,Citation21,Citation35]. ConT remains the main treatment method for symptomatic BTNs [Citation21]. Although ET is a minimally invasive method, few surgeons and patients selected this approach in this study. The efficacy of TA for alleviating symptoms and cosmetic problems was similar to that of ConT and ET. However, the operation time, hospitalization time, and total hospitalization cost were lower in the TA group than in the ConT and ET groups. These results are consistent with those of previous studies that compared patients treated with RFA, MWA, HIFU, or ConT [Citation19,Citation20,Citation36–38]. The main advantage of ConT and ET is complete nodule removal. The calculation of total treatment time included pre-operative and post-operative ultrasound examination and ablation time. Accordingly, the total treatment time was longer than that in previous studies [Citation38,Citation39]. Obvious shrinkage of nodules treated with RFA, LA, or MWA has been reported at 6 months and subsequent follow-ups [Citation14,Citation17,Citation40–42]. Based on the goal of obtaining a similar treatment effect, less invasive treatment is a more suitable choice for benign nodules. As the most minimally invasive technique, TA has evident advantages, such as low surgical technique requirements, no need for general anesthesia, quick post-operative recovery, and no surgical scars on the skin.
The telephone survey results revealed that patients’ long-term overall satisfaction in the TA group was comparable to that in the ConT and ET groups. Further, in the TA group, fewer patients reported post-operative discomfort, and thyroid function achieved better protection. Thyroidectomy removes part of the thyroid tissue, whereas TA merely treats the local nodules, thus affording better protection of the patients’ thyroid tissue. Moreover, more patients reported satisfaction with cosmetic relief after TA, which was predominantly due to the absence of scars on the skin. Accordingly, TA may be the first choice for patients who prefer minimally invasive surgery, scarless skin, and long-term satisfaction after surgery.
However, several inherent flaws of TA compared to ConT and ET should be emphasized. First, the diagnosis of nodules is obtained by FNA or CNB because pathology specimens are not obtained in patients with TA therapy, which may lead to false-negative results. Second, the nodules, especially larger nodules, may not resolve completely. Indeed, only 6.8% of nodules in this study resolved completely over the 1–3 years of follow-up in this study. An incomplete response is associated with technical efficacy, VRR, and nodule regrowth [Citation43–47]. Accordingly, a complete response should be achieved as soon as possible [Citation46]. CEUS was routinely used during the operation to detect viable zones, which was effective for avoiding residual areas. Third, there is a lack of long-term evidence on efficacy and there is a higher risk of regrowth in patients with larger nodules [Citation31,Citation39,Citation48]. Studies have reported a nodule regrowth rate as high as 20–38% after RFA and LA [Citation49]. The high regrowth rate is particularly evident for larger nodules [Citation49]. However, the regrowth rate was low (0.8%) in this study. The nodule volume at baseline was smaller, and VRR was higher in this study than in previous reports [Citation46,Citation49]. Negro et al. [Citation50] suggested that a 12-month VRR < 50% was a predictive risk factor for regrowth. Moreover, a high complete response rate may help to reduce regrowth [Citation46]. Accordingly, it is necessary to obtain a high complete response and VRR during the procedure. Fourth, the size, number, and location of nodules are the main factors affecting surgery decision-making [Citation51]. A previous review suggested that large nodular goiter, multinodular thyroid disease, or deeply positioned lesion were less appropriate indications for TA [Citation52]. Moreover, TA may not be the first choice for nodules located behind the sternum [Citation53]. More evidence-based studies are warranted to verify the efficacy of TA for the treatment of multiple thyroid nodules.
There are several limitations to this study. First, this was a singer-center retrospective study that was underscored by inherent flaws, such as patient heterogeneity, operator experience, and selection bias. However, we used the PSM to reduce these limitations and achieve the best comparisons. Prospective studies in patients treated with TA or ConT/ET are needed. Second, patients’ quality of life (QoL) was not evaluated in this study, which should be evaluated in future prospective studies using the SF-36 [Citation54], thyroid disease-specific questionnaire ‘ThyPRO-39hin’ [Citation6,Citation55] or health-related QoL (SF-12) questionnaire [Citation8]. Third, two different TA approaches were used in this study, and we did not compare the differences in patient characteristics and treatment outcomes between RFA and MWA. As such, our analysis lacked homogeneous data. The number of patients in the two groups was unbalanced (RFA vs. MWA, 41 vs. 88), which may have affected the validity of the results. Previous studies have demonstrated that RFA, LTA, and MWA exhibited comparable efficacy and safety for the treatment of patients with BTNs, whereas RFA and LTA are associated with the strongest evidence-based data regarding thyroid nodule volume reduction [Citation13,Citation56,Citation57]. More randomized controlled trials are warranted to compare the treatment outcomes between these TA techniques. Fourth, as we used a telephone survey for follow-up, it was difficult to accurately establish the number of patients with permanent hypothyroidism.
In conclusion, our study demonstrates that TA is an effective and safe method for the treatment of patients with symptomatic BTNs based on VRR, symptom and cosmetic relief, and complication rates. Compared to ConT and ET, TA is associated with shorter treatment and hospitalization time, lower total hospitalization cost, better protection of thyroid function, and comparable overall patient satisfaction. Therefore, TA can be regarded as an alternative for patients with symptomatic BTNs who are unsuitable or unwilling to receive ConT and ET treatment.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Burman KD, Wartofsky L. Clinical practice. Thyroid nodules. N Engl J Med. 2015;373(24):2347–2356.
- Pemayun TG. Current diagnosis and management of thyroid nodules. Acta Med Indones. 2016;48(3):247–257.
- Wong R, Farrell SG, Grossmann M. Thyroid nodules: diagnosis and management. Med J Aust. 2018;209(2):92–98.
- Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency ablation for the management of thyroid nodules: a critical appraisal of the literature. Clin Endocrinol. 2017;87(6):639–648.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Johri G, Chand G, Mishra A, et al. Endoscopic versus conventional thyroid surgery: a comparison of quality of life, cosmetic outcomes and overall patient satisfaction with treatment. World J Surg. 2020;44(12):4118–4126.
- Deandrea M, Trimboli P, Garino F, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: a longitudinal 5-year observational study. J Clin Endocrinol Metab. 2019;104(9):3751–3756.
- Lang BH, Woo YC, Wong C. High-intensity focused ultrasound for treatment of symptomatic benign thyroid nodules: a prospective study. Radiology. 2017;284(3):897–906.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Ben Hamou A, Ghanassia E, Espiard S, et al. Safety and efficacy of thermal ablation (radiofrequency and laser): should we treat all types of thyroid nodules? Int J Hyperthermia. 2019;36(1):666–676.
- Liu YJ, Qian LX, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med. 2017;242(15):1515–1523.
- Li XL, Xu HX, Lu F, et al. Treatment efficacy and safety of ultrasound-guided percutaneous bipolar radiofrequency ablation for benign thyroid nodules. Br J Radiol. 2016;89(1059):20150858.
- Yue WW, Wang SR, Lu F, et al. Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: a propensity score matching study. Endocrine. 2017;55(2):485–495.
- Mauri G, Papini E, Bernardi S, et al. Image-guided thermal ablation in autonomously functioning thyroid nodules. A retrospective multicenter three-year follow-up study from the Italian minimally invasive treatment of the thyroid (MITT) group. Eur Radiol. 2021;32(3):1738–1746.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid2016 NODULES-2016 UPDATE. Endocr Pract. 2016;22(5):622–639.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99(10):3653–3659.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33(8):911–919.
- Guan SH, Wang H, Teng DK. Comparison of ultrasound-guided thermal ablation and conventional thyroidectomy for benign thyroid nodules: a systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):442–449.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. Am J Neuroradiol. 2015;36(7):1321–1325.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Lee JH, Kim YS, Lee D, et al. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010;34(7):1488–1493.
- Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81(5):905–910.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Xiaoyin T, Ping L, Dan C, et al. Risk assessment and hydrodissection technique for radiofrequency ablation of thyroid benign nodules. J Cancer. 2018;9(17):3058–3066.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Boland M, Sweeney MR, Scallan E, et al. Emerging advantages and drawbacks of telephone surveying in public health research in Ireland and the U.K. BMC Public Health. 2006;6:208.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Hu K, Wu J, Dong Y, et al. Comparison between ultrasound-guided percutaneous radiofrequency and microwave ablation in benign thyroid nodules. J Cancer Res Ther. 2019;15(7):1535–1540.
- Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab. 2017;61(2):173–179.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2017;33(3):295–299.
- Xu D, Ge M, Yang A, et al. Expert consensus workshop report: guidelines for thermal ablation of thyroid tumors (2019 edition). J Cancer Res Ther. 2020;16(5):960–966.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Negro R, Trimboli P. Thermal ablation for benign, non-functioning thyroid nodules: a clinical review focused on outcomes, technical remarks, and comparisons with surgery. Electromagn Biol Med. 2020;39(4):347–355.
- Dong P, Wu XL, Sui GQ, et al. The efficacy and safety of microwave ablation versus lobectomy for the treatment of benign thyroid nodules greater than 4 cm. Endocrine. 2021;71(1):113–121.
- Jin H, Fan J, Liao K, et al. A propensity score matching study between ultrasound-guided percutaneous microwave ablation and conventional thyroidectomy for benign thyroid nodules treatment. Int J Hyperthermia. 2018;35(1):232–238.
- Lang BHH, Wong CKH, Ma EPM, et al. A propensity-matched analysis of clinical outcomes between open thyroid lobectomy and high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Surgery. 2019;165(1):85–91.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicenter prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label, trial (Lara trial). Thyroid. 2020;30(6):847–856.
- Cesareo R, Manfrini S, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for thyroid nodules: 12-month results of a randomized trial (Lara II study). J Clin Endocrinol Metab. 2021;106(6):1692–1701.
- Shi YF, Zhou P, Zhao YF, et al. Microwave ablation compared with laser ablation for treating benign thyroid nodules in a propensity-score matching study. Front Endocrinol. 2019;10:874.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Cai W, Liu S, Yu X, et al. Is partial ablation appropriate for benign thyroid nodules? A retrospective study with long-term follow-up after microwave ablation. Int J Hyperthermia. 2021;38(1):923–930.
- Bernardi S, Cavallaro M, Colombin G, et al. Initial ablation ratio predicts volume reduction and retreatment after 5 years from radiofrequency ablation of benign thyroid nodules. Front Endocrinol. 2020;11:582550.
- Sim JS, Baek JH. Unresolved clinical issues in thermal ablation of benign thyroid nodules: regrowth at long-term follow-up. Korean J Radiol. 2021;22(8):1436–1440.
- Zhao CK, Xu HX, Lu F, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. CH. 2017;65(4):393–405.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. 2020;30(12):1759–1770.
- Negro R, Greco G, Deandrea M, et al. Twelve-month volume reduction ratio predicts regrowth and time to regrowth in thyroid nodules submitted to laser ablation: a 5-year follow-up retrospective study. Korean J Radiol. 2020;21(6):764–772.
- Bo XW, Lu F, Xu HX, et al. Thermal ablation of benign thyroid nodules and papillary thyroid microcarcinoma. Front Oncol. 2020;10:580431.
- Papini E, Pacella CM, Hegedus L. Diagnosis of endocrine disease: thyroid ultrasound (US) and US-assisted procedures: from the shadows into an array of applications. Eur J Endocrinol. 2014;170(4):R133–146.
- Chen AY, Bernet VJ, Carty SE, et al. American thyroid association statement on optimal surgical management of goiter. Thyroid. 2014;24(2):181–189.
- Yue WW, Wang SR, Li XL, et al. Quality of life and cost-effectiveness of radiofrequency ablation versus open surgery for benign thyroid nodules: a retrospective cohort study. Sci Rep. 2016;6:37838.
- Watt T, Hegedüs L, Groenvold M, et al. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol. 2010;162(1):161–167.
- Korkusuz Y, Gröner D, Raczynski N, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol. 2018;28(3):929–935.
- Guo DM, Chen Z, Zhai YX, et al. Comparison of radiofrequency ablation and microwave ablation for benign thyroid nodules: a systematic review and meta-analysis. Clin Endocrinol. 2021;95(1):187–196.
